Summary
Background
Cellular mechanisms aimed at repairing protein damage and maintaining homeostasis, widely understood to be triggered by the damage itself, have recently been shown to be under cell nonautonomous control in the metazoan C. elegans. The heat shock response (HSR) is one such conserved mechanism, activated by cells upon exposure to proteotoxic conditions such as heat. Previously, we had shown that this conserved cytoprotective response is regulated by the thermosensory neuronal circuitry of C. elegans. Here, we investigate the mechanisms and physiological relevance of neuronal control.
Results
By combining optogenetic methods with live visualization of the dynamics of the heat shock transcription factor (HSF1), we show that excitation of the AFD thermosensory neurons is sufficient to activate HSF1 in another cell, even in the absence of temperature increase. Excitation of the AFD thermosensory neurons enhances serotonin release. Serotonin release elicited by direct optogenetic stimulation of serotonergic neurons activates HSF1 and upregulates molecular chaperones through the metabotropic serotonin receptor SER-1. Consequently, excitation of serotonergic neurons alone can suppress protein misfolding in C. elegans peripheral tissue.
Conclusions
These studies imply that thermosensory activity coupled to serotonergic signaling is sufficient to activate the protective HSR prior to frank proteotoxic damage. The ability of neurosensory release of serotonin to control cellular stress responses and activate HSF1 has powerful implications for the treatment of protein conformation diseases.
Introduction
Serotonin (5-hydroxytryptamine, 5-HT) is implicated in the stress response of all animals, and its enhanced release modulates physiological and metabolic adaptation to adverse conditions [1, 2]. Anticipation of danger can trigger serotonin release [3]. In addition, experimentally manipulating serotonin signaling in mammalian models can increase stress hormone production and elicit aversive behaviors, even in the absence of the actual stressor [1,2]. Thus, although it is unclear whether enhanced release of serotonin is specific to particular stressors or whether it is a nonspecific response associated with increased arousal, serotonergic signaling allows organisms to rapidly adjust their physiology, metabolism, and behavior in expectation of imminent danger.
Exposure to unfavorable environments can also cause macromolecular damage [4, 5]. However, cellular mechanisms aimed at repairing damage and maintaining homeostasis are widely understood to be triggered by the damage itself, either through damage to the cell that activates the response [4, 6–8] or, as has been suggested by recent experiments, through the activation of a stress response in other “sending” cells of an organism that are subject to proteotoxic conditions [9]. Indeed, there is little evidence to date for the preemptive activation of cellular stress responses prior to macromolecular damage. One key homeostatic mechanism by which cells protect themselves against protein damage is through the heat shock response (HSR) and activation of the highly conserved transcription factor, heat shock factor 1 (HSF1) [6, 7, 10]. HSF1 upregulates heat shock protein (HSP) genes that act as molecular chaperones to maintain protein conformation under stress, refold misfolded proteins, and target irreversibly damaged proteins for degradation [6, 7, 10]. Thus, HSF1 activation suppresses protein misfolding and toxicity in numerous animal models of protein conformational diseases, such as Alzheimer’s, Parkinson’s, and Huntington’s diseases [6,10,11].
We have previously shown that in C. elegans, the HSR is regulated cell nonautonomously by the animals’ AFD thermo-sensory neuronal circuitry [12, 13]. In addition, other groups have also shown nonautonomous regulation of HSF1 and other cellular stress responses by the nervous system [9, 12–16]. Thermosensory neurons in C. elegans are exquisitely sensitive and detect changes as small as 0.05°C above ambient temperature [17]; hence, they can arguably be excited by temperature increments well below those that cause cellular damage. Therefore, a central question that arises from the observation that thermosensory neurons control the HSR of peripheral tissue is whether the nervous system plays an instructive role in activating the HSR upon sensing temperature increase, prior to macromolecular damage, or whether it plays a more general, permissive role in allowing the stress response to be triggered by macromolecular damage. An instructive role involving signaling pathways from neuronal cells to peripheral tissue could activate protective mechanisms against macromolecular damage in anticipation of its actual occurrence. This would not only be adaptive for organisms but, if identified, could suggest powerful strategies to counteract diseases of protein conformation.
Here, we tested whether excitation of the AFD thermosensory neuronal circuitry was sufficient to activate the HSR in distant tissues, even in the absence of heat, through serotonergic signaling. To do this, we used optogenetics to excite specific neurons combined with live imaging of HSF1 dynamics in noninnervated gonad nuclei of live, intact animals. We found that excitation of thermosensory and serotonergic neurons alone can activate the HSR in other cells through regulated serotonin release and protect these cells from protein aggregation.
Results
Optogenetic Excitation of C. elegans AFD Thermosensory Neurons Activates HSF1
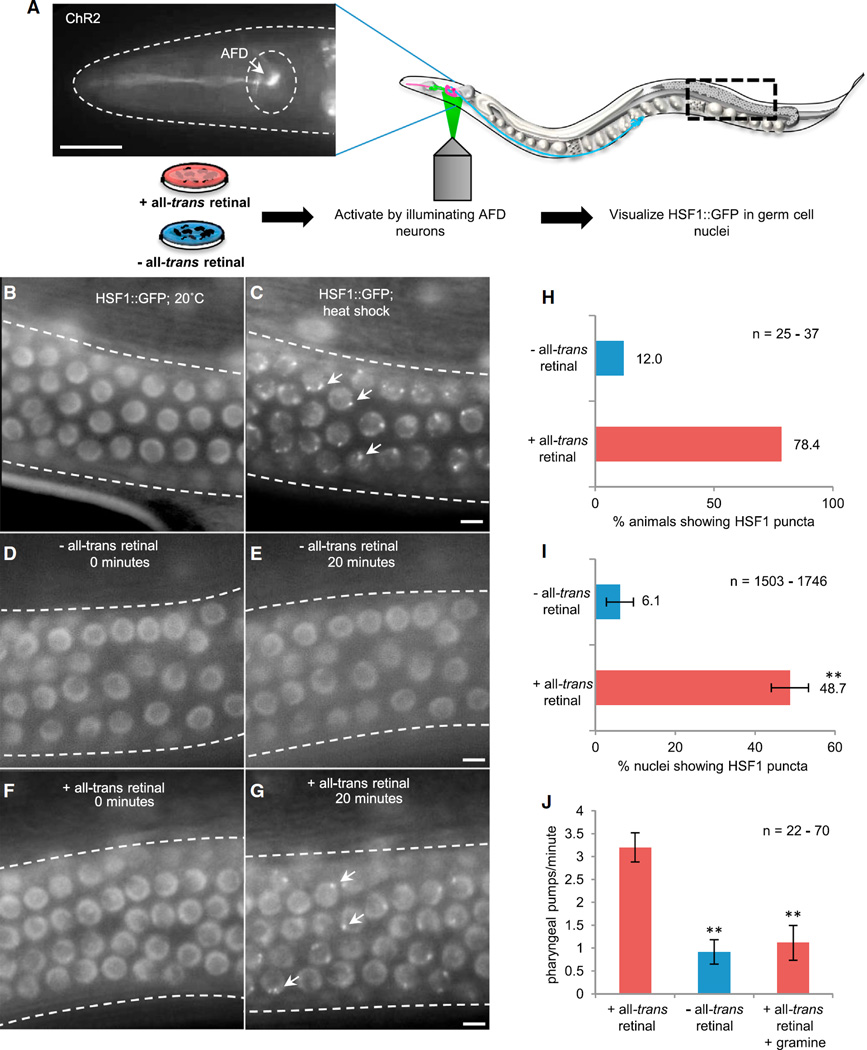
To assess whether thermosensory activity was sufficient to induce the HSR in another cell, we used optogenetics [18] to locally stimulate the two AFD thermosensory neurons in C. elegans while maintaining animals at 20°C (Figure 1A). Optogenetic activation was conducted in a previously characterized transgenic strain that expresses the blue-light activated cation channel channelrhodopsin-2 (ChR2) only in the AFD neurons (AFD::ChR2). Live imaging of HSF1 dynamics following optogenetic activation was conducted in germ cell nuclei, which are not innervated. To visualize HSF1, we used transgenic C. elegans ubiquitously expressing a single copy of HSF1::GFP under its own promoter (see Supplemental Experimental Procedures available online). In this strain, HSF1 is present diffusely throughout nuclei at ambient temperatures of 20°C–22°C and upon heat shock relocalizes rapidly to stress-induced nuclear puncta (Figures 1B, 1C, S1A, and S1B). These HSF1 nuclear stress granules are a well-accepted readout for HSF1 activation in multiple model systems [19–21], and many have been shown to correspond to transcriptionally active sites in C. elegans [22]. Light activation of ChR2 requires the small molecule all-trans retinal (ATR), which in C. elegans is supplied through food [18]. The need to supplement exogenous ATR for the activation of ChR2 allowed us to control for the nonspecific effects of handling, light exposure, phototoxicity, immobilization, etc. that might activate HSF1, as we could mock activate animals expressing ChR2 and HSF1::GFP but grown in the absence of ATR by exposure to the exact same light regimen and use them as controls.
