Abstract
Esophageal cancer is the eighth most common type of cancer in the world, and the sixth most common cause of mortality from cancer. Alcohol consumption is the major risk factor for esophageal cancer, due to the worldwide prevalence and high carcinogenicity of the ethanol metabolite. In epidemiological studies, the efficiency of alcohol intake to enhance the risk of esophageal cancer is altered by daily ethanol consumption, type of alcoholic beverages ingested, time since quitting drinking, age of drinking initiation, differences in population and subtypes of esophageal cancer. Corresponding factors, including gene polymorphisms, tobacco smoking, oral microorganisms and folate deficiency, reveal a synergistic effect in concurrent alcohol users that may lead to an increased risk of developing esophageal cancer. Consequently, esophageal cancer prevention involves multiple aspects, including quitting drinking and smoking, maintaining an adequate oral health and ingesting adequate quantities of folate, particularly in genetically high-risk populations.
Keywords: esophageal cancer, risk factors, alcohol, epidemiology, corresponding factors
1. Introduction
Esophageal cancer is the eighth most common type of cancer in the world and the sixth most common cause of mortality from cancer, according to the results of the GLOBOCAN project, published by the International Agency for Research on Cancer in 2012 (1). Alcohol consumption has been demonstrated to be a major risk factor for developing esophageal cancer, particularly esophageal squamous cell carcinoma (ESCC) in men (2–4). Globally, prevailing alcohol intake has been an increasingly dire health problem due to the carcinogenicity of ethanol (5,6). Chronic ethanol ingestion leads to nutritional deficiencies and generation of reactive oxygen species (ROS). In addition, ethanol acts as a solvent of carcinogens (7). Acetaldehyde, the primary metabolite of ethanol, is highly mutagenic, due to its ability to form exocyclic DNA adducts (8). The diversity of carcinogenetic mechanisms may reflect the wide interaction between ethanol and cofactors from the inner and outer environment (9). The present study focuses on how alcohol consumption associates with the risk of esophageal cancer and interacts with corresponding factors, mainly from an epidemiological aspect.
2. Association between alcohol consumption and the risk of esophageal cancer
Daily ethanol consumption
The risk of developing esophageal cancer has been indicated to increase with an increase in alcohol intake (10–18). Fan et al (18) demonstrated a positive association between the total amount of ethanol intake during lifetime and the risk of esophageal cancer. In another study, heavy ethanol intake (>53.3 g/day) was significantly associated with the risk of esophageal cancer, even in a relatively short duration (≤20 years) (12). Castellsagué et al (19) demonstrated that, compared with individuals with a decreased daily alcohol consumption for numerous years, those drinking large amounts of alcohol for a shorter period of time tend to carry an increased risk of developing esophageal cancer. Therefore, compared with a long duration of alcohol consumption, an increased daily amount of alcohol consumption may be a more effective risk factor of esophageal cancer.
Type of alcoholic beverages
As the major component of alcoholic beverages, ethanol is the determinant of the risk of esophageal cancer (20). Acetaldehyde, though present in trace amounts in beverages, may be another risk factor, due to its strong carcinogenicity (21). The most prevalent beverage in a region tends to have the greatest relative risk (22). Baijiu (a type of hard liquor with a high alcohol content) in China, wine in Italy, calvados in France and spirits in South America enhance the development of esophageal cancer, due to their high ethanol content and great popularity in each particular region (12,19,22,23). In contrast, wine consumption was observed to reduce the risk of esophageal cancer in a previous cohort study (17). Despite the regional variations in drinking habits, certain antitumor substances, including flavonoids, that are contained in wine may explain the inconsistencies reported across different studies (24). In previous studies, beer had a relatively mild effect on the risk of esophageal cancer, even with large consumption, compared with other beverages, due to its low ethanol content (17–19,22).
Years since quitting drinking
There are controversies regarding the association between the time since quitting drinking and the risk of developing esophageal cancer. Castellsagué et al (19) and Wu et al (25) demonstrated a virtually negative association in men. The conclusions of the study by Zambon et al (14) were more complex. Compared with persistent drinkers, drinking cessation was associated with certain increased risk in the first 10 subsequent years and with a non-significant risk reduction thereafter (14). These differences may be due to variations in reference and population stratification in case-control studies.
Age of drinking initiation
This variant has an uncertain effect on the risk of esophageal cancer. In the study by Castellsagué et al (19), along with increasing daily alcohol intake, there was a greater tendency for individuals that started drinking at an older age to develop esophageal cancer. However, Zambon et al (14) showed no association between the age of drinking initiation and the risk of developing esophageal cancer.
Differences in population
Different populations involved in various studies affect the strength of the association between alcohol and esophageal cancer. Alcohol consumption may be a great risk factor for esophageal cancer in Caucasian populations (14,19,26); however, the effect of alcohol consumption on the risk of developing esophageal cancer appears to be much weaker in Asian populations (25,27), particularly in certain regions with high incidence of esophageal cancer, including Linxian (China), where alcohol consumption is not a major risk factor for esophageal cancer (28). This weak association may be attributed to strong confounding factors, including gene polymorphism and other carcinogens that dilute the effect of alcohol (11,28). In studies concerning gender, the association between alcohol consumption on the risk of developing esophageal cancer was weaker in women compared with men, which may be partly explained by a short history of alcohol exposure and low alcohol prevalence among women (19,25).
Subtypes of esophageal cancer
A large number of studies have indicated a strong positive association between alcohol intake and ESCC (11,12,15,17,18,29). However, the association between alcohol intake and esophageal adenocarcinoma (EAC) is attenuated. The two cohort studies by Steevens et al (17,30) revealed that alcohol consumption did not promote the risk of developing EAC or Barrett's esophagus (precancerous lesions of EAC). Other analyses reached similar conclusions (29,31,32). The study by Akiyama et al (33) showed a moderate increase in the risk of Barrett's esophagus in Japanese male drinkers. In the meta-analysis conducted by Lubin et al (15), odd ratios (ORs) with drinking-years exhibited an inverse association with alcohol consumption in <5 drinks/day consumers, and no association in heavier consumers. The distinct outcome of EAC and ESCC may be explained by the different pathogenesis of these two subtypes of esophageal cancer (34).
3. Interactions between alcohol consumption and the corresponding factors
Gene polymorphism
Since numerous genes participate in the catabolism of ethanol directly or indirectly, gene polymorphism greatly affects the carcinogenicity of ethanol in various populations (35). In several epidemiological studies, even genes that are barely associated with ethanol metabolism revealed similar effects (Table I).
Table I.
Interaction between alcohol consumption and gene polymorphisms in the risk of esophageal cancer.
| Odds ratio/relative risk (95% confidence interval) | |||||
|---|---|---|---|---|---|
| Genes | Gene polymorphismsa | Alcohol consumptionb | Synergismc | First author, year | Refs. |
| ADH1B*1 | 4.25 (0.41–43.80) | 7.01 (3.77–13.00) | 38.60 (13.3–113.00) | Yokoyama, 2002 | (41) |
| 2.37 (1.40–4.01) | 6.21 (2.39–16.30) | 25.20 (10.90–53.40) | Zhang, 2010 | (42) | |
| 0.97 (0.21–3.62) | 2.72 (0.55–79.60) | 3.70 (0.34–282.00) | Wang, 2011 | (44) | |
| 1.39 (0.84–2.31) | 1.67 (1.18–2.37) | 3.58 (2.20–5.84) | Wu, 2013 | (45) | |
| ADH1C*2 | 0.81 (0.17–3.99) | 6.64 (3.66–12.10) | 23.80 (7.67–74.10) | Yokoyama, 2002 | (41) |
| ALDH2*2 | 1.44 (0.22–9.54) | 10.40 (2.85–37.80) | 88.90 (24.0–74.10) | Yokoyama, 2002 | (41) |
| 1.70 (1.05–2.75) | 4.22 (2.03–8.77) | 21.50 (6.44–71.60) | Zhang, 2010 | (42) | |
| 1.59 (0.87–3.71) | 1.03 (0.07–27.50) | 7.05 (0.48–331.00) | Wang, 2011 | (44) | |
| 0.74 (0.53–1.02) | 1.35 (0.99–1.85) | 2.34 (1.52–3.61) | Wu, 2013 | (45) | |
| CYP2E1*C1 | 0.56 (0.20–1.59) | 1.93 (0.43–2.41) | 7.64 (2.82–11.30) | Guo, 2008 | (62) |
| 2.70 (1.31–5.57) | 1.94 (0.82–4.60) | 7.10 (3.44–14.70) | Qin, 2008 | (63) | |
| MTHFR C677T | 2.19 (1.03–4.73) | 3.42 (1.28–8.93) | 5.77 (2.11–15.70) | Zhao, 2011 | (75) |
| GSTM1 deletion | 1.56 (0.80–3.04) | 2.74 (1.01–7.55) | 6.27 (2.30–17.70) | Wang, 2004 | (82) |
| CYP1A1 Val/Val | 2.01 (0.94–3.43) | 3.02 (1.31–7.03) | 7.71 (2.39–32.20) | Wang, 2004 | (82) |
Individuals who are homozygotes or heterozygotes for one of the listed genes but not alcohol-drinkers.
