Abstract
O-linked N-acetylglucosamine transferase (OGT) catalyzes O-linked glycosylation (O-GlcNAcylation). O-GlcNAcylation is a post-translational carbohydrate modification of diverse nuclear and cytosolic proteins by the addition of O-linked β-N-acetylglucosamine. It was recently demonstrated that OGT and the level of O-GlcNAcylation are upregulated in esophageal cancer; however, the physiological consequences of this upregulation remain unknown. The current study reports that OGT knockdown by short hairpin RNA (shRNA) did not affect cell viability; however, cell migration in esophageal cancer Eca-109 cells was significantly reduced. OGT-specific shRNA vectors efficiently decreased the protein and mRNA levels of OGT and the RL2 level (a marker of O-GlcNAcylation levels) in Eca-109 esophageal cancer cells. In addition, colony formation and cell proliferation assays demonstrated that OGT-specific shRNA decreased the proliferation of Eca-109 cells; however, there was no significant statistical difference between OGT-specific shRNA and control shRNA. Notably, transwell assays demonstrated that the migratory ability of Eca-109 cells was significantly suppressed following knockdown of the OGT gene. Correspondingly, western blot analyses demonstrated that OGT knockdown significantly downregulated the expression of matrix metalloproteinase 9 (MMP9) in Eca-109 cells. These results suggest that OGT may promote the migration, invasion and metastasis of esophageal cancer cells by enhancing the stability or expression of MMP9.
Keywords: polypeptide, β-N-acetylglucosaminyltransferase (OGT), matrix metalloproteinase 9, esophageal cancer, metastasis
Introduction
Esophageal cancer is one of the most common types of cancer worldwide with >480,000 new cases diagnosed annually (1,2). Globally, the disease accounts for ~400,000 cancer-associated mortalities annually (3) with a 5-year survival rate of <20% (4). According to the National Comprehensive Cancer Network guidelines (5), surgery is the optimal treatment choice, however, chemotherapy and radiotherapy may also be administered (6). Squamous cell carcinoma is one of the most common types of cancer and this reacts poorly to common chemotherapy compared with other types of cancer, such as adenocarcinoma (7). Novel studies are required to provide evidence for effective therapy on this disease. Glycolysis and the uptake of glucose are enhanced in cancer cells in order to meet the increased energy requirements of the rapidly proliferating cells, including esophageal caner cells, which are known of elevated Wurberg Effects. A fraction of the glucose in cancer cells is metabolized by the hexosamine biosynthetic pathway (HBP) (8–10). The HBP regulates enzymatic O-linked glycosylation (O-GlcNAcylation), a post-translational carbohydrate modification of diverse nuclear and cytosolic proteins by the addition of O-linked β-N-acetylglucosamine (O-GlcNAc). O-GlcNAcylation of a protein alters the protein's stability, intracellular localization and function. O-GlcNAcylation serves important roles in an array of normal biological processes, and its dysregulation is involved in a variety of human diseases, including diabetes mellitus (11,12) and various neurological disorders (13). Several GlcNAcylated tumor-associated proteins have recently been identified, including c-Myc (14) and p53 (15), each one of the most important oncogenes and tumor suppressor genes, respectively. These findings suggest that O-GlcNAcylation serves a significant role in oncogenesis and tumor progression (16–18). In our previous study, OGT and a marker of O-GlcNAcylation levels, mouse monoclonal anti-O-linked N-acetylglucosamine antibody (RL2), were observed to be upregulated in esophageal cancer (19). However, the physiological consequences of this upregulation remain to be determined.
In the current study, RNA interference (RNAi) was used to knock down OGT in esophageal cancer Eca-109 cells, and the cell proliferation and migration capabilities were subsequently assessed. The results demonstrated that cell viability was unaffected by RNAi knockdown of OGT in Eca-109 esophageal cancer cells; however, the knockdown significantly reduced cell migration and markedly decreased matrix metalloproteinase 9 (MMP9) levels in knockdown cells.
Materials and methods
Reagents
Polyclonal rabbit anti-human OGT antibody was purchased from ProteinTech Group, Inc. (Chicago, IL, USA; catalog no., 11576-2-AP) and mouse monoclonal anti-human MMP9 antibody was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA; catalog no., sc-12759). Mouse monoclonal RL2 antibody (catalog no., MA1-072) and the Invitrogen™ BLOCK-iT™ Pol II miR RNAi Expression Vector Kit (catalog no., K4935-00) were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Transwell plates were purchased from Corning Incorporated (Corning, NY, USA).
