The extent of axonal injury after brain trauma cannot yet be assessed reliably in living subjects. Magnoni et al. report that microdialysis measurements of the cytoskeletal protein tau correlate well with locally reduced anisotropy revealed by diffusion tensor imaging. This cross-validation increases confidence in both methods to assess axonal injury.
Keywords: traumatic brain injury, traumatic axonal injury, diffusion tensor imaging, microdialysis, tau
The extent of axonal injury after brain trauma cannot yet be assessed reliably in living subjects. Magnoni et al. report that microdialysis measurements of the cytoskeletal protein tau correlate well with locally reduced anisotropy revealed by diffusion tensor imaging. This cross-validation increases confidence in both methods to assess axonal injury.
Abstract
Axonal injury is a major contributor to adverse outcomes following brain trauma. However, the extent of axonal injury cannot currently be assessed reliably in living humans. Here, we used two experimental methods with distinct noise sources and limitations in the same cohort of 15 patients with severe traumatic brain injury to assess axonal injury. One hundred kilodalton cut-off microdialysis catheters were implanted at a median time of 17 h (13–29 h) after injury in normal appearing (on computed tomography scan) frontal white matter in all patients, and samples were collected for at least 72 h. Multiple analytes, such as the metabolic markers glucose, lactate, pyruvate, glutamate and tau and amyloid-β proteins, were measured every 1–2 h in the microdialysis samples. Diffusion tensor magnetic resonance imaging scans at 3 T were performed 2–9 weeks after injury in 11 patients. Stability of diffusion tensor imaging findings was verified by repeat scans 1–3 years later in seven patients. An additional four patients were scanned only at 1–3 years after injury. Imaging abnormalities were assessed based on comparisons with five healthy control subjects for each patient, matched by age and sex (32 controls in total). No safety concerns arose during either microdialysis or scanning. We found that acute microdialysis measurements of the axonal cytoskeletal protein tau in the brain extracellular space correlated well with diffusion tensor magnetic resonance imaging-based measurements of reduced brain white matter integrity in the 1-cm radius white matter-masked region near the microdialysis catheter insertion sites. Specifically, we found a significant inverse correlation between microdialysis measured levels of tau 13–36 h after injury and anisotropy reductions in comparison with healthy controls (Spearman’s r = −0.64, P = 0.006). Anisotropy reductions near microdialysis catheter insertion sites were highly correlated with reductions in multiple additional white matter regions. We interpret this result to mean that both microdialysis and diffusion tensor magnetic resonance imaging accurately reflect the same pathophysiological process: traumatic axonal injury. This cross-validation increases confidence in both methods for the clinical assessment of axonal injury. However, neither microdialysis nor diffusion tensor magnetic resonance imaging have been validated versus post-mortem histology in humans. Furthermore, future work will be required to determine the prognostic significance of these assessments of traumatic axonal injury when combined with other clinical and radiological measures.
Introduction
Traumatic brain injury (TBI) is a common cause of mortality and disability among people worldwide (Coronado et al., 2012). Traumatic axonal injury is commonly observed after human and experimental TBI (Adams et al., 1982; Gennarelli et al., 1982; Blumbergs et al., 1995; Smith et al., 2000, 2003) and diffuse axonal injury is a predominant pathology in fatal TBI and disabled survivors from traumatic brain injury (Strich, 1956; Adams et al., 2011). Experimental and clinical data indicate that axonal pathology plays a fundamental role in the pathophysiology of TBI, and may be a key predictor of outcome (Medana and Esiri, 2003). However, the true burden of traumatic axonal damage in survivors is not known with certainty, in large part due to lack of validated methods for assessing white matter injury in the living human brain. Specific measures of white matter damage, if validated, will fill this gap, helping to overcome the limitations of existing severity classifications for traumatic brain injury, and allowing future improved stratification of patients for therapeutic interventions targeting axonal injury.
