Abstract
Aims: Methionine sulfoxide reductase B3 (MsrB3), which stereospecifically repairs methionine-R-sulfoxide, is an important Msr protein that is associated with auditory function in mammals. MsrB3 deficiency leads to profound congenital hearing loss due to the degeneration of stereociliary bundles and the apoptotic death of cochlear hair cells. In this study, we investigated a fundamental treatment strategy in an MsrB3 deficiency mouse model and confirmed the biological significance of MsrB3 in the inner ear using MsrB3 knockout (MsrB3−/−) mice. Results: We delivered a recombinant adeno-associated virus encoding the MsrB3 gene directly into the otocyst at embryonic day 12.5 using a transuterine approach. We observed hearing recovery in the treated ears of MsrB3−/− mice at postnatal day 28, and we confirmed MsrB3 mRNA and protein expression in cochlear extracts. Additionally, we demonstrated that the morphology of the stereociliary bundles in the rescued ears of MsrB3−/− mice was similar to those in MsrB3+/+ mice. Innovation: To our knowledge, this is the first study to demonstrate functional and morphological rescue of the hair cells of the inner ear in the MsrB3 deficiency mouse model of congenital genetic sensorineural hearing loss using an in utero, virus-mediated gene therapy approach. Conclusion: Our results provide insight into the role of MsrB3 in hearing function and bring us one step closer to hearing restoration as a fundamental therapy. Antioxid. Redox Signal. 24, 590–602.
Introduction
Molecular oxygen, which is used for numerous processes during aerobic metabolism in cells, is related to the generation of reactive oxygen species (ROS) (18). ROS, such as superoxide anion, hydrogen peroxide, and hydroxyl radical, can oxidize cell components, including nucleic acids, intracellular proteins, and lipids, and induce cell death (22, 45, 47). Both the polypeptide backbone and amino acid residue side chains of proteins can also be targeted (5, 47, 48), and sulfur-containing amino acids such as methionine (Met) and cysteine (Cys) are the most susceptible to oxidation by ROS (5). However, some of these modifications can be repaired by defense systems that protect against oxidative stress (23, 33, 34, 41, 44, 46). Met residues are converted to Met-S-sulfoxide and Met-R-sulfoxide by oxidative stress and are repaired by stereospecific enzymes such as Met sulfoxide reductase A (MsrA) and B (MsrB1, MsrB2, and MsrB3) (23, 26, 46, 49, 50, 53). In addition, the Met/Msr system may aid other redox systems to maintain cellular redox homeostasis (26).
Innovation.
Methionine sulfoxide reductase B3 (MsrB3) is important for the maintenance of hair cells, and the mutations in the MsrB3 gene are associated with human autosomal recessive nonsyndromic hearing loss. The present work provides the first demonstration of functional and morphological rescue of the hair cells of the inner ear in a mouse model of congenital genetic sensorineural hearing loss using an in utero, virus-mediated gene therapy approach. The results of this study provide an exciting and significant step toward the treatment of congenital hearing loss, a step that addresses the underlying cause of deafness.
Due to the physiological importance of the Met/Msr system in mammals, several studies in animal models have shown that Msr deficiency leads to various oxidative stress-related disorders, such as neurodegenerative diseases, cystic fibrosis, and aging (1, 8, 12, 24, 32, 35–37). However, until now, the congenital hearing loss that results from a genetic defect in the methionine sulfoxide reductase B3 (MSRB3) gene in humans had been considered a unique monogenic disease. Ahmed et al. reported that the MSRB3 gene is associated with human DFNB74, which is a locus for autosomal recessive nonsyndromic hearing loss (1). They identified two mutations, p.Cys89Gly and p.Arg19X, in eight Pakistani families and demonstrated that these mutations result in the loss of MSRB3 enzymatic activity (1). We previously generated an MsrB3 knockout mouse model (MsrB3−/−) and identified a profound hearing loss phenotype that was similar to that observed in hearing loss patients with the MSRB3 mutations (24). Moreover, we determined that MsrB3 is highly expressed in hair cells, which are the auditory sensory receptors that serve as the key element of sound transduction. We also showed that MsrB3 deficiency results in the degeneration of stereociliary bundles, which is then followed by hair cell apoptosis (24).
Within the inner ear, the cochlea contains the organ of Corti, which is the receptor organ for hearing. Damage to the hair cells of the human cochlea by genetic or environmental factors results in irreversible hearing loss because mammals cannot regenerate hair cells (52). The inability to regenerate hair cells causes sensorineural hearing loss (SNHL), which results from functional abnormalities in the hair cells of the organ of Corti or in auditory nerve cells and accounts for ∼90% of hearing loss cases (9–11, 13, 19, 39, 43).
Recently, several groups have reported the results of gene therapy studies that involved therapeutic gene transfer into the cochleae of rodents (2, 3, 31, 55). These studies demonstrated the potential of this therapeutic modality for the treatment of SNHL caused by genetic mutations (2, 3, 31, 55). This approach appears most promising as a means to replace nonfunctional gene products via the delivery of functional copies of the affected gene (e.g., complementary DNA; cDNA) in SNHL patients who suffer from nonsyndromic deafness with an autosomal recessive inheritance pattern. To successfully treat this category of patients via a gene therapy approach, the exogenously delivered gene should be expressed before the onset of hearing loss. Because the majority of these patients are profoundly deaf from birth, it is reasonable to choose an administration time that corresponds to early inner ear development in the embryo. Bedrosian et al. and others demonstrated that in utero gene transfer of recombinant adeno-associated virus (rAAV) vectors into the mouse otocyst at embryonic day (E) 12–12.5 was a safe and effective way of targeting sensory hair cell progenitors in the developing cochlea (4, 51). However, there have been no in vivo demonstrations that in utero gene therapy using rAAVs can rescue hearing function in an animal model with a profound, human-like SNHL phenotype.
In the present study, we applied gene therapy by administering the rAAV that expresses the MsrB3 gene into the embryonic otocyst of MsrB3−/−mice via transuterine microinjection. We examined the results of this in vivo approach on the hair cell morphology and hearing of these animals.
Results
Validation of rAAV-mediated gene delivery into the cochlea via a transuterine approach
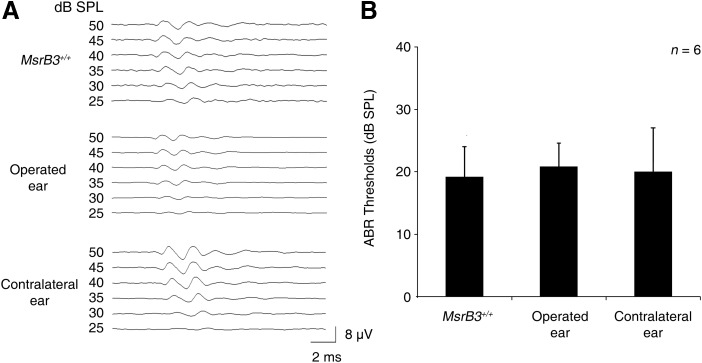
To evaluate the safety and feasibility of rAAV injection into the cochlea via a transuterine approach, we delivered rAAV2/1-GFP directly into the otocyst of MsrB3+/+ mice at E12.5 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). We then measured auditory brainstem response (ABR) thresholds in both ears of the treated mice at postnatal day (P) 28 to identify any ototoxicity inherent to the procedure. The average ABR thresholds for click stimuli were 19.2, 20.8, and 20 dB in the untreated ear, operated left ear, and contralateral right ear, respectively, of MsrB3+/+ mice (Fig. 1A). No significant differences in auditory function were observed between the operated ear and the contralateral ear or between the operated ears and the ears of MsrB3+/+ mice (n = 6 for each group, p > 0.5; Fig. 1B). Symptoms of vestibular dysfunction that could have been caused by surgical damage, such as circling and head tossing, were not observed (data not shown).