Cell culture and RNAi
The human esophageal cancer Eca-109 cell line was obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.). Cells were incubated at 37°C in an atmosphere of 5% CO2. Three shRNAs were designed with the assistance of the online RNAi software BLOCK-iT™ RNAi Designer (http://rnaidesigner.lifetechnologies.com/rnaiexpress/). The names and sequences of the three shRNA segments are listed in Table I. Eca-109 cells were transfected with an RNAi-OGT-EmGFP Vector according to the BLOCK-iT™ Pol II miR RNAi Expression Vector Kit manufacturer's instructions. Negative control cells were composed of Eca-109 cells transfected with negative control shRNA, as described in Table I.
Table I.
shRNA segment names and sequences.
| Sequence | ||
|---|---|---|
| shRNA segment | Top strand | Bottom strand |
| RNAi-OGTA | 5′-TGCTGGATGTGCCAACTCAGC | 5′-CCTGGATGTGCCAACAGCTAA |
| TAACCGTTTTGGCCACTGACTG | CCGTCAGTCAGTGGCCAAAACG | |
| ACGGTTAGCTGTTGGCACATC-3′ | GTTAGCTGAGTTGGCACATCC-3′ | |
| RNAi-OGTB | 5′-TGCTGTAGAGTAGGCATCAGC | 5′-CCTGTAGAGTAGGCAAGCAA |
| AAAGGGTTTTGGCCACTGACTG | AGGGTCAGTCAGTGGCCAAAAC | |
| ACCCTTTGCTTGCCTACTCTA-3′ | CCTTTGCTGATGCCTACTCTAC-3′ | |
| RNAi-OGTC | 5′-TGCTGAATACTGCTCAGCAAC | 5′-CCTGAATACTGCTCAAACTTC |
| TTCAGGTTTTGGCCACTGACTG | AGGTCAGTCAGTGGCCAAAACC | |
| ACCTGAAGTTTGAGCAGTATT-3′ | TGAAGTTGCTGAGCAGTATTC-3′ | |
| Negative control | 5′-GAAATGTACTGCGCGTGGAG | |
| ACGTTTTGGCCACTGACTGACG | ||
| TCTCCACGCAGTACATTT-3′ | ||
shRNA, short hairpin RNA; RNAi, RNA interference; OGT, O-linked N-acetylglucosamine transferase.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the Eca-109 cells using TRIzol reagent according to the manufacturers' instructions (Takara Bio, Otsu, Japan), and cDNA was synthesized using GoScript™ Reverse Transcription System, according to the manufacturer's instructions (Promega Corporation, Madison, WI, USA; catalog no., A5001). In total, 950 ng of total RNA from each sample was used for the synthesis of cDNA. The target gene mRNA levels relative to the GAPDH control were determined by qPCR in an Applied Biosystems™ 7900 HT Fast Real-Time PCR System (Thermo Fisher Scientific, Inc.) using the GoTaq® qPCR Master Mix (Promega Corporation; catalog no., A6001). The data were analyzed using the 2−ΔΔCq method (20). The genes of interest were OGT1, OGT2, OGT3 and MMP9. The primers were designed using BLOCK-iT™ RNAi Designer software (Invitrogen; Thermo Fisher, Inc.) and synthesized by BGI Shenzhen (Shenzhen, China). The following primer sequences were used: MMP9, forward 5′-CCTGGAGACCTGAGAACCAATCT-3′ and reverse 5′-CCACCCGAGTGTAACCATAGC-3′. PCR was performed under the following conditions: Initial denaturation at 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec, and elongation at 60°C for 30 sec. Each of the cDNA samples from each group were assessed for gene expression in duplicate. All the tests were repeated for 3 times.
Western blot
Total protein was extracted on ice from Eca-109 cells with cell lysis buffer (Cell Signaling Technology, Inc., Danvers, MA, USA). Equal amounts of protein were separated by 10% SDS-PAGE and then transferred to a polyvinylidene difluoride membrane (catalog no., P2813; Sigma-Aldrich, St. Louis, MO, USA). Membranes were blocked with 2% fat-free milk in phosphate-buffered saline (PBS) at room temperature for 1 h and then probed with primary antibodies (dilution, 1:200) overnight at 4°C in PBS containing 0.1% Tween 20 (PBST) and 1% fat-free milk. Following the overnight incubation, membranes were washed four times in PBST and then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (dilution, 1:200; catalog no., 1662408; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Signals were developed using enhanced chemiluminescence reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Densitometric analysis was performed to quantify the results using Image Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA).