There is increasing evidence suggesting that a cascade of early events including disruption of axonal membranes, cytoskeletal breakdown and loss of microtubules, in addition to the interruption of axonal transport, are all mechanisms of traumatic axonal injury, and these mechanisms can trigger late axonal degeneration (Büki and Povlishock, 2006). As a consequence, it has been hypothesized that cytoskeletal proteins from damaged axons would be released into the cerebral interstitial fluid compartment after TBI (Siman et al., 2004). Recent studies using microdialysis, an invasive but generally safe technique for the dynamic monitoring of brain metabolic changes in patients with TBI, confirmed this hypothesis showing that the brain extracellular fluid of patients with TBI contains increased concentrations of tau, neurofilament light chain and neurofilament heavy chain, all of which are normally cytosolic axonal proteins (Marklund et al., 2009; Petzold et al., 2011; Magnoni et al., 2012). Notably, elevated levels of both tau (Magnoni et al., 2012) and neurofilament heavy chain cleavage products (Petzold et al., 2011) predicted worse clinical outcomes. These data support the hypothesis that microdialysis measurements of cytoskeletal proteins in the extracellular space of the injured human brain represent a useful approach to assess the severity of traumatic axonal injury, and possibly to predict outcome. However, the relationship between brain interstitial fluid levels of tau and other proteins and the severity of traumatic axonal injury was not directly verified in these previous studies. The ultimate gold standard would be to compare microdialysis measures to direct visualization of axonal injury at autopsy. However, with modern intensive care, relatively few of our patients die in the early days to weeks following microdialysis catheter implantation. We therefore decided to use diffusion tensor magnetic resonance imaging (DTI) as an independent non-invasive method to assess axonal pathology in human TBI patients. DTI is a sensitive and robust method to assess white matter structural integrity following TBI (Arfanakis et al., 2002; Inglese et al., 2005; Nakayama et al., 2006; Salmond et al., 2006; Kraus et al., 2007; Newcombe et al., 2007; Niogi et al., 2008; Sidaros et al., 2008; Perlbarg et al., 2009; Niogi and Mukherjee, 2010; Kinnunen et al., 2011; Galanaud et al., 2012; Hulkower et al., 2013). DTI measures the directional diffusion of water, which in normal white matter is restricted by the orientation of axon bundles. The resulting anisotropy (directional asymmetry) of water diffusion is higher in intact white matter compared to altered white matter (Le Bihan, 2003). Studies in animal models of traumatic brain injury have shown a strong correspondence between DTI abnormalities, such as reduced anisotropy, and histopathological features of pericontusional traumatic axonal injury and non-contusional diffuse white matter injury (Mac Donald et al., 2007a, b; Li et al., 2011, 2013; Bennett et al., 2012).
Further, we attempted to cross-validate DTI versus microdialysis measurements of tau in human patients with TBI. In principle, two methods of assessing traumatic axonal injury in the same patient should yield similar results. From the scientific perspective, cross-validation of two independent methods would greatly increase the confidence of interpretation of both methods, as it is very unlikely that two entirely distinct sets of technical caveats and noise sources would give the same result. From a clinical perspective, microdialysis and DTI have complementary strengths and limitations. Microdialysis provides regional whereas DTI provides whole brain information. Microdialysis is low risk but still requires an invasive neurosurgical procedure, whereas DTI is entirely safe in most patients. DTI typically can be done only once or a few times at most (see Newcombe et al., 2015), whereas microdialysis provides continuous time-resolved assessments. DTI is typically performed at relatively late times after injury, whereas microdialysis can be performed early after injury. Thus, if both measures provide similar information, this would add to the flexibility of the clinician in choosing which assessment to perform depending on the circumstances.
Materials and methods
Patients
Twenty patients admitted to intensive care for severe closed-head traumatic brain injury between 2007 and 2012 were prospectively recruited for the study. All research protocols were approved by the hospital ethics committee at the Ospedale Maggiore Policlinico in Milan. The patients, next of kin or legally authorized representatives provided written informed consent to the study. Inclusion criteria have been described previously (Magnoni et al., 2012). Briefly, 20 adult patients with acute severe brain injury (Glasgow Coma Scale ≤ 8), with a clinical indication for placement of invasive intracranial pressure monitoring, were recruited and underwent cerebral microdialysis monitoring. Two patients, in whom MRI was contraindicated because of the presence of ferromagnetic metal implants, were excluded. Two more patients were excluded from analysis because the microdialysis catheters were placed in contused haemorrhagic tissue, in which quantitative analysis of DTI was not feasible. One patient, who underwent late surgical evacuation of contusion followed by decompressive craniectomy, was also excluded because the CT scan showing the location of the microdialysis catheter and the DTI scan could not be co-registered. The final sample size was therefore 15 patients. Microdialysis measurements of tau and amyloid-β levels from four of these patients have previously been reported (Magnoni et al., 2012).