FIG. 1.
Auditory function following transuterine rAAV-mediated gene delivery in MsrB3+/+ mice. (A) Click-evoked ABR response of an untreated ear (upper), an operated ear (middle), and a contralateral ear (lower) of an MsrB3+/+ mouse at P28 after administration of the rAAV2/1-GFP treatment. The amplitude of the response is measured in microvolts (μV). The time is expressed in milliseconds (ms) and is indicated on the x-axis. (B) The average click-evoked ABR thresholds of each group are shown. No significant differences were observed between the groups (n = 6 for each group, p > 0.5). Student's t-tests were conducted for statistical comparisons. The data are shown as the mean ± SD. ABR, auditory brainstem response; GFP, green fluorescent protein; MsrB3, methionine sulfoxide reductase B3; rAAV, recombinant adeno-associated virus.
Next, we delivered rAAV2/1-MsrB3-GFP into the otocysts of MsrB3+/+ mice (Supplementary Fig. S1A) and observed strong green fluorescent protein (GFP) expression in the organ of Corti at P0 (Fig. 2A). Additionally, whole-mount immunostaining of the sensory epithelium revealed that the transduction efficiency was high, as indicated by the high level of GFP expression in >90% and 83% of the inner hair cells (IHCs) and outer hair cells (OHCs), respectively, at P28 (n = 4 for each group, Fig. 2B, C).
FIG. 2.
Expression of GFP in MsrB3+/+ mice following rAAV-mediated gene transfer into the organ of Corti. (A) Immunostaining was performed to detect rAAV-transduced cells. Fluorescence images show the organ of Corti from an MsrB3+/+ mouse that is immunostained for GFP (green) and phalloidin (red) at P0. (B) Fluorescence images show that the GFP-positive cells consisted primarily of IHCs and OHCs at P28. (C) The graph represents the transduction efficiency of IHCs and OHCs in each of the three turns, the apical, middle, and basal turns, within a 160-μm region of the organ of Corti. No significant differences were observed between the groups (p > 0.1, n = 4 for each group). Student's t-tests were conducted for statistical comparisons. The data are shown as the mean ± SD. IHC, inner hair cell; OHC, outer hair cell. Scale bars: 20 μm.
Hearing restoration in MsrB3−/− mice after delivery of rAAV2/1-MsrB3-GFP into the otocyst
Because virus-mediated gene delivery into the otocyst did not damage the cochlea and successfully induced transgene expression in hair cells, in which the MsrB3 gene is normally expressed, we investigated whether virus-mediated expression of the MsrB3 gene restored hearing function.
To determine whether virus-mediated expression of the MsrB3 gene restored hearing function, we injected rAAV2/1-MsrB3-GFP into the otocyst of MsrB3−/− mice at E12.5 using the transuterine approach. We then measured ABR thresholds when the mice were 4 weeks old (Fig. 3). The ears of untreated and rAAV2/1-GFP-treated MsrB3−/− mice did not exhibit responses to click stimuli or tone bursts, which is consistent with the previously reported phenotype of the MsrB3−/− mouse (24) (Fig. 3A, middle panels). In contrast, the treated ear that was injected with the MsrB3 gene exhibited waveforms that were typical of a normal hearing threshold and that were similar to those observed in MsrB3+/+ mice (Fig. 3A, left and right panel). At all frequencies measured, ABR thresholds differed significantly between the ears of the rAAV2/1-GFP-treated MsrB3−/− mice and those treated with rAAV2/1-MsrB3-GFP, whereas at 16 and 32 kHz, there was a slight distinction between the ears of the MsrB3+/+ mice and those of MsrB3−/− mice that were treated with rAAV2/1-MsrB3-GFP (n = 5 for each group, *p < 0.05; Fig. 3B).
FIG. 3.
Restoration of auditory function following rAAV2/1-mediated MsrB3 gene transfer into the otocysts of MsrB−/− mice. (A) Example of click-evoked ABR response of a post-treatment MsrB3−/− mouse ear compared with that of an MsrB3+/+ mouse ear at P28. The waveform of the rAAV2/1-MsrB3-GFP-treated ear was changed after treatment. (B) Graphical representation of the ABR data that show the average ABR thresholds for click and tone burst (4, 8, 16, and 32 kHz) stimuli of the five rescued MsrB3−/− mice. Asterisks indicate statistical differences between the ears of the MsrB3+/+ mice and the rAAV2/1-MsrB3-GFP-treated ears of the MsrB3−/− mice (*p < 0.05). p-Values were determined by Student's t-tests. The data are shown as the mean ± SD. n = 5 for each group.
Then, we determined the duration of the hearing recovery in the ears of the rescued MsrB3−/− mice. In all of the rescued mice, the improved hearing performance was maintained for at least 4 weeks after the treatment. However, hearing thresholds began to deteriorate beginning with the high frequencies at ∼7 weeks of age, and the reduced hearing function remained at 8 weeks after treatment (Supplementary Fig. S2A).
Exogenous expression of MsrB3 in the inner ear in mice that were injected with rAAV2/1-MsrB3-GFP into the otocyst
To investigate whether the hearing restoration resulted from the delivery of the MsrB3 gene, we next performed histological analyses of the rescued MsrB3−/− mice (Fig. 4). Whole-mount immunostaining of the ears of MsrB3−/− mice that were treated with rAAV2/1-MsrB3-GFP was performed at P28 and revealed robust GFP expression in the cells of the organ of Corti (Fig. 4A). The GFP signal was strong in the IHCs and OHCs along the cochlear turns, and the average number of GFP-positive IHCs and OHCs was in the range of 89–91% and 84–92%, respectively (n = 4 for each group, Fig. 4B), indicating a similar level of transduction efficiency as that observed in the rAAV2/1-MsrB3-GFP-injected MsrB3+/+ mice.
FIG. 4.
Localization and transduction efficiency of MsrB3 and GFP following gene transfer. (A) An anti-GFP antibody was used to label the organ of Corti of the treated left ears of 28-day-old MsrB3−/− mice, and F-actin was labeled using Alexa 555-conjugated phalloidin. The tissue samples were then imaged with a fluorescence microscope. The low-magnification images show the distribution of GFP expression following the delivery of rAAV2/1-MsrB3-GFP into the otocysts of MsrB3−/− mice (upper panel). The high-magnification images show that GFP (green) was detected in both the IHCs and OHCs (lower panel) in the rescued MsrB3−/− mice. (B) Graphical representation of the percentage of GFP-positive cells that were transfected with rAAV2/1-MsrB3-GFP within a 160-μm region of IHCs and OHCs in each of the three turns, including the apical, middle, and basal turn. No significant differences were observed between the groups (p > 0.05, n = 4 for each group). p-Values were determined by Student's t-tests. The data are shown as the mean ± SD. (C) MsrB3 and GFP were specifically expressed in both types of hair cells within the cochlea (left panel). The higher-resolution images show that MsrB3 and GFP were colocalized in both the IHCs and OHCs in the organ of Corti (right panel). The scale bars represent 25 μm in (A), 100 μm in (C, left panel), and 20 μm in (C, right panel).