Cell proliferation assay
Cells were seeded into 96-well plates at a density of 5×104/ml in 100 µl of medium, and grown for 24 h. Following transfection for 24–72 h, the CellTiter 96® AQueous One Solution Cell Proliferation Assay System (Promega Corporation) was added (20 µl/well) and incubated for 90 min. Finally, the optical densities (OD) were measured at 492 nm with a scanning microplate reader (EnSpire® Multimode Plate Reader; Perkin Elmer, Waltham, MA, USA).
Colony formation assay
A total of 200 cells were plated in a 10-cm Petri dish. Two weeks following transfection, the cells were washed with PBS, fixed with cold methanol for 15 min at −20°C, and stained with 1% crystal violet (Thermo Fisher Scientific, Inc.) in 25% methanol for 15 min. The dishes were thoroughly washed with water, and the blue colonies were counted.
Transwell chamber assay
Transwell chamber migration assays were performed using Nunc 24-well 8.0-µm pore Transwell plates (Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. Eca-109 cells were plated at a density of 5×104cells/ml in each well with RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.) free of FBS, and 500 µl culture medium containing 10% FBS was added to the bottom of the 24-well plate. Following incubation for 24 h, non-invading cells were removed from the upper surface of the membrane using a cotton-tipped swab. The invading cells were subsequently fixed in methanol for 10 min and stained with 0.1% crystal violet hydrate (Sigma-Aldrich) for 30 min. The stained cells were counted as cells per field at 10× magnification in 3 fields (CX31 microscope; Olympus Corporation, Tokyo, Japan).
Statistical analysis
All data are presented as the mean ± standard error of the mean of at least three individual experiments. Student's t-test was used to analyze the differences between groups. Statistical significance was determined using SPSS software version 13.0 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate statistically significant differences.
Results
Silencing of OGT by RNAi in Eca-109 cells
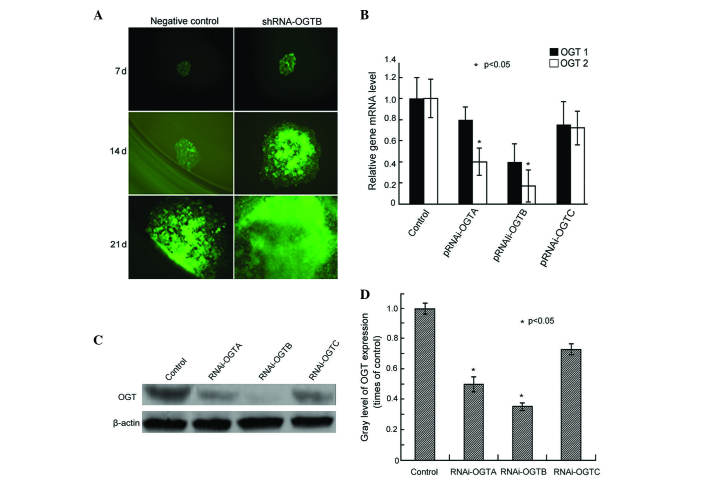
Three pairs of double-stranded OGT oligonucleotides (RNAi-OGTA, RNAi-OGTB and RNAi-OGTC) were designed and cloned into the transfection vector RNAi-OGT-EmGFP. To assess the transfection efficiency, Eca-109 cells were transfected with RNAi-OGT-EmGFP and EmGFP fluorescence was observed after 7-, 14- and 21-day incubations. After 7 days of incubation, >90% of the cells fluoresced green, and the cells remained strongly fluorescent until at least 21 days (Fig. 1A), indicating efficient transfection of the construct.
Figure 1.