Microdialysis
CMA71 catheters (M Dialysis) with a nominal molecular weight cut-off of 100 kDa were inserted by trained neurosurgeons in conjunction with the insertion of an intracranial pressure monitoring device. All catheters were placed in the right frontal white matter. In three patients the catheter insertion took place at the end of a neurosurgical procedure for the removal of haemorrhagic contusions followed by decompressive craniectomy. Microdialysis catheters were placed at a minimum of 10 and maximum of 29 h after injury. CT scans were used to confirm the placement of the microdialysis catheters (Fig. 1 and Supplementary Fig. 1). The microdialysis catheters were consistently placed in normal appearing tissue on CT scans. Notably, none of the catheters were perilesional, and in patients with large contusions, the catheters were inserted contralaterally. CNS perfusion fluid (M Dialysis) + 1.5% sterile human albumin was perfused at a rate of 0.3 µl/min, as previously described (Brody et al., 2008). Sample collection was started 1–2 h after catheter insertion. Samples were collected every 1–2 h for at least 72 h (range 111–138, median 119 h). Samples were analysed for the commonly measured small molecule analytes glucose, lactate, pyruvate and glutamate with a commercial bedside kinetic enzymatic analyser (Iscus, M Dialysis), immediately placed on ice, and frozen at −80°C within 12 h of collection. All catheters were removed at the bedside by a trained neurosurgeon before MRI scanning.
Figure 1.
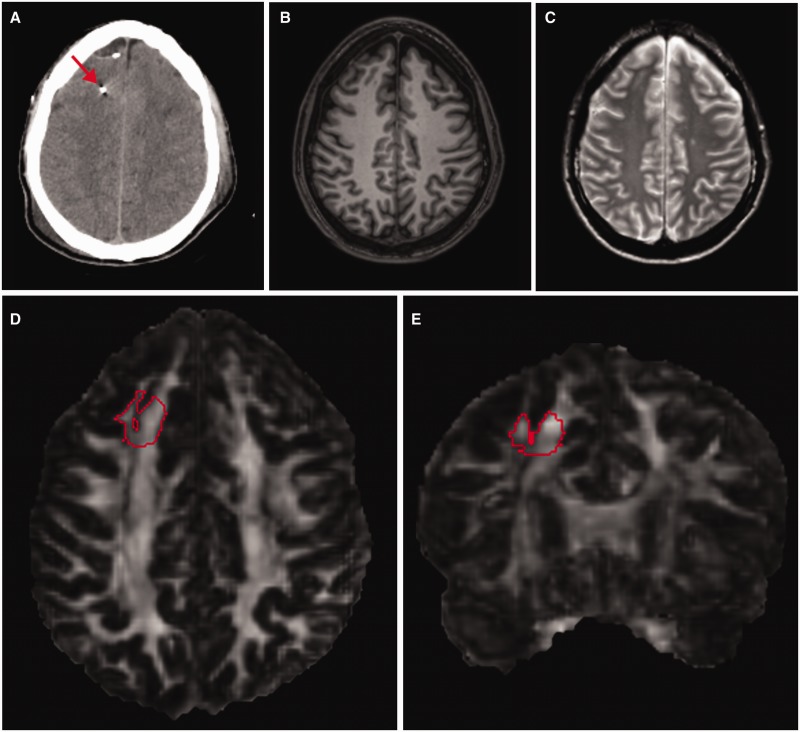
Example of DTI analysis in a region of interest around the location where the microdialysis catheter had been. (A) Axial view of the co-registered CT scan of the brain from a representative patient, showing the microdialysis gold-tipped catheter (arrow) placed in right frontal white matter. (B and C) Axial view of the co-registered T1- and T2-weighted MRI scans of the same patient, showing that brain tissue appears normal on conventional MRI around the region where the microdialysis catheter had been. Axial (D) and coronal (E) view of the co-registered fractional anisotropy map of the same patient performed 18 days after the injury and 11 days after the microdialysis catheter had been removed. The 3D region of interest (in red) used for DTI analysis was manually traced on the fractional anisotropy map around the coordinates in standardized space where the microdialysis catheter had been. The region of interest was then thresholded to include only white matter.