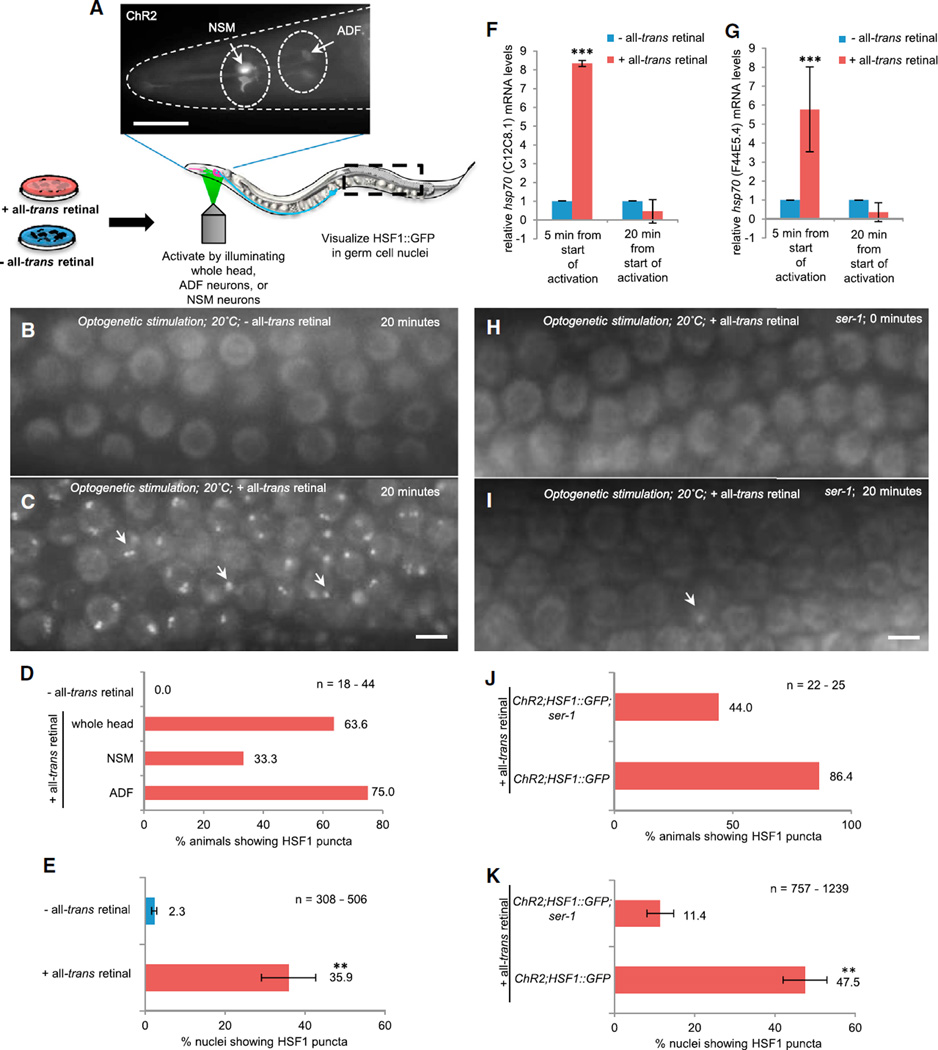
Figure 1. Optogenetic Stimulation of AFD Thermosensory Neurons in C. elegans Activates HSF1 and Elicits Serotonin Release in the Absence of Stress.
(A) Experimental setup: localized illumination of AFD neurons in individual C. elegans expressing ChR2 in AFD neurons (the scale bar represents 50 µm). Wavelength = 490 nm. Experimental animals were grown in the presence of all-trans retinal (+ATR) and control animals in the absence of all-trans retinal (−ATR). HSF1::GFP was monitored in germ cell nuclei.
(B and C) HSF1::GFP localization at 20°C (B) and following heat shock at 34°C for 10 min (C) (arrows indicate HSF1 nuclear stress granules/puncta). The scale bar represents 5 µm.
(D and E) HSF1::GFP in control (−ATR) animals at 0 min (D) and 20 min (E) after the start of optogenetic stimulation. The scale bar represents 5 µm.
(F and G) HSF1::GFP in experimental (+ATR) animals at 0 min (F) and 20 min (G) after the start of optogenetic stimulation. The scale bar represents 5 µm.
(H) Percentage of control (−ATR) and experimental (+ATR) animals showing HSF1 nuclear puncta after stimulation of AFD neurons. n = 25–37 animals.
(I) Percentage of nuclei with HSF1 puncta in control (−ATR) and experimental (+ATR) animals following optogenetic stimulation. n = 25–37 animals; 1,503–1,746 nuclei. Values indicate mean ± SEM, t test; **p < 0.01.
(J) Pharyngeal pumping rates in control (−ATR) and experimental (+ATR) animals following optogenetic stimulation and pharyngeal pumping in (+ATR) experimental animals treated with 100 µM gramine and optogenetically stimulated. n = 22–70 animals. Values indicate mean ± SEM, t test; **p < 0.01.
See also Figure S1.
We first verified that neither ATR treatment nor manipulations required for the optogenetic experiments activated the HSR. We did this by examining the localization of HSF1 and measuring hsp70 mRNA levels in mock-activated, control animals (Figures S1C–S1E). Next, to discount the possibility that the manipulation of the animals as required for optogenetic activation caused protein misfolding for unknown, albeit improbable, reasons, we assessed the effects of mock activation on two metastable proteins expressed in C. elegans tissue: an expanded polyglutamine (polyQ) expressed in body wall muscle cells [23] and an endogenous temperature-sensitive dynamin allele known to be expressed in neurons, intestine, and pharyngeal muscle [24]. The polyglutamine Q35::YFP is a construct containing 35 glutamine residues fused to YFP that aggregates in an age-dependent manner in body wall muscle cells. Q35::YFP can serve as a folding reporter to assess endogenous misfolding, as its aggregation increases upon misfolding of endogenous C. elegans proteins [25] and upon heat shock (Figure S1F). The temperature-sensitive dynamin mutants, dyn-1(ky51), show decreased motility upon misfolding of dynamin that can occur upon a temperature shift or other misfolding events [24]. Therefore, the numbers of aggregates in Q35::YFP animals and the motility of dyn-1(ky51) animals would provide us with a measure of misfolding, were it to occur due to our experimental protocol. Following mock activation, both Q35::YFP-expressing animals and dyn-1(ky51) animals continued to show similar levels of mis-folding as control animals not subjected to mock activation, indicating that the experimental procedure itself did not trigger overt misfolding (Figures S1G and S1H).
Having established that our methods were not proteotoxic, we proceeded to test whether optogenetic stimulation of the AFD neurons could activate HSF1. In all animals, prior to the light-induced excitation of the AFD neurons, HSF1 was present diffusely throughout nuclei (Figures 1D and 1F). This diffuse localization persisted in the control animals grown in the absence of ATR, even after illumination of the AFD neurons with 490 nm light (Figure 1E). Only 12% (n = 25) of the animals displayed any evidence of HSF1::GFP nuclear puncta (Figure 1H), with as few as 6.1% 6 3.4% nuclei (n = 1,503) showing HSF1 puncta (Figure 1I), perhaps corresponding to basal HSF1 activity. In contrast, light stimulation of the AFD thermosensory neurons resulted in the formation of HSF1::GFP puncta in 78.4% of the experimental animals (n = 37) grown on ATR (Figures 1F–1I). This occurred as early as 10 min postactivation, and by 20 min, HSF1 was activated in 25%–79.3% of nuclei per gonad (average = 48.7% ± 4.7% gonadal nuclei; n = 1,746; Figure 1I). These observations show that excitation of the AFD neurons alone is sufficient to initiate a signaling mechanism to activate HSF1 in distal tissues, even in the absence of temperature increase.
AFD Thermosensory Neuronal Activity Enhances Serotonin Release from the ADF and NSM Serotonergic Neurons
In C. elegans, as in mammals, neurosensory regulation of serotonin levels controls many aversive behavioral and physiological responses to noxious stimuli [26–28]. Given the role of serotonin in regulating adaptive physiological changes in anticipation of danger [1–3], we examined whether thermosensory neuronal control of the HSR could be mediated by serotonin. Although the AFD thermosensory neurons are themselves not serotonergic, we had previously seen that during heat stress, the neuronal control of the HSR was dependent on chemosensory input that modulated the metabolic state of the animal [12]. If serotonin release triggered by thermosensory neuronal activity was a signaling mechanism for the nonautonomous activation of the HSR, we predicted that (1) activation of the AFD thermosensory neurons, either optogenetically or upon exposure to increasing temperatures, should enhance serotonin release, (2) inhibiting AFD thermosensory neuronal activity should diminish this release of serotonin, and (3) inhibiting serotonin signaling should diminish the HSR. We tested each of these predictions.
Optogenetic excitation of the AFD thermosensory neurons caused an increase in pharyngeal pumping within the first minute of light exposure (Figure 1J). Enhanced pumping is a well-characterized consequence of serotonin release by serotonergic neurons [29–31]. This occurred only in AFD::ChR2 animals grown on ATR and not in animals grown in the absence of ATR (Figure 1J), and it was consistent and significant, despite the lower pharyngeal pumping rates typically seen in animals that are immobilized, as for our experiments [31]. The increase in pharyngeal pumping stimulated by excitation of the AFD neurons could be inhibited with 100 µM gramine [30], a competitive serotonin antagonist (Figure 1J). Thus, optogenetic excitation of AFD thermosensory neurons was sufficient to cause serotonin release.