Then, we confirmed expression of the MsrB3 and of GFP in the ears of the rescued MsrB3−/− mice because the expression of these genes was driven by individual cytomegalovirus (CMV) promoters within the same rAAV2/1 backbone. As shown in Figure 4C, expression of the MsrB3 and of GFP was specifically observed in both types of hair cells within the cochlea. The expression was also largely overlapping within the IHCs and OHCs, indicating successful rAAV-mediated transgene delivery into the hair cells of the rescued MsrB3−/− mice.
We also used reverse transcription–polymerase chain reaction (RT-PCR) to examine the mRNA expression levels of the transduced MsrB3 gene in the ears of MsrB3−/− mice that were treated with rAAV2/1-MsrB3-GFP (Fig. 5A). Compared with rAAV2/1-GFP-treated ears and the untreated ears of MsrB3−/− mice, the ears of the MsrB3−/− mice that were treated with rAAV2/1-MsrB3-GFP exhibited some MsrB3 expression, but the expression level was lower than that observed in MsrB3+/+ mice. This result confirmed that the MsrB3 gene was introduced into the cochlea and properly expressed in the MsrB3−/− mice and that this expression was associated with the restoration of hearing function.
FIG. 5.
Quantification of exogenous MsrB3 expression levels using RT-PCR and Western blot analysis. (A) RT-PCR analysis of MsrB3 expression, which confirmed the success of rAAV-mediated gene transfer. Total RNA was extracted from the inner ears of MsrB3+/+ and rescued MsrB3−/− mice at P28. MsrB3 mRNA was found in the ears of the MsrB3+/+ mice and MsrB3+/− mice and in the rAAV2/1-MsrB3-GFP-treated ears of the MsrB3−/− mice. Gapdh served as an internal control. (B) Western blot analysis of inner ears extracted from MsrB3+/+ mice, MsrB3+/− mice, the rescued ears of MsrB3−/− mice, and the untreated ears of MsrB3−/− mice at P28. β-actin was used as a quantitative loading control. MsrB3 protein was also detected in the rescued ears of the MsrB3−/− mice. (C) Graphical representation of the relative MsrB3 levels, which were normalized to β-actin, in the ears of MsrB3+/− mice and in the rAAV2/1-MsrB3-GFP-treated and untreated ears of MsrB3−/− mice compared with those in the ears of MsrB3+/+ mice. Asterisks denote significant differences between the rAAV2/1-MsrB3-GFP-treated ears of the MsrB3−/− mice and the untreated ears of MsrB3+/− or MsrB3−/− mice (n = 4 for each group, *p < 0.001). p-Values were determined by Student's t-tests. The data are shown as the mean ± SD. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; RT-PCR, reverse transcription–polymerase chain reaction.
To quantify the level of MsrB3 protein that was produced following delivery of the viral vector, we performed a Western blot analysis of inner ear protein extracts from MsrB3+/+ mice, MsrB3+/− mice, and MsrB3−/− mice that were treated with rAAV2/1-MsrB3-GFP and untreated MsrB3−/− mice (Fig. 5B). The level of MsrB3 protein in the inner ears of the MsrB3+/− mice was 43 ± 4.2% of the level observed in the MsrB3+/+ mice. Moreover, MsrB3 was not expressed in the inner ears of the untreated MsrB3−/− mice (Fig. 5C). Strikingly, the level of MsrB3 protein expression in the inner ears of the rescued MsrB3−/− mice was found to be 15 ± 1.6% of that observed in the MsrB3+/+ mice.
Taken together, our results demonstrate that delivery of the MsrB3 gene via transuterine injection of a viral vector successfully produced stable expression of the transgene in the targeted area (i.e., hair cells at an early developmental stage) and that this expression ultimately rescued the hearing of the MsrB3−/− mice.
Localization of the exogenous MsrB3 gene within the organ of Corti of the rescued MsrB3−/− mice
To compare the distribution of exogenous MsrB3 delivered by rAAV2/1 with that of endogenous MsrB3, at P28, we performed immunohistochemistry on the ears of MsrB3+/+ mice as well as on the ears of untreated MsrB3−/− mice, MsrB3−/− mice that were treated with rAAV2/1-GFP, and MsrB3−/− mice that were treated with rAAV2/1-MsrB3-GFP (Fig. 6). We immunohistochemically detected Myo7a using a green label. The GFP signal was not confused with the green label for Myo7a because following AAV viral infection, the GFP signal was not detected in paraffin-embedded sections without antibody enhancement even though GFP was coexpressed with MsrB3. MsrB3 expression was localized in both IHCs and OHCs and was colocalized with Myo7a expression in the ears of the MsrB3−/− mice that were treated with rAAV2/1-MsrB3-GFP; this pattern was similar to that observed in the ears of the MsrB3+/+ mice (Fig. 6A–C, J–L), although endogenous MsrB3 is more broadly expressed, including from the inner sulcus cells to the outer sulcus cells (24). MsrB3 expression was not observed in the untreated ears or rAAV2/1-GFP-treated ears of MsrB3−/− mice (Fig. 6D–I). These results demonstrate that delivery of the exogenous MsrB3 gene at E12.5 successfully targeted expression to both the IHCs and OHCs of the organ of Corti in the ears of the rescued MsrB3−/− mice that were treated with rAAV2/1-MsrB3-GFP.
FIG. 6.
Immunohistochemical analysis of MsrB3 expression in cochlear sections. Immunohistochemistry was performed on serially sliced inner ear samples from the ears of MsrB3+/+ mice and from the untreated, rAAV2/1-GFP-treated, and rAAV2/1-MsrB3-GFP-treated ears of MsrB3−/− mice at P28. The expression of MsrB3 protein (red) and Myo7a (green) was assessed in MsrB3+/+ mice (A–C). No MsrB3 expression was found in the untreated ears or rAAV2/1-GFP-treated ears of the MsrB3−/− mice (D–I). In contrast, the rescued ears of the MsrB3−/− mice exhibited local expression of exogenous MsrB3 in the IHCs and OHCs, and the expression of exogenous MsrB3 overlapped with that of Myo7a (J–L). Scale bars represent 10 μm.