Microdialysis analytical methods
A sequential ELISA method was used to measure multiple analytes in each microdialysis sample. The general principles and accuracy of sequential ELISA methods have been described previously (Magnoni et al., 2012). Total tau levels were measured by ELISA using a commercial kit (#KHB0042, Invitrogen). Recombinant human tau standards provided by the manufacturer were run with each set of assays to generate standard curves. The sensitivity of this ELISA was 32 pg/ml. Amyloid-β (amyloid-β1–x) levels were measured by ELISA, as previously reported (Brody et al., 2008), or using a commercial kit for human amyloid-β (#JP27729, IBL International). The sensitivities of these ELISAs were typically 4.9 and 7.81 pg/ml, respectively. The two amyloid-β ELISAs performed otherwise comparably. Urea was measured using the QuantiChrom™ (BioAssay Systems) colorimetric urea assay kit, as a measure of catheter performance. A standard curve was run with each set of assays using urea standards provided by the manufacturer. Analysis of tau, amyloid-β, and urea were performed blinded to clinical and imaging data. Recovery of tau has been previously reported and is 1–2% (Magnoni et al., 2012).
Magnetic resonance imaging, including diffusion tensor imaging
All patients underwent the imaging protocol at Milan Ospedale Policlinico using a Philips 3 T Achieva scanner and an 8-channel head coil. The protocol included 3D T1-weighted, and T2-weighted morphological sequences plus DTI. The scanning parameters for T1 images were as follows: slice thickness 1 mm, field of view 324 × 240 mm, repetition time/echo time 8.1/3.7 ms, acquisition matrix 240 × 240. T2 images were acquired in the axial plane with 3 mm slice thickness, field of view was 340 × 256 mm, and repetition time/echo time 3000/80 ms, acquisition matrix 256 × 256. The DTI data were collected by a single-shot, spin echo-based and diffusion-weighted echo planar imaging sequence. The DTI parameters were as follows: single b0 followed by 32 non-collinear directions, b-value of 1000 s/mm2, field of view 256 × 256 mm, acquisition matrix 128 × 128, 70 axial slices, 2-mm slice thickness, repetition time 12 851 ms, echo time 56 ms. The total scan time was 35 min.
Eleven patients were scanned 2 to 9 weeks after injury (Table 1). Patients were all sedated and mechanically ventilated during subacute phase MRI acquisition. Many patients in the subacute phase were agitated and movement in the scanner would have been excessive without sedation. Neurointensive care specialists were present during all imaging sessions, and clinical parameters (ECG, SpO2, non-invasive blood pressure and end tidal CO2) were monitored using a wireless MRI compatible monitoring system. No adverse events related to the scans were observed.
Table 1.
Clinical characteristics of patients
| ID | Agea (years) M/F | GCS | Marshall CT Classification | Classification of TAI on conventional MRIb | Surgery Y/N | Time to scans after TBI w/m |
|---|---|---|---|---|---|---|
| 1 | 44 M | 7 | Evacuated mass lesion | Grade 2 | Y + DC | 4 w |
| 2 | 20 F | 5 | Diffuse injury III | Grade 3 | N | 4 w |
| 3 | 30 M | 5 | Diffuse injury III | Grade 2 | N | 9 w/36 m |
| 4 | 38 M | 7 | Diffuse Injury II | Grade 3 | N | 6 w/36 m |
| 5 | 46 M | 7 | Diffuse injury II | Grade 2 | N | 3 w/18 m |
| 6 | 21 M | 5 | Evacuated mass lesion | Grade 2 | Y + DC | 4 w/24 m |
| 7 | 35 F | 7 | Evacuated mass lesion | Grade 1 | Y | 2 w/17 m |
| 8 | 20 F | 5 | Diffuse injury II | Grade 2 | N | 2 w/33 m |
| 9 | 46 M | 5 | Diffuse injury II | Grade 2 | N | 2 w |
| 10 | 19 M | 5 | Diffuse injury II | Grade 2 | N | 2 w/12 m |
| 11 | 36 M | 5 | Evacuated mass lesion | Grade 2 | Y + DCc | 2 w |
| 12 | 16 F | 4 | Diffuse Injury II | Grade 3 | N | 36 m |
| 13 | 40 M | 6 | Diffuse injury II | Grade 2 | N | 36 m |
| 14 | 29 F | 5 | Diffuse injury II | Grade 3 | N | 24 m |
| 15 | 30 M | 5 | Diffuse injury II | Grade 2 | N | 12 m |
GCS = initial post-stabilization total Glasgow Coma Score; Diffuse injury II = cisterns present, midline shift <5 mm, and/or small <25 cm3 high or mixed density lesions; Diffuse Injury III = compressed or absent cisterns but midline shift <5 mm and/or small <25 cm3 high or mixed density lesions; DC = decompressive craniectomy; w = weeks after injury; m = months after injury; M = male; F = female; Y = yes; N = no.
aAge at the time of MRI.
bMRI classification of traumatic axonal injury, adapted from Adams and colleagues (Gentry et al., 1988; Adams et al., 1989).
cIn this patient surgical evacuation of a cerebral haematoma followed by decompressive craniectomy was performed on the second day of monitoring.