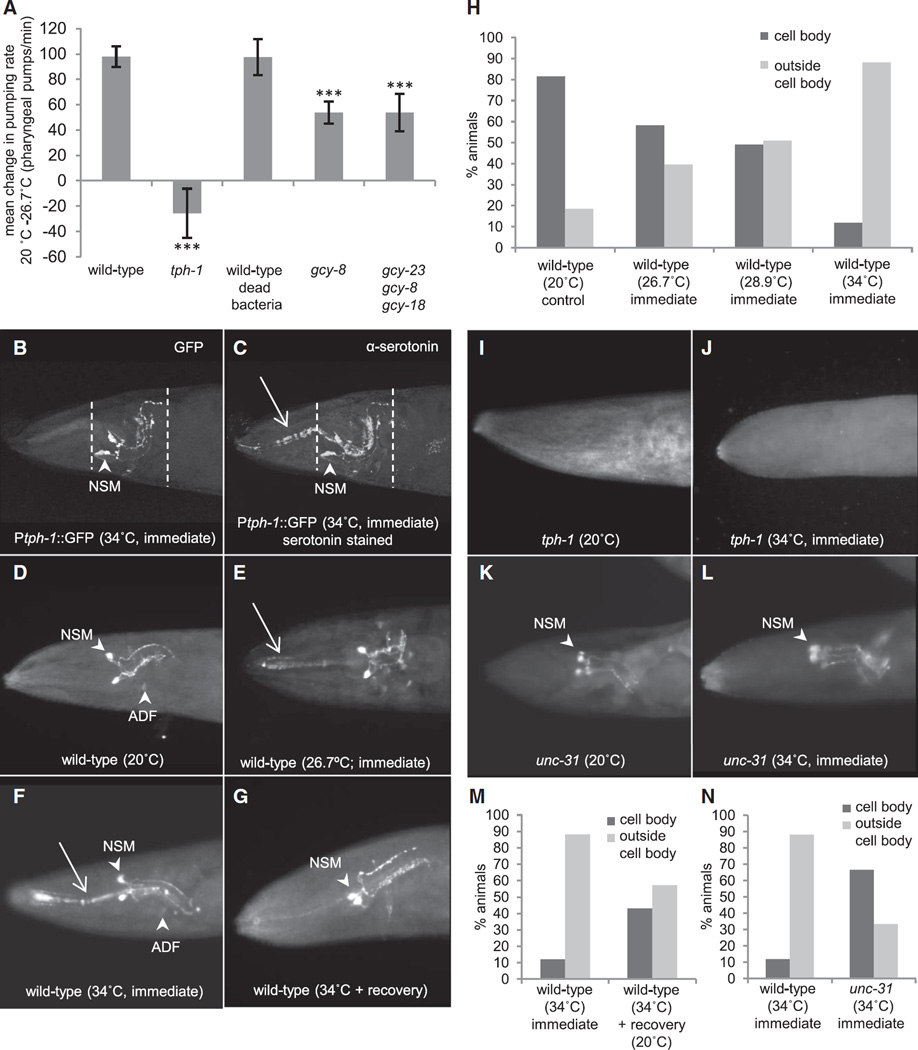
Serotonin was also released in an AFD-dependent manner upon increases in temperature. Animals were subjected to a controlled increase in temperature of 1°C/min (Figure S2B), a rate known to activate the AFD thermosensory neurons [17, 32], and pharyngeal pumping was measured [29]. At 20°C–22°C, wild-type animals exhibited a pumping rate of 204 ± 24 pumps per minute, similar to what has been previously reported (Figures S2A and S2C) [29]. As early as 5 min upon temperature increase to 26.7°C, there was a dramatic increase in pumping rates to an average rate of 304 ± 50 (Figures 2A, S2A, and S2C), representing an approximately 50% increase in the number of pumps per minute. This increase was transient but occurred in all animals (Figures S2A and S2C). Animals deficient in serotonin synthesis because of mutations in the only C. elegans tryptophan hydroxylase gene, tph-1 [33], did not increase their pumping rates upon temperature increase, confirming that the temperature-dependent increases in pumping occurred as a consequence of serotonergic signaling (Figures 2A, S2A, and S2D). Disrupting the function of the AFD thermosensory neurons by mutations in the guanylyl cyclase genes gcy-8, gcy-18, and gcy-23 [32, 34] greatly diminished the increase in pharyngeal pumping upon temperature upshift (Figures 2A, S2A, S2F, and S2G), confirming the role of the AFD neurons in eliciting temperature-dependent serotonin release. On the other hand, bacteria that serve as food for C. elegans and are known to modulate serotonin release [28, 29] were not responsible for the temperature-enhanced pumping as animals grown on dead bacteria showed a comparable increase (from 167 ± 20, 20°C to 265 ± 39, 26.7°C; Figures 2A, S2A, and S2E). These data show that serotonin was released in response to sensed increases in temperature in an AFD-dependent manner.
Figure 2. Serotonin Is Released upon Temperature Increase.
(A) The change in mean pumping rates when temperature increases from ambient (20°C–22°C) to 26.7°C in the following animals: wild-type animals under normal growth conditions, tph-1(mg280) II, wild-type animals transferred to dead bacteria, gcy-8(oy44) IV, and gcy-23(nj37) gcy-8(oy44) gcy-18(nj38) IV. n = 9 for wild-type on dead bacteria; n = 20–25 for all others. Values indicate mean ± SEM, t test; ***p < 0.001.
(B and C) Collapsed confocal z stack image showing serotonin staining in mgIs42 animals expressing GFP in serotonergic neurons under a tph-1 promoter. Animals were fixed immediately following a 15 min exposure to 34°C.
(B) Ptph-1::GFP marks serotonergic neuronal cell bodies (arrowhead), present within areas confined by dashed lines.
(C) Serotonin staining is detected both within the neuronal cell bodies and in areas outside the dashed lines (arrow).
(D–G) Serotonin immunolocalization in wild-type animals at 20°C (n = 119) (D), exposed for 5 min to temperature ramp rate of 1°C/min (corresponding to 26.7°C; n = 96) (E), exposed to 34°C for 15 min (n = 119) (F), and exposed to 34°C for 15 min and recovered at 20°C for 1 hr (n = 7) (G).
(H) Percentage of wild-type animals, scored blinded (see Experimental Procedures), showing serotonin localization within or outside neuronal cell bodies at 20°C, 26.7°C, 28.9°C, and 34°C.
(I–L) Serotonin immunolocalization in tph-1(mg280) II at 20°C (n = 15) (I), tph-1(mg280) II at 34°C for 15 min (n = 9) (J), unc-31(e928) IV; C12C8.1p::mCherry at 20°C (n = 13) (K), and unc-31(e928) IV; C12C8.1p::mCherry at 34°C for 15 min (n = 15) (L). Arrowheads indicate cell bodies; arrows indicate serotonin localization outside cell bodies.
(M) Percentage of wild-type animals exposed to 34°C for 15 min and recovered at 20°C for 1 hr scored blinded for serotonin localization.
(N) Percentage of unc-31; C12C8.1p::mCherry animals exposed to 34°C for 15 min scored blinded for serotonin localization compared to wild-type.
See also Figure S2.
We confirmed that serotonin was indeed released by visualizing endogenous serotonin localization in animals at 20°C and upon increasing temperature. Specifically, we examined animals exposed to the temperature ramp rate of 1 °C/min for 5 and 10 min (26.7°C and 28.9°C, where 26.7°C corresponds to the peak of pharyngeal pumping) and upon heat shock at 34°C for 15 min. In wild-type C. elegans raised at 20°C, antibodies against serotonin stained cell bodies of a pair of neurosecretory motor neurons (NSMs), a pair of ADF chemosensory neurons (Figure 2D), the HSN neurons that innervate vulval muscle cells, and, less consistently, a single RIH neuron and a pair of AIM interneurons [28, 29, 35]. When animals were exposed to increases in temperature, serotonin staining expanded into areas outside serotonergic neuronal cell bodies (Figures 2E, 2F, and 2H). This was evidenced by immunolocalizing serotonin in animals expressing GFP under the tph-1 promoter (Ptph-1::GFP), where GFP marked the boundaries of serotonergic neuronal cell bodies. In these animals, following heat shock, serotonin staining could be detected outside the confines of GFP (Figures 2B and 2C). We examined whether changes in tph-1 expression itself could account for the change in localization of serotonin with increased temperatures [27, 28], as tph-1 can be expressed in other tissues, such as pharyngeal muscle cells of the procorpus and meta-corpus during embryogenesis [36]. However, neither Ptph-1::GFP localization (Figures 3A and 3B) nor GFP mRNA levels changed following heat shock (Figure 3C). The altered localization of serotonin following heat shock was transient, as many animals showed control immunolocalization patterns by 1 hr following recovery at 20°C after exposure to 34°C for 15 min (Figures 2F, 2G, 2H, and 2M). We confirmed that the immunolocalization pattern was specific for serotonin by using tph-1(mg280) II mutant animals that do not make serotonin (Figures 2I and 2J). In addition, a loss-of-function mutation in unc-31 that disrupts dense core vesicle-dependent neurosecretion required for serotonin secretion [35] diminished serotonin relocalization upon heat shock (88% versus 33% animals showed localization outside serotonergic cell bodies; Figures 2K, 2L, and 2N).