Individuals who are heavy drinkers but non-carriers of one of the listed genes, or heterozygotes for that gene.
Synergism between gene polymorphisms and alcohol consumption in individuals who are heavy alcohol-drinkers and homozygotes or heterozygotes for one of the listed genes. ADH, alcohol dehydrogenase; ALDH, aldehyde dehydrogenase; CYP, cytochrome P450; MTHFR, methylenetetrahydrofolate reductase; GST, glutathione S-transferase.
Alcohol dehydrogenases (ADH) and aldehyde dehydrogenases (ALDH) are the major enzymes that participate in the metabolism of ethanol. Ethanol is oxidated to acetaldehyde by ADH, and the subsequent oxidation of acetaldehyde to acetate is catalyzed by ALDH. Polymorphism of these gene families caused by point mutations alters enzymatic activity, resulting in potential individual variations in acetaldehyde exposure (36–38).
The ADH1B*2 (Arg48His) allele encodes a more active subunit of ADH1B, compared with the ADH1B*1 allele, the homodimer of which has a ~40-fold greater maximum velocity than the ADH1B*1/2*1 form of ADH1B (39,40). The enzyme encoded by the ADH1C*1 (Ile350Val) allele has a 2.5-times faster speed of acetaldehyde production than the enzyme encoded by the ADH1C*2 allele (39,40). ADH1B*1 has been previously demonstrated to enhance the risk of esophageal cancer in Asian populations (41–45). By contrast, the association between ADH1C and the risk of esophageal cancer is contradictory. Yokoyama et al (41) and Muto et al (46) demonstrated that ADH1C*2 increased the risk of developing esophageal cancer; however, the study by Wu et al (45) showed no enhanced risk. Furthermore, ADH1C*1, but not ADH1C*2, enhanced the risk of esophageal cancer in certain studies conducted in western countries (47,48). This contradiction may be explained by different linkage disequilibrium patterns among various populations (40,48). ADH7 is mainly expressed in the upper gastrointestinal tract (49), and certain studies showed that single nucleotide polymorphisms of ADH7 were associated with esophageal cancer in alcohol drinkers (50–52).
The ALDH2*2 (Glu487Lys) allele, which encodes an inactive subunit of ALDH, occurs most frequently in Asian countries, including China, Japan, Korea, Mongolia and Indochina (53). As the major enzyme affecting blood acetaldehyde concentration, the ALDH2*1*2 and ALDH2*2*2 forms of ALDH produced a 6- and 19-fold increased acetaldehyde concentrations, respectively, compared with the ALDH2*1*1 form (54). The enhancing effect of the ALDH2*2 allele on the risk of ESCC in Japanese men was demonstrated in previous reports by Yokoyama et al (55,56). A previous meta-analysis including seven case-control studies in Asia revealed a positive association between the risk of developing esophageal cancer and the level of alcohol consumption in subjects carrying the ALDH2*1*2 genotype (57). The ALDH2*2 and ADH1B*1 alleles acted in a multiplicative manner to enhance the risk of esophageal cancer (41,42). Notably, although the ALDH2*1*2 genotype increased the risk of developing esophageal cancer, the ALDH2*2*2 genotype reduced the risk in a previous study (42). It may be hypothesized that increased blood levels of acetaldehyde due to the ALDH2*2 homodimer results in the ‘alcohol flushing response’, which includes facial flushing, nausea and tachycardia, preventing people from heavy drinking, thus decreasing the possibility of esophageal cancer (58,59). Individuals that possessed the ALDH2*1/2*2 genotype and also carried ADH2*1/2*1 did not exhibit flushing following drinking. These individuals tended to be heavy drinkers and had the greatest risk of developing esophageal cancer (58).
Cytochrome P450 2E1 (CYP2E1) is the major enzyme in the microsomal ethanol oxidation system (60). Induced by ethanol consumption, CYP2E1 metabolically activates procarcinogens and produces noxious ROS during ethanol oxidation (61). Compared with CYP2E1*c1 (Pst I-/Rsa I+), the mutant CYP2E1*c2 (Pst I+/Rsa I-) is considered to display a decreased activity (38). Individuals with the CYP2E1*c1*c1 genotype possessed a much greater risk of developing ESCC compared with those carrying the CYP2E1*c2 allele among Chinese drinkers; Furthermore, CYP2E1*c1*c1 exhibits a synergistic interaction with ALDH2*1*2 and methylenetetrahydrofolate reductase (MTHFR)677 (C/T+T/T) (62,63). However, in several cases, particularly in Caucasians, gene polymorphisms of CYP2E1 have no association with esophageal cancer (64–67).
Polymorphisms of the enzymes involved in the folate metabolic pathways also impact the development of esophageal cancer. MTHFR, a key enzyme in folate metabolism, is important for DNA methylation (68,69). Altered activity of MTHFR may cause DNA hypomethylation, a process associated with carcinogenesis (70,71). The most commonly studied MTHFR mutant is the change from C to T at nucleotide 677 of the MTHFR gene, which results in an alanine to valine substitution in the MTHFR enzyme (72). The homozygote and heterozygote of 667T exhibit a 30 and 65% activity, respectively, compared with the 100% activity exhibited by the 677CC genotype (73). Previous case-control studies and meta-analyses have revealed that the MTHFR C677T allele increased the risk of developing ESCC (74–78). In coordination with ADLH2*2, individuals with C677T demonstrated elevated ORs (74). The MTHFR 677T allele collaborated with alcohol consumption to increase the risk of esophageal cancer in former, moderate and heavy drinkers (75). However, the interaction between MTHFR 677T and alcohol intake is not consistent in certain meta-analyses (77,78). A previous study conducted in Japan reached the opposite conclusion, and reported that the MTHFR 677TT genotype significantly decreased esophageal cancer risk in heavy drinkers (79). This inconsistency may be due to the regional variations in folate consumption among different populations, which would suggest a significant gene-nutrient interaction between folate consumption and MTHFR genotype (78–80).
Certain gene polymorphisms with little association with the metabolism of alcohol somewhat alter the risk of esophageal cancer. According to the review by Hiyama et al (81), carcinogen metabolism-associated genes [such as cytochrome P450 family 1 subfamily A member 1 (CYP1A1), glutathione S-transferases (GSTs) and natural antisense transcripts], DNA repair genes (such as X-ray repair complementing defective repair in Chinese hamster cells 1 and xeroderma pigmentosum group D), cell cycle control genes (such as tumor protein p53 and cyclin D1) and oncogenes (such as v-myc avian myelocytomatosis viral oncogene lung carcinoma derived homolog) showed varying degrees of association with esophageal cancer. However, inconsistencies exist in various studies, and certain studies showed that the aforementioned genes interacted with alcohol consumption in the development of esophageal cancer. The GSTM1 deletion, GSTP1 341C/T+341T/T and CYP1A1 Val/Val genotypes were indicated to possess much greater ORs in alcohol drinkers compared with non-drinkers in two case-control studies (82,83).