Ultrastructure of stereociliary bundles in the IHCs and OHCs of the rescued MsrB3−/− mice
In a previous study, we demonstrated that MsrB3−/−mice exhibit progressive degeneration of stereociliary bundles starting at P8 and that this degeneration is followed by a loss of hair cells that results in profound deafness (24). Therefore, we assessed morphological rescue of the stereociliary bundles in the ears of the rescued MsrB3−/− mice at the ultrastructural level using scanning electron microscopy (SEM; Fig. 7). In the organ of Corti of 28-day-old MsrB3+/+ mice, the stereociliary bundles of the IHCs exhibited a normal approximately linear shape, and those of the OHCs exhibited a V-like shape, consistent with our previous results (Fig. 7A, left column). Degeneration of the stereociliary bundles of both OHCs and IHCs was observable at P28 in the ears of MsrB3−/− mice compared with those of MsrB3+/+ mice (Fig. 7A, middle column). The stereociliary bundles in the ears of the rescued MsrB3−/− mice, in which the MsrB3 gene was delivered into the otocyst, exhibited OHC and IHC morphologies that were similar to those of the MsrB3+/+ mice (Fig. 7A, right column). Previous studies have reported that more severe hair cell degeneration appears in the apical turn than in the middle and basal turns in MsrB3−/− mice. Magnified views of the stereociliary bundles indicated that the structure of OHCs and IHCs was similar throughout all cochlear turns in the ears of the rescued MsrB3−/− mice, similar to the MsrB3+/+ mice (Fig. 7B). However, at 5 weeks of age, stereociliary bundle degeneration was observed in all of the cochlear turns, and this degeneration was more severe at 7 weeks of age (Supplementary Fig. S2B). This observation correlated with the progressive loss of hearing ability in the rescued ears of the MsrB3−/− mice (Supplementary Fig. S2). Overall, our results indicate that hearing function recovered due to restoration of the stereociliary bundles of hair cells in the treated ears of the rescued MsrB3−/− mice until 4 weeks of age and that both hearing ability and the morphological integrity of the stereociliary bundles deteriorated after 4 weeks of age.
FIG. 7.
Ultrastructural morphology of the stereociliary bundles. MsrB3+/+, MsrB3−/−, and rescued MsrB3−/− mice were sacrificed at P28 for scanning electron microscopy analysis. The images show stereociliary bundles along the three cochlear turns, including the apical, middle, and basal turn. (A) Overview image of a large portion of the organ of Corti that shows the stereociliary bundles of the three rows of OHCs and one row of IHCs in the ears of MsrB3+/+ (left column) mice, the ears of MsrB3−/− mice (middle column), and the ears of rescued MsrB3−/− mice (right column). (B) Representative higher-resolution images of the OHCs (left panel) and IHCs (right panel). IHC, inner hair cell; OHC, outer hair cell. The scale bars represent 10 μm in (A) and 1 μm in (B).
Discussion
SNHL is the most common type of hearing loss in humans, and in >50% of all newborns with SNHL, the hearing loss is caused by genetic factors. However, there are no fundamental therapies to correct the underlying cellular deficit in these cases. To date, the hearing loss is managed by providing patients with appropriate amplification systems, such as hearing aids or cochlear implants. Currently, cochlear implantation (CI) is the only effective treatment option for profound deafness (10, 30, 31, 40). However, CI has many drawbacks, including the surgical burden, the variable auditory outcome, and the inherent problems of implantable devices.
Advances in gene therapy that would enable the regeneration of hair cells or the restoration of auditory function have been considered as a promising way to improve therapies for hearing restoration in a clinical setting (www.wiley.co.uk/genmed/clinical); nevertheless, few studies have reported positive gene therapy results for hearing loss (2, 31). Miwa et al. reported that supplemental expression of the connexin 30 (Cx30)-encoding gene via its electroporation into the otocyst during the embryonic stage could ameliorate deafness caused by the loss of Cx30 expression (31). However, in practice, critical obstacles, such as surgical damage and low expression efficiency, preclude clinical application of electroporation-based gene transfer to the otocyst. Akil et al. demonstrated the restoration of hearing in vesicular glutamate transporter 3 (Vglut3) knockout mice after reinsertion of the Vglut3 gene using rAAV-mediated transfer during the postnatal period, suggesting a potential treatment option for genetic forms of hearing loss (2). However, newborns are able to hear, indicating that the functional and anatomical development of the human ear is largely complete at birth. Therefore, treatments for congenital hearing loss in humans should ideally be targeted to the embryonic period.
In this study, we demonstrated that delivery of rAAV2/1-GFP into the otocyst at E12.5 led to high transduction efficiency, as demonstrated by GFP expression, in cells in the organ of Corti, including IHCs, OHCs, and supporting cells, similar to previous reports (20, 21). Therefore, rAAV2/1-mediated gene delivery could be a useful therapeutic approach for hearing loss that results directly from hair cell defects.
We also showed that a single transuterine injection of rAAV2/1-MsrB3-GFP was sufficient to restore hearing in MsrB3−/− mice based on ABR and histological studies. The improvement of ABR thresholds that was observed in the rAAV2/1-MsrB3-GFP-treated ears compared with the untreated ears of the MsrB3−/− mice persisted for at least 4 weeks after birth. This functional recovery correlated with the levels of exogenous MsrB3 transcript and protein, as demonstrated by RT-PCR and Western blot analyses, respectively. Interestingly, the level of exogenous MsrB3 protein in the rescued ears of the MsrB3−/− mice was only ∼15% that observed in the ears of the MsrB3+/+ mice, but this level was sufficient to rescue hearing function. These results are consistent with the results of a previous rAAV-mediated gene therapy study that demonstrated that ∼15% of the normal level of phosphodiesterase expression was sufficient to prevent retinal degeneration in mice (38). Additionally, Lee et al. reported that heterozygous or homozygous patients who harbored the c.919-2A>G splice mutation in the SLC26A4 gene exhibited better residual hearing compared with those who carried the missense mutation, p.H723R, which is the most common cause of hearing loss (28). A residual level of normal mRNA (6–17%) is thought to be the underlying cause of the better hearing abilities of patients with splice mutations compared with missense mutations. Together, these results suggest that a low level of gene expression is sufficient to restore some degree of function in recessive hereditary disorders that are characterized by loss-of-function pathogenicity.
We also observed that the morphology of the stereociliary bundles in the rescued ears was close to normal and that this largely normal morphology was maintained at P28. Because we previously demonstrated that the stereociliary bundles of hair cells in the apical turn were more severely degenerated than those in the other turns in MsrB3−/− mice, we assessed the extent of the rescue of the stereociliary bundles along the cochlear turns in the rescued ears. Interestingly, the stereociliary bundles of the hair cells in all of the cochlear turns of the rescued ears of the MsrB3−/− mice were shaped normally and appeared similar to those in the MsrB3+/+ mice. Additionally, we consistently observed the preferential expression of rAAV2/1 toward the cochlear apical turn, although all turns exhibited high transduction efficiency. This result suggests that elevated expression of exogenous MsrB3 in the cochlear apical turn may have further supported the formation of normal stereociliary bundles, and this expression pattern could explain why threshold improvements for low frequencies persisted longer than those for high frequencies in the rescued ears of the MsrB3−/− mice.