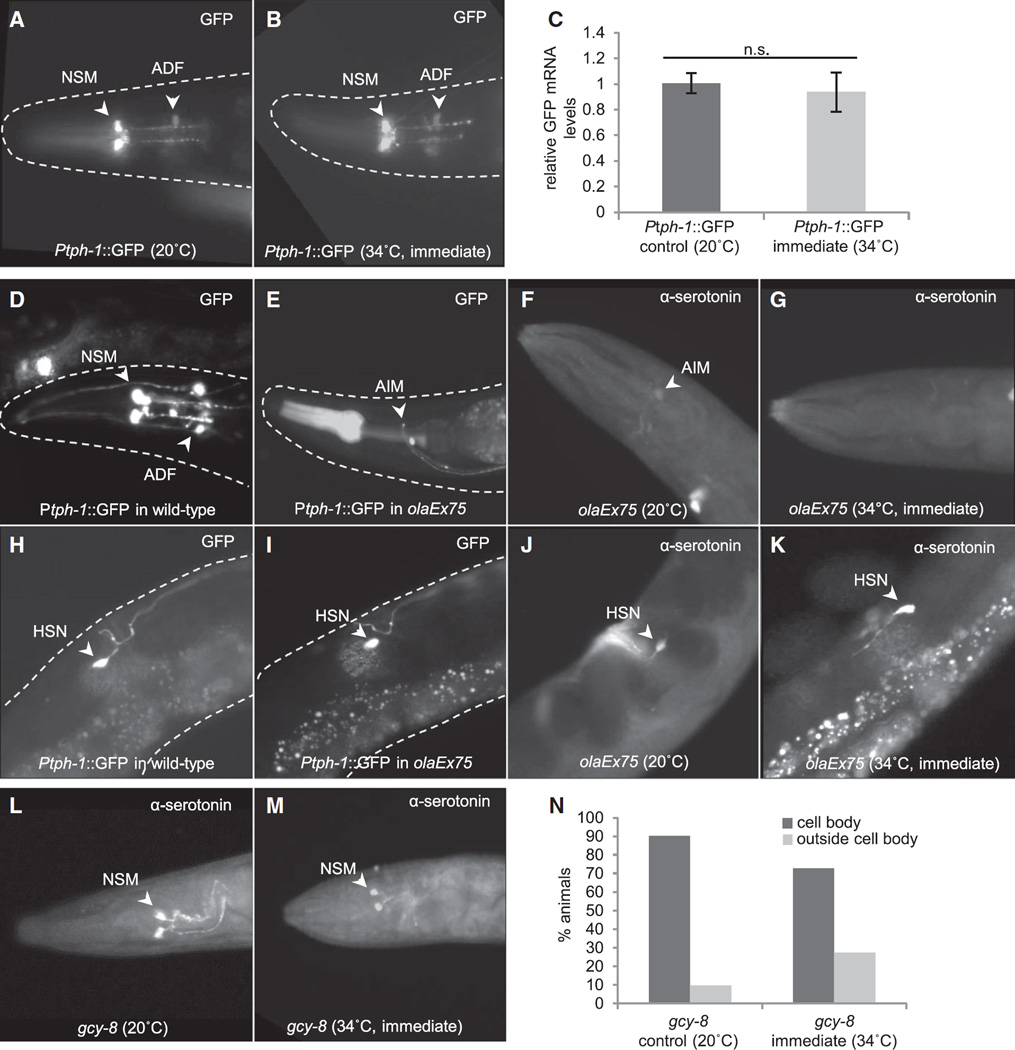
Figure 3. Serotonin Is Released from Serotonergic ADF and NSM Neurons upon Temperature Increase in an AFD-Dependent Manner.
(A and B) Localization of Ptph-1::GFP at 20°C (A) and following exposure to 34°C for 15 min (B).
(C) GFP mRNA levels measured by qRT-PCR in Ptph-1::GFP animals before and immediately after 15 min heat exposure (34°C). Values indicate mean ± SEM.
(D and E) The expression of a split caspase under a minimal tph-1 promoter ablates the ADF and NSM neurons in olaEx75-expressing animals; Ptph-1::GFP localization in serotonergic neurons in heads of wild-type animals (D) and olaEx75-expressing animals (E).
(F and G) Serotonin immunolocalization in olaEx75-expressing animals at 20°C (n = 11) (F) and immediately following a 15 min exposure to 34°C (n = 15) (G).
(H and I) Ptph-1::GFP expression in wild-type (H) and olaEx75-expressing (I) animals showing the presence of HSN neurons.
(J and K) Serotonin immunolocalization in HSN neurons is preserved in olaEx75-expressing animals at both 20°C (n = 11) (J) and following 15 min exposure to 34°C (n = 15) (K).
(L and M) Serotonin immunolocalization in gcy-8(oy44) IV animals at 20°C (n = 31) (L) and following 15 min exposure to 34°C (n = 11) (M).
(N) Percentage of gcy-8(oy44) animals scored for serotonin localization (20°C and 34°C, 15 min). Arrowheads indicate cell bodies.
We then investigated which of the serotonergic neurons were responsible for the temperature-dependent serotonin release by genetically ablating specific serotonergic neurons. The cell bodies of the NSM neurons are buried in cavities inside the pharyngeal muscle cells, whereas the ADF neuronal cell bodies lie just outside the pharynx, and both were possible candidates to contribute to the observed serotonin staining pattern [36, 37]. Genetic ablation of the NSM and ADF neurons only, achieved by directing the expression of a split caspase to these cells (Figures 3D–3K; [38]), resulted in the loss of serotonin relocalization following heat shock (Figure 3F and 3G). As with temperature-enhanced pharyngeal pumping, the heat shock-induced immunolocalization of serotonin outside the neuronal cell bodies required AFD thermosensory neuronal function: only 27% of the gcy-8 thermosensory mutant animals compared with 88% of wild-type animals showed serotonin release following exposure to increased temperature (Figures 3L–3N). These studies together support the conclusion that, as with optogenetic activation, temperature-induced AFD thermosensory activity also elicits the release of serotonin. This occurs from serotonergic NSM and/or ADF neurons.
Serotonin Is Necessary for the HSR
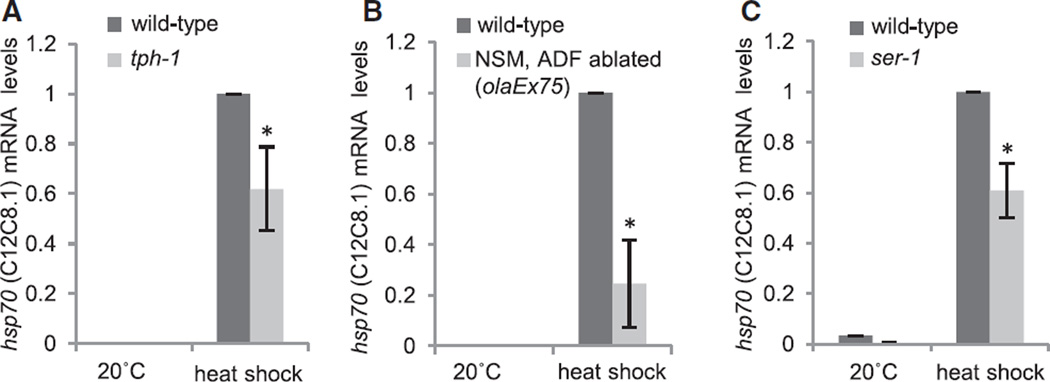
We next tested whether serotonin was necessary for heat shock-dependent induction of hsp70 mRNA. Animals deficient in serotonin signaling and wild-type animals with intact serotonin signaling systems were subjected to a brief heat shock, corresponding to the regimen that induced the relocalization of serotonin as described above. hsp70 mRNA accumulation was then quantified using quantitative RT-PCR (qRT-PCR). Both the tph-1 mutants (Figure 4A) and animals lacking serotonergic ADF and NSM neurons (Figure 4B) showed significant decreases in hsp70 mRNA accumulation upon heat shock compared to wild-type animals. In C. elegans, four metabotropic G protein-coupled receptors (GPCRs), SER-1, SER-4, SER-5 and SER-7, and one serotonin-gated chloride channel, MOD-1, are known to bind serotonin [39, 40]. Among these receptors, loss-of-function mutations in SER-1 decreased hsp70 upregulation upon heat shock in a manner similar to the loss of tph-1 (Figures 3C and S3), suggesting that the serotonin signal may be transmitted through this ortholog of the mammalian metabotropic 5-HT2 receptor to regulate the HSR. These experiments collectively indicate that serotonergic signaling is necessary for hsp70 mRNA expression upon heat shock and could therefore serve as a signaling mechanism for HSR activation.
Figure 4. Serotonergic Signaling Is Necessary for hsp70 Induction upon Heat Shock.