Tobacco smoking
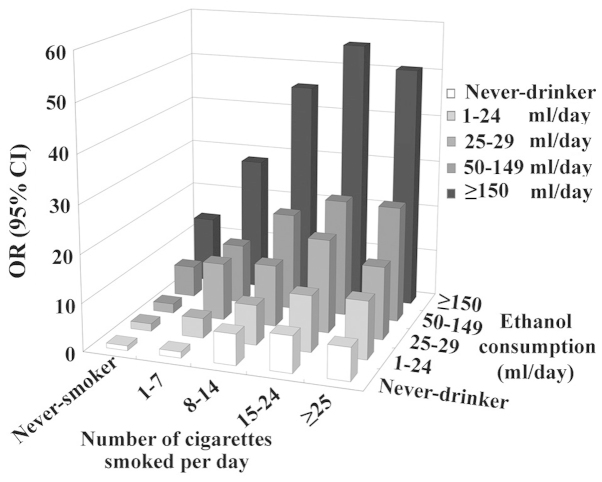
Similarly to alcohol beverages, tobacco has spread all over the world, with >1 billion current smokers (84). The majority of alcohol drinkers are also tobacco smokers (85). Epidemiological studies involving the combined effects of tobacco and alcohol have consistently revealed the existence of a positive synergistic effect between these two factors on the risk of developing esophageal cancer (14,17–19,25,86–88) (Fig. 1). The two variants usually exhibit a mutual dose-response association (14,17,19), and a combined OR reached a value of 130 in an Italian study (14). However, the effect of this combination appeared to be much weaker in Asian populations, with a combined OR of <10 at the highest alcohol and tobacco use (18,25). Similarly, women had a less combined OR compared with men (19,25). The variation between populations may be attributed to regional and gender differences in terms of tobacco prevalence (25,89). Reportedly, ~66.9% of men but only 4.2% of women are tobacco smokers in China, whereas the prevalence of smoking among men and women was estimated to be 35 and 22%, respectively, in developed countries, and 50 and 9%, respectively, in developing countries (25,89).
Figure 1.
Combined exposure to alcohol consumption and cigarette smoking, and risk of developing esophageal cancer, as reported by Castellsagué et al (19). OR, odds ratio; CI, confidence interval.
Tobacco smoke contains >60 carcinogens, including tobacco-specific nitrosamines such as 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and N'-nitrosonornicotine, and polycyclic aromatic hydrocarbons (PAHs) such as benzo[a]pyrene and acetaldehyde (84,90,91). Tobacco smoke interacts with ethanol in the initiation of cancer in several ways: i) Ethanol activates the CYP members that metabolize tobacco procarcinogens to carcinogens (91); ii) ethanol increases cellular membrane permeability and acts as a solvent to facilitate the penetration of molecules like PAHs into the intracellular domain of mucosal epithelial cells (91); and iii) tobacco smoke, as a direct source of acetaldehyde or as a regulator of the population of oral bacteria, cooperatively elevates acetaldehyde exposure in a direct or indirect way by 7-fold, compared with alcohol drinking alone (92).
Oral microorganisms
Epidemiological evidence of the associations between salivary acetaldehyde and esophageal cancer remains limited, but several experiments conducted in animals may aid to elucidate the local carcinogenic effects of acetaldehyde on the mucosa of the upper digestive tract. Previous studies suggested that long-term alcohol consumption may induce increased cell proliferation in the oral and esophageal mucosa of rats (93,94). As a more direct model for reflecting the effect of salivary acetaldehyde, rats that drank water with an increased concentration of acetaldehyde showed hyperplasia and hyperproliferation in the epithelia of their upper gastrointestinal tract (95).
Oral microbes and prolonged ethanol use are two major factors in the generation of salivary acetaldehyde. This hypothesis has been previously demonstrated in vivo by Homann et al (96). In this study, moderate ethanol ingestion resulted in carcinogenic amounts of acetaldehyde in the saliva; whereas using an antiseptic mouthwash with chlorhexidine prior to ethanol exposure, in vivo acetaldehyde production decreased by >50%, with a parallel evident decrease in bacterial counts. Yokoyama et al (97) showed that following 3 weeks of abstinence, the microorganism count and salivary acetaldehyde production decreased in alcoholics. These results indicate a certain mutual effect between ethanol and oral microorganisms. Chronic alcohol consumption may increase bacterial concentrations through affecting salivary gland morphology and decreasing salivary flow (98,99).
As another promoter of microbial acetaldehyde production, tobacco smoking may exhibit a strong association with increased salivary acetaldehyde during alcohol drinking. Smokers that smoke while drinking have 7-fold increased salivary acetaldehyde levels compared with non-smokers (100). With the exception of the direct contribution of acetaldehyde by tobacco smoke, the alteration of oral microorganisms by smoking is also a major source of the increased concentration of salivary acetaldehyde (99). As previously reported, increased yeast infections and conversions from Gram-negative to Gram-positive bacteria has been demonstrated in smokers (99,101,102). However, oral bacteria may activate the nitrosamines from tobacco smoking to carcinogenic adducts by forming hydroxylated products (103–105).
Although >700 bacterial species inhabit the oral cavity (105), only the prevalence of several aerobic Gram-positive bacteria and yeast have been proved to play a leading role in acetaldehyde production (106). This phenomenon may due to the increased ADH activity, which has been confirmed in Streptococcus gordonii V2016 (107), Neisseria (108) and Streptococcus salivarius (109), the prevalence of aerobic Gram-positive bacteria and yeast are important in acetaldehyde production (109), due to their increased ADH activity. Other species, including hemolytic Streptococcus viridans var., Stomatococcus sp. and Corynebacterium sp., are not highly efficient in producing acetaldehyde; however, these species were significantly associated with increased acetaldehyde levels in saliva (110). Yeasts such as Candida albicans were indicated to have great capacity to produce carcinogenic acetaldehyde (111).
Folate deficiency
In previous studies, people ingesting a greater quantity and variety of fresh vegetable and fruits were less likely to develop esophageal cancer (112–115). Among numerous anticarcinogenic nutrients contained in plant foods, folate intake has been widely demonstrated to be closely associated with cancer of the brain, lung, esophagus, pancreas, colorectum, breast, cervix and breast in previous epidemiological studies (116). Three meta-analyses involving worldwide case-control studies conducted between 1988 and 2011 reached a consensus that folate intake may effectively protect individuals from ESCC and EAC, with a pooled OR and relative risk (RR) between 0.50 and 0.66 (78,117,118). In a large cohort study, a decreased intake of folate compared with the daily median intake (405 µg) demonstrated an inverse association with the risk of developing ESCC, but increased intake showed no association; furthermore, there were no significant associations between dietary folate and risk of EAC (119). One hypothesis proposed that folate intake was not linearly associated with cancer risk, with a protective effect only in moderate folate intake but no protection or even tumor promotion in low or excessive ingestion (120). In that study, the cohort had a relatively high median of folate consumption, which may explain this inconsistency with other studies.
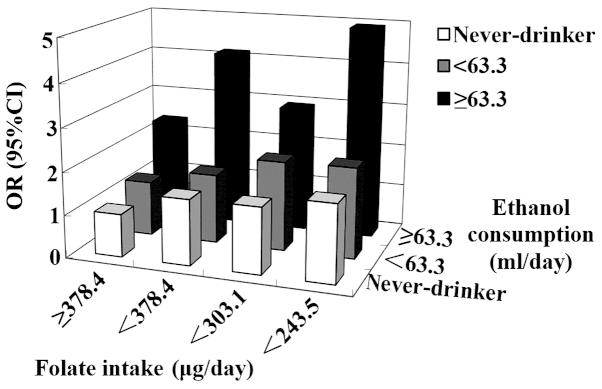
A significant interaction has been observed between folate and alcohol intake in upper digestive tract cancer. In the case-control study by Ibiebele et al (121), individuals with increased alcohol and decreased folate intake demonstrated a 3-fold OR of ESCC compared with individuals with decreased alcohol and increased folate intake. Matsuo et al (122) reported a similar association in oral and pharyngeal cancer (Fig. 2). Another large prospective cohort study additionally confirmed the antagonistic interaction between alcohol and folate intake in oral cancer (123).
Figure 2.
Combined exposure to alcohol consumption and folate intake, and risk of developing oral and pharyngeal cancer, as reported by Matsuo et al (122). OR, odds ratio; CI, confidence interval.