Effects of transfection of RNAi-OGT in Eca-109 esophageal cancer cells. (A) Eca-109 cells were transfected with RNAi-OGT-EmGFP (RNAi-OGTA, RNAi-OGTB or RNAi-OGTC) for 7, 14 and 21 days, and EmGFP fluorescence was assessed using fluorescence microscopy. Representative fluorescent images of RNAi-OGTB-transfected cells (green) are shown. (B) Eca-109 cells were transfected with RNAi-OGTA, RNAi-OGTB or RNAi-OGTC for 72 h, and OGT mRNA levels of were determined by reverse transcription-quantitative polymerase chain reaction. The RNAi transfected cells had a significantly decreased expression of OGTA and OGTB compared with the negative control cells. OGT1, cells transfected with negative control shRNA; OGT2, cells transfected with RNAi-OGTA, RNAi-OGTB or RNAi-OGTC. (C) Eca-109 cells were transfected with RNAi-OGTA, RNAi-OGTB or RNAi-OGTC for 72 h, and OGT protein levels were determined by western blotting with β-actin as the loading control. (D) Gradation value ratio of OGT on western blotting (C) demonstrated that the RNAi transfected cells had a significantly decreased expression of OGTA and OGTAB compared with the negative control cells. Gray levels calculated following densitometric analysis of western blots. *P<0.05 vs. cells infected with negative control shRNA. RNAi, RNA interference; OGT, O-linked N-acetylglucosamine transferase.
To assess the RNAi efficiency of the RNAi-OGT constructs, the OGT mRNA levels were measured using RT-qPCR following three days of transfection. The results indicated that RNAi-OGTA, RNAi-OGTB and RNAi-OGTC decreased OGT mRNA levels compared with negative control shRNA transfected cells. Furthermore, the strongest OGT knockdown was induced by RNAi-OGTB (Fig. 1B). Consistent with OGT mRNA level alterations, western blot analysis demonstrated that OGT protein levels were also markedly downregulated by RNAi-OGTA, RNAi-OGTB and RNAi-OGTC. Once again, the strongest OGT knockdown was induced by RNAi-OGTB (Fig. 1C and D). Therefore, the subsequent functional analyses of OGT knockdown in Eca-109 cells were performed using the RNAi-OGTB construct.
OGT knockdown by RNAi decreases O-GlcNAcylation in Eca-109 cells
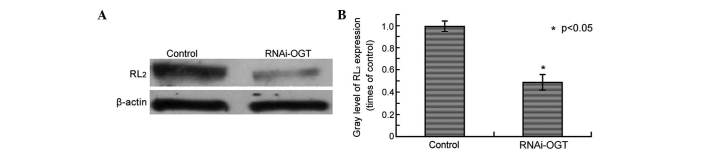
In our previous study, OGT and the O-GlcNAcylation marker RL2 were observed to be highly expressed in esophageal cancer (19). The present study aimed to determine whether RNAi silencing of OGT leads to decreased O-GlcNAcylation in esophageal cancer cells. RL2 protein levels were assessed by western blot analysis of Eca-109 cells transfected with RNAi-OGTB for 72 h, revealing that, compared with cells transfected with the control shRNA, RNAi-OGTB-transfected cells exhibited significantly decreased RL2 protein levels (P=0.002; Fig. 2).
Figure 2.
RNAi silencing of OGT resulted in decreased RL2 protein in Eca-109 cells. (A) Eca-109 cells were transfected with RNAi-OGTB for 72 h. RL2 protein levels were determined by western blotting with β-actin as the loading control. (B) Gradation value ratio of RL2 between the control and RNAi-OGT blots (A). Gray levels calculated following densitometric analysis of western blots. *P<0.001 vs. cells infected with negative control shRNA. RNAi, RNA interference; OGT, O-linked N-acetylglucosamine transferase; RL2, O-linked N-acetylglucosamine.
RNAi knockdown of OGT does not affect the viability of Eca-109 cells
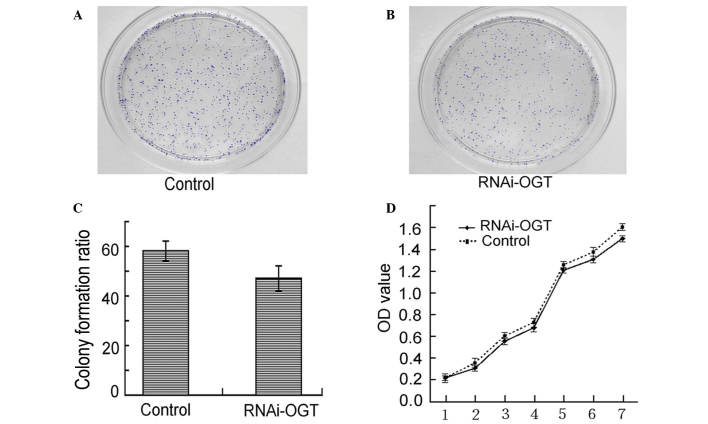
Colony formation assays demonstrated that RNAi-OGTB transfection of Eca-109 cells led to decreased colony formation. However, the observed decrease in colony formation did not reach statistical significance when compared with the negative control shRNA transfected cells (P=0.232; Fig. 3A–C). Similarly, cell proliferation assays revealed that RNAi-OGTB transfection of Eca-109 cells did not significantly inhibit cell proliferation when compared with negative control shRNA transfected cells (P=0.728; Fig. 3D). These results indicate that the RNAi knockdown of OGT had no effect on Eca-109 cell viability.