(A–C) Quantification of hsp70 (C12C8.1) mRNA levels by qRT-PCR following heat shock (34°C, 15 min) of wild-type and tph-1(mg280) II animals (A), wild-type and olaEx75-expressing animals (B), and wild-type and ser-1(ok345)X animals (C). RNA was extracted 30 min following recovery after heat shock. Values indicate mean fold change ± SEM, t test; *p < 0.05. See also Figure S3.
Optogenetic Excitation of Serotonergic ADF and NSM Neurons Activates the HSR
If serotonin release triggered by thermosensory activity was indeed a signal to nonautonomously activate HSF1 in other cells, we would predict that stimulating serotonin release directly from the NSM and ADF neurons in wild-type animals without exciting thermosensory neurons should be sufficient to activate HSF1 in distant tissues. To test this, we again used optogenetics [18] to stimulate only the NSM and ADF neurons while maintaining animals at 20°C (Figure 5A). This was done by locally illuminating the NSM and ADF neurons in a previously characterized transgenic strain lite-1(ce314) X; ljIs102, which expresses ChR2 only in these neurons [40]. We confirmed that the optogenetic activation of these serotonergic neurons caused the relocalization of serotonin into areas outside serotonergic neuronal cell bodies, as observed upon heat shock (Figures S4A–S4C). We also confirmed that, as previously reported [40], optogenetic excitation of serotonergic neurons decreased locomotory rates (number of body bends) and increased pharyngeal pumping rates [29, 30] (Figures S4D and S4E). As described above, in all animals, prior to the light-induced excitation of the NSM and ADF neurons, HSF1 was present diffusely throughout nuclei (Figure S1C). This diffuse localization persisted in the control mock-activated animals grown in the absence of ATR (Figure 5B), and none (n = 22) of these animals displayed the relocalization of HSF1::GFP to nuclear stress granules/puncta (Figures 5D and 5E). In contrast, as seen upon excitation of the AFD thermosensory neurons, optogenetic activation of the ADF and NSM neurons caused 64% (n = 44) of the experimental animals grown on ATR to exhibit relocalization of HSF1::GFP into nuclear stress granules within 10–20 min postlight activation (Figures 5C and 5D). The number of nuclei with nuclear stress granules varied between animals and ranged from 6% to 90% per gonad (n = 506 nuclei), with an average of 35.9% ± 6.8% (Figure 5E). Thus, optogenetic stimulation of the serotonergic neurons alone, even without exciting the AFD thermosensory neurons, is sufficient to activate HSF1 in distant tissues. Furthermore, optogenetic stimulation of either the ADF or the NSM neurons was effective in activating HSF1, although the ADF neurons appeared more effective (75% versus 33.3%; Figure 5D).
Figure 5. Optogenetic Stimulation of ADF and NSM Neurons Is Sufficient to Activate the HSR in Other Cells through the Serotonin Receptor SER-1.
(A) Experimental setup: localized illumination of the whole head, serotonergic ADF neurons, or NSM neurons in individual C. elegans expressing ChR2 in these neurons (the scale bar represents 50 µm). Wavelength = 490 nm. Experimental animals were grown in the presence of +ATR; control animals were grown in the absence of −ATR. HSF1::GFP was monitored in germ cell nuclei.
(B and C) Germ cell nuclei showing HSF1::GFP localization in control (−ATR) (B) and experimental (+ATR) (C) animals at 20 min after the start of optogenetic stimulation (the scale bar represents 5 mm; arrows indicate HSF1 puncta).
(D) Percentage of animals showing HSF1 nuclear puncta after stimulation of whole head (+ATR and −ATR) or only the ADF neurons or NSM neurons (+ATR). n = 18–44 animals per condition.
(E) Percentage of nuclei with HSF1 nuclear puncta in control (−ATR) and experimental (+ATR) animals following optogenetic stimulation. n = 308–506 nuclei. Values indicate mean ± SEM, t test; **p < 0.01.
(F and G) hsp70 (C12C8.1) (F) and hsp70 (F44E5.4) (G) mRNA levels measured using qRT-PCR in experimental (+ATR) animals relative to control (−ATR) animals. Animals were harvested 5 min and 20 min from the start of optogenetic stimulation. Values indicate mean ± SD, t test; ***p < 0.001.
(H and I) Germ cell nuclei showing HSF1::GFP localization in experimental (+ATR) ser-1(ok345)X animals at 0 min (H) and 20 min (I) after the start of optogenetic stimulation (the scale bar represents 5 µm).
(J) Percentage of animals showing HSF1 nuclear puncta after stimulation of the ADF serotonergic neurons in experimental (+ATR) wild-type and ser-1(ok345)X animals. n = 22–25 animals per condition.
(K) Percentage of nuclei with HSF1 puncta in experimental (+ATR) wild-type and ser-1(ok345)X animals. n = 757–1,239 nuclei. Values indicate mean ± SEM, **p < 0.01.
See also Figure S4.
Although HSF1 nuclear stress granules form concomitant with the induction of heat shock gene transcription, HSF1 stress granules do not typically localize to hsp70 promoters [19–21]. We therefore tested whether, along with the activation of HSF1 evidenced by nuclear stress granule formation, optogenetic stimulation of the ADF and NSM neurons also increased hsp70 mRNA levels. To allow for harvesting of animals for mRNA extraction in these experiments, we performed optogenetic activation on free-behaving animals on small lawns of OP50 [39]. In experimental (+ATR), but not in control (2ATR), animals, stimulation of the ADF and NSM neurons resulted in a 5- to 8-fold induction of hsp70 mRNA (Figures 5F and 5G). However, hsp70 mRNA upregulation was transient, and mRNA levels declined after 20 min postoptogenetic stimulation (Figures 5F and 5G). These results taken together indicate that serotonin release from the ADF and/or NSM neurons is sufficient to activate HSF1 and initiate hsp70 mRNA transcription. In subsequent experiments, we focused on activating only the ADF neurons.
SER-1 Is Necessary for HSF1 Activation
Serotonergic neurons not only release serotonin but also are responsible for the secretion of other small molecules, such as INS-1 [41]. Therefore, to confirm that serotonin rather than another signaling molecule was the trigger for the nonautonomous activation of HSF1, we examined whether the activation of HSF1 upon optogenetic stimulation of the ADF neurons could be abolished by mutations in the metabotropic serotonin receptor, SER-1. Indeed, in animals carrying a deletion in ser-1, both the numbers of animals activating HSF1 and the extent of HSF1 activation were markedly reduced (Figures 5H and 5I). Thus, 47.5% of nuclei in the wild-type animals showed the formation of HSF1 nuclear stress granules within 20 min following optogenetic activation of ADF serotonergic neurons (n = 757 nuclei); however, only 11.4% of nuclei in the ser-1(ok345) deletion mutant animals showed any sign of HSF1 puncta (n = 1,239 nuclei) (Figures 5J and 5K). These data confirm that cell nonautonomous neuronal control of HSF1 occurs through serotonin secretion, although they do not preclude the involvement of other collaborating ligands.