Folate is important in DNA metabolism, since it mediates the synthesis of S-adenosylmethionine, a methyl donor used in biological methylation reactions and de novo deoxynucleoside triphosphate synthesis (116). Folate depletion may be oncogenic through altered DNA/RNA methylation, disruption of DNA integrity and disruption of DNA repair (116). Alcohol ingestion is a primary cause of folate deficiency (124,125). In addition to dietary inadequacy, alcohol may decrease internal folate levels through intestinal malabsorption, decreased hepatic storage and increased renal excretion (124,125). Folate homeostasis depends on transporter proteins, including reduced folate carrier, proton-coupled folate transporter, folate-binding protein, mitochondrial folate transporter and enzymes such as folylpolyglutamate synthetase (126–129). Chronic ethanol exposure may downregulate gene expression, thus impairing the transportation of folate across membranes (126–129). With the exception of causing folate deficiency, ethanol intake interferes widely in folate-dependent intermediary metabolism by inhibiting enzymes in the one-carbon metabolism, particularly methionine synthase and its associated products of metabolism, thus disturbing the synthesis of nucleotides (130). By contrast, folate deficiency facilitates the adverse effects of alcohol in methionine metabolism and promotes alcohol-induced oxidative cell injury (124).
4. Conclusion
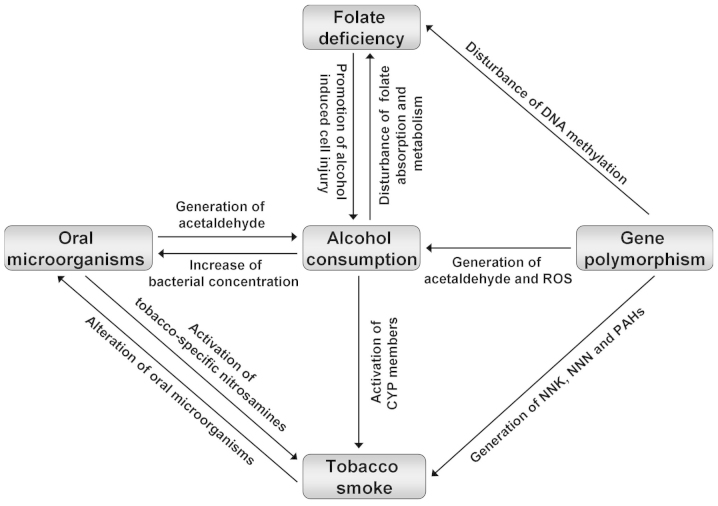
Alcohol consumption significantly increases the risk of esophageal cancer, exhibiting a dose-response association with daily intake and an altered efficiency in various beverage types, populations and cancer subtypes. Gene polymorphisms, tobacco smoking, oral microorganisms and folate deficiency act as collaborators with concurrent alcohol use (Fig. 3). Current evidence suggests that, rather than mere alcohol consumption, the synergy of alcohol consumption and corresponding factors is important for the development of esophageal cancer. Therefore, quitting alcohol drinking and tobacco smoking, maintaining an adequate oral hygiene and ingesting adequate levels of plant foods may effectively protect high-risk individuals from developing esophageal cancer.
Figure 3.
Interactions between alcohol consumption and its corresponding factors. ROS, reactive oxygen species; CYP, cytochrome P450; PAHs, polycyclic aromatic hydrocarbons; NNK, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone; NNN, N-nitrosonornicotine.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Comparative Risk Assessment collaborating group (Cancers): Causes of cancer in the world: Comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang JB, Fan JH, Liang H, Li J, Xiao HJ, Wei WQ, Dawsey SM, Qiao YL, Boffetta P. Attributable causes of esophageal cancer incidence and mortality in China. PLoS One. 2012;7:e42281. doi: 10.1371/journal.pone.0042281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, et al. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404–1413. doi: 10.1093/jnci/djg047. [DOI] [PubMed] [Google Scholar]
- 5.Jones L, Bates G, McCoy E, Bellis MA. Relationship between alcohol-attributable disease and socioeconomic status, and the role of alcohol cons-umption in this relationship: A systematic review and meta-analysis. BMC Public Health. 2015;15:400. doi: 10.1186/s12889-015-1720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pöschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–165. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 8.Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: Implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–193. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 10.Oze I, Matsuo K, Wakai K, Nagata C, Mizoue T, Tanaka K, Tsuji I, Sasazuki S, Inoue M, Tsugane S. Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan: Alcohol drinking and esophageal cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2011;41:677–692. doi: 10.1093/jjco/hyr026. [DOI] [PubMed] [Google Scholar]
- 11.Islami F, Fedirko V, Tramacere I, Bagnardi V, Jenab M, Scotti L, Rota M, Corrao G, Garavello W, Schüz J, et al. Alcohol drinking and esophageal squamous cell carcinoma with focus on light-drinkers and never-smokers: A systematic review and meta-analysis. Int J Cancer. 2011;129:2473–2484. doi: 10.1002/ijc.25885. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai N, Wakai T, Akazawa K, Ling Y, Wang S, Shan B, Okuhara Y, Hatakeyama Y, Kataoka H. Heavy alcohol intake is a risk factor for esophageal squamous cell carcinoma among middle-aged men: A case-control and simulation study. Mol Clin Oncol. 2013;1:811–816. doi: 10.3892/mco.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao YT, McLaughlin JK, Blot WJ, Ji BT, Benichou J, Dai Q, Fraumeni JF., Jr Risk factors for esophageal cancer in Shanghai, China. I. Role of cigarette smoking and alcohol drinking. Int J Cancer. 1994;58:192–196. doi: 10.1002/ijc.2910580209. [DOI] [PubMed] [Google Scholar]
- 14.Zambon P, Talamini R, La Vecchia C, Dal Maso L, Negri E, Tognazzo S, Simonato L, Franceschi S. Smoking, type of alcoholic beverage and squamous-cell oesophageal cancer in northern Italy. Int J Cancer. 2000;86:144–149. doi: 10.1002/(SICI)1097-0215(20000401)86:1<144::AID-IJC23>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 15.Lubin JH, Cook MB, Pandeya N, Vaughan TL, Abnet CC, Giffen C, Webb PM, Murray LJ, Casson AG, Risch HA, et al. The importance of exposure rate on odds ratios by cigarette smoking and alcohol consumption for esophageal adenocarcinoma and squamous cell carcinoma in the Barrett's Esophagus and Esophageal Adenocarcinoma Consortium. Cancer Epidemiol. 2012;36:306–316. doi: 10.1016/j.canep.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polesel J, Dal Maso L, Bagnardi V, Zucchetto A, Zambon A, Levi F, La Vecchia C, Franceschi S. Estimating dose-response relationship between ethanol and risk of cancer using regression spline models. Int J Cancer. 2005;114:836–841. doi: 10.1002/ijc.20756. [DOI] [PubMed] [Google Scholar]
- 17.Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Alcohol consumption, cigarette smoking and risk of subtypes of oesophageal and gastric cancer: A prospective cohort study. Gut. 2010;59:39–48. doi: 10.1136/gut.2009.191080. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Yuan JM, Wang R, Gao YT, Yu MC. Alcohol, tobacco, and diet in relation to esophageal cancer: The Shanghai Cohort Study. Nutr Cancer. 2008;60:354–363. doi: 10.1080/01635580701883011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castellsagué X, Muñoz N, De Stefani E, Victora CG, Castelletto R, Rolón PA, Quintana MJ. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82:657–664. doi: 10.1002/(SICI)1097-0215(19990827)82:5<657::AID-IJC7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 20.Holmes RS, Vaughan TL. Epidemiology and pathogenesis of esophageal cancer. Semin Radiat Oncol. 2007;17:2–9. doi: 10.1016/j.semradonc.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Linderborg K, Joly JP, Visapää JP, Salaspuro M. Potential mechanism for Calvados-related oesophageal cancer. Food Chem Toxicol. 2008;46:476–479. doi: 10.1016/j.fct.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Bosetti C, La Vecchia C, Negri E, Franceschi S. Wine and other types of alcoholic beverages and the risk of esophageal cancer. Eur J Clin Nutr. 2000;54:918–920. doi: 10.1038/sj.ejcn.1601113. [DOI] [PubMed] [Google Scholar]