Figure 3.
Inhibition of OGT using RNAi-OGT decreased the colony formation and proliferation ability of the Eca-109 cells. Colony formation assay of the (A) control and (B) RNAi-OGT groups. (C) Colony formation ratio between the control and RNAi-OGT groups was calculated from the amount of colonies in 3 regions of 1 cm2 in size on each plate, which was compared between the two groups used Student's t test (P=0.339). (D) Cellular proliferation curves (P=0.317). RNAi, RNA interference; OGT, O-linked N-acetylglucosamine transferase; OD, optical density.
RNAi knockdown of OGT inhibits Eca-109 cell migration
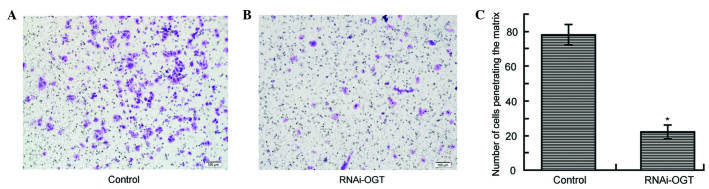
To investigate whether RNAi knockdown of OGT affects cell migration, Transwell assays were performed using Eca-109 cells transfected with RNAi-OGTB for 72 h. In comparison with the control (Fig. 4A), RNAi-OGTB transfection significantly decreased cell migration (P<0.001; Fig. 4B and C), suggesting that OGT promotes cell migration.
Figure 4.
Inhibition of OGT using RNAi-OGT decreased the cellular migratory ability of ECA-109 cells. Transwell migration assays were performed using Eca-109 cells transfected with (A) RNA-Control or (B) RNAi-OGTB for 72 h. (C) The number of cells penetrating the matrix for the control and RNAi-OGT groups, revealing a significant reduction in the RNAi-OGT group (*P=0.024 vs. cells infected with negative control shRNA). RNAi, RNA interference; OGT, O-linked N-acetylglucosamine transferase.
RNAi knockdown of OGT downregulates MMP9 expression in Eca-109 cells
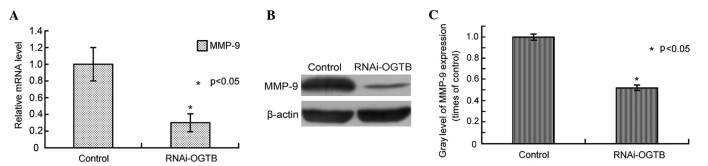
To investigate the underlying mechanism by which OGT downregulation inhibits cell migration, MMP9 mRNA and protein levels were assessed by RT-qPCR and western blot analysis, respectively, in Eca-109 cells transfected with RNAi-OGTB for 72 h. Compared to the control, RNAi-OGTB transfection significantly downregulated MMP9 mRNA levels (P<0.001; Fig. 5A). Correspondingly, western blot analysis also demonstrated the marked downregulation of MMP9 by RNAi-OGTB transfection (P=0.003; Fig. 5B and C).
Figure 5.
RNAi knockdown of OGT decreased MMP9 expression in Eca-109 cells. (A) Eca-109 cells were transfected with RNAi-OGTB for 72 h and MMP9 mRNA levels were determined by reverse transcription-polymerase chain reaction (P<0.001) (B) MMP9 protein levels were determined by western blotting, and the levels of MMP-9 expression were lower in OGT-B inhibiton group. β-Actin was the loading control. (C) Gradation value ratio of the MMP9 protein levels between the control and RNAi-OGTB blots. Gray levels calculated following densitometric analysis of western blots. *P<0.01 vs. cells infected with negative control shRNA. RNAi, RNA interference; OGT, O-linked N-acetylglucosamine transferase; MMP9, matrix metalloproteinase 9.
Discussion
In this study, OGT was successfully knocked down using RNAi in human Eca-109 esophageal cancer cells. OGT downregulation resulted in a decrease of the O-GlcNAcylation marker RL2. OGT knockdown did not significantly alter cell viability; however, it did significantly reduce Eca-109 cell migration. Moreover, MMP9 mRNA and protein expression was significantly decreased following RNAi knockdown of OGT in Eca-109 cells. These findings suggest that OGT may promote the migration, invasion and metastasis of esophageal cancer cells by enhancing the stability or expression of MMP9.