Patients with TBI who had been included in a previous microdialysis study (Magnoni et al., 2012) were re-approached for evaluation with an identical imaging protocol in the chronic phase, based on the consideration that fractional anisotropy abnormalities (reductions) can be observed long after injury (Inglese et al., 2005; Nakayama et al., 2006; Salmond et al., 2006; Kraus et al., 2007; Niogi et al., 2008; Sidaros et al., 2008; Kinnunen et al., 2011), likely resulting from a persistent fractional anisotropy depression (Sidaros et al., 2008; Mac Donald et al., 2011). Patients were excluded if they had contraindications to MRI, ventricular shunts, or any other metallic implants. Patients who had undergone major neurosurgical procedures at any time after injury were also not included in the chronic phase scanning because of the resultant difficulty in co-registering images. Among the other 10 who were approached, three did not consent to the study and three were unable to participate because of the severity of their disability or because they had relocated. This yielded an additional four patients who consented to undergo the MRI scan in the chronic phase (Table 1).
In addition, a repeat chronic phase scan was performed in 7 of 11 patients who were scanned initially 2–9 weeks after injury (Table 1). This involved an identical MRI protocol on the same MRI scanner and using the same 8-channel head coil, 1 to 3 years after injury. Two of the remaining four patients who did not undergo the follow-up scan had died, one had relocated, and one was unavailable. None of the patients undergoing chronic phase scans were sedated for the imaging protocol.
For each patient, we matched five controls (healthy volunteers) by age and sex. Several patients were similar in age and sex, thus the study required 32 control subjects, who underwent an identical imaging protocol.
An experienced neuroradiologist (A.C.) reviewed the conventional MRI sequences (T1 and T2-weighted images) and performed qualitative analysis, using an MRI-based classification of white matter damage derived from the neuropathological classification of traumatic axonal injury severity originally proposed by Adams and colleagues (Gentry et al., 1988; Adams et al., 1989; Skandsen et al., 2010). According to this classification, patients were classified as having lobar and/or cerebellar white matter lesions only (stage 1), corpus callosum (stage 2) and brainstem focal lesions (stage 3).
Overall, image quality was good, and there were no apparent motion artefacts. Tensor calculation and spatial co-registration of MRI were done at Washington University as previously described, using in-house software (Shimony et al., 2009; Mac Donald et al., 2011). Briefly, co-registration of each image set was performed using vector gradient measure maximization. The first acquired, unsensitized (b = 0) DTI volume was registered to the T2 image; stretch and shear were enabled (9-parameter affine transform) to partially compensate for subject motion and eddy current distortion. T2 was then co-registered similarly to T1. Finally, atlas transformation was computed via the T1-weighted image, which was registered to an atlas representative target. CT scan images were co-registered with the same modalities. Accuracy of co-registration was confirmed visually in all cases (Supplementary Fig. 1).
DTI region of interest analysis around microdialysis catheter insertion sites
Analyze software, version 10.1 (Bioimaging Imaging Resource) was used to extract quantitative DTI parameters, including voxel number, axial diffusivity, radial diffusivity, and mean diffusivity for regions of interest. The coordinates corresponding to the exact position of each microdialysis catheters were calculated for every patient on the atlas-registered CT scan showing the gold-tip of the catheter. Quantitative DTI analysis was performed by analysts blinded to clinical assessment and tau data. The analysts manually traced 10 mm radius spherical regions of interest centred on the catheter coordinates (Fig. 1). Regions of interest were segmented to exclude grey matter and CSF voxels and retain only brain white matter voxels by applying a fractional anisotropy threshold (<0.17). In one subject (Patient 9) a small haemorrhage was detectable around the catheter for intracranial pressure monitoring, in proximity to the microdialysis catheter. The haemorrhagic tissue was manually excluded during region of interest analysis (Supplementary Fig. 1).