- 23.Launoy G, Milan C, Day NE, Faivre J, Pienkowski P, Gignoux M. Oesophageal cancer in France: Potential importance of hot alcoholic drinks. Int J Cancer. 1997;71:917–923. doi: 10.1002/(SICI)1097-0215(19970611)71:6<917::AID-IJC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Neuhouser ML. Dietary flavonoids and cancer risk: Evidence from human population studies. Nutr Cancer. 2004;50:1–7. doi: 10.1207/s15327914nc5001_1. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, Zhao JK, Zhang ZF, Han RQ, Yang J, Zhou JY, Wang XS, Zhang XF, Liu AM, van't Veer P, et al. Smoking and alcohol drinking increased the risk of esophageal cancer among Chinese men but not women in a high-risk population. Cancer Causes Control. 2011;22:649–657. doi: 10.1007/s10552-011-9737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vioque J, Barber X, Bolumar F, Porta M, Santibáñez M, de la Hera MG, Moreno-Osset E. PANESOES Study Group: Esophageal cancer risk by type of alcohol drinking and smoking: A case-control study in Spain. BMC Cancer. 2008;8:221. doi: 10.1186/1471-2407-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Tang L, Sun G, Tang Y, Xie Y, Wang S, Hu X, Gao W, Cox SB, Wang JS. Etiological study of esophageal squamous cell carcinoma in an endemic region: A population-based case control study in Huaian, China. BMC Cancer. 2006;6:287. doi: 10.1186/1471-2407-6-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, Mark SD, Qiao YL, Taylor PR. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–463. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 29.Freedman ND, Murray LJ, Kamangar F, Abnet CC, Cook MB, Nyrén O, Ye W, Wu AH, Bernstein L, Brown LM, et al. Alcohol intake and risk of oesophageal adenocarcinoma: A pooled analysis from the BEACON Consortium. Gut. 2011;60:1029–1037. doi: 10.1136/gut.2010.233866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steevens J, Schouten LJ, Driessen AL, Huysentruyt CJ, Keulemans YC, Goldbohm RA, van den Brandt PA. A prospective cohort study on overweight, smoking, alcohol consumption, and risk of Barrett's esophagus. Cancer Epidemiol Biomarkers Prev. 2011;20:345–358. doi: 10.1158/1055-9965.EPI-10-0636. [DOI] [PubMed] [Google Scholar]
- 31.Tramacere I, Pelucchi C, Bagnardi V, Rota M, Scotti L, Islami F, Corrao G, Boffetta P, La Vecchia C, Negri E. A meta-analysis on alcohol drinking and esophageal and gastric cardia adenocarcinoma risk. Ann Oncol. 2012;23:287–297. doi: 10.1093/annonc/mdr136. [DOI] [PubMed] [Google Scholar]
- 32.Thrift AP, Kramer JR, Richardson PA, El-Serag HB. No significant effects of smoking or alcohol consumption on risk of Barrett's esophagus. Dig Dis Sci. 2014;59:108–116. doi: 10.1007/s10620-013-2892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akiyama T, Inamori M, Iida H, Mawatari H, Endo H, Hosono K, Yoneda K, Fujita K, Yoneda M, Takahashi H, et al. Alcohol consumption is associated with an increased risk of erosive esophagitis and Barrett's epithelium in Japanese men. BMC Gastroenterol. 2008;8:58. doi: 10.1186/1471-230X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17:38–44. doi: 10.1016/j.semradonc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 35.Druesne-Pecollo N, Tehard B, Mallet Y, Gerber M, Norat T, Hercberg S, Latino-Martel P. Alcohol and genetic polymorphisms: Effect on risk of alcohol-related cancer. Lancet Oncol. 2009;10:173–180. doi: 10.1016/S1470-2045(09)70019-1. [DOI] [PubMed] [Google Scholar]
- 36.Edenberg HJ. The genetics of alcohol metabolism: Role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 37.Brennan P, Lewis S, Hashibe M, Bell DA, Boffetta P, Bouchardy C, Caporaso N, Chen C, Coutelle C, Diehl SR, et al. Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer: A HuGE review. Am J Epidemiol. 2004;159:1–16. doi: 10.1093/aje/kwh003. [DOI] [PubMed] [Google Scholar]
- 38.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 39.Bosron WF, Li TK. Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology. 1986;6:502–510. doi: 10.1002/hep.1840060330. [DOI] [PubMed] [Google Scholar]
- 40.Osier M, Pakstis AJ, Kidd JR, Lee JF, Yin SJ, Ko HC, Edenberg HJ, Lu RB, Kidd KK. Linkage disequilibrium at the ADH2 and ADH3 loci and risk of alcoholism. Am J Hum Genet. 1999;64:1147–1157. doi: 10.1086/302317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokoyama A, Kato H, Yokoyama T, Tsujinaka T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M, et al. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferase M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis. 2002;23:1851–1859. doi: 10.1093/carcin/23.11.1851. [DOI] [PubMed] [Google Scholar]
- 42.Zhang GH, Mai RQ, Huang B. Meta-analysis of ADH1B and ALDH2 polymorphisms and esophageal cancer risk in China. World J Gastroenterol. 2010;16:6020–6025. doi: 10.3748/wjg.v16.i47.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee CH, Lee JM, Wu DC, Goan YG, Chou SH, Wu IC, Kao EL, Chan TF, Huang MC, Chen PS, et al. Carcinogenetic impact of ADH1B and ALDH2 genes on squamous cell carcinoma risk of the esophagus with regard to the consumption of alcohol, tobacco and betel quid. Int J Cancer. 2008;122:1347–1356. doi: 10.1002/ijc.23264. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Ji R, Wei X, Gu L, Chen L, Rong Y, Wang R, Zhang Z, Liu B, Xia S. Esophageal squamous cell carcinoma and ALDH2 and ADH1B polymorphisms in Chinese females. Asian Pac J Cancer Prev. 2011;12:2065–2068. [PubMed] [Google Scholar]
- 45.Wu M, Chang SC, Kampman E, Yang J, Wang XS, Gu XP, Han RQ, Liu AM, Wallar G, Zhou JY, et al. Single nucleotide polymorphisms of ADH1B, ADH1C and ALDH2 genes and esophageal cancer: A population-based case-control study in China. Int J Cancer. 2013;132:1868–1877. doi: 10.1002/ijc.27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muto M, Nakane M, Hitomi Y, Yoshida S, Sasaki S, Ohtsu A, Yoshida S, Ebihara S, Esumi H. Association between aldehyde dehydrogenase gene polymorphisms and the phenomenon of field cancerization in patients with head and neck cancer. Carcinogenesis. 2002;23:1759–1765. doi: 10.1093/carcin/23.10.1759. [DOI] [PubMed] [Google Scholar]
- 47.Terry MB, Gammon MD, Zhang FF, Vaughan TL, Chow WH, Risch HA, Schoenberg JB, Mayne ST, Stanford JL, West AB, et al. Alcohol dehydrogenase 3 and risk of esophageal and gastric adenocarcinomas. Cancer Causes Control. 2007;18:1039–1046. doi: 10.1007/s10552-007-9046-0. [DOI] [PubMed] [Google Scholar]
- 48.Hashibe M, Boffetta P, Zaridze D, Shangina O, Szeszenia-Dabrowska N, Mates D, Janout V, Fabiánová E, Bencko V, Moullan N, et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 2006;15:696–703. doi: 10.1158/1055-9965.EPI-05-0710. [DOI] [PubMed] [Google Scholar]