In a previous report, we identified that MsrB3 is important for the maintenance of hair cells rather than for their differentiation or development, suggesting that the degeneration of stereociliary bundles in hair cells is the primary cause of the hearing loss that resulted from the absence of MsrB3 enzymatic activity in the MsrB3−/− mice (24). Although the precise pathogenic mechanism by which MsrB3 deficiency results in hearing loss and the specific target proteins that are reduced by the MsrB3 enzyme have not yet been identified, we speculate that the accumulation of oxidative stress is not the major cause of MsrB3 deficiency-induced hair cell degeneration; instead, the failure of redox regulation of specific target proteins in the hair cell is a primary factor as demonstrated by the absence of significant differences in MsrB enzyme activity and carbonylated protein levels between the inner ears of MsrB3−/− and MsrB3+/+ mice. Additionally, the MsrB3−/− mice showed more severe hair cell defects in the apical turn of the cochlea. This pattern of defects is in contrast with the general characteristics of oxidative stress-mediated hearing loss, which predominantly affects the hair cells in the basal turn of the cochlea (24). Redox regulation provides signals that are necessary for important cellular processes, such as immune responses, hormone synthesis, Ca2+ homeostasis, and cytoskeletal remodeling, among others (54). We focused on studies of redox balance regulation of actin dynamics because actin dynamics play an essential role in the stereociliary bundles of hair cells. Recently, Hung et al. showed that the monooxygenase, Mical, acts as an actin regulator by inducing the disassembly of F-actin via the selective oxidization of two Mets (the 44th and 47th Met, residue numbers from rabbit) of F-actin (15, 16). Lee et al. followed up this study by demonstrating that MsrB1 and MsrB2 reduce the oxidized Mets in Mical-treated F-actin and therefore act as Mical antagonists in the control of actin disassembly and assembly (27). Interestingly, MsrB3 is localized at the base of the stereocilia, suggesting that MsrB3 may act as a regulator of actin dynamics (24). Based on these reports, we propose that MsrB3 deficiency may result in the accumulation of oxidized Met and induce the disassembly of F-actin and stereocilia degeneration in the hair cells of MsrB3−/− mice. In addition, the role of MsrB3 in supporting cells and the role of potassium and magnesium channels, which are known to be regulated by Met redox signaling (6, 7, 14, 42), should also be considered in further studies to more fully understand MsrB3-related hearing loss and to enable the development of improved therapies for MsrB3 deficiency.
In this study, our findings demonstrated that the restoration of the stereociliary bundles of hair cells and of hearing function in the MsrB3−/− mice resulted from successful MsrB3 gene transfer into the otocyst. However, this study had several limitations. First, the rescued hearing loss in the rAAV2/1-MsrB3-GFP-treated ears of the MsrB3−/− mice persisted for 4 weeks after birth, but hearing subsequently deteriorated, and the deterioration of hearing also correlated with the degeneration of hair cell stereociliary bundles. Similar variability in the duration of post-treatment hearing restoration was also reported by Akil et al., who found that a variable level of hearing loss developed after 7 weeks in knockout mice that were rescued by rAAV-mediated gene therapy (2). There are several potential explanations for why the hearing ability of the rescued mice did not persist. However, we can rule out an influence of either treatment time or delivery approach on the hearing loss variability because our studies and the study of Akil et al. used different time points of treatment (embryonic vs. postnatal) and delivery techniques (transuterine vs. cochleostomy and injection through the round window membrane). One possible explanation for the variability in the duration of post-treatment hearing restoration is the tropism of the rAAV serotype used in the gene therapy. Studies by us and others have shown that rAAV2/1 is the most suitable serotype to use as a delivery vector when targeting hair cells in gene therapies for SNHL (4, 51). When we analyzed the localization of MsrB3, we observed a small difference in the expression pattern of MsrB3 in the inner ear between the ears of the rescued MsrB3−/− mice and the ears of MsrB3+/+ mice. Indeed, MsrB3 expression was restricted to the hair cells and was rarely observed in the supporting cells of the treated ears. Therefore, we hypothesize that although hearing was rescued during the early postnatal period when MsrB3 expression was localized to the inner ear hair cells, more widespread expression of MsrB3 might be necessary to maintain hearing in the adult mouse inner ear. To overcome this variability, (1) it may be necessary to treat with a combination of different rAAV serotypes to target different type of cells in the cochlea and (2) readministration of rAAV in treated MsrB3−/− mice during the early postnatal period may also improve the therapeutic stability of the hearing restoration. Second, the study included the limitations that are inherent to the in utero gene delivery method in terms of clinical application. Further studies are required to develop a suitable method for in utero gene delivery in humans. However, our study demonstrates that rAAV-mediated gene therapy could be a promising strategy for rescuing the function of the sensory hair cells of the mammalian inner ear in hereditary deafness.
In summary, to our knowledge, this is the first study to demonstrate functional and morphological rescue of the hair cells of the inner ear in a mouse model of congenital SNHL caused by a deafness gene using an in utero rAAV-mediated gene therapy strategy. Although further studies of the maintenance of the restored hearing function and the development of methods to apply gene therapy in humans are needed, there is no doubt that our study has led patients with sensorineural deafness one step closer toward the restoration of hearing function.
Materials and Methods
Recombinant adeno-associated virus
All of the rAAVs used in this study contained the inverted terminal repeat of AAV serotype 2 and the capsid of AAV serotype 1 (rAAV2/1) and were purchased from SignaGen Laboratories (Rockville, MD). Prepackaged rAAVs were used that contained either a GFP gene (rAAV2/1-GFP), driven by a CMV or CMV early enhancer/chicken β-actin (CAG) promoter, or a mouse MsrB3 cDNA along with GFP (rAAV2/1-MsrB3-GFP), driven by the CMV promoter (Supplementary Fig. S1A). In this study, the rAAV titers were defined as vector genome copies per milliliter (VG/ml). The titer of the rAAV2/1-CAG-GFP stock was 1.2 × 1013 VG/ml, and the titers of both the rAAV2/1-CMV-GFP and rAAV2/1-MsrB3-GFP stocks were 1.31 × 1013 VG/ml. The rAAVs were stored at −80°C and thawed immediately before the surgery.
Animals
Pregnant females of the Institute for Cancer Research (ICR) strain (12–14 weeks of age) were purchased from Hyochang Science (Daegu, Republic of Korea) and used as wild-type (WT) controls to validate the safety and efficiency of rAAV2/1-GFP- and rAAV2/1-MsrB3-GFP-mediated gene delivery into the otocyst at E12.5. MsrB3−/− mice were used for rAAV-mediated gene transfer of the MsrB3 gene. Details regarding the MsrB3−/− mice have been published elsewhere (24).
Surgical procedures
All surgical procedures were performed on a dedicated surgical work surface using sterile techniques. Pregnant ICR or MsrB3−/− mice were anesthetized at 12.5 days postcoitus by intramuscular (i.m.) injection of tiletamine–zolazepam (1.8 mg/100 g) and xylazine hydrochloride (0.7 mg/100 g) mixtures. After the belly hair was removed from the surgical site, we incised the abdominal skin along the midline over ∼15–20 mm. To maintain the body temperature of the pregnant mice, we used a heating pad and infused prewarmed (37°C) normal saline into the abdomen at various times during the surgery. Approximately 0.6–1 μl of rAAV2/1-GFP or rAAV2/1-MsrB3-GFP was microinjected into the left otocyst (Supplementary Fig. S1B) of the E12.5 embryos using a glass micropipette connected to a 25-μl Hamilton syringe (Hamilton, Bonaduz, Switzerland). The rAAV solution contained fast green FCF dye (Sigma-Aldrich, St. Louis, MO) to accurately visualize the position of the glass micropipette during the injection. The contralateral right otocyst of each embryo served as an internal control because in each embryo, the virus was delivered unilaterally to the left otocyst. After the embryo was injected, we irrigated the abdomen with prewarmed normal saline, closed the abdominal wound with surgical sutures, and covered it with a povidone/iodine-impregnated wound dressing.