Optogenetic Activation of Serotonergic Neurons Protects Protein Homeostasis
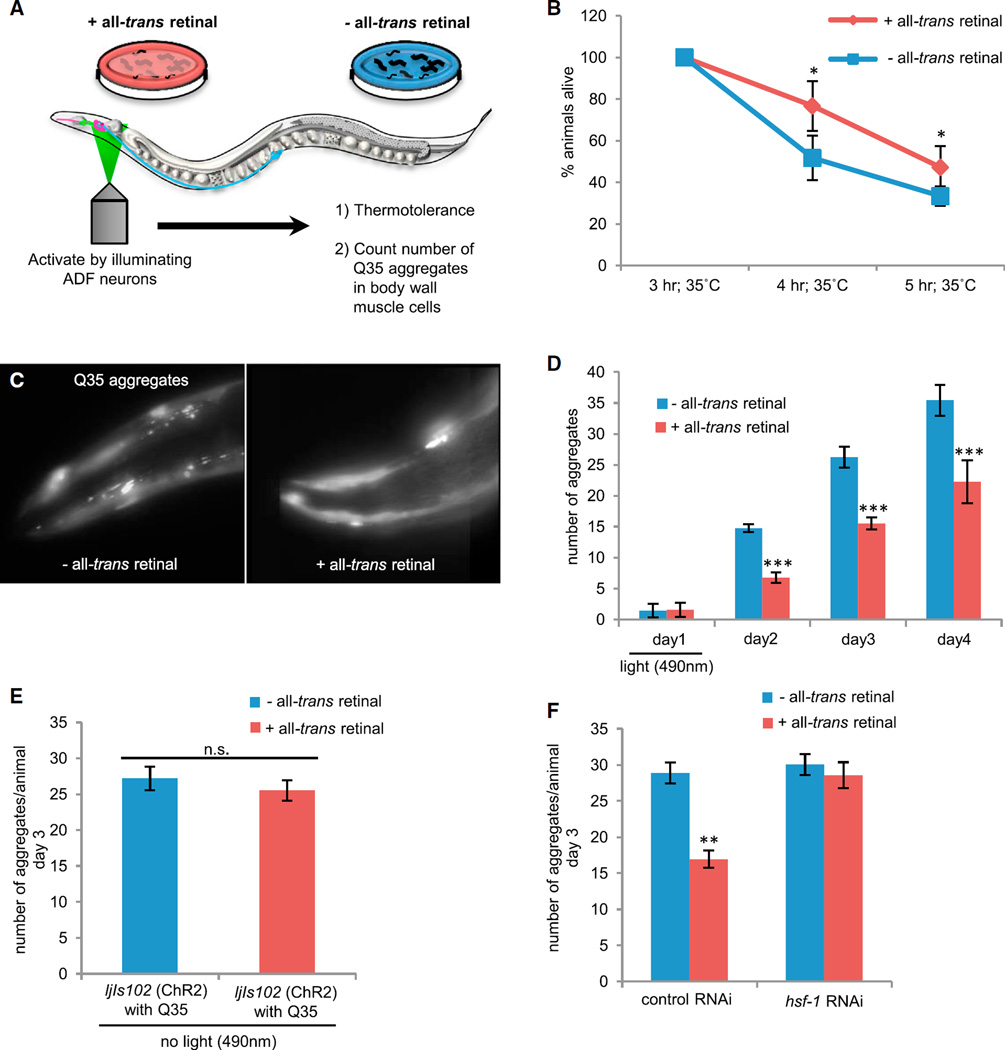
HSF1 transcriptional activation protects organisms against lethal stress and the accumulation of misfolded and aggregated proteins [6, 7]. Consistent with this, we found that optogenetic stimulation of serotonergic neurons in experimental (+ATR) animals increased organismal survival upon subsequent exposure to a lethal dose of heat stress when compared to controls (−ATR) (Figures 6A and 6B). We next examined whether optogenetic activation of the ADF neurons was sufficient to cell nonautonomously suppress misfolding and aggregation of Q35::YFP in body wall muscle cells of C. elegans [13, 23]. Remarkably, a single pulse of optogenetic stimulation of the ADF neurons alone was sufficient to reduce the age-dependent accumulation of Q35 aggregates (Figures 6A, 6C, and 6D). Although all animals started with a similar number of aggregates, control animals (−ATR) and experimental animals (+ATR) that had not been optogenetically activated (Figures 6D and 6E) accumulated an average of 26.2 aggregates by day 3 of adulthood. In contrast, optogenetic activation of ADF neurons reduced aggregation by 41% to an average of 15.5 aggregates per animal (Figures 6C and 6D), and aggregate accumulation remained lower with age. This reduction in the number of Q35 aggregates was HSF1 dependent as knockdown of HSF1 with RNA interference restored the number to control levels (Figure 6F).
Figure 6. Optogenetic Stimulation of ADF and NSM Neurons Increases Thermotolerance and Suppresses polyQ Aggregation in Body Wall Muscle Cells.
(A) Experimental set up (as described in Figure 5 legend).
(B) Thermotolerance of experimental (+ATR) versus control (−ATR) animals following optogenetic stimulation. n = 80–83 animals per condition; two-way ANOVA; *p < 0.05.
(C) Q35::YFP aggregates visualized in head-muscle cells of control (−ATR) and experimental (+ATR) animals 3 days following optogenetic stimulation.
(D) The number of Q35::YFP aggregates in control (−ATR) and experimental (+ATR) animals over time. n = 7–10 animals per time point per experimental manipulation, t test; ***p < 0.001.
(E) The number of Q35::YFP aggregates in control (−ATR) and experimental (+ATR) animals in the absence of optogenetic stimulation.
(F) The number of Q35::YFP aggregates following RNAi-mediated knockdown of HSF1 in experimental (+ATR) animals following optogenetic stimulation. n = 26, t test; **p < 0.01.
Discussion
To date, the signaling mechanisms underlying nonautonomous control of the HSR and its biological relevance were unknown. Our experiments identify the enhanced release of serotonin resulting from thermosensory neuronal activity as a signaling mechanism sufficient to initiate the protective activation of HSF1 in remote tissues. Because in our optogenetic experiments this activation occurs in the absence of any detectable indications of stress, we hypothesize that, as in numerous psychophysical stress paradigms where serotonin release allows the animal to preemptively adapt its physiology to threatening conditions [1–3], serotonin release upon neurosensory perception of temperature change allows organisms to initiate protective protein quality control mechanisms prior to the oncoming onslaught of proteotoxic damage (Figure 7). Notably, hsp70 accumulation upon optogenetic activation of neuronal circuitry is orders of magnitude less than what is seen following actual exposure to high temperatures or other proteotoxic conditions. Given the instability of hsp70 mRNA under normal, nonproteotoxic conditions, this is perhaps not too surprising [42, 43]. These data support our hypothesis that serotonin release may be an initiating event in hsp70 induction important for the adaptation of the organism to the subsequent protein damage that could be inflicted if noxious conditions persist.
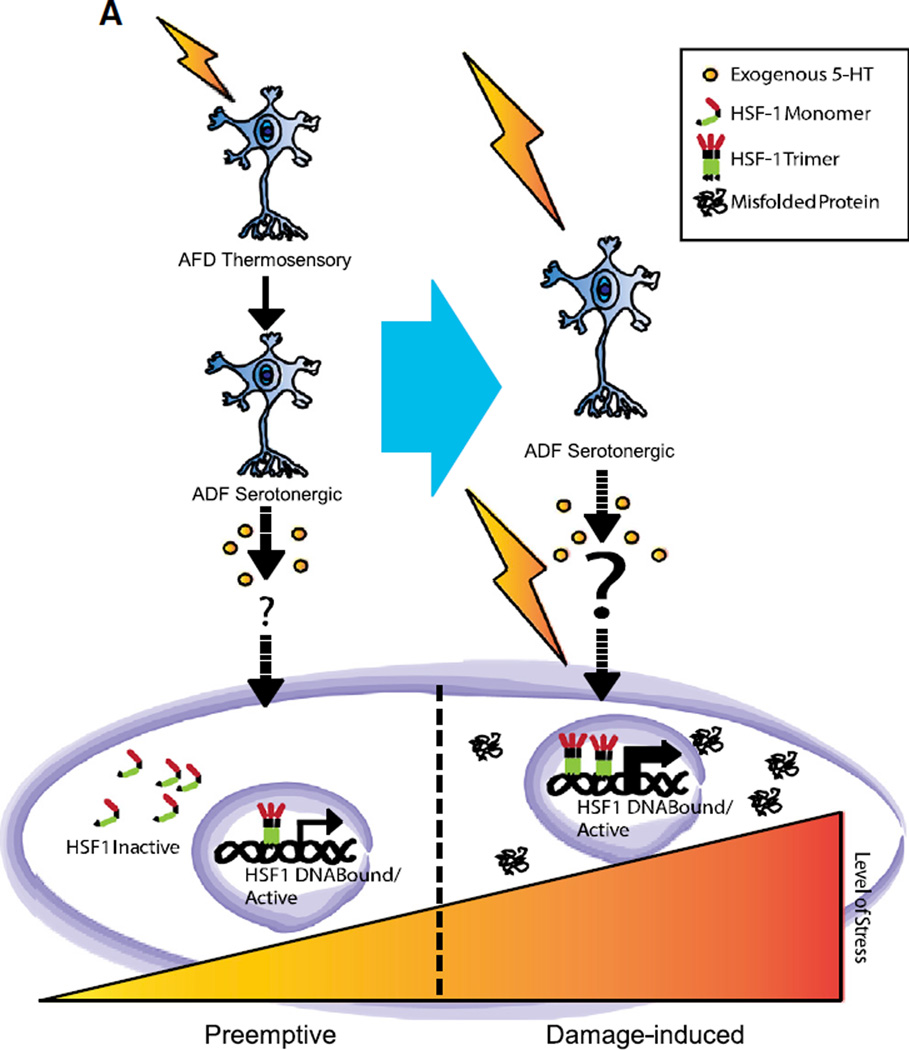
Figure 7. Model for HSF1 Activation upon Neurosensory Release of Serotonin.
The HSR of a cell can be activated in C. elegans in two distinct phases. An initial preemptive neurosensory phase involves the excitation of AFD thermosensory neurons that typically respond to innocuous temperature shifts. This elicits serotonin release, directly or indirectly activates HSF1 to transiently increase hsp70 mRNA levels, and occurs in the absence of protein misfolding. Subsequently, prolonged exposure to heat can cause protein misfolding, and the resulting damage induces HSF1-dependent hsp70 transcription as has been well described. The role of serotonin in this latter phase is not known.