- 49.Jairam S, Edenberg HJ. An enhancer-blocking element regulates the cell-specific expression of alcohol dehydrogenase 7. Gene. 2014;547:239–244. doi: 10.1016/j.gene.2014.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J, Wei J, Xu X, Pan W, Ge Y, Zhou C, Liu C, Gao J, Yang M, Mao W. Replication study of ESCC susceptibility genetic polymorphisms locating in the ADH1B-ADH1C-ADH7 cluster identified by GWAS. PLoS One. 2014;9:e94096. doi: 10.1371/journal.pone.0094096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hashibe M, McKay JD, Curado MP, Oliveira JC, Koifman S, Koifman R, Zaridze D, Shangina O, Wünsch-Filho V, Eluf-Neto J, et al. Multiple ADH genes are associated with upper aerodigestive cancers. Nat Genet. 2008;40:707–709. doi: 10.1038/ng.151. [DOI] [PubMed] [Google Scholar]
- 52.Oze I, Matsuo K, Suzuki T, Kawase T, Watanabe M, Hiraki A, Ito H, Hosono S, Ozawa T, Hatooka S, et al. Impact of multiple alcohol dehydrogenase gene polymorphisms on risk of upper aerodigestive tract cancers in a Japanese population. Cancer Epidemiol Biomarkers Prev. 2009;18:3097–3102. doi: 10.1158/1055-9965.EPI-09-0499. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Borinskaya S, Yoshimura K, Kal'ina N, Marusin A, Stepanov VA, Qin Z, Khaliq S, Lee MY, Yang Y, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet. 2009;73:335–345. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizoi Y, Yamamoto K, Ueno Y, Fukunaga T, Harada S. Involvement of genetic polymorphism of alcohol and aldehyde dehydrogenases in individual variation of alcohol metabolism. Alcohol Alcohol. 1994;29:707–710. [PubMed] [Google Scholar]
- 55.Yokoyama A, Muramatsu T, Ohmori T, Makuuchi H, Higuchi S, Matsushita S, Yoshino K, Maruyama K, Nakano M, Ishii H. Multiple primary esophageal and concurrent upper aerodigestive tract cancer and the aldehyde dehydrogenase-2 genotype of Japanese alcoholics. Cancer. 1996;77:1986–1990. doi: 10.1002/(SICI)1097-0142(19960515)77:10<1986::AID-CNCR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 56.Yokoyama A, Muramatsu T, Ohmori T, Yokoyama T, Okuyama K, Takahashi H, Hasegawa Y, Higuchi S, Maruyama K, Shirakura K, Ishii H. Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics. Carcinogenesis. 1998;19:1383–1387. doi: 10.1093/carcin/19.8.1383. [DOI] [PubMed] [Google Scholar]
- 57.Lewis SJ, Smith GD. Alcohol, ALDH2, and esophageal cancer: A meta-analysis which illustrates the potentials and limitations of a Mendelian randomization approach. Cancer Epidemiol Biomarkers Prev. 2005;14:1967–1971. doi: 10.1158/1055-9965.EPI-05-0196. [DOI] [PubMed] [Google Scholar]
- 58.Yokoyama T, Yokoyama A, Kato H, Tsujinaka T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M, et al. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol Biomarkers Prev. 2003;12:1227–1233. [PubMed] [Google Scholar]
- 59.Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A. The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis DF, Bird MG, Parke DV. Molecular modelling of CYP2E1 enzymes from rat, mouse and man: An explanation for species differences in butadiene metabolism and potential carcinogenicity, and rationalization of CYP2E substrate specificity. Toxicology. 1997;118:93–113. doi: 10.1016/S0300-483X(96)03583-4. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 62.Guo YM, Wang Q, Liu YZ, Chen HM, Qi Z, Guo QH. Genetic polymorphisms in cytochrome P4502E1, alcohol and aldehyde dehydrogenases and the risk of esophageal squamous cell carcinoma in Gansu Chinese males. World J Gastroenterol. 2008;14:1444–1449. doi: 10.3748/wjg.14.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin JM, Yang L, Chen B, Wang XM, Li F, Liao PH, He L. Interaction of methylenetetrahydrofolate reductase C677T, cytochrome P4502E1 polymorphism and environment factors in esophageal cancer in Kazakh population. World J Gastroenterol. 2008;14:6986–6992. doi: 10.3748/wjg.14.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morita S, Yano M, Shiozaki H, Tsujinaka T, Ebisui C, Morimoto T, Kishibuti M, Fujita J, Ogawa A, Taniguchi M, et al. CYP1A1, CYP2E1 and GSTM1 polymorphisms are not associated with susceptibility to squamous-cell carcinoma of the esophagus. Int J Cancer. 1997;71:192–195. doi: 10.1002/(SICI)1097-0215(19970410)71:2<192::AID-IJC11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 65.Lucas D, Ménez C, Floch F, Gourlaouen Y, Sparfel O, Joannet I, Bodénez P, Jezequel J, Gouérou H, Berthou F, et al. Cytochromes P4502E1 and P4501A1 genotypes and susceptibility to cirrhosis or upper aerodigestive tract cancer in alcoholic caucasians. Alcohol Clin Exp Res. 1996;20:1033–1037. doi: 10.1111/j.1530-0277.1996.tb01943.x. [DOI] [PubMed] [Google Scholar]
- 66.Rossini A, Rapozo DC, Soares Lima SC, Guimarães DP, Ferreira MA, Teixeira R, Kruel CD, Barros SG, Andreollo NA, Acatauassú R, et al. Polymorphisms of GSTP1 and GSTT1, but not of CYP2A6, CYP2E1 or GSTM1, modify the risk for esophageal cancer in a western population. Carcinogenesis. 2007;28:2537–2542. doi: 10.1093/carcin/bgm222. [DOI] [PubMed] [Google Scholar]
- 67.Dura P, Berkers T, van Veen EM, Salomon J, te Morsche RH, Roelofs HM, Kristinsson JO, Wobbes T, Witteman BJ, Tan AC, et al. Polymorphisms in alcohol-metabolizing enzymes and esophageal carcinoma susceptibility: A Dutch Caucasian case-control study. J Hum Genet. 2013;58:742–748. doi: 10.1038/jhg.2013.95. [DOI] [PubMed] [Google Scholar]
- 68.Trimmer EE. Methylenetetrahydrofolate reductase: Biochemical characterization and medical significance. Curr Pharm Des. 2013;19:2574–2593. doi: 10.2174/1381612811319140008. [DOI] [PubMed] [Google Scholar]
- 69.Ly A, Hoyt L, Crowell J, Kim YI. Folate and DNA methylation. Antioxid Redox Sign. 2012;17:302–326. doi: 10.1089/ars.2012.4554. [DOI] [PubMed] [Google Scholar]
- 70.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, Matthews RG, Boers GJ, den Heijer M, Kluijtmans LA, van den Heuvel LP. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 71.Stern LL, Mason JB, Selhub J, Choi SW. Genomic DNA hypomethylation, a characteristic of most cancers, is present in peripheral leukocytes of individuals who are homozygous for the C677T polymorphism in the methylenetetrahydrofolate reductase gene. Cancer Epidemiol Biomarkers Prev. 2000;9:849–853. [PubMed] [Google Scholar]
- 72.Goyette P, Sumner JS, Milos R, Duncan AM, Rosenblatt DS, Matthews RG, Rozen R. Human methylenetetrahydrofolate reductase: Isolation of cDNA, mapping and mutation identification. Nat Genet. 1994;7:195–200. doi: 10.1038/ng0694-195. [DOI] [PubMed] [Google Scholar]
- 73.Bailey LB, Gregory JF., III Polymorphisms of methylenetetrahydrofolate reductase and other enzymes: Metabolic significance, risks and impact on folate requirement. J Nutr. 1999;129:919–922. doi: 10.1093/jn/129.5.919. [DOI] [PubMed] [Google Scholar]
- 74.Li QD, Li H, Wang MS, Diao TY, Zhou ZY, Fang QX, Yang FY, Li QH. Multi-susceptibility genes associated with the risk of the development stages of esophageal squamous cell cancer in Feicheng County. BMC Gastroenterol. 2011;11:74. doi: 10.1186/1471-230X-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao P, Lin F, Li Z, Lin B, Lin J, Luo R. Folate intake, methylenetetrahydrofolate reductase polymorphisms, and risk of esophageal cancer. Asian Pac J Cancer Prev. 2011;12:2019–2023. [PubMed] [Google Scholar]