Types of protein modification include phosphorylation, ubiquitination, glycosylation, nitrosylation and O-GlcNAcylation. O-GlcNAcylation is a kind of post-translational modification that targets diverse nuclear and cytosolic proteins by the cycling of a single O-linked β-N-acetylglucosamine on the hydroxyl groups of the serine and threonine residues of target proteins (17,18,21). O-GlcNAcylation is dynamically regulated by the polypeptides OGT and β-N-acetylglucosaminidase (OGA): OGT catalyzes the addition of O-linked β-N-acetylglucosamine from uridine diphosphate N-acetylglucosamine onto the hydroxyl group of a serine or threonine residue on the target protein substrate (22), whilst OGA is a neutral hexosaminidase with a catalytic site similar to a family of 84 glycoside hydrolases that specifically catalyze the removal of β-linked GlcNAc on their substrates (23–25). O-GlcNAcylation is involved in cellular signal transduction pathways and regulates cell growth, proliferation and migration (26). Increased OGT expression and O-GlcNAcylation levels have been observed to promote tumorigenesis in numerous types of tissue, including lung, colon and breast cancers (27,28). Our group previously demonstrated that the expression of OGT was positively related to the level of O-GlcNAcylation, and that OGT expression and O-GlcNAcylation levels were increased in esophageal cancer when compared to normal esophageal tissues (19). These results led us to hypothesize that elevated OGT expression may promote the tumorigenesis and progression of esophageal cancer. To investigate this hypothesis, an RNAi knockdown of OGT in the Eca-109 human esophageal cell line was created.
The results of the present study demonstrated that neither the RNAi knockdown of OGT expression nor the decrease of O-GlcNAcylation significantly inhibited cellular proliferation. This negative result may be due to the incomplete depletion of OGT in these cells; in previous studies, complete deletion of OGT led to cell death (29,30). High levels of O-GlcNAcylation in breast cancer have recently been observed to promote metastasis, particularly to lymph nodes (31). Furthermore, in our previous report, high levels of O-GlcNAcylation were identified to be associated with lymph node metastasis in esophageal carcinoma (19). These results suggest that OGT may promote cancer cell metastasis. In accordance with this suggestion, the Transwell assay performed in the current study demonstrated that RNAi knockdown of OGT significantly reduced cell migration. Since matrix metalloproteinases (MMPs), including MMP9, serve crucial roles in cell migration (32,33), the effect of RNAi knockdown of OGT in Eca-109 cells on MMP9 expression was investigated. This revealed that MMP9 was significantly decreased in the RNAi-OGTB-transfected Eca-109 cells when compared with negative control shRNA transfected cell group, suggesting that knocking down the OGT gene in esophageal cancer may affect cellular migratory ability by decreasing MMP9, a phenomenon which may be predicted to be relative to the level of O-GlcNAcylation. Supporting this hypothesis, high OGT expression and high levels of O-GlcNAcylation have been observed to promote the metastasis of breast cancer cells (34). Also consistent with the current results, MMP9 has been reported to be involved in esophageal carcinoma metastasis (35), and high levels of O-GlcNAcylation have been associated with lymph node metastasis in esophageal carcinoma (19). However, the molecular mechanism by which O-GlcNAcylation affects MMP9 is largely unknown. O-GlcNAcylation has been reported to regulate extracellular signal-regulated kinases (ERKs) through MAPK/ERK kinases and Raf (36), and ERK regulates MMP2 and MMP9 (37,38). These facts imply a potential mechanism, in which OGT knockdown results in the downregulation of O-GlcNAcylation, which in turn results in the downregulation of MMP9 through the ERK pathway.
In summary, the current study demonstrated that RNAi is able to successfully downregulate OGT, and that OGT downregulation suppresses O-GlcNAcylation in Eca-109 cells. OGT knockdown did not affect cell viability; however, it did significantly reduce cell migration. Supporting this result, OGT knockdown was accompanied by a decrease in MMP9 expression. These findings suggest that O-GlcNAcylation may promote the migration, invasion and metastasis of esophageal cancer cells by enhancing the stability or expression of MMP9.