For each patient, the same coordinates corresponding to the location of the microdialysis catheter were placed on the scans of each of five controls, and then the same manual outlining procedure was performed on the control scans. Careful visual inspection for anatomical correspondence was carried out in every subject. Any misalignment was manually corrected using the microdialysis catheters’ coordinates as reference. Multiple radii have been tested, based on previous studies using microdialysis and PET (Hutchinson et al., 2009).
The robustness of the region of interest analysis was tested calculating the intra- and inter-rater reliability for fractional anisotropy and voxel number. Intra-rater reliabilities were 98.7% (range 95–100%) and 98.5 (range 94–100%), respectively performed in a blinded fashion separated by several months, whereas blinded inter-rater reliabilities were 99.3% (range 97–100%) and 99.1% (range 96–100%), respectively for the two analysts (J.S. and B.S.).
DTI analysis of other brain regions of interest
We used DTIStudio (www.MRI.studio.org), an atlas-based region of interest analysis package, to obtain additional anisotropy measures from right and left brain regions commonly demonstrating diffuse axonal injury following brain trauma, including frontal gyrus white matter, corona radiata, corpus callosum, internal capsules and cerebral peduncles. Briefly, images for each subject and controls were aligned to a template atlas in a fully automated fashion, as previously described (Oishi et al., 2008). White matter was then segmented using a fractional anisotropy threshold of >0.20 for peripheral regions such as frontal gyrus white matter, followed by a minimum manual adjustment if necessary. The DTIStudio output provided fractional anisotropy, mean diffusivity, axial and radial diffusivity data and total voxels for each region. In two patients with prominent contusions and haemorrhages, region of interest analysis was not reliable in all regions; fractional anisotropy measures were not obtained from 6 of 20 regions in Patient 1, and 2 of 20 regions for Patient 3 (Supplementary material).
Statistical methods
All data were analysed using GraphPad Prism version 6 (GraphPad Software). Microdialysis data were typically not normally distributed, so Mann-Whitney U-tests were used for unpaired group comparisons, and Wilcoxon matched-pairs signed rank tests were used for paired comparisons. Spearman rank-order correlations were used for analyses of the relationships between variables, and Friedman tests were used for comparisons across time followed by Dunn’s multiple comparisons test, when applicable. One-sided tests were used when hypotheses were prespecified and were reported as significant for P < 0.05. Two-sided tests were used for other comparisons and reported as significant after correction for multiple comparisons. Specific P-values reported have not been corrected for multiple comparisons.
To assess tau and amyloid-β dynamics in a homogenous fashion across all patients with regard to the time of injury, 24-h time bins starting from 12 h after trauma were used for primary analyses. In addition, to compare the results with previous findings, we also used time bins based on intervals after the initiation of microdialysis (Magnoni et al., 2012). For DTI assessments in individual subjects, the abnormalities consistent with traumatic axonal injury were defined as values for fractional anisotropy that were >2 SD (standard deviations) below the mean of the values for their matched healthy controls (n = 5 for each patient). Abnormalities in the other DTI indices, such as mean, radial, axial diffusivity, voxel number and age differences were similarly defined as values beyond 2 SD the mean of the control values.
To assess the relationship between axonal injury and tau levels in the regions of interest, we performed correlation analyses between measures of anisotropy and mean tau levels in various time windows. For the analysis, we used the z-scores of the DTI indices, a measure which indicates by how many standard deviations the data were below or above the mean DTI values from the same anatomical region of interest in five age-matched normal control subjects scanned on the same scanner, using the same sequence.
Results
Patient characteristics and conventional imaging findings
We performed the complete protocol in 15 patients with severe acute TBI from the Neurointensive Care Unit at the Ospedale Maggiore Policlinico in Milan. Demographics and clinical characteristics of the subjects are summarized in Table 1. Initial Glasgow Coma Scores ranged from 4–7, indicated severe TBI. Age ranged from 16–46 years (at the time of injury), and there were five females and 10 males. According to the Marshall CT classification, 11 patients had diffuse brain injuries on the initial CT scan. Nine patients were classified as Diffuse Injury II, with only small lesions and effaced sulci observed (e.g. Fig. 1A), and two patients had more severe diffuse injury, with non-visible brain basal cisterns, a radiological signs of raised intracranial pressure (Diffuse Injury III). In four patients the principal lesions were haemorrhagic contusions and/or extra-cerebral haematomas that required early surgical treatment, including decompressive craniectomy in three (Supplementary Fig. 1). None of the patients had a normal CT scan. Qualitative analysis of conventional MRI showed signal abnormalities consistent with axonal injury in all patients (Table 1). One patient only had visible white matter lesions in the lobar hemispheres or cerebellum only, and was therefore classified as having less severe traumatic axonal injury (Grade 1), compared to the majority of subjects, who had additional lesions in the corpus callosum (Grade 2, n = 10) and in the brainstem (Grade 3, n = 4). Intracranial pressure was well controlled with appropriate medical treatment (in 12 patients) and/or early decompressive craniectomy (in two patients). Only one patient had sustained severe intracranial hypertension despite decompressive craniectomy (Patient 11).