Auditory brainstem response measurement
To assess auditory function, we performed ABR measurements using an ABR workstation (System 3; Tucker Davis Technology (TDT), Inc., Alachua, FL) as previously described (29). All tests were conducted in a soundproofed room. Briefly, before ABR measurement, the animals were anesthetized by i.m. injection of tiletamine–zolazepam (1.8 mg/100 g) and xylazine hydrochloride (0.7 mg/100 g) mixtures and placed on a heating pad to maintain their body temperature at 37°C. The animal's body temperature was monitored using a rectal thermometer. To record the ABRs, subcutaneous needle electrodes were inserted into the vertex (+charge), mastoid (−e), and hind leg (ground). Acoustic stimuli consisted of either a tone burst stimulus with a 1-ms rise/fall time and a 5-ms plateau at frequencies of 4, 8, 16, and 32 kHz or transient click stimuli and were applied monaurally through a speaker. The stimulus signals were generated by SigGenRP and an RP2.1 real-time processor, and then transmitted through a programmable attenuator (PA5, TDT), a speaker driver (ED1, TDT), and an electrostatic speaker (EC1, TDT). At every frequency, 500 repetitions of each stimulus were generated, starting from a 90-dB sound pressure level and decreasing in 5-dB steps to the acoustic threshold. The phase of the stimulus was reversed upon each presentation to reduce artifacts caused by repetitive stimuli.
Immunostaining of cochlear whole mounts
The organ of Corti was prepared from the inner ears of the ICR and MsrB3−/− mice. The isolated inner ears were quickly fixed by injecting 4% paraformaldehyde (PFA, pH 7.4) in phosphate-buffered saline (PBS) through the oval window, and then immersing them in the same fixative for 2 h at 4°C. After Reissner's membrane and the lateral wall and tectorial membrane of the cochlea were removed, the organ of Corti was dissected into individual turns. The tissues were permeabilized with 0.1% Triton X-100 in PBS (PBS-Tx) for 30 min, blocked using a blocking solution comprising 5% normal goat serum diluted in PBS-Tx for 1 h at room temperature (RT), and stained with either a mouse anti-GFP antibody (1:400; Millipore Filter Corporation, Bedford, MA) or a rabbit anti-MsrB3 antibody (1:200; Sigma-Aldrich) diluted in the blocking solution at 4°C overnight. The next day, the tissue sections were washed with PBS and then incubated for 1 h at RT with an Alexa Fluor 488 (fluorescein)-conjugated goat anti-rabbit IgG secondary antibody (1:1000; Invitrogen, La Jolla, CA) diluted in the blocking solution. Next, F-actin was labeled with an Alexa Fluor® 555-conjugated phalloidin stain (1:1000; Molecular Probes, Eugene, OR) in PBS-Tx for 3 h at RT. The samples were washed with PBS to remove any residual antibodies, mounted with Fluoromount (Sigma-Aldrich), and sealed with a microscope coverslip (Marienfeld Laboratory, Lauda-Königshofen, Germany). The mounted specimens were imaged using a laser scanning confocal microscope (LSM 700; Carl Zeiss, Thornwood, NY). Then, single-transfected IHCs and OHCs were counted in a 160-μm portion of each turn.
RT-PCR analysis
The inner ears were dissected from WT, MsrB3−/−, and treated MsrB3−/− mice. Total RNA was extracted from the whole inner ear using an RNeasy® Micro Kit (Qiagen, Hilden, Germany). We synthesized single-stranded cDNA from the extracted total RNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The cDNA samples were used for polymerase chain reaction (PCR) amplification with specific primers for mouse MsrB3 (NM 177092.4) and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) (NM001289726.1). For RT-PCR, we used primers that specifically amplified a portion of exon 7 of the MsrB3 gene because exon 7 was eliminated in the MsrB3−/− mice. This PCR allowed us to compare samples from the MsrB3+/+ mice with those from the untreated right ears and treated left ears of the rescued MsrB3−/− mice. The Gapdh gene was used as a positive control and was amplified in all samples. The following PCR primer sequences were used: MsrB3 forward, CTC CCC TCA GGG TCA TGT AGG; MsrB3 reverse, AGC ACC ACA CTG AGA ACA GC; Gapdh forward, GGT GCT GAG TAT GTC GTG GA; and Gapdh reverse, CTA AGC AGT TGG TGG TGC AG. All of the PCR products were separated via agarose gel electrophoresis (1.5% agarose gels containing ethidium bromide) and then visualized under ultraviolet (UV) light.
Western blot analysis
Each inner ear was homogenized in lysis buffer containing a 1× protease inhibitor cocktail (Calbiochem, La Jolla, CA) for 30 min at 4°C and then centrifuged at 13,000 rpm for 30 min. Then, the content of the supernatant was resolved via 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The proteins were detected using a rabbit anti-MsrB3 primary antibody (1:500; Sigma-Aldrich) and a goat anti-rabbit IgG-HRP (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) secondary antibody. A rabbit anti-β-actin antibody (1:2000; Cell Signaling Technology, Beverly, MA) and a goat anti-rabbit IgG-HRP antibody (1:2000; Santa Cruz Biotechnology) were used to detect β-actin, which was used as a loading control. Protein expression was visualized using an enhanced chemiluminescence system (Thermo Fisher Scientific, Pittsburgh, PA). The intensity of each MsrB3 and β-actin band was measured using ImageJ software (http://rsb.info.nih.gov/ij/), and MsrB3 signals were quantified by normalization to the expression of β-actin.
Paraffin sections
Following ABR testing, the mice were perfused with 4% PFA in PBS, and the inner ears were isolated. For paraffin sections, the inner ears were fixed with 4% PFA in PBS for 24 h at 4°C and then decalcified in 10% ethylenediaminetetraacetic acid (EDTA) in PBS for another 24 h at 4°C. The specimens were dehydrated with a graded ethanol series, permeabilized with xylene, and embedded in paraffin at RT. The paraffin-embedded inner ears were then serially sectioned into 5-μm-thick slices using a microtome (Leica RM2235; Leica Microsystems, Wetzlar, Germany) and mounted on Superfrost Plus microscope slides (Thermo Fisher Scientific) for staining. All slides were maintained at 4°C until use.
Immunohistochemistry
The expression of rAAV2/1-MsrB3-GFP in the inner ear was determined via immunofluorescence in paraffin sections. The slides of paraffin-embedded inner ear sections were incubated for 1 h at 65°C, deparaffinized with xylene, and rehydrated with a graded ethanol series. The tissue sections were permeabilized with PBS-Tx for 30 min, blocked using a blocking solution comprising 5% normal goat serum and PBS-Tx for 1 h at RT, and incubated at 4°C overnight with a mouse anti-GFP antibody (1:400; Millipore Filter Corporation), a mouse anti-Myo7a antibody (1:500; Developmental Studies Hybridoma Bank, Iowa City, IA), or a rabbit anti-MsrB3 antibody (1:200; Sigma-Aldrich) diluted in the blocking solution. The next day, the tissue sections were washed with PBS and then incubated for 1 h at RT with the appropriate secondary antibody, which was either an Alexa Fluor 488 (fluorescein)-conjugated goat anti-mouse IgG antibody (1:1000; Invitrogen) or an Alexa Fluor 555-conjugated goat anti-rabbit IgG antibody (1:1000; Invitrogen) diluted in the blocking solution. To visualize nuclei, the sections were washed and stained with a 1 μg/ml 4′-6-diamidino-2-phenylindole (DAPI) solution diluted in methanol for 5 min at RT. The samples were washed with PBS to remove any residual antibodies, mounted with Fluoromount (Sigma-Aldrich), and sealed with a microscope coverslip (Marienfeld Laboratory). All slides were observed under a laser scanning confocal microscope (LSM 700; Carl Zeiss).