Our data show that the AFD thermosensory neurons communicate with the ADF and NSM serotonergic neurons. How this occurs is not known. There is evidence for crosstalk between serotonergic ADF and thermosensory AFD neurons of C. elegans involving conserved signaling pathways, and these may play a role [12, 44–46]. For instance, while the lack of serotonin does not disrupt thermotaxis, serotonin is required for the adaptive plasticity of C. elegans thermotaxis behavior through activation of the protein kinase C homolog [46]. AFD neurons are themselves not serotonergic, nor do they synapse directly onto serotonergic neurons. Thus, AFD neurons could act through downstream interneurons, such as the AIY, to communicate with serotonergic neurons via synaptic mechanisms or electrical/chemical coupling across gap junctions. Alternatively, the excitation of AFD neurons may cause local paracrine release of neuroendocrine factors, which act on the serotonergic neurons to elicit serotonin release. These mechanisms are not mutually exclusive and remain to be investigated. We also do not know where serotonin is localized following its enhanced release or whether its effects on germ cell nuclei are direct or indirect. The NSM neurons have sensory endings in the pharyngeal lumen and are well poised to communicate with the rest of the worm’s body via secretion into the pseudocoelomic fluid [37, 39], and SER-1 is expressed in many tissues, including pharyngeal muscle, neuronal processes in the nerve ring, neurons in the tail, ventral cord motor neurons in vulval muscle, uterine cells, and perhaps in the posterior intestine [47, 48], where it could play a role in the secondary transmission of the stress signal to germ cell nuclei. In addition, the AIM and RIH neurons express the serotonin reuptake channel MOD-5 and are known to uptake extrasynaptic serotonin [35]; however, since deletion of mod-5 does not cause a decrease in the HSR, this pathway may not be integral to the signaling mechanism.
An intriguing possibility is that enhanced serotonin release could act as a node for the nonautonomous regulation of HSF1 in response to multiple noxious environmental stimuli. Serotonergic signaling is involved in the innate immune response to pathogens and response to hypoxia [27, 28]. It would be of interest to see whether activation of the sensory neurons involved in pathogen recognition or oxygen tension alone can also preemptively activate HSF1 through serotonin release.
The decrease in polyQ aggregation following stimulation of serotonergic neurons is also striking. The most dramatic decrease occurs within the first 2 days following optogenetic stimulation, presumably due to the transient activation of HSF1. However, aggregation continues to occur at apparently lower rates. At the molecular level, we do not know whether this corresponds to decreasing the pool of oligomers, increasing degradation rates, or both. Although technically challenging in our current assays, it would be interesting to determine whether a more-patterned regimen of optogenetic stimulation could completely prevent exacerbation of aggregation altogether. Serotonergic signaling pathways are highly conserved between C. elegans and mammals, suggesting that the nonautonomous regulation of protein homeostasis seen here may also be conserved. Indeed, serotonin is integral to thermoregulation in mammals [49, 50], and serotonergic syndrome, a life-threatening human condition associated with excessive serotonergic activity, causes fever, a stimulus for HSF1 activation [51, 52]. Indeed, modulating serotonin levels by administration of SSRIs can provide enhanced protection against neurodegeneration in vertebrate models of protein conformational diseases [53]. Conversely, dysregulation of serotonin signaling alters aging rates in C. elegans and is associated with Alzheimer’s disease and ALS [53, 54]. In summary, if protein quality control mechanisms in multicellular organisms can be activated cell nonautonomously and preemptively through the regulation of sensory neuronal modalities and serotonin release as shown here, another treatment of neurodegenerative diseases could involve modulation of neurosensory systems: a method that can be used alone or in combination with small molecules to effectively target protein misfolding.
Experimental Procedures
Detailed experimental procedures are described in Supplemental Experimental Procedures.
Growth Conditions
All strains were grown and maintained at 20°C. Extreme care was taken to maintain ambient temperature in the room very close to 20°C. Two transgenic strains were generated for these experiments: (1) a strain, AM1061, that expressed a hsf-1 minigene fused to GFP integrated into the ttTi5605 II locus and (2) a strain, DCR186, that expressed the olaEx75 [Ptph-1:: caspase-3(p12)::nz; Ptph-1::cz::caspase-3(p17);Punc-122::mCherry] transgene, which induced the caspase-mediated ablation of NSM and ADF neurons. Strains expressing ChR2 in the AFD thermosensory neurons and ADF and NSM serotonergic neurons were kind gifts from Paul Sternberg and Michael R. Koelle, respectively. The remaining strains were obtained from the Caenorhabditis Genetics Center (CGC).
Measuring Pumping Rates
The pumping rate of each worm was recorded under a MZ10F stereo microscope (Leica) at 8× magnification or under the Axio Observer A1 inverted microscope (Zeiss) at 40× magnification, as needed.
Serotonin Whole-Worm Immunostaining Protocol
Anti-serotonin staining was performed following a modified protocol developed by the Loer laboratory (http://home.sandiego.edu/~cloer/loerlab/anti5htshort.html). Serotonin localization patterns were scored blinded and quantified using Ptph-1::GFP expression to define serotonergic neuronal cell bodies. Specifically, collapsed confocal z stack images were taken for each worm to visualize both GFP (Ptph-1::GFP) and serotonin. Colocalization of serotonin and GFP expression was seen within neuronal cell bodies, but only anti-serotonin staining was observed outside the neuronal cell bodies. Each worm was divided into the following three areas: area 1 (from metacorpus to buccal cavity), area 2 (serotonergic neuronal cell bodies), and area 3 (from pharyngeal terminal bulb toward posterior body). Images were classified in a binary manner, based on the following criteria: (1) location (presence of staining in area 1); (2) continuity of staining in area 1; (3) intensity higher than that of background. For criterions (1) and (3), the background fluorescence in area 1 was quantified as mean pixel intensity. Animals with continuous staining in area 1, which had an average intensity of 150 mean pixel intensity over background, were scored as positive for staining outside serotonergic neuronal cell bodies.
Heat Shock Protocol
The animals were subjected to temperature increase in two ways: (1) heat shock on a Peltier stage (PeCon GmbH Temp Controller) and (2) heat shock in a water bath.
RNA Extraction and qRT-PCR
RNA extraction was conducted according to previously published methods [1]. The relative amounts of hsp mRNA were determined using the delta delta CT (ddCT) method. Actin mRNA was used as an internal control. All relative changes of hsp mRNA were normalized to that of wild-type heat-shocked animals, except where otherwise noted. Technical duplicates or triplicates were used to obtain CT values. Experiments were repeated at least three times. Values shown indicate mean ± SEM. All quantitative PCR (qPCR) reactions were assessed by melt-curve analysis performed at the end of the reaction and included no reverse transcriptase (no-RT) controls.
Optogenetic Experiments
Optogenetic activation was conducted according to previously published methods as per the requirements of the experiment. The main concerns were to minimize stress from the methodology itself and to obtain enough sample material. Thus, for HSF1 activation and Q35 protection assays, optogenetic stimulation was conducted on immobilized animals under the Axio Observer A1 inverted microscope (Zeiss) using Andor iQ 2.9.1 software (Andor) and X-Cite XLED1 1.1.0.2 software (Lumen Dynamics) to control the light source. For hsp70 mRNA measurements and thermotolerance assays, free-behaving ijIs102; lite-1(ce314) animals were activated at 6.3× magnification using a MZ10 F microscope with an EL6000 light source (Leica). Optogenetic serotonin release following light stimulation was confirmed by measuring pharyngeal pumping rates or body bends per minute.
Thermotolerance Experiments
All experiments following exposure to lethal heat stress were scored blinded.
Statistical Analysis
Where applicable, significance was tested using Student’s t tests or, for the thermotolerance assay, a two-way ANOVA. p values are indicated as follows: *p < 0.05, **p < 0.01, ***p < 0.001. A Fisher’s exact test in conjunction with a post hoc power analysis was used for determining the appropriateness of our smaller sample sizes to support our conclusions.
Supplementary Material
Acknowledgments
We would like to acknowledge the members of the V.P. laboratory, Jessica Nelson, Josh Weiner, Sarit Smolikove, Tali Gidalevitz, Anat Ben-Zvi, Michael Petrascheck, Sandra Encalada, and Daniel Colon-Ramos for helpful comments. The DCR186 strain was a kind gift from Daniel Colon-Ramos. Nematode strains were obtained from the CGC, funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). L.A.M.-V. was supported by the Diversity Supplement to NS057931 (Colón-Ramos/L.A.M.-V.). L.C. was funded by the Fulbright Franco-American Commission. R.I.M. was funded by grants from the NIH (NIGMS, NIA, NINDS), the Ellison Medical Foundation, and the Daniel F. and Ada L. Rice Foundation. V.P. was funded by the Ellison Medical Foundation (AG-NS-1056-13).
Footnotes
Supplemental Information
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2014.11.040.
Author Contributions
M.C.T, M.R.C., F.K.O., and V.P. designed and performed the experiments and wrote the manuscript. L.C. and R.I.M. made and contributed the AM1061 strain. L.A.M.-V. made the DCR186 strain. H.W.M.S. contributed the anti-serotonin antibodies. All authors commented on the manuscript.