Acknowledgements
The present study was funded by the Scientific and Technological Research of Shaanxi Province of China (grant no. 2007K09-01), and was approved by the Ethics Review Committee of the Second Affiliated Hospital of Xi'an Jiaotong University (Xi'an, China).
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Pakzad R, Mohammadian-Hafshejani A, Khosravi B, Soltani S, Pakzad I, Mohammadian M, Salehiniya H, Momenimovahed Z. The incidence and mortality of esophageal cancer and their relationship to development in Asia. Ann Transl Med. 2016;4:29. doi: 10.3978/j.issn.2305-5839.2016.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37(Suppl 8):S4–S66. doi: 10.1016/S0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 4.van Rensburg SJ. Esophageal cancer, micronutrient malnutrition, and silica fragments. Lancet. 1982;2:1098–1099. doi: 10.1016/S0140-6736(82)90022-8. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network: NCCN Guidelines and Clinical Resources: Guidelines for Treatment of Cancer by Site. 2015 Version 7. [Google Scholar]
- 6.Brescia AA, Broderick SR, Crabtree TD, Puri V, Musick JF, Bell JM, Kreisel D, Krupnick AS, Patterson GA, Meyers BF. Adjuvant therapy for positive nodes after induction therapy and resection of esophageal cancer. Ann Thorac Surg. 2016;101:200–210. doi: 10.1016/j.athoracsur.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Ma J, Han Y, Liu J, Zhou W, Hong L, Fan D. Targeted therapy in esophageal cancer. Expert Rev Gastroenterol Hepatol. 2016 Feb 19; doi: 10.1586/17474124.2016.1140036. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 8.Zeidan Q, Hart GW. The intersections between O-GlcNAcylation and phosphorylation: Implications for multiple signaling pathways. J Cell Sci. 2010;123:13–22. doi: 10.1242/jcs.053678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 10.Copeland RJ, Bullen JW, Hart GW. Cross-talk between GlcNAcylation and phosphorylation: Roles in insulin resistance and glucose toxicity. Am J Physiol Endocrinol Metab. 2008;295:E17–E28. doi: 10.1152/ajpendo.90281.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Housley MP, Rodgers JT, Udeshi ND, Kelly TJ, Shabanowitz J, Hunt DF, Puigserver P, Hart GW. O-GlcNAc regulates FoxO activation in response to glucose. J Biol Chem. 2008;283:16283–16292. doi: 10.1074/jbc.M802240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vosseller K, Wells L, Lane MD, Hart GW. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 2002;99:5313–5318. doi: 10.1073/pnas.072072399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang JY, Gu JL, Shi JH, Liu F, Shen Q. The inhibitory effect of OGT gene expression on the level of tau phosphorylation. Prog Biochem Biophys. 2009;36:346–352. doi: 10.3724/SP.J.1206.2008.00326. [DOI] [Google Scholar]
- 14.Vervoorts J, Lüscher-Firzlaff J, Lüscher B. The ins and outs of MYC regulation by posttranslational mechanisms. J Biol Chem. 2006;281:34725–34729. doi: 10.1074/jbc.R600017200. [DOI] [PubMed] [Google Scholar]
- 15.Yang WH, Kim JE, Nam HW, Ju JW, Kim HS, Kim YS, Cho JW. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability. Nat Cell Biol. 2006;8:1074–1083. doi: 10.1038/ncb1470. [DOI] [PubMed] [Google Scholar]
- 16.Barone BB, Yeh HC, Snyder CF, Peairs KS, Stein KB, Derr RL, Wolff AC, Brancati FL. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. Jama. 2008;300:2754–2764. doi: 10.1001/jama.2008.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J Biol Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 18.Hart GW, Housley MP, Slawson C. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 2007;446:1017–1022. doi: 10.1038/nature05815. [DOI] [PubMed] [Google Scholar]
- 19.Qiao Z, Dang C, Zhou B, Li S, Zhang W, Jiang J, Zhang J, Kong R, Ma Y. O-linked N-acetylglucosamine transferase (OGT) is overexpressed and promotes O-linked protein glycosylation in esophageal squamous cell carcinoma. J Biomed Res. 2012;26:268–273. doi: 10.7555/JBR.26.20110121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Love DC, Hanover JA. The hexosamine signaling pathway: Deciphering the ‘O-GlcNAc code’. Sci STKE. 2005;2005:re13. doi: 10.1126/stke.3122005re13. [DOI] [PubMed] [Google Scholar]