- 76.Li D, Diao Y, Li H, Fang X, Li H. Association of the polymorphisms of MTHFR C677T, VDR C352T and MPO G463A with risk for esophageal squamous cell dysplasia and carcinoma. Arch Med Res. 2008;39:594–600. doi: 10.1016/j.arcmed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Langevin SM, Lin D, Matsuo K, Gao CM, Takezaki T, Stolzenberg-Solomon RZ, Vasavi M, Hasan Q, Taioli E. Review and pooled analysis of studies on MTHFR C677T polymorphism and esophageal cancer. Toxicol Lett. 2009;184:73–80. doi: 10.1016/j.toxlet.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu YX, Wang B, Wan MH, Tang WF, Huang FK, Li C. Meta-analysis of the relationship between the metholenetetrahydrofolate reductase C677T genetic polymorphism, folate intake and esophageal cancer. Asian Pac J Cancer Prev. 2011;12:247–252. [PubMed] [Google Scholar]
- 79.Yang CX, Matsuo K, Ito H, Shinoda M, Hatooka S, Hirose K, Wakai K, Saito T, Suzuki T, Maeda T, Tajima K. Gene-environment interactions between alcohol drinking and the MTHFR C677T polymorphism impact on esophageal cancer risk: Results of a case-control study in Japan. Carcinogenesis. 2005;26:1285–1290. doi: 10.1093/carcin/bgi076. [DOI] [PubMed] [Google Scholar]
- 80.Song C, Xing D, Tan W, Wei Q, Lin D. Methylene-tetrahydrofolate reductase polymorphisms increase risk of esophageal squamous cell carcinoma in a Chinese population. Cancer Res. 2001;61:3272–3275. [PubMed] [Google Scholar]
- 81.Hiyama T, Yoshihara M, Tanaka S, Chayama K. Genetic polymorphisms and esophageal cancer risk. Int J Cancer. 2007;121:1643–1658. doi: 10.1002/ijc.23044. [DOI] [PubMed] [Google Scholar]
- 82.Wang AH, Sun CS, Li LS, Huang JY, Chen QS, Xu DZ. Genetic susceptibility and environmental factors of esophageal cancer in Xi'an. World J Gastroenterol. 2004;10:940–944. doi: 10.3748/wjg.v10.i7.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li D, Dandara C, Parker MI. The 341C/T polymorphism in the GSTP1 gene is associated with increased risk of oesophageal cancer. BMC Genet. 2010;11:47. doi: 10.1186/1471-2156-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. WHO International Agency for Research on Cancer Monograph Working Group: A review of human carcinogens - Part E: Tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/S1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 85.Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! Shouldn't we do something about it? Alcohol Alcohol. 2007;42:167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- 86.Prabhu A, Obi KO, Rubenstein JH. The synergistic effects of alcohol and tobacco consumption on the risk of esophageal squamous cell carcinoma: A meta-analysis. Am J Gastroenterol. 2014;109:822–827. doi: 10.1038/ajg.2014.71. [DOI] [PubMed] [Google Scholar]
- 87.Yaegashi Y, Onoda T, Morioka S, Hashimoto T, Takeshita T, Sakata K, Tamakoshi A. Joint effects of smoking and alcohol drinking on esophageal cancer mortality in Japanese men: Findings from the Japan collaborative cohort study. Asian Pac J Cancer Prev. 2014;15:1023–1029. doi: 10.7314/APJCP.2014.15.2.1023. [DOI] [PubMed] [Google Scholar]
- 88.Chen J, Zhang N, Wakai T, Wei L, He Y, Kumagai N, Kitsu K, Wang S, Akazawa K. Effect of the interaction between the amount and duration of alcohol consumption and tobacco smoking on the risk of esophageal cancer: A case-control study. Exp Ther Med. 2010;1:991–997. doi: 10.3892/etm.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, Taylor CE, Becker K, Xu J. Smoking in China: Findings of the 1996 National Prevalence Survey. JAMA. 1999;282:1247–1253. doi: 10.1001/jama.282.13.1247. [DOI] [PubMed] [Google Scholar]
- 90.Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S, Egashira A, Saeki H, Kakeji Y, Morita M, Sakaguchi Y, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: Molecular mechanisms of carcinogenesis. Int J Clin Oncol. 2010;15:135–144. doi: 10.1007/s10147-010-0057-6. [DOI] [PubMed] [Google Scholar]
- 91.Lopes CF, de Angelis BB, Prudente HM, de Souza BV, Cardoso SV, de Azambuja Ribeiro RI. Concomitant consumption of marijuana, alcohol and tobacco in oral squamous cell carcinoma development and progression: Recent advances and challenges. Arch Oral Biol. 2012;57:1026–1033. doi: 10.1016/j.archoralbio.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 92.Salaspuro M. Interactions of alcohol and tobacco in gastrointestinal cancer. J Gastroenterol Hepatol. 2013;28:1252. doi: 10.1111/j.1440-1746.2012.07017.x. [DOI] [PubMed] [Google Scholar]
- 93.Simanowski UA, Suter P, Stickel F, Maier H, Waldherr R, Smith D, Russell RM, Seitz HK. Esophageal epithelial hyperproliferation following long-term alcohol consumption in rats: Effects of age and salivary gland function. J Natl Cancer Inst. 1993;85:2030–2033. doi: 10.1093/jnci/85.24.2030. [DOI] [PubMed] [Google Scholar]
- 94.Maier H, Weidauer H, Zöller J, Seitz HK, Flentje M, Mall G, Born IA. Effect of chronic alcohol consumption on the morphology of the oral mucosa. Alcohol Clin Exp Res. 1994;18:387–391. doi: 10.1111/j.1530-0277.1994.tb00030.x. [DOI] [PubMed] [Google Scholar]
- 95.Homann N, Kärkkäinen P, Koivisto T, Nosova T, Jokelainen K, Salaspuro M. Effects of acetaldehyde on cell regeneration and differentiation of the upper gastrointestinal tract mucosa. J Natl Cancer Inst. 1997;89:1692–1697. doi: 10.1093/jnci/89.22.1692. [DOI] [PubMed] [Google Scholar]
- 96.Homann N, Jousimies-Somer H, Jokelainen K, Heine R, Salaspuro M. High acetaldehyde levels in saliva after ethanol consumption: Methodological aspects and pathogenetic implications. Carcinogenesis. 1997;18:1739–1743. doi: 10.1093/carcin/18.9.1739. [DOI] [PubMed] [Google Scholar]
- 97.Yokoyama A, Tsutsumi E, Imazeki H, Suwa Y, Nakamura C, Yokoyama T. Contribution of the alcohol dehydrogenase-1B genotype and oral microorganisms to high salivary acetaldehyde concentrations in Japanese alcoholic men. Int J Cancer. 2007;121:1047–1054. doi: 10.1002/ijc.22792. [DOI] [PubMed] [Google Scholar]
- 98.Maier H, Born IA, Veith S, Adler D, Seitz HK. The effect of chronic ethanol consumption on salivary gland morphology and function in the rat. Alcohol Clin Exp Res. 1986;10:425–427. doi: 10.1111/j.1530-0277.1986.tb05117.x. [DOI] [PubMed] [Google Scholar]
- 99.Salaspuro MP. Acetaldehyde, microbes, and cancer of the digestive tract. Crit Rev Clin Lab Sci. 2003;40:183–208. doi: 10.1080/713609333. [DOI] [PubMed] [Google Scholar]
- 100.Salaspuro V, Salaspuro M. Synergistic effect of alcohol drinking and smoking on in vivo acetaldehyde concentration in saliva. Int J Cancer. 2004;111:480–483. doi: 10.1002/ijc.20293. [DOI] [PubMed] [Google Scholar]
- 101.Holmstrup P, Bessermann M. Clinical, therapeutic, and pathogenic aspects of chronic oral multifocal candidiasis. Oral Surg Oral Med Oral Pathol. 1983;56:388–395. doi: 10.1016/0030-4220(83)90349-3. [DOI] [PubMed] [Google Scholar]
- 102.Colman G, Beighton D, Chalk AJ, Wake S. Cigarette smoking and the microbial flora of the mouth. Aust Dent J. 1976;21:111–118. doi: 10.1111/j.1834-7819.1976.tb02833.x. [DOI] [PubMed] [Google Scholar]