In vivo microdialysis measurements
Brain microdialysis catheters were implanted at a median time of 17 h (13–29 h) after injury in normal appearing (on CT scan) right frontal white matter in all patients (Fig. 1A and Supplementary Fig. 1). None of the catheters were perilesional and in patients with large contusions, the catheters were inserted on the contralateral side. There were no intracranial haemorrhages or other adverse events related to microdialysis procedures, though there was a small haemorrhage around the nearby intracranial pressure monitor in one patient (Patient 9; Supplementary Fig. 1). In three patients small non-haemorrhagic abnormalities were detectable on conventional MRI in regions corresponding to the insertion of microdialysis catheters and/or intracranial pressure monitors (Supplementary Fig. 1 and Supplementary Table 1).
Tau levels were typically highest at the start of microdialysis and generally fell monotonically over time (Supplementary Fig. 2A), as previously reported (Magnoni et al., 2012). Overall, the median tau levels over the first 24 h of microdialysis sampling were markedly elevated: 12 813pg/ml (25th–75th percentile: 4858–18744 pg/ml). In contrast, amyloid-β levels measured in the same samples were lowest initially (25th–75th percentile: 575–1079 pg/ml, median 756 pg/ml) and rose over time (Supplementary Fig. 2B), which is in line with previous data (Magnoni et al., 2012). In this cohort, no catheters were placed in pericontusional regions, so overall tau levels were slightly lower than previously reported (Magnoni et al., 2012). We identified almost identical tau and amyloid-β dynamics when analysing the data from the perspective of time after injury starting 12 h after TBI (Supplementary Fig. 3). Tau, in particular, was markedly elevated (Friedman test, P < 0.0001) between 13–36 after injury (25th–75th percentile: 5900–18 303 pg/ml, median 13 441pg/ml) and significantly lower (Friedman test, followed by Dunn's multiple comparisons test, P < 0.5) 37–60 h after injury (25th–75th percentile: 622–11 421 pg/ml, median 2050 pg/ml) and 61–84 h after injury (25th–75th percentile: 627–5195 pg/ml, median 1894 pg/ml, Friedman test, followed by Dunn's multiple comparisons test, P < 0.0001). Furthermore, data were analysed at 6-h intervals from catheter insertion. These data indicate that in general, tau levels stabilize at 12–18 h after probe insertion (Supplementary Fig. 4A). However, there was a large variability in individual patients’ profiles, as in some subjects tau levels stabilized after a few hours, whereas in others it did not (Supplementary Fig. 4B and C).
The same microdialysis samples were assayed for brain metabolic markers and the small molecule glutamate (Hillered et al., 2005). These data are summarized in Supplementary Table 1. Overall, we found abnormalities in nine patients; the remaining subjects had entirely normal microdialysis data (Reinstrup et al., 2000; Hillered et al., 2006). Notably, the most frequently altered metabolite was lactate to pyruvate ratio, levels of which were pathologically high (>25) in seven cases, followed by glucose, which was abnormally low (<0.8 mM) in four patients, lactate which was >4 mM in four cases, and glutamate which was >25 μM in three patients. Only one patient (Patient 5) had severely elevated lactate to pyruvate ratio (i.e. >40) reflecting severely compromised brain metabolism (Vespa et al., 2007; Marcoux et al., 2008). Notably, Patient 5 also had elevated glutamate and abnormalities on conventional MRI in the region corresponding to the microdialysis catheter (Supplementary Table 1). None of the patients had truly ischaemic microdialysis values (i.e. elevated lactate to pyruvate ratio with low absolute pyruvate levels) nor signs of local infarction on MRI scans (Supplementary Fig. 1). Urea measurements were available in all but four patients (Supplementary Table 1). There were no significant correlations between brain urea and tau or between brain urea and amyloid-β.
Quantification of axonal injury by DTI