Abstract
Cardiac tissue engineering is a strategy to replace damaged contractile tissue and model cardiac diseases to discover therapies. Current cardiac and vascular engineering approaches independently create aligned contractile tissue or perfusable vasculature, but a combined vascularized cardiac tissue remains to be achieved. Here, we sought to incorporate a patterned microvasculature into engineered heart tissue, which balances the competing demands from cardiomyocytes to contract the matrix versus the vascular lumens that need structural support. Low-density collagen hydrogels (1.25 mg/mL) permit human embryonic stem cell-derived cardiomyocytes (hESC-CMs) to form a dense contractile tissue but cannot support a patterned microvasculature. Conversely, high collagen concentrations (density ≥6 mg/mL) support a patterned microvasculature, but the hESC-CMs lack cell–cell contact, limiting their electrical communication, structural maturation, and tissue-level contractile function. When cocultured with matrix remodeling stromal cells, however, hESC-CMs structurally mature and form anisotropic constructs in high-density collagen. Remodeling requires the stromal cells to be in proximity with hESC-CMs. In addition, cocultured cardiac constructs in dense collagen generate measurable active contractions (on the order of 0.1 mN/mm2) and can be paced up to 2 Hz. Patterned microvascular networks in these high-density cocultured cardiac constructs remain patent through 2 weeks of culture, and hESC-CMs show electrical synchronization. The ability to maintain microstructural control within engineered heart tissue enables generation of more complex features, such as cellular alignment and a vasculature. Successful incorporation of these features paves the way for the use of large scale engineered tissues for myocardial regeneration and cardiac disease modeling.
Introduction
A recent report from the American Heart Association attributes one in six deaths every year to coronary heart disease, which commonly presents with tissue ischemia and/or myocardial infarction.1 With the exception of whole heart transplantation, there are currently no treatments to prevent the decline in cardiac function that occurs after an ischemic assault and cardiomyocyte death. Transplantation with human embryonic stem cell-derived cardiomyocytes (hESC-CMs) has shown promise in a number of animal models to regenerate the damaged heart tissue.2–6
An attractive alternative is the tissue engineering approach, where three-dimensional (3D) structural control and tissue maturation before implantation are possible.7 Many groups have had considerable success forming contractile cardiac constructs within native soft biological hydrogels, such as fibrin,8,9 collagen,10–12 and decellularized heart matrix,13 as well as in scaffold-free conditions.14,15 Engineering large scale cardiac tissues, however, continues to be hindered by a lack of vasculature and poor structural and functional maturation of stem cell-derived cardiomyocytes.
The heart is the most metabolically demanding organ in the body. Every cardiomyocyte is adjacent to at least one capillary to fuel contractile function.16 Vasculature and blood flow are critical in not only supplying nutrients and oxygen but also in modulating cardiomyocyte maturation,17 subtype specification,18 and ischemic protection.19 In engineered cardiac tissues, vascular structures have been generated by combining myocytes, endothelial cells, and a mural cell population, such as mesenchymal stem cells or fibroblasts.11,14,15,20–22 Under these conditions, de novo lumens and cord-like structures form through endothelial cell self-assembly. These “tri-cellular” constructs were further shown to have increased active twitch force,23 suggesting the importance of paracrine signals from nonmyocytes.
When transplanted, the self-assembled vessels in these constructs integrate to some degree with host circulation;15,21,22 however, they lack an orderly branching hierarchy, and graft–host integration is slow relative to the immediate perfusion needs of the construct.24,25 Attempts have been made to enhance vascular density within the constructs by tuning matrix composition, matrix structure, biochemical signals, or mechanical cues.26–35 Nonetheless, outstanding challenges exist due to sluggish perfusion in the self-assembled vessels, which limits the function and integration of the cardiac constructs. An ideal construct would have both perfusable vasculature that recapitulates the hemodynamic environment of coronary vessels and appropriate characteristics for myocardial function such as a compliance-matched matrix, myofibril maturation and alignment, electrical propagation, and force generation.
In this study, we developed a cardiac tissue construct that has sufficient mechanical strength to support fabrication and patency of a perfusable vascular network and optimized cellular composition to promote cardiomyocyte survival, organization, and maturation. We showed that although dense collagen (≥6 mg/mL) is required for microchannel fabrication, it does not support cardiomyocyte structure and function. We demonstrated that coculturing with a stromal cell population promoted matrix remodeling and cardiomyocyte alignment and allowed for the formation of a functional vascularized cardiac tissue in dense collagen. Our work represents an important step toward generating perfusable vascularized cardiac tissues in vitro and suggests a key role for stromal cells in matrix organization and cardiac maturation.
Materials and Methods
Cell culture
Spontaneously beating cardiomyocytes (Supplementary Video S1; Supplementary Data are available online at www.liebertpub.com/tea) were generated from high-density monolayers of RUES2 human embryonic stem cells (hESCs) using a combination of activin A, BMP4, and small molecule Wnt activation/inhibition as described previously3 and in the Supplementary Data. For a subset of experiments, cardiomyocytes were generated from transgenic RUES2 hESCs that constitutively express GCaMP3, a fluorescent calcium reporter. Zinc-Finger nuclease (ZFN)-mediated targeting was used to construct this line as previously described.5 Cardiomyocyte cultures averaged 64.1% ± 19.3% cardiac troponin T (cTnT) positive by flow cytometry, and this preparation is referred to as “cardiomyocytes” or hESC-CM throughout the article.
Four different stromal cells were used in this study, including two human bone marrow-derived stromal cell lines (HS27a and HS5),36 human dermal fibroblasts (hDFs; Lonza), and primarily isolated human fetal heart perivascular cells. See Supplementary Data for more detail on cardiomyocyte differentiation and stromal cell culture.
Cardiac construct formation and culture
Tissue molds were fabricated through molding of uncured polydimethylsiloxane (PDMS) (Dow Corning Sylgard 184 silicone elastomer) into a laser-etched acrylic negative template with 17 × 3 mm wells containing 1 mm diameter posts at each end to provide uniaxial strain (Fig. 1A). The molded PDMS was baked at 65°C for at least 1 h and then peeled away for autoclave sterilization. Immediately before construct formation, the PDMS wells were treated with a hand-held corona treater (BD-20; Electrotechnic Products, Inc.) to make surfaces hydrophilic.
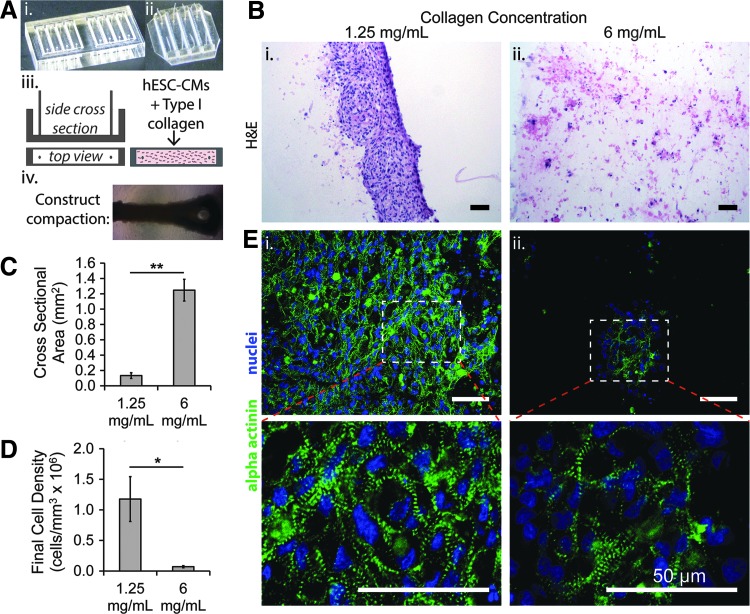
FIG. 1.