- 22.Haltiwanger RS, Holt GD, Hart GW. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine: Peptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1990;265:2563–2568. [PubMed] [Google Scholar]
- 23.Dong DL, Hart GW. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol Chem. 1994;269:19321–19330. [PubMed] [Google Scholar]
- 24.Cetinbas N, Macauley MS, Stubbs KA, Drapala R, Vocadlo DJ. Identification of Asp174 and Asp175 as the key catalytic residues of human O-GlcNAcase by functional analysis of site-directed mutants. Biochemistry. 2006;45:3835–3844. doi: 10.1021/bi052370b. [DOI] [PubMed] [Google Scholar]
- 25.Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. Dynamic O-glycosylation of nuclear and cytosolic proteins: Cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 26.Rogacka D, Piwkowska A, Jankowski M, Kocbuch K, Dominiczak MH, Stepiński JK, Angielski S. Expression of GFAT1 and OGT in podocytes: Transport of glucosamine and the implications for glucose uptake into these cells. J Cell Physiol. 2010;225:577–584. doi: 10.1002/jcp.22242. [DOI] [PubMed] [Google Scholar]
- 27.Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X, Cong Q, Yu W. O-GlcNAcylation is a novel regulator of lung and colon cancer malignancy. Biochim Biophys Acta. 2011;1812:514–519. doi: 10.1016/j.bbadis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Caldwell SA, Jackson SR, Shahriari KS, Lynch TP, Sethi G, Walker S, Vosseller K, Reginato MJ. Nutrient sensor O-GlcNAc transferase regulates breast cancer tumorigenesis through targeting of the oncogenic transcription factor FoxM1. Oncogene. 2010;29:2831–2842. doi: 10.1038/onc.2010.41. [DOI] [PubMed] [Google Scholar]
- 29.Ngoh GA, Facundo HT, Zafir A, Jones SP. O-GlcNAc signaling in the cardiovascular system. Circ Res. 2010;107:171–185. doi: 10.1161/CIRCRESAHA.110.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slawson C, Copeland RJ, Hart GW. O-GlcNAc signaling: A metabolic link between diabetes and cancer? Trends Biochem Sci. 2010;35:547–555. doi: 10.1016/j.tibs.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C, Yang J, Han F, Lu X, Yu W. GlcNAcylation plays an essential role in breast cancer metastasis. Cancer Res. 2010;70:6344–6351. doi: 10.1158/0008-5472.CAN-09-1887. [DOI] [PubMed] [Google Scholar]
- 32.Akter H, Park M, Kwon OS, Song EJ, Park WS, Kang MJ. Activation of matrix metalloproteinase-9 (MMP-9) by neurotensin promotes cell invasion and migration through ERK pathway in gastric cancer. Tumour Biol. 2015;36:6053–6062. doi: 10.1007/s13277-015-3282-9. [DOI] [PubMed] [Google Scholar]
- 33.Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, Faber C, Schäfers M, Körner H, Opdenakker G, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015;10:1040–1054. doi: 10.1016/j.celrep.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 34.Slawson C, Pidala J, Potter R. Increased N-acetyl-beta-glucosaminidase activity in primary breast carcinomas corresponds to a decrease in N-acetylglucosamine containing proteins. Biochim Biophys Acta. 2001;1537:147–157. doi: 10.1016/S0925-4439(01)00067-9. [DOI] [PubMed] [Google Scholar]
- 35.Wang YZ, Wu JN, Sun RQ, Wu WX. Expression of MMP-9 and MMP-9 mRNA in esophageal carcinoma its correlation with antioncogene p53. Jiang Su Da Xue Xue Bao. 2005;2:113–116. (In Chinese) [Google Scholar]
- 36.Kneass ZT, Marchase RB. Protein O-GlcNAc modulates motility-associated signaling intermediates in neutrophils. J Biol Chem. 2005;280:14579–14585. doi: 10.1074/jbc.M414066200. [DOI] [PubMed] [Google Scholar]
- 37.Hong S, Park KK, Magae J, Ando K, Lee TS, Kwon TK, Kwak JY, Kim CH, Chang YC. Ascochlorin inhibits matrix metalloproteinase-9 expression by suppressing activator protein-1-mediated gene expression through the erk1/2 signaling pathway: Inhibitory effects of ascochlorin on the invasion of renal carcinoma cells. J Biol Chem. 2005;280:25202–25209. doi: 10.1074/jbc.M413985200. [DOI] [PubMed] [Google Scholar]
- 38.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. Faseb J. 1999;13:781–792. [PubMed] [Google Scholar]