Scanning electron microscopy
The inner ears were immediately harvested from the euthanized WT, MsrB3−/−, and rescued MsrB3−/− mice and carefully perfused through the oval window with a solution of 2% PFA dissolved in 0.1 M sodium cacodylate buffer (pH 7.4) containing 2.5% glutaraldehyde. The prepared specimens were immersed in the same fixative for 1 h at RT. The lateral wall, tectorial membrane, and Reissner's membrane were removed under a dissecting microscope, and the organ of Corti was then dissected and fixed overnight at 4°C in a fixation mixture comprising 0.1 M sodium cacodylate buffer (pH 7.4), 2 mM calcium chloride, 2.5% glutaraldehyde, and 3.5% sucrose. Following fixation, the prepared specimens were washed thrice for 20 min at 4°C with 0.1 M sodium cacodylate buffer containing 2 mM calcium chloride. We used the method of Hunter-Duvar (17) for postfixation analysis. Briefly, the specimens were immersed in a 1% osmium tetroxide (OsO4)–thiocarbohydrazide (TCH) solution for 1 h at 4°C and placed in 1% TCH for 20 min at RT. These steps were repeated thrice. Then, the specimens were dehydrated in a graded ethanol series, dried using a critical point dryer (HCP-2; Hitachi, Tokyo, Japan), attached on the stub, and coated with platinum using a sputter coater (E1030; Hitachi). The coated specimens were sealed with the stub holder. The specimens were examined under cold-field emission SEM (SU8220; Hitachi) that was operated at 5 or 10 kV.
Statistical analyses
Statistical analyses were performed using two-tailed Student's t-tests with a significance criterion of p < 0.05. The data were analyzed by comparing the treated and the untreated contralateral sides or the therapy and control groups.
Study approval
All animal procedures, including care and handling, were conducted in accordance with the guidelines of the Institutional Animal Care issued by the Committee of Animal Research at Kyungpook National University.
Supplementary Material
Abbreviations Used
- ABR
auditory brainstem response
- CAG
CMV early enhancer/chicken β-actin
- cDNA
complementary DNA
- CI
cochlear implantation
- CMV
cytomegalovirus
- Cx30
connexin 30
- DAPI
4′-6-diamidino-2-phenylindole
- E
embryonic day
- EDTA
ethylenediaminetetraacetic acid
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- GFP
green fluorescent protein
- ICR
Institute for Cancer Research
- IHC
inner hair cell
- i.m.
intramuscular
- ITR
inverted terminal repeat
- MsrA
methionine sulfoxide reductase A
- MsrB3
methionine sulfoxide reductase B3
- OHC
outer hair cell
- OsO4
osmium tetroxide
- P
postnatal day
- PBS
phosphate-buffered saline
- PBS-Tx
triton X-100 in PBS
- PCR
polymerase chain reaction
- PFA
paraformaldehyde
- rAAV
recombinant adeno-associated virus
- ROS
reactive oxygen species
- RT
room temperature
- RT-PCR
reverse transcription–polymerase chain reaction
- SEM
scanning electron microscopy
- SNHL
sensorineural hearing loss
- TCH
thiocarbohydrazide
- TDT
Tucker Davis Technology
- Vglut3
vesicular glutamate transporter 3
- WT
wild-type
Acknowledgments
The authors especially thank Dr. Dennis Drayna and Dr. Doris Wu for comments on the manuscript. The research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant Number: HI14C2119).
Author Contributions
M.-A.K. performed the experiments, analyzed the data, and wrote the manuscript; H.-J.C. performed the experiments and wrote the manuscript; S.-H.B., B.L., S.-K.O., and T.-J.K. performed the experiments; Z.-Y.R., H.-Y.K., and J.-H.C. provided important reagents and mice; and K.-Y.L. and U.-K.K. designed the study, analyzed the data, and wrote the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ahmed ZM, Yousaf R, Lee BC, Khan SN, Lee S, Lee K, Husnain T, Rehman AU, Bonneux S, Ansar M, Ahmad W, Leal SM, Gladyshev VN, Belyantseva IA, Van Camp G, Riazuddin S, Friedman TB, and Riazuddin S. Functional null mutations of MSRB3 encoding methionine sulfoxide reductase are associated with human deafness DFNB74. Am J Hum Genet 88: 19–29, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akil O, Seal RP, Burke K, Wang C, Alemi A, During M, Edwards RH, and Lustig LR. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 75: 283–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson PJ, Wise AK, Flynn BO, Nayagam BA, and Richardson RT. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One 9: e102077, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedrosian JC, Gratton MA, Brigande JV, Tang W, Landau J, and Bennett J. In vivo delivery of recombinant viruses to the fetal murine cochlea: transduction characteristics and long-term effects on auditory function. Mol Ther 14: 328–335, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berlett BS. and Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272: 20313–20316, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Cao G, Lee KP, van der Wijst J, de Graaf M, van der Kemp A, Bindels RJ, and Hoenderop JG. Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J Biol Chem 285: 26081–26087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciorba MA, Heinemann SH, Weissbach H, Brot N, and Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci U S A 94: 9932–9937, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabbita SP, Aksenov MY, Lovell MA, and Markesbery WR. Decrease in peptide methionine sulfoxide reductase in Alzheimer's disease brain. J Neurochem 73: 1660–1666, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Gillespie LN, Richardson RT, Nayagam BA, and Wise AK. Treating hearing disorders with cell and gene therapy. J Neural Eng 11: 065001, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Groves AK. The challenge of hair cell regeneration. Exp Biol Med (Maywood) 235: 434–446, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heffer LF, Sly DJ, Fallon JB, White MW, Shepherd RK, and O'Leary SJ. Examining the auditory nerve fiber response to high rate cochlear implant stimulation: chronic sensorineural hearing loss and facilitation. J Neurophysiol 104: 3124–3135, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henderson LB, Doshi VK, Blackman SM, Naughton KM, Pace RG, Moskovitz J, Knowles MR, Durie PR, Drumm ML, and Cutting GR. Variation in MSRA modifies risk of neonatal intestinal obstruction in cystic fibrosis. PLoS Genet 8: e1002580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hildebrand MS, Newton SS, Gubbels SP, Sheffield AM, Kochhar A, de Silva MG, Dahl HH, Rose SD, Behlke MA, and Smith RJ. Advances in molecular and cellular therapies for hearing loss. Mol Ther 16: 224–236, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Hoshi T. and Heinemann S. Regulation of cell function by methionine oxidation and reduction. J Physiol 531: 1–11, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung RJ, Pak CW, and Terman JR. Direct redox regulation of F-actin assembly and disassembly by Mical. Science 334: 1710–1713, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJ, and Terman JR. Mical links semaphorins to F-actin disassembly. Nature 463: 823–827, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter-Duvar IM. A technique for preparation of cochlear specimens for assessment with the scanning electron microscope. Acta Otolaryngol Suppl 351: 3–23, 1978 [DOI] [PubMed] [Google Scholar]
- 18.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77: 755–776, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jecmenica J, Bajec-Opancina A, and Jecmenica D. Genetic hearing impairment. Childs Nerv Syst 31: 515–519, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Jero J, Mhatre AN, Tseng CJ, Stern RE, Coling DE, Goldstein JA, Hong K, Zheng WW, Hoque AT, and Lalwani AK. Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther 12: 539–548, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Konishi M, Kawamoto K, Izumikawa M, Kuriyama H, and Yamashita T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J Gene Med 10: 610–618, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Krause KH. Aging: a revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp Gerontol 42: 256–262, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Kryukov GV, Kumar RA, Koc A, Sun Z, and Gladyshev VN. Selenoprotein R is a zinc-containing stereo-specific methionine sulfoxide reductase. Proc Natl Acad Sci U S A 99: 4245–4250, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwon TJ, Cho HJ, Kim UK, Lee E, Oh SK, Bok J, Bae YC, Yi JK, Lee JW, Ryoo ZY, Lee SH, Lee KY, and Kim HY. Methionine sulfoxide reductase B3 deficiency causes hearing loss due to stereocilia degeneration and apoptotic cell death in cochlear hair cells. Hum Mol Genet 23: 1591–1601, 2014 [DOI] [PubMed] [Google Scholar]
- 25.This reference has been deleted.