References
- 1.Chaouloff F, Berton O, Mormède P. Serotonin and stress. Neuropsychopharmacology. 1999;21(Suppl):28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 2.Joëls M, Baram TZ. The neuro-symphony of stress. Nat. Rev. Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF. Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. J. Neurosci. 2011;31:14107–14115. doi: 10.1523/JNEUROSCI.3095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukau B. Regulation of the Escherichia coli heat-shock response. Mol. Microbiol. 1993;9:671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 5.Somero GN. Proteins and temperature. Annu. Rev. Physiol. 1995;57:43–68. doi: 10.1146/annurev.ph.57.030195.000355. [DOI] [PubMed] [Google Scholar]
- 6.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat. Rev. Mol. Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindquist S. The heat-shock response. Annu. Rev. Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 8.Vabulas RM, Raychaudhuri S, Hayer-Hartl M, Hartl FU. Protein folding in the cytoplasm and the heat shock response. Cold Spring Harb. Perspect. Biol. 2010;2:a004390. doi: 10.1101/cshperspect.a004390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Oosten-Hawle P, Morimoto RI. Transcellular chaperone signaling: an organismal strategy for integrated cell stress responses. J. Exp. Biol. 2014;217:129–136. doi: 10.1242/jeb.091249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- 11.Bonini NM. Chaperoning brain degeneration. Proc. Natl. Acad. Sci. USA. 2002;99(Suppl 4):16407–16411. doi: 10.1073/pnas.152330499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320:811–814. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prahlad V, Morimoto RI. Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proc. Natl. Acad. Sci. USA. 2011;108:14204–14209. doi: 10.1073/pnas.1106557108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumsta C, Ching TT, Nishimura M, Davis AE, Gelino S, Catan HH, Yu X, Chu CC, Ong B, Panowski SH, et al. Integrin-linked kinase modulates longevity and thermotolerance in C. elegans through neuronal control of HSF-1. Aging Cell. 2014;13:419–430. doi: 10.1111/acel.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maman M, Carvalhal Marques F, Volovik Y, Dubnikov T, Bejerano-Sagie M, Cohen E. A neuronal GPCR is critical for the induction of the heat shock response in the nematode C. elegans. J. Neurosci. 2013;33:6102–6111. doi: 10.1523/JNEUROSCI.4023-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153:1435–1447. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark DA, Biron D, Sengupta P, Samuel ADT. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J. Neurosci. 2006;26:7444–7451. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Husson SJ, Gottschalk A, Leifer AM. Optogenetic manipulation of neural activity in C. elegans: from synapse to circuits and behaviour. Biol. Cell. 2013;105:235–250. doi: 10.1111/boc.201200069. [DOI] [PubMed] [Google Scholar]
- 19.Sandqvist A, Sistonen L. Nuclear stress granules: the awakening of a sleeping beauty? J. Cell Biol. 2004;164:15–17. doi: 10.1083/jcb.200311102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jolly C, Usson Y, Morimoto RI. Rapid and reversible re-localization of heat shock factor 1 within seconds to nuclear stress granules. Proc. Natl. Acad. Sci. USA. 1999;96:6769–6774. doi: 10.1073/pnas.96.12.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarge KD, Murphy SP, Morimoto RI. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA-binding activity, and nuclear localization and can occur in the absence of stress. Mol. Cell. Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton EA, Lamitina T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell. 2013;12:112–120. doi: 10.1111/acel.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morley JF, Brignull HR, Weyers JJ, Morimoto RI. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark SG, Shurland DL, Meyerowitz EM, Bargmann CI, van der Bliek AM. A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans. Proc. Natl. Acad. Sci. USA. 1997;94:10438–10443. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gidalevitz T, Ben-Zvi A, Ho KH, Brignull HR, Morimoto RI. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- 26.Chang AJ, Chronis N, Karow DS, Marletta MA, Bargmann CI. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 2006;4:e274. doi: 10.1371/journal.pbio.0040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pocock R, Hobert O. Hypoxia activates a latent circuit for processing gustatory information in C. elegans. Nat. Neurosci. 2010;13:610–614. doi: 10.1038/nn.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438:179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 29.Song BM, Faumont S, Lockery S, Avery L. Recognition of familiar food activates feeding via an endocrine serotonin signal in Caenorhabditis elegans. Elife. 2013;2:e00329. doi: 10.7554/eLife.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avery L, Horvitz HR. Effects of starvation and neuroactive drugs on feeding in Caenorhabditis elegans. J. Exp. Zool. 1990;253:263–270. doi: 10.1002/jez.1402530305. [DOI] [PubMed] [Google Scholar]
- 31.Keane J, Avery L. Mechanosensory inputs influence Caenorhabditis elegans pharyngeal activity via ivermectin sensitivity genes. Genetics. 2003;164:153–162. doi: 10.1093/genetics/164.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasserman SM, Beverly M, Bell HW, Sengupta P. Regulation of response properties and operating range of the AFD thermosensory neurons by cGMP signaling. Curr. Biol. 2011;21:353–362. doi: 10.1016/j.cub.2011.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sze JY, Victor M, Loer C, Shi Y, Ruvkun G. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature. 2000;403:560–564. doi: 10.1038/35000609. [DOI] [PubMed] [Google Scholar]
- 34.Inada H, Ito H, Satterlee J, Sengupta P, Matsumoto K, Mori I. Identification of guanylyl cyclases that function in thermosensory neurons of Caenorhabditis elegans. Genetics. 2006;172:2239–2252. doi: 10.1534/genetics.105.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jafari G, Xie Y, Kullyev A, Liang B, Sze JY. Regulation of extrasynaptic 5-HT by serotonin reuptake transporter function in 5-HT-absorbing neurons underscores adaptation behavior in Caenorhabditis elegans. J. Neurosci. 2011;31:8948–8957. doi: 10.1523/JNEUROSCI.1692-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Axäng C, Rauthan M, Hall DH, Pilon M. Developmental genetics of the C. elegans pharyngeal neurons NSML and NSMR. BMC Dev. Biol. 2008;8:38. doi: 10.1186/1471-213X-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- 38.Sze JY, Zhang S, Li J, Ruvkun G. The C. elegans POU-domain transcription factor UNC-86 regulates the tph-1 tryptophan hydroxylase gene and neurite outgrowth in specific serotonergic neurons. Development. 2002;129:3901–3911. doi: 10.1242/dev.129.16.3901. [DOI] [PubMed] [Google Scholar]
- 39.Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans. WormBook. 2007:1–15. doi: 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gürel G, Gustafson MA, Pepper JS, Horvitz HR, Koelle MR. Receptors and other signaling proteins required for serotonin control of locomotion in Caenorhabditis elegans. Genetics. 2012;192:1359–1371. doi: 10.1534/genetics.112.142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris G, Korchnak A, Summers P, Hapiak V, Law WJ, Stein AM, Komuniecki P, Komuniecki R. Dissecting the serotonergic food signal stimulating sensory-mediated aversive behavior in C. elegans. PLoS ONE. 2011;6:e21897. doi: 10.1371/journal.pone.0021897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen R, Lindquist S. The Drosophila hsp70 message is rapidly degraded at normal temperatures and stabilized by heat shock. Gene. 1988;72:161–168. doi: 10.1016/0378-1119(88)90138-2. [DOI] [PubMed] [Google Scholar]
- 43.Petersen RB, Lindquist S. Regulation of HSP70 synthesis by messenger RNA degradation. Cell Regul. 1989;1:135–149. doi: 10.1091/mbc.1.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adachi R, Osada H, Shingai R. Phase-dependent preference of thermosensation and chemosensation during simultaneous presentation assay in Caenorhabditis elegans. BMC Neurosci. 2008;9:106. doi: 10.1186/1471-2202-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ailion M, Thomas JH. Dauer formation induced by high temperatures in Caenorhabditis elegans. Genetics. 2000;156:1047–1067. doi: 10.1093/genetics/156.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Zhao Y, Huang X, Lin X, Guo Y, Wang D, Li C, Wang D. Serotonin control of thermotaxis memory behavior in nematode Caenorhabditis elegans. PLoS ONE. 2013;8:e77779. doi: 10.1371/journal.pone.0077779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao H, Hapiak VM, Smith KA, Lin L, Hobson RJ, Plenefisch J, Komuniecki R. SER-1, a Caenorhabditis elegans 5-HT2-like receptor, and a multi-PDZ domain containing protein (MPZ-1) interact in vulval muscle to facilitate serotonin-stimulated egg-laying. Dev. Biol. 2006;298:379–391. doi: 10.1016/j.ydbio.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 48.Dempsey CM, Mackenzie SM, Gargus A, Blanco G, Sze JY. Serotonin (5HT), fluoxetine, imipramine and dopamine target distinct 5HT receptor signaling to modulate Caenorhabditis elegans egg-laying behavior. Genetics. 2005;169:1425–1436. doi: 10.1534/genetics.104.032540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azmitia EC. Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res. Bull. 2001;56:413–424. doi: 10.1016/s0361-9230(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 50.Feldberg W, Myers RD. A new concept of temperature regulation by amines in the hypothalamus. Nature. 1963;200:1325. doi: 10.1038/2001325a0. [DOI] [PubMed] [Google Scholar]
- 51.Brown IR, and Rush SJ. In vivo activation of neural heat shock transcription factor HSF1 by a physiologically relevant increase in body temperature. J. Neurosci. Res. 1996;44:52–57. doi: 10.1002/(SICI)1097-4547(19960401)44:1<52::AID-JNR7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 52.Iqbal MM, Basil MJ, Kaplan J, Iqbal MT. Overview of serotonin syndrome. Ann. Clin. Psychiatry. 2012;24:310–318. [PubMed] [Google Scholar]
- 53.Mattson MP, Maudsley S, Martin B. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res. Rev. 2004;3:445–464. doi: 10.1016/j.arr.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Ye X, Linton JM, Schork NJ, Buck LB, Petrascheck M. A pharmacological network for lifespan extension in Caenorhabditis elegans. Aging Cell. 2014;13:206–215. doi: 10.1111/acel.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.