- 103.Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: Bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 104.Shapiro KB, Hotchkiss JH, Roe DA. Quantitative relationship between oral nitrate-reducing activity and the endogenous formation of N-nitrosoamino acids in humans. Food Chem Toxicol. 1991;29:751–755. doi: 10.1016/0278-6915(91)90183-8. [DOI] [PubMed] [Google Scholar]
- 105.Ahn J, Chen CY, Hayes RB. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23:399–404. doi: 10.1007/s10552-011-9892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pavlova SI, Jin L, Gasparovich SR, Tao L. Multiple alcohol dehydrogenases but no functional acetaldehyde dehydrogenase causing excessive acetaldehyde production from ethanol by oral streptococci. Microbiology. 2013;159:1437–1446. doi: 10.1099/mic.0.066258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muto M, Hitomi Y, Ohtsu A, Shimada H, Kashiwase Y, Sasaki H, Yoshida S, Esumi H. Acetaldehyde production by non-pathogenic Neisseria in human oral microflora: Implications for carcinogenesis in upper aerodigestive tract. Int J Cancer. 2000;88:342–350. doi: 10.1002/1097-0215(20001101)88:3<342::AID-IJC4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 108.Väkeväinen S, Tillonen J, Blom M, Jousimies-Somer H, Salaspuro M. Acetaldehyde production and other ADH-related characteristics of aerobic bacteria isolated from hypochlorhydric human stomach. Alcohol Clin Exp Res. 2001;25:421–426. doi: 10.1111/j.1530-0277.2001.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 109.Homann N, Tillonen J, Meurman JH, Rintamäki H, Lindqvist C, Rautio M, Jousimies-Somer H, Salaspuro M. Increased salivary acetaldehyde levels in heavy drinkers and smokers: A microbiological approach to oral cavity cancer. Carcinogenesis. 2000;21:663–668. doi: 10.1093/carcin/21.4.663. [DOI] [PubMed] [Google Scholar]
- 110.Homann N, Tillonen J, Rintamäki H, Salaspuro M, Lindqvist C, Meurman JH. Poor dental status increases acetaldehyde production from ethanol in saliva: A possible link to increased oral cancer risk among heavy drinkers. Oral Oncol. 2001;37:153–158. doi: 10.1016/S1368-8375(00)00076-2. [DOI] [PubMed] [Google Scholar]
- 111.Tillonen J, Homann N, Rautio M, Jousimies-Somer H, Salaspuro M. Role of yeasts in the salivary acetaldehyde production from ethanol among risk groups for ethanol-associated oral cavity cancer. Alcohol Clin Exp Res. 1999;23:1409–1415. doi: 10.1111/j.1530-0277.1999.tb04364.x. [DOI] [PubMed] [Google Scholar]
- 112.Tang L, Lee AH, Xu F, Zhang T, Lei J, Binns CW. Fruit and vegetable consumption and risk of esophageal cancer: A case-control study in north-west China. Dis Esophagus. 2014;27:777–782. doi: 10.1111/dote.12157. [DOI] [PubMed] [Google Scholar]
- 113.Jeurnink SM, Büchner FL, Bueno-de-Mesquita HB, Siersema PD, Boshuizen HC, Numans ME, Dahm CC, Overvad K, Tjønneland A, Roswall N, et al. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2012;131:E963–E973. doi: 10.1002/ijc.27517. [DOI] [PubMed] [Google Scholar]
- 114.Lucenteforte E, Garavello W, Bosetti C, Talamini R, Zambon P, Franceschi S, Negri E, La Vecchia C. Diet diversity and the risk of squamous cell esophageal cancer. Int J Cancer. 2008;123:2397–2400. doi: 10.1002/ijc.23761. [DOI] [PubMed] [Google Scholar]
- 115.Freedman ND, Park Y, Subar AF, Hollenbeck AR, Leitzmann MF, Schatzkin A, Abnet CC. Fruit and vegetable intake and esophageal cancer in a large prospective cohort study. Int J Cancer. 2007;121:2753–2760. doi: 10.1002/ijc.22993. [DOI] [PubMed] [Google Scholar]
- 116.Choi SW, Mason JB. Folate and carcinogenesis: An integrated scheme. J Nutr. 2000;130:129–132. doi: 10.1093/jn/130.2.129. [DOI] [PubMed] [Google Scholar]
- 117.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: A meta-analysis. Gastroenterology. 2006;131:1271–1283. doi: 10.1053/j.gastro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 118.Tio M, Andrici J, Cox MR, Eslick GD. Folate intake and the risk of upper gastrointestinal cancers: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:250–258. doi: 10.1111/jgh.12446. [DOI] [PubMed] [Google Scholar]
- 119.Xiao Q, Freedman ND, Ren J, Hollenbeck AR, Abnet CC, Park Y. Intakes of folate, methionine, vitamin B6, and vitamin B12 with risk of esophageal and gastric cancer in a large cohort study. Br J Cancer. 2014;110:1328–1333. doi: 10.1038/bjc.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ulrich CM. Folate and cancer prevention: A closer look at a complex picture. Am J Clin Nutr. 2007;86:271–273. doi: 10.1093/ajcn/86.2.271. [DOI] [PubMed] [Google Scholar]
- 121.Ibiebele TI, Hughes MC, Pandeya N, Zhao Z, Montgomery G, Hayward N, Green AC, Whiteman DC, Webb PM. Study of Digestive Health; Australian Cancer Study: High intake of folate from food sources is associated with reduced risk of esophageal cancer in an Australian population. J Nutr. 2011;141:274–283. doi: 10.3945/jn.110.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Matsuo K, Rossi M, Negri E, Oze I, Hosono S, Ito H, Watanabe M, Yatabe Y, Hasegawa Y, Tanaka H, et al. Folate, alcohol, and aldehyde dehydrogenase 2 polymorphism and the risk of oral and pharyngeal cancer in Japanese. Eur J Cancer Prev. 2012;21:193–198. doi: 10.1097/CEJ.0b013e32834c9be5. [DOI] [PubMed] [Google Scholar]
- 123.Shanmugham JR, Zavras AI, Rosner BA, Giovannucci EL. Alcohol-folate interactions in the risk of oral cancer in women: A prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2010;19:2516–2524. doi: 10.1158/1055-9965.EPI-10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Halsted CH, Villanueva JA, Devlin AM, Chandler CJ. Metabolic interactions of alcohol and folate. J Nutr. 2002;132(Suppl 8):2367S–2372S. doi: 10.1093/jn/132.8.2367S. [DOI] [PubMed] [Google Scholar]
- 125.Hamid A, Wani NA, Kaur J. New perspectives on folate transport in relation to alcoholism-induced folate malabsorption - association with epigenome stability and cancer development. FEBS J. 2009;276:2175–2191. doi: 10.1111/j.1742-4658.2009.06959.x. [DOI] [PubMed] [Google Scholar]
- 126.Biswas A, Senthilkumar SR, Said HM. Effect of chronic alcohol exposure on folate uptake by liver mitochondria. Am J Physiol Cell Physiol. 2012;302:C203–C209. doi: 10.1152/ajpcell.00283.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wani NA, Hamid A, Kaur J. Alcohol-associated folate disturbances result in altered methylation of folate-regulating genes. Mol Cell Biochem. 2012;363:157–166. doi: 10.1007/s11010-011-1168-8. [DOI] [PubMed] [Google Scholar]
- 128.Said HM, Mee L, Sekar VT, Ashokkumar B, Pandol SJ. Mechanism and regulation of folate uptake by pancreatic acinar cells: Effect of chronic alcohol consumption. Am J Physiol Gastrointest Liver Physiol. 2010;298:G985–G993. doi: 10.1152/ajpgi.00068.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wani NA, Nada R, Khanduja KL, Kaur J. Decreased activity of folate transporters in lipid rafts resulted in reduced hepatic folate uptake in chronic alcoholism in rats. Genes Nutr. 2013;8:209–219. doi: 10.1007/s12263-012-0318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mason JB, Choi SW. Effects of alcohol on folate metabolism: Implications for carcinogenesis. Alcohol. 2005;35:235–241. doi: 10.1016/j.alcohol.2005.03.012. [DOI] [PubMed] [Google Scholar]