Three-dimensional cardiac constructs with hESC-CMs only in low- and high-density collagen. (A) Schematic of construct formation in rectangular strained system: An acrylic negative template (i) was used to generate PDMS molds with posts at each end (ii) that was then seeded with collagen premixed with hESC-CMs (iii). The resulting cardiac construct remodeled and compacted the gel after 2–3 days (iv). (B) Representative images of hemotoxylin and eosin (H&E) staining of constructs in (i) 1.25 mg/mL and (ii) 6 mg/mL collagen after culture for 14 days. (C, D) Final cross-sectional area (C) and cellularity (D) of constructs after 2 week culture (*p < 0.05, **p < 0.01; N = 4–7 biological replicates). (E) Confocal images of sarcomeric α actinin and nuclei in (i) 1.25 mg/mL and (ii) 6 mg/mL collagen at 40× (top panel) and ×3 optical zoom (bottom panel). Scale bars: 50 μm. hESC-CMs, human embryonic stem cell-derived cardiomyocytes; PDMS, polydimethylsiloxane.
Cells were mixed with preneutralized collagen gel (2.5 or 10 mg/mL, see Supplementary Data for collagen stock preparation) to achieve final collagen concentration of 1.25 or 6 mg/mL, respectively. Final cell–gel mixtures of 60 μL were pipetted into each well and allowed to gel for 45 min at 37°C. After gelation, constructs were given RPMI-B27 (with insulin) and fed every other day for 14 days. All cocultured constructs were made with a 1:1 cell density ratio of cardiomyocytes to stromal cells. Final cardiomyocyte density in all constructs was ∼16 × 106 per mL.
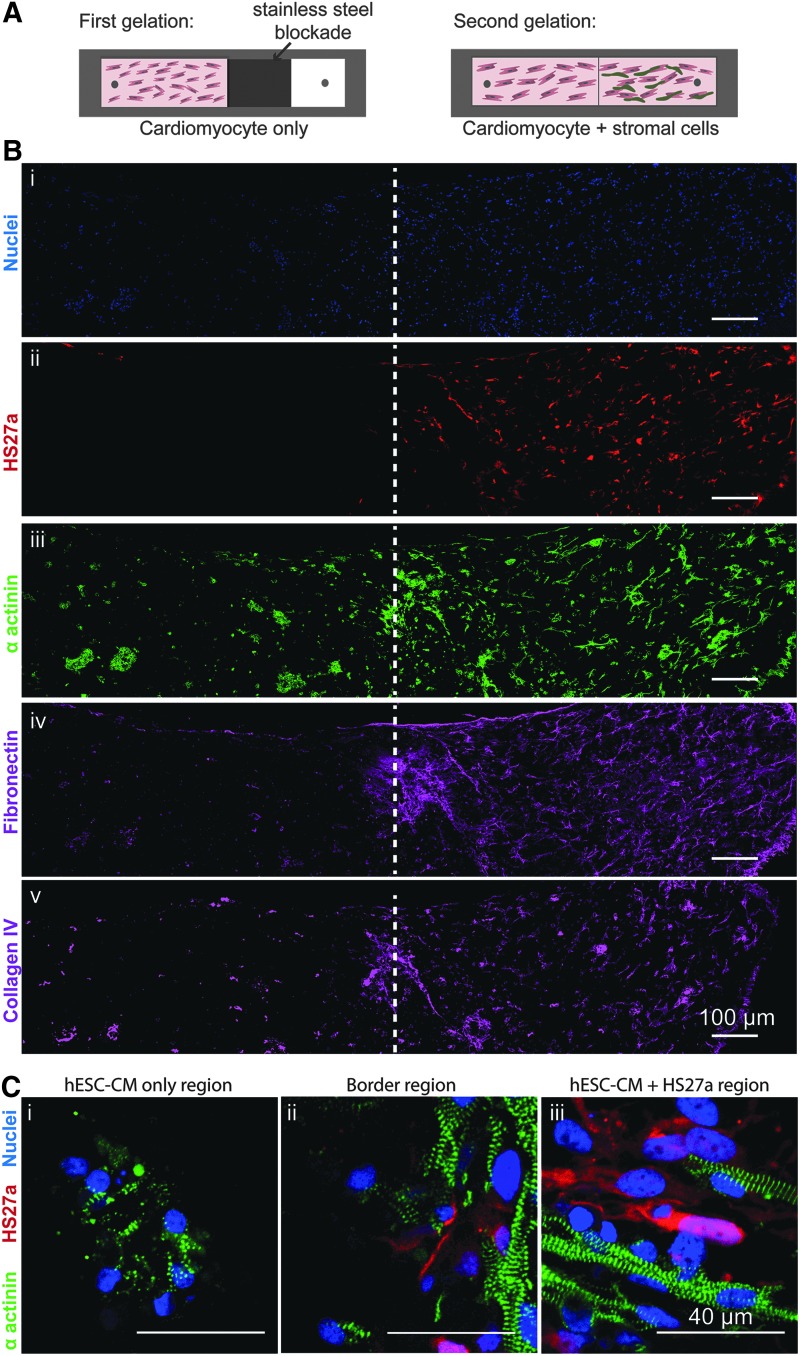
Constructs for sharp boundary experiments were made by two-step gelation: first, a stainless steel block was placed in one half of the well to allow the cardiomyocyte-only side to undergo 30 min of gelation, then the block was removed and the coculture mixture (cardiomyocytes and HS27a stromal cells in liquid collagen) was added, followed by an additional 30 min gelation period. All constructs were cultured for 14 days, fixed, histologically processed, and stained (Supplementary Data). Constructs were fixed while under constant strain in the tissue molds.
Contractile force generation analysis
Functional analysis was carried out on constructs after 2 weeks of maturation as previously described11 (Supplementary Data). In brief, construct sections were stretched in 5% increments up to 20–25% of their total length, and their resulting passive tension and spontaneous active force traces were recorded and analyzed using customized LabView software. Stress–strain relationships were recorded for each construct at basal conditions with no electrical stimulation. After that, the constructs were stimulated at maximum stretch (25%) at 1, 1.5, 2, and 3 Hz using a field stimulator with 5V and 40 ms pulse duration. The spontaneous beating rate of each construct was determined by taking the inverse of time between active contractions at maximum stretch in unstimulated preparations.
Microvessel fabrication
GCaMP3 hESC-CMs (16 × 106 per mL, final) and stromal cells (16 × 106 per mL, final) were mixed with dense collagen (6 mg/mL final concentration). Microfeatures were embedded in the cell-containing gel using techniques of injection molding and soft lithography as previously described.37 Collagen with density less than 4 mg/mL fails to maintain mechanical integrity for the microstructures. The final constructs have dimensions of 8 × 17 × 1 mm and parallel posts flanking the construct to provide uniaxial strain. A patterned network was molded into the top device by using a microfabricated PDMS stamp with channel dimensions 125 × 125 μm on the bottom surface of the top device. After gelation, the devices were assembled together to create an enclosed microfluidic network.