- 26.Lee BC, Dikiy A, Kim HY, and Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta 1790: 1471–1477, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BC, Peterfi Z, Hoffmann FW, Moore RE, Kaya A, Avanesov A, Tarrago L, Zhou Y, Weerapana E, Fomenko DE, Hoffmann PR, and Gladyshev VN. MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol Cell 51: 397–404, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ, Jung J, Shin JW, Song MH, Kim SH, Lee JH, Lee KA, Shin S, Kim UK, Bok J, Lee KY, Choi JY, and Park HJ. Correlation between genotype and phenotype in patients with bi-allelic SLC26A4 mutations. Clin Genet 86: 270–275, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Yoo SJ, Lee S, Song HJ, Huh MI, Jin SU, Lee KY, Lee J, Cho JH, and Chang Y. Functional activity mapping of rat auditory pathway after intratympanic manganese administration. Neuroimage 60: 1046–1054, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Minoda R, Miwa T, Ise M, and Takeda H. Potential treatments for genetic hearing loss in humans: current conundrums. Gene Ther 22: 603–609, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Miwa T, Minoda R, Ise M, Yamada T, and Yumoto E. Mouse otocyst transuterine gene transfer restores hearing in mice with connexin 30 deletion-associated hearing loss. Mol Ther 21: 1142–1150, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moskovitz J. Methionine sulfoxide reductases: ubiquitous enzymes involved in antioxidant defense, protein regulation, and prevention of aging-associated diseases. Biochim Biophys Acta 1703: 213–219, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Moskovitz J, Berlett BS, Poston JM, and Stadtman ER. The yeast peptide-methionine sulfoxide reductase functions as an antioxidant in vivo. Proc Natl Acad Sci U S A 94: 9585–9589, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moskovitz J, Rahman MA, Strassman J, Yancey SO, Kushner SR, Brot N, and Weissbach H. Escherichia coli peptide methionine sulfoxide reductase gene: regulation of expression and role in protecting against oxidative damage. J Bacteriol 177: 502–507, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oien DB, Ortiz AN, Rittel AG, Dobrowsky RT, Johnson MA, Levant B, Fowler SC, and Moskovitz J. Dopamine D(2) receptor function is compromised in the brain of the methionine sulfoxide reductase A knockout mouse. J Neurochem 114: 51–61, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oien DB, Osterhaus GL, Latif SA, Pinkston JW, Fulks J, Johnson M, Fowler SC, and Moskovitz J. MsrA knockout mouse exhibits abnormal behavior and brain dopamine levels. Free Radic Biol Med 45: 193–200, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pal R, Oien DB, Ersen FY, and Moskovitz J. Elevated levels of brain-pathologies associated with neurodegenerative diseases in the methionine sulfoxide reductase A knockout mouse. Exp Brain Res 180: 765–774, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Pang JJ, Boye SL, Kumar A, Dinculescu A, Deng W, Li J, Li Q, Rani A, Foster TC, Chang B, Hawes NL, Boatright JH, and Hauswirth WW. AAV-mediated gene therapy for retinal degeneration in the rd10 mouse containing a recessive PDEbeta mutation. Invest Ophthalmol Vis Sci 49: 4278–4283, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patuzzi RB, Yates GK, and Johnstone BM. Outer hair cell receptor current and sensorineural hearing loss. Hear Res 42: 47–72, 1989 [DOI] [PubMed] [Google Scholar]
- 40.Rivolta MN. New strategies for the restoration of hearing loss: challenges and opportunities. Br Med Bull 105: 69–84, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Rodrigo MJ, Moskovitz J, Salamini F, and Bartels D. Reverse genetic approaches in plants and yeast suggest a role for novel, evolutionarily conserved, selenoprotein-related genes in oxidative stress defense. Mol Genet Genomics 267: 613–621, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Santarelli LC, Wassef R, Heinemann SH, and Hoshi T. Three methionine residues located within the regulator of conductance for K+ (RCK) domains confer oxidative sensitivity to large-conductance Ca2+-activated K+ channels. J Physiol 571: 329–348, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shepherd RK. and Hardie NA. Deafness-induced changes in the auditory pathway: implications for cochlear implants. Audiol Neurootol 6: 305–318, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Singh VK. and Moskovitz J. Multiple methionine sulfoxide reductase genes in Staphylococcus aureus: expression of activity and roles in tolerance of oxidative stress. Microbiology 149: 2739–2747, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Sinha K, Das J, Pal PB, and Sil PC. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87: 1157–1180, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Skaar EP, Tobiason DM, Quick J, Judd RC, Weissbach H, Etienne F, Brot N, and Seifert HS. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc Natl Acad Sci U S A 99: 10108–10113, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stadtman ER. Protein oxidation and aging. Free Radic Res 40: 1250–1258, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Stadtman ER. and Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25: 207–218, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Stadtman ER, Moskovitz J, Berlett BS, and Levine RL. Cyclic oxidation and reduction of protein methionine residues is an important antioxidant mechanism. Mol Cell Biochem 234–235: 3–9, 2002 [PubMed] [Google Scholar]
- 50.Stadtman ER, Moskovitz J, and Levine RL. Oxidation of methionine residues of proteins: biological consequences. Antioxid Redox Signal 5: 577–582, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Stone IM, Lurie DI, Kelley MW, and Poulsen DJ. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther 11: 843–848, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Stone JS, Oesterle EC, and Rubel EW. Recent insights into regeneration of auditory and vestibular hair cells. Curr Opin Neurol 11: 17–24, 1998 [DOI] [PubMed] [Google Scholar]
- 53.Ugarte N, Petropoulos I, and Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal 13: 539–549, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Wilson C. and Gonzalez-Billault C. Regulation of cytoskeletal dynamics by redox signaling and oxidative stress: implications for neuronal development and trafficking. Front Cell Neurosci 9: 381, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yagi M, Kanzaki S, Kawamoto K, Shin B, Shah PP, Magal E, Sheng J, and Raphael Y. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol 1: 315–325, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.