The network was seeded with 10 μL human umbilical vein endothelial cell (passage 5) suspension at a density of 10 × 106 per mL in endothelial growth medium (EGM; Lonza). After 1 h of attachment under static conditions, EGM was perfused through the microvessels under gravity-driven flow through attached reservoirs (initial pressure of 1 cm H2O, replenished every 12 h). Constructs were additionally submerged in a 3:1 mixture of cardiomyocyte media (RPMI 1640 basal medium with B27 Supplement [Invitrogen] containing insulin) and EGM.
At days 7 and 14 of culture, GCaMP3 fluorescence was observed and recorded with a Canon HD video camera (HF S20) to assess cardiomyocyte function and tissue-level electrical properties. After 14 days, the constructs were fixed and stained for cTnT (1:100; mouse antihuman; Fisher) to label cardiomyocytes and CD31 (1:25; rabbit antihuman; Abcam) to label endothelial cells, followed by imaging in situ with confocal fluorescence microscopy. All antibodies and solutions were perfused through the network and used to submerge the construct.
Statistics
Single variable analysis between two samples was compared by Student's t-test assuming unequal variance. Unless otherwise noted, at least three biological replicates were performed with two to three technical replicates in each. A number of biological replicates were used to determine significance. Results are presented as mean ± SEM. For all results, significance was defined as *p < 0.05, **p < 0.01.
Detailed methods are included in Supplementary Data.
Results
Cardiomyocytes alone in dense collagen do not form functional cardiac tissue
The incorporation of perfusable microvessels in collagen-based constructs requires the use of dense collagen to maintain structural integrity of the fabricated channels.38 To evaluate the influence of collagen density on the structure and function of cardiac constructs in a higher throughput manner, we used rectangular wells (Fig. 1A) to generate cardiac tissue constructs under uniaxial strain with hESC-CMs in both low (1.25 mg/mL) and high (6 mg/mL) collagen concentrations (initial complex moduli of 81.0 ± 14.8 and 281.8 ± 17.1 Pa, respectively—Supplementary Data).
After 2 weeks in culture, hESC-CMs in 1.25 mg/mL remodeled the collagen gel and formed compact tissues, with cross-sectional areas of 0.133 ± 0. 038 mm2 (Fig. 1A-iv, B-i). In 6 mg/mL collagen, hESC-CMs were unable to compact the matrix, which resulted in isolated cell islands and a lack of cell–cell contact (Fig. 1B-ii). The cross-sectional area of the constructs was 1.247 ± 0.142 mm2, nearly 10-fold higher than in low-density collagen (Fig. 1C). The greater degree of compaction in low-density collagen led to a final cell density of 1.2 ± 0.4 × 106 per mm3, ∼20-fold higher than in 6 mg/mL (Fig. 1D).
hESC-CMs showed markedly different morphology in collagen of different densities. In collagen of 1.25 mg/mL, cardiomyocytes expressed α actinin robustly throughout the construct in a striated pattern, indicating sarcomere formation in the majority of cells (Fig. 1E-i). In collagen of 6 mg/mL, regions of striated α actinin expression were visible, but these regions were sparse and isolated (Fig. 1E-ii). To evaluate electrical synchronization of the tissues, we performed fluorescent imaging of calcium transients. In collagen of 1.25 mg/mL, there was synchronous excitation and contraction throughout the whole constructs (Supplementary Video S2). In collagen of 6 mg/mL, electrical activation events were isolated with no propagation, indicating the cardiomyocytes were alive and able to undergo spontaneous excitation, but were not connected or electrically synchronized (Supplementary Video S3).
Collectively, these results suggest that cardiomyocytes in dense collagen retained necessary molecular and structural components for electromechanical function, but were unable to organize into an integrated tissue.
Stromal cell coculture promotes cardiomyocyte organization and matrix remodeling in both low- and high-density collagen
Stromal cells have been shown to be critical for matrix synthesis and remodeling during normal development and cardiac regeneration.15,39 In a normal adult mammalian heart, stromal and fibroblast populations contribute to approximately half of the total cell numbers.39,40 To improve the structure and function of our cardiac constructs, we cocultured cardiomyocytes with human stromal cells (bone marrow-derived HS27a line36) in both low- and high-density collagen. In both collagen matrices, cocultured constructs were significantly more organized, with higher cellularity and greater alignment (Fig. 2). hESC-CMs had more organized striated α actinin and a larger degree of alignment in cocultured constructs than in cardiomyocyte-only constructs (Fig. 2A-ii, B-ii, E).
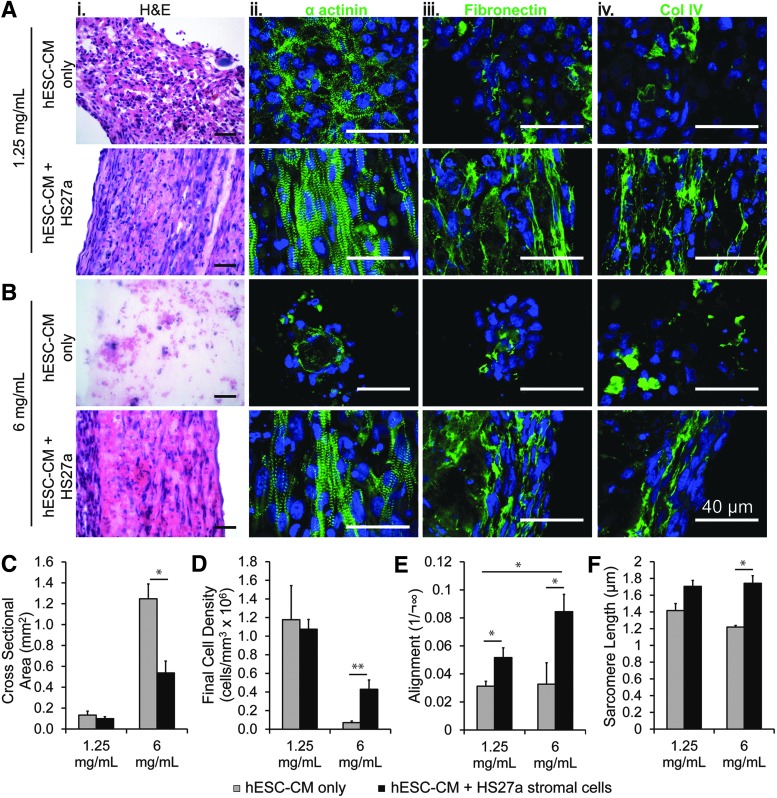
FIG. 2.
Effect of HS27a stromal cell incorporation on cardiac constructs. (A, B) Representative histological sections of constructs in (A) 1.25 mg/mL and (B) 6 mg/mL collagen after culture for 14 days (top panels: cardiomyocyte-only condition; bottom panels: cocultured cardiomyocytes and stromal cell condition); representative images are shown for constructs stained for H&E (i), sarcomeric α actinin (ii), fibronectin (iii), and collagen IV (iv). (C–F) Quantitative comparison of (C) final cross-sectional area, (D) final cell density, (E) cardiomyocyte alignment (inverse of dispersion angle measured on α actinin striations), and (F) sarcomere length (*p < 0.05; **p < 0.01; N = 2–4 biological replicates). Scale bars: 40 μm.
Coculture increased expression and organization of matrix proteins, predominately fibronectin and collagen IV, both of which appeared to align along the direction of axial strain and cellular elongation (Fig. 2A-iii, iv, B-iii, iv). The matrix constituents heparin sulfate, laminin, and versican were not detected in these cocultured constructs by immunohistochemistry (data not shown). In dense collagen particularly, coculture led to a significant improvement in tissue compaction and homogeneity with a nearly 60% decrease in cross-sectional area (Fig. 2C) and a sixfold increase in cell density (Fig. 2D) compared with cardiomyocyte-only conditions.
Myofibrils showed significantly enhanced alignment along the direction of uniaxial strain for the coculture conditions in both low and high collagen matrix (Fig. 2E). Cardiomyocyte sarcomere length also increased significantly with coculture in high-density collagen (Fig. 2F), indicating greater myofilament organization and maturation. These findings suggest that stromal cells promote matrix synthesis and remodeling required for hESC-derived cardiomyocytes to elongate and structurally mature.
Cocultured dense collagen constructs are electromechanically functional
An essential requirement for engineering functional myocardial tissue is its ability to propagate an electrical signal followed by whole construct contraction and force generation. Cocultured constructs with hESC-CMs and stromal cells in both 6 mg/mL and 1.25 mg/mL collagen showed calcium wave propagation and contraction throughout the whole construct, indicating sufficient cell–cell contacts and maturation of hESC-CMs (Supplementary Video S4 and S5, respectively).
We further mounted the constructs between a force transducer and a motor (Fig. 3A) and measured the length dependence of force generation for each construct by stretching incrementally with 5–10% steps and recording twitch force at each step length (Fig. 3B). All constructs produced increasing twitch force with increased strain, demonstrating the tissue-level correlate of the Frank–Starling relationship, where increased length triggers increased force of contraction (Supplementary Fig. S1). Cocultured constructs in 6 mg/mL collagen produced a peak active force of 0.1 mN/mm2, which is comparable to cocultured constructs generated in low collagen (Supplementary Fig. S1) as well as previously reported values for collagen-based engineered cardiac tissue.7,11,41
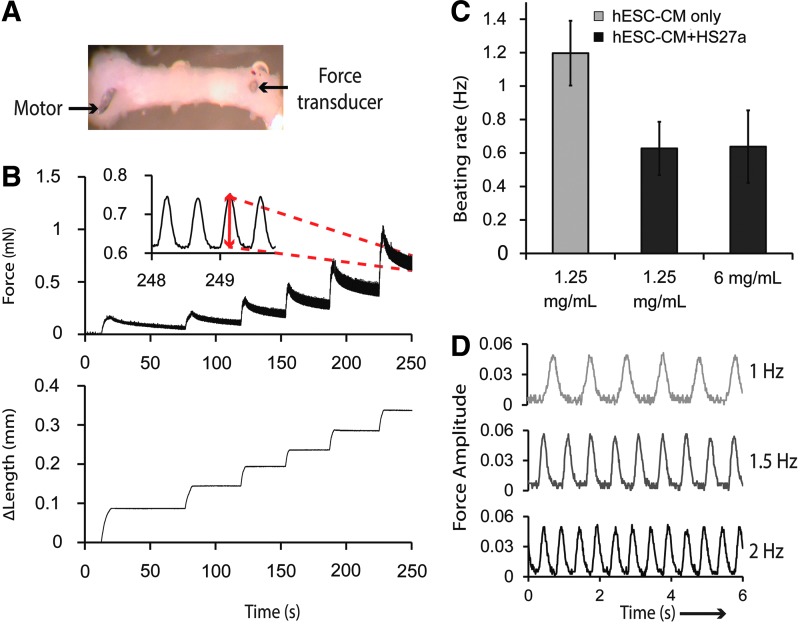
FIG. 3.
Functional characterization of cardiac constructs after 2 week culture. (A) A construct mounted between a force transducer and a motor. (B) Representative force trace displaying passive tension and active twitch force during incremental step-wise length increases for a cocultured construct in 6 mg/mL collagen. Inset: enlarged view of active force trace at maximum strain with red arrow indicating the amplitude of active twitch force (∈ = 0.25). (C) Spontaneous beating rate of constructs measured at time of force measurement acquisition at 37°C (N = 3 biological replicates) (D) Sample stimulation traces at ∈ = 0.25 of cocultured constructs in 6 mg/mL paced at 1, 1.5, and 2 Hz. Color images available online at www.liebertpub.com/tea
A functional engineered cardiac construct is expected to follow a range of stimulation frequencies to electrically synchronize with the host during future therapeutic applications. We measured the spontaneous beating rate of cardiac constructs since automaticity is a key indicator of cardiac subtype and electrophysiological maturation.18,42,43 In constructs with cardiomyocyte-only conditions in 1.25 mg/mL collagen, the average spontaneous beating rate is 1.2 Hz. Coculture conditions resulted in a slight decrease of spontaneous beating rate to ∼0.6 Hz in both 1.25 and 6 mg/mL collagen, although the decrease is not statistically significant (Fig. 3C).
Importantly, all three groups, including cocultured constructs in dense collagen, were able to reliably follow an electrical stimulation pace up to 2 Hz (Fig. 3D) while maintaining the same magnitude of force generation (Supplementary Fig. S2). In response to 3 Hz stimulation, all construct groups were unable to follow the pace consistently. These findings are consistent with previous studies showing that fibroblasts reduce the intrinsic beating frequency of cardiomyocytes, and nonmyocytes play a role in modulating the electrophysiological maturation of stem cell-derived cardiomyocytes during differentiation.44,45
The type of stromal cells in cocultured constructs influences their interactions with cardiomyocytes and matrix remodeling
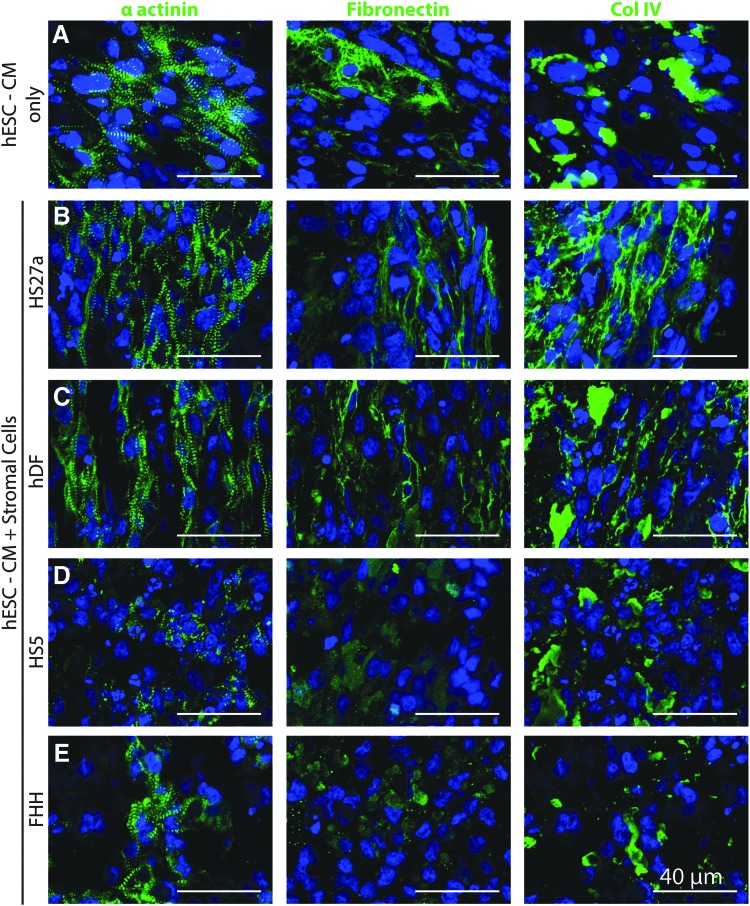
Different populations of “stromal cells” or “fibroblasts” have drastic differences in their phenotype, gene expression, and signaling.46 To understand how stromal cells from different sources affect remodeling and cellular alignment in cardiac constructs, we generated cardiac constructs by coculturing cardiomyocytes with four different fibroblast or stromal cell lines in low-density collagen for 4 days. These stromal cells included two immortalized human bone marrow-derived stromal cell lines (named HS27a and HS536), primary hDFs, and primary isolated fetal human heart NG2+/PDGFRβ+ cells (FHH). HS27a and HS5 have been extensively characterized and although both lines secrete a plethora of ECM proteins, they have distinct functions due to differing expression of matrix proteases and cytokines.36,47,48
hDFs are known to secrete vast amounts of extracellular matrix and matrix degradation proteins normally and during wound healing.49 FHH cells were selected based on their expression of pericyte markers NG2 and PDGFRβ, suggesting a more pericyte-like function of vascular stabilization.50,51 When these different stromal cells were cocultured with cardiomyocytes in collagen constructs, distinct differences were observed regarding cellular and matrix alignment.
Compared to constructs with cardiomyocyte-only conditions, HS27a and hDF cocultured constructs showed greatly improved cardiomyocyte alignment and matrix synthesis (Fig. 4A–C), similar to results shown in Figure 2. However, the HS5 and FHH cocultured constructs had no improvement in cardiomyocyte structure and organization and no evident cellular or matrix alignment (Fig. 4D, E). Furthermore, coculturing with HS5 resulted in poor construct integrity with a breakdown of matrix and structure after ∼4 days in culture. Cocultured constructs also had upregulated mRNA expression of fibronectin (FN1) and collagen I (COL1A2) with the most pronounced increases seen in HS27a and hDF cocultured constructs (Supplementary Fig. S3). Taken together, these results suggest that HS27a and hDF promote matrix remodeling that is suitable for promoting cardiomyocyte growth and organization.
FIG. 4.
Effect of different stromal cells on cardiac construct remodeling. Representative immunofluorescent images of α actinin (left), fibronectin (middle), and collagen IV (right) on cryosections of constructs formed by (A) hESC-CMs only or with (B–E) coculture conditions using various stromal cell lines: (B) bone marrow-derived HS27a stromal cells, (C) human dermal fibroblasts (hdF), (D) bone marrow-derived HS5 stromal cells, and (E) fetal human heart NG2+/PDGFRβ+ cells (FHH). All constructs here are generated in 1.25 mg/mL collagen and processed after 4 days of culture. N = 3–4 biological replicates (each with two to three technical replicates). Color images available online at www.liebertpub.com/tea
Cardiomyocyte alignment requires proximity to stromal cells
To understand how stromal cells provide cues for cardiomyocyte alignment and maturation, we generated a composite construct in 6 mg/mL collagen with a sharp boundary between two halves: one half containing only cardiomyocytes and the other half cocultured with HS27a stromal cells (Fig. 5A). After 14 days of culture, stromal cells remained in the cocultured half region with minimal migration (Fig. 5B-i, ii). Consistent with the stand-alone construct conditions, the cocultured half of the construct had higher and more integrated cellularity (Fig. 5B-i, ii) with stronger and more uniform α actinin expression in cardiomyocytes compared with the cardiomyocyte-only region (Fig. 5B-iii). More organized matrix proteins such as fibronectin and collagen IV (Fig. 5B-iv, v) were deposited in the cocultured half of the construct.
FIG. 5.
Effect of stromal cells in composite constructs with a sharp boundary between a cocultured and cardiomyocyte-only region. (A) Schematic of constructs generated using a two-step gelation process, where collagen seeded with cardiomyocytes is allowed to gel in one half of the rectangular tissue mold followed by a second gelation of collagen containing cardiomyocytes and HS27a stromal cells. (B) Large view confocal immunofluorescence images of serial cryosections near the stromal cell interface (white dotted line): (i) Hoechst staining (ii) HS27a stromal cells (marked with red fluorescent protein), (iii) α actinin, (iv) fibronectin, and (v) collagen IV. (C) Higher magnification image of (i) cardiomyocyte-only region, (ii) the border region, and (iii) the cocultured region. N = 3 biological replicates. Color images available online at www.liebertpub.com/tea
Further away from the boundary between the two halves, the cardiomyocytes displayed a similar structural organization as the two distinct culture conditions shown previously: disarrayed sarcomere striations in cardiomyocyte-only conditions (Fig. 5C-i) compared with robust sarcomere formation and cellular alignment in the direction of uniaxial strain in the cocultured region (Fig. 5C-iii). At the boundary between the two halves, cardiomyocyte alignment sharply declined between the stromal and nonstromal regions (Fig. 5C-ii). This suggests that proximity of stromal cells was required for proper matrix remodeling and cardiomyocyte structural organization in 3D constructs, and paracrine factors alone were not sufficient to induce such effects.
Engineered microvasculature within functional cardiac constructs
The ability to generate cardiac constructs in a dense collagen matrix allowed us to embed microchannels within the construct to form patterned vasculature. We modified our previous soft lithographic injection molding technique for engineering microvessels37 and generated 3D functional cardiac tissue with embedded endothelialized channels (Fig. 6A). Namely, the system was miniaturized to lessen cardiomyocyte demand and facilitate higher throughput testing. Consequently, the device is now open on the top and bottom to allow for sufficient media around constructs; and horizontal posts located just outside of both the inlet and outlet created uniaxial strain (Fig. 6A).
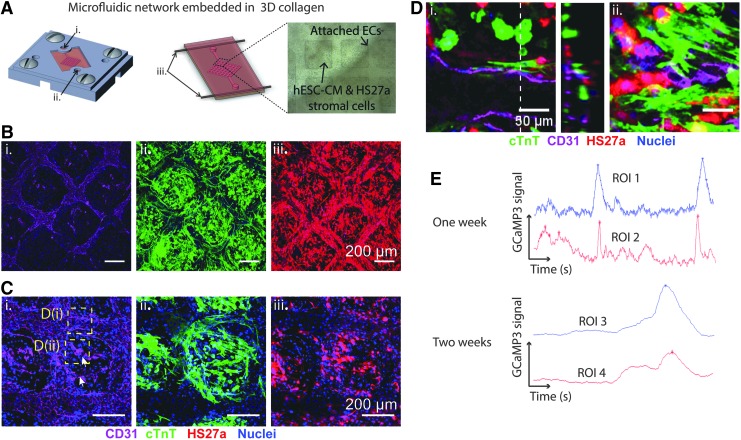
FIG. 6.
Cocultured cardiac constructs with patterned microvasculature cultured for 14 days. (A) Fabrication of microfluidic device with embedded microchannel in cardiomyocyte-embedded collagen matrix: (i) inlet, (ii) outlet, (iii) horizontal posts for uniaxial strain (see Materials and Methods section for more detail). (B) Z-stack projection images of (i) CD31, (ii) cTnT, and (iii) HS27a stromal cells. (C) Z-stack projection images at a higher magnification view showing (i) endothelial sprouts, (ii) elongated CM and (iii) interstitial HS27a stromal cells. (D) Zoomed in regions (i) and (ii) from (C-i) with cross-section view of patterned channel (middle panel). (E) Sample GCaMP3 traces after 1 and 2 weeks of culture for two regions of interest. cTnT, cardiac troponin T.
Constructs generated using this fabrication method and optimized coculture conditions had intact network geometry immediately after fabrication. We then perfused endothelial cells through the network and observed attachment on the microchannels within 1 h after seeding, with complete attachment overnight (Fig. 6A). The structural integrity of the network was maintained throughout 14 days of culture, and attached endothelial cells formed CD31+ junctions within the patterned network (Fig. 6B-i). The interstitial space between the patterned channels contained dense populations of both stromal cells and cardiomyocytes (Fig. 6B-ii, iii).
Cardiomyocyte morphology was elongated with uniform distribution of cTnT-positive cells throughout the interstitial collagen space (Fig. 6B, C-ii). Endothelial sprouts were observed to extend from the patterned network into the bulk of the construct (Fig. 6C-i arrowheads). Importantly, we found that throughout the remodeling process and 2 week culture, the endothelial-lined channels were able to maintain their patency (Fig. 6D-i). Stromal cells, and in some places the cardiomyocytes, followed the vasculature and elongated along the longitudinal direction of the microvessels (Fig. 6D-i) and the endothelial sprouts (Fig. 6D-ii). In comparison, control constructs generated using the same device but without an endothelialized microvessel had poor cardiac morphology with a lack of structural organization of both cells and constructs (Supplementary Fig. S4).
To evaluate the function of cardiac constructs with patterned vasculature, we performed calcium imaging using GCaMP3 fluorescence after 1 and 2 weeks in culture and analyzed the traces at two distinct regions of interest to assess electrical synchronization throughout the construct. We observed synchronous electrical wave propagation after just 1 week in culture, and increased synchronization between two identified regions of the construct after 2 weeks of culture with much of the background signal from isolated electrical activity gone (Fig. 6E; Supplementary Video S6). These data demonstrate that our optimized cellular and matrix conditions enable the generation of functional cardiac constructs with embedded endothelialized microchannels and potentially fully vascularized cardiac tissue.
Discussion
In recent years, many methods of generating perfusable, interconnected networks of vessels have emerged as viable approaches to vascularization of engineered tissue. For example, 3D printing of sacrificial vascular networks into a 3D grid,52 the generation of aligned branched microvessels with alginate fibers embedded in the matrix,53 and microfabricated vessels37 are all exciting approaches with vast implications in microvascular engineering and promising potential for the generation of prevascularized tissues.
Success, however, has been limited in combining these vascular fabrication techniques with standard 3D cardiac tissue cultures. This limitation stems, in part, from incompatible matrix and mechanical conditions that do not provide favorable conditions for both cardiac function and incorporation of vasculature. The formation of compact cardiac tissue with synchronous contractions requires remodeling to occur so that the cells may form proper cell–cell junctions and establish electrical communication, but this remodeling tends to collapse embedded channels due a lack of mechanical support.24
In this study, we used coculture with cardiomyocytes and stromal cells in dense collagen and successfully demonstrated that we can promote cardiomyocyte organization and function and simultaneously maintain the mechanical integrity of microvessels in 3D cardiac constructs. The additional stromal cells promoted significant matrix remodeling, which presumably was through the combination of ECM synthesis and proteolytic degradation through synthesis and release of matrix metalloproteinases.54 We showed that cardiomyocytes aligned along the same direction as the matrix proteins, which was also the direction of uniaxial strain applied on the 3D constructs. Previous studies have shown that fibroblast and stromal cell populations remodel matrix and induce matrix alignment in response to uniaxial strain.55–57 These aligned matrix proteins possibly serve as the topographical cues for the cardiomyocytes to align and mature.44,58
Results from our composite constructs (Fig. 5) demonstrated that paracrine signaling through soluble factors was unlikely to be the predominate mechanism at hand. Rather the proximity and interactions between stromal cells and cardiomyocytes promoted cardiomyocyte organization in cocultured constructs. This further supports the suggested mechanism that local stromal cell-induced matrix synthesis and remodeling allow for adjacent cardiomyocytes to initiate cell–ECM contact, undergo elongation, and structurally mature.
Stromal cells from different origins showed different capacities to facilitate sufficient matrix alignment and cardiac construct formation. Coculture with both hDFs and marrow-derived HS27a stromal cells led to remodeling and cardiac structural maturation, whereas coculture with HS5 stromal cells and NG2+/PDGFRβ+ fetal human heart cells did not. hDFs are known to be highly proficient at remodeling matrix, particularly in 3D cultures.59 HS27a and HS5, although both originated from the human bone marrow, appeared to have drastic differences in their ability to remodel matrix and support cardiac morphology.
HS27a has been shown to have much higher expression of stromal cell-derived factor 1, regulators of G protein signaling, and tissue inhibitors of matrix metalloproteases than HS5.48 In addition, unlike HS5 stromal cells, HS27a stromal cells strongly express the Notch surface ligand Jagged147,48 whose signaling is known to play an important role during cardiac repair, namely by inducing cardiomyocyte proliferation and growth.60,61 HS5 stromal cells, however, express much higher levels of inflammatory cytokines, such as IL-6 and IL-8, than HS27a stromal cells.36,47,48 Prolonged exposure to such inflammatory signals can have adverse effects in the heart such as myocardial hypertrophy that are known predictors of heart failure.62–64
Our use of different stromal and fibroblast lines revealed that not all stromal cells are compatible with cardiac construct formation. Instead, specific stromal cell lines are required to produce sufficient matrix deposition and assembly to elicit alignment and a beneficial response of the cardiomyocytes. Fibroblasts, and particularly dermal fibroblasts, are well known to adapt to external forces and play a key role in modulating mechanical tensions within the tissue through ECM remodeling, cytoskeletal rearrangement, and cellular contraction.65 Indeed, this characteristic of dermal fibroblasts has already been used for a wide range of tissue engineering applications where matrix remodeling and organization are required.66,67
Our finding that the immortalized HS27a stromal cells are similarly effective is advantageous for their use in optimizing engineered tissues. Moving forward, the use of cardiac or stem cell-derived fibroblasts would allow researchers to match the sources of each input cell type to better mimic a cardiac environment.
In addition to cellular alignment and global improvement in cardiac structure, coculture with stromal cells led to myofilament maturation within the cardiomyocytes. Cardiomyocyte-only constructs had sarcomere lengths ranging from 1.2 to 1.4 μm, which is characteristic of immature thick filaments and sarcomeres. In coculture conditions, cardiomyocytes had sarcomere lengths that ranged from 1.7 to 1.8 μm, more similar to that of a mature adult-like cardiomyocyte.68,69
Although structural maturation was observed, the magnitude of force production was still <1% of adult cardiac tissue, which generates a twitch force on the order of 100 mN/mm2.70 This could be attributed to the functional immaturity of stem cell-derived cardiomyocytes and to the relatively low density of cardiomyocytes in the constructs. Longer term culture68 and higher cellular densities could potentially further promote the functional maturation of hESC-CMs and engineered cardiac tissue.
Optimized cellular and matrix culture conditions enabled us to successfully generate microvessels in 3D cardiac tissues. We demonstrated vascular patency and cardiac function in constructs for up to 2 weeks: the vasculature displayed remodeling capability and cardiomyocytes developed proper cardiac morphology and function with necessary cell–cell contacts.
We showed that endothelial cells sprout outward from the patterned vasculature into the surrounding matrix, suggesting the potential of our platform to generate fully vascularized constructs with patterned small arteriole conduits as well as capillaries. This sprouting and endothelial outgrowth may be the result of crosstalk between endothelial cells and cardiomyocytes/stromal cells or from possible hypoxic gradients generated toward the distal regions of the constructs. Endothelial sprouting may also vary for different sources of endothelial cells, and an alternative endothelial cell source could be incorporated into the microvessel to promote additional angiogenesis.71
Our vascularized cardiac tissue provides a useful system to understand the effects of endothelial cells, hydrodynamic stresses, and transport processes on cardiomyocyte maturation and function. We hypothesize that the presence of planar perfusable vessels in the cardiac tissue will guide additional vascular connections within the surrounding construct and further promote integration to the host vasculature once implanted. This study provided a proof-of-principle demonstration of engineered cardiac tissue with patterned vasculature.
Additional studies will be pursued to further characterize the impact of the microvascular network on cardiomyocytes in terms of survival, thickness of viable construct, cardiac morphology, and function. The current system could be further modified to pattern multilayered 3D vasculature throughout the construct by stacking layers of microvessels together or using alternative microfabrication techniques such as 3D printing. As these approaches mature, prevascularized cardiac tissue can be made in large scale and potentially be used for the treatment of ischemic heart disease.
Supplementary Material
Acknowledgments
We thank the Lynn and Mike Garvey Imaging Laboratory in the Institute for Stem Cell and Regenerative Medicine and the Nanotech User Facility, both at the University of Washington. We thank Dr. Lilo D. Pozzo and Dr. Gregory Newbloom for experimental assistance with rheological measurements. We thank Dr. Lil Pabon, Dr. Hans Reinecke, and Dr. Stephen Schwartz for helpful discussions. We acknowledge Dr. Torok-Storb for providing HS27a and HS5 cells. We acknowledge the financial support of National Institute of Health grants DP2DK102258 (to Y.Z.), K99 HL115123 (to K.L.C.), R01 HL084642, P01 HL094374, P01 GM081619, U01 HL100405, and the Foundation Leducq Transatlantic Network of Excellence (to C.E.M.), R01 HL111197 (to M.R.), and training grants T32EB001650 and T32HL007312 (to M.A.R.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Roger V.L., Go A.S., Lloyd-Jones D.M., Benjamin E.J., Berry J.D., Borden W.B., et al. Executive summary: heart disease and stroke statistics-2012 update: a report from the American heart association. Circulation 125, 188, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Chong J.J.H., Yang X., Don C.W., Minami E., Liu Y.-W., Weyers J.J., et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 510, 273, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K., et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 25, 1015, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Caspi O., Huber I., Kehat I., Habib M., Arbel G., Gepstein A., et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol 50, 1884, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Shiba Y., Fernandes S., Zhu W.-Z., Filice D., Muskheli V., Kim J., et al. Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489, 322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Laake L.W., Passier R., Monshouwer-Kloots J., Verkleij A.J., Lips D.J., Freund C., et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res 1, 9, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann W.-H., and Eschenhagen T. Cardiac tissue engineering for replacement therapy. Heart Fail Rev 8, 259, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Mol A., van Lieshout M.I., Dam-de Veen C.G., Neuenschwander S., Hoerstrup S.P., Baaijens F.P.T., et al. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials 26, 3113, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Thomson K.S., Korte F.S., Giachelli C.M., Ratner B.D., Regnier M., and Scatena M. Prevascularized microtemplated fibrin scaffolds for cardiac tissue engineering applications. Tissue Eng Part A 19, 967, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paul J.D., Coulombe K.L.K., Toth P.T., Zhang Y., Marsboom G., Bindokas V.P., et al. SLIT3-ROBO4 activation promotes vascular network formation in human engineered tissue and angiogenesis in vivo. J Mol Cell Cardiol 64, 124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tulloch N.L., Muskheli V., Razumova M.V., Korte F.S., Regnier M., Hauch K.D., et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res 109, 47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmermann W.-H., Melnychenko I., Wasmeier G., Didié M., Naito H., Nixdorff U., et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med 12, 452, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Duan Y., Liu Z., O'Neill J., Wan L.Q., Freytes D.O., and Vunjak-Novakovic G. Hybrid gel composed of native heart matrix and collagen induces cardiac differentiation of human embryonic stem cells without supplemental growth factors. J Cardiovasc Transl Res 4, 605, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens K.R., Kreutziger K.L., Dupras S.K., Korte F.S., Regnier M., Muskheli V., et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A 106, 16568, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreutziger K.L., Muskheli V., Johnson P., Braun K., Wight T.N., and Murry C.E. Developing vasculature and stroma in engineered human myocardium. Tissue Eng Part A 17, 1219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoker M.E., Gerdes A.M., and May J.F. Regional differences in capillary density and myocyte size in the normal human heart. Anat Rec 202, 187, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Lepic E., Burger D., Lu X., Song W., and Feng Q. Lack of endothelial nitric oxide synthase decreases cardiomyocyte proliferation and delays cardiac maturation. Am J Physiol Cell Physiol 291, C1240, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Zhu W.Z., Xie Y., Moyes K.W., Gold J.D., Askari B., and Laflamme M.A. Neuregulin/ErbB signaling regulates cardiac subtype specification in differentiating human embryonic stem cells. Circ Res 107, 776, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedhli N., Huang Q., Kalinowski A., Palmeri M., Hu X., Russell R.R., et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation 123, 2254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caspi O., Lesman A., Basevitch Y., Gepstein A., Arbel G., Huber I., et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res 100, 263, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Sekine H., Shimizu T., Hobo K., Sekiya S., Yang J., Yamato M., et al. Endothelial cell coculture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation 118, S145, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Sasagawa T., Shimizu T., Sekiya S., Haraguchi Y., Yamato M., Sawa Y., et al. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials 31, 1646, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Naito H., Melnychenko I., Didié M., Schneiderbanger K., Schubert P., Rosenkranz S., et al. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circulation 114, I72, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Montgomery M., Zhang B., and Radisic M. Cardiac tissue vascularization: from angiogenesis to microfluidic blood vessels. J Cardiovasc Pharmacol Ther 19, 382, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Coulombe K.L.K., and Murry C.E. Vascular Perfusion of Implanted Human Engineered Cardiac Tissue. Proc IEEE Annu Northeast Bioeng Conf 1, 2014, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdalla S., Makhoul G., Duong M., Chiu R.C.J., and Cecere R. Hyaluronic acid-based hydrogel induces neovascularization and improves cardiac function in a rat model of myocardial infarction. Interact Cardiovasc Thorac Surg 17, 767, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang N.F., Yu J., Sievers R., Li S., and Lee R.J. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng 11, 1860, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Madden L.R., Mortisen D.J., Sussman E.M., Dupras S.K., Fugate J.A., Cuy J.L., et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A 107, 15211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson K.S., Dupras S.K., Murry C.E., Scatena M., and Regnier M. Proangiogenic microtemplated fibrin scaffolds containing aprotinin promote improved wound healing responses. Angiogenesis 17, 195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saif J., Schwarz T.M., Chau D.Y.S., Henstock J., Sami P., Leicht S.F., et al. Combination of injectable multiple growth factor-releasing scaffolds and cell therapy as an advanced modality to enhance tissue neovascularization. Arterioscler Thromb Vasc Biol 30, 1897, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Hao X., Silva E.A., Månsson-Broberg A., Grinnemo K.H., Siddiqui A.J., Dellgren G., et al. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovasc Res 75, 178, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Xiao Y., Zhang B., Liu H., Miklas J.W., Gagliardi M., Pahnke A., et al. Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab Chip 14, 869, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raghavan S., Nelson C.M., Baranski J.D., Lim E., and Chen C.S. Geometrically controlled endothelial tubulogenesis in micropatterned gels. Tissue Eng Part A 16, 2255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baranski J.D., Chaturvedi R.R., Stevens K.R., Eyckmans J., Carvalho B., Solorzano R.D., et al. Geometric control of vascular networks to enhance engineered tissue integration and function. Proc Natl Acad Sci U S A 110, 7586, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moya M.L., Hsu Y.-H., Lee A.P., Hughes C.C.W., and George S.C. In vitro perfused human capillary networks. Tissue Eng Part C Methods 19, 730, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roecklein B.A., and Torok-Storb B. Functionally distinct human marrow stromal cell lines immortalized by transduction with the human papilloma virus E6/E7 genes. Blood 85, 997, 1995 [PubMed] [Google Scholar]
- 37.Zheng Y., Chen J., Craven M., Choi N.W., Totorica S., Diaz-Santana A., et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc Natl Acad Sci U S A 109, 9342, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cross V.L., Zheng Y., Won Choi N., Verbridge S.S., Sutermaster B.A., Bonassar L.J., et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials 31, 8596, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan D., Takawale A., Lee J., and Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 5, 15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camelliti P., Borg T.K., and Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 65, 40, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Kofidis T., Akhyari P., Boublik J., Theodorou P., Martin U., Ruhparwar A., et al. In vitro engineering of heart muscle: artificial myocardial tissue. J Thorac Cardiovasc Surg 124, 63, 2002 [DOI] [PubMed] [Google Scholar]
- 42.He J.-Q., Ma Y., Lee Y., Thomson J.A., and Kamp T.J. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res 93, 32, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Dorn T., Goedel A., Lam J.T., Haas J., Tian Q., Herrmann F., et al. Direct nkx2-5 transcriptional repression of isl1 controls cardiomyocyte subtype identity. Stem Cells 33, 1113, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim C., Majdi M., Xia P., Wei K.A., Talantova M., Spiering S., et al. Non-cardiomyocytes influence the electrophysiological maturation of human embryonic stem cell-derived cardiomyocytes during differentiation. Stem Cells Dev 19, 783, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kizana E., Ginn S.L., Smyth C.M., Boyd A., Thomas S.P., Allen D.G., et al. Fibroblasts modulate cardiomyocyte excitability: implications for cardiac gene therapy. Gene Ther 13, 1611, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Zalewski A., Shi Y., and Johnson A.G. Diverse origin of intimal cells: smooth muscle cells, myofibroblasts, fibroblasts, and beyond? Circ Res 91, 652, 2002 [DOI] [PubMed] [Google Scholar]
- 47.Torok-Storb B., Iwata M., Graf L., Gianotti J., Horton H., and Byrne M.C. Dissecting the marrow microenvironment. Ann N Y Acad Sci 872, 164, 1999 [DOI] [PubMed] [Google Scholar]
- 48.Graf L., Iwata M., and Torok-Storb B. Gene expression profiling of the functionally distinct human bone marrow stromal cell lines HS-5 and HS-27a. Blood 100, 1509, 2002 [DOI] [PubMed] [Google Scholar]
- 49.Carlson M.A., and Longaker M.T. The fibroblast-populated collagen matrix as a model of wound healing: a review of the evidence. Wound Repair Regen 12, 134, 2004 [DOI] [PubMed] [Google Scholar]
- 50.von Tell D., Armulik A., and Betsholtz C. Pericytes and vascular stability. Exp Cell Res 312, 623, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Armulik A., Genové G., and Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21, 193, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Miller J.S., Stevens K.R., Yang M.T., Baker B.M., Nguyen D.H., Cohen D.M., et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat Mater 11, 768, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vollert I., Seiffert M., Bachmair J., Sander M., Eder A., Conradi L., et al. In vitro perfusion of engineered heart tissue through endothelialized channels. Tissue Eng Part A 20, 854, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Larsen M., Artym V.V., Green J.A., and Yamada K.M. The matrix reorganized: extracellular matrix remodeling and integrin signaling. Curr Opin Cell Biol 18, 463, 2006 [DOI] [PubMed] [Google Scholar]
- 55.De Jonge N., Kanters F.M.W., Baaijens F.P.T., and Bouten C.V.C. Strain-induced collagen organization at the micro-level in fibrin-based engineered tissue constructs. Ann Biomed Eng 41, 763, 2013 [DOI] [PubMed] [Google Scholar]
- 56.Nichol J.W., Engelmayr G.C., Cheng M., and Freed L.E. Co-culture induces alignment in engineered cardiac constructs via MMP-2 expression. Biochem Biophys Res Commun 373, 360, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomopoulos S., Fomovsky G.M., and Holmes J.W. The development of structural and mechanical anisotropy in fibroblast populated collagen gels. J Biomech Eng 127, 742, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Wang P.Y., Yu J., Lin J.H., and Tsai W.B. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater 7, 3285, 2011 [DOI] [PubMed] [Google Scholar]
- 59.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 13, 264, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Sassoli C., Pini A., Mazzanti B., Quercioli F., Nistri S., Saccardi R., et al. Mesenchymal stromal cells affect cardiomyocyte growth through juxtacrine Notch-1/Jagged-1 signaling and paracrine mechanisms: clues for cardiac regeneration. J Mol Cell Cardiol 51, 399, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Li Y., Hiroi Y., and Liao J.K. Notch signaling as an important mediator of cardiac repair and regeneration after myocardial infarction. Trends Cardiovasc Med 20, 228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aoyagi T., and Matsui T. The cardiomyocyte as a source of cytokines in cardiac injury. J Cell Sci Ther 2012(S5), pii:, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sano M., Fukuda K., Kodama H., Pan J., Saito M., Matsuzaki J., et al. Interleukin-6 family of cytokines mediate angiotensin II-induced cardiac hypertrophy in rodent cardiomyocytes. J Biol Chem 275, 29717, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Hirota H., Yoshida K., Kishimoto T., and Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci 92, 4862, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silver F.H., Siperko L.M., and Seehra G.P. Mechanobiology of force transduction in dermal tissue. Ski Res Technol 9, 3, 2003 [DOI] [PubMed] [Google Scholar]
- 66.Masuda S., Matsuura K., Anazawa M., Iwamiya T., Shimizu T., and Okano T. Formation of vascular network structures within cardiac cell sheets from mouse embryonic stem cells. Regen Ther 2, 6, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong T., McGrath J.A., and Navsaria H. The role of fibroblasts in tissue engineering and regeneration. Br J Dermatol 156, 1149, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Lundy S.D., Zhu W.-Z., Regnier M., and Laflamme M.A. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev 22, 1991, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Korte F.S., Dai J., Buckley K., Feest E.R., Adamek N., Geeves M.A., et al. Upregulation of cardiomyocyte ribonucleotide reductase increases intracellular 2 deoxy-ATP, contractility, and relaxation. J Mol Cell Cardiol 51, 894, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kreutziger K.L., Piroddi N., McMichael J.T., Tesi C., Poggesi C., and Regnier M. Calcium binding kinetics of troponin C strongly modulate cooperative activation and tension kinetics in cardiac muscle. J Mol Cell Cardiol 50, 165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palpant N.J., Pabon L., Roberts M., Hadland B., Jones D., Jones C., et al. Inhibition of -catenin signaling respecifies anterior-like endothelium into beating human cardiomyocytes. Development 142, 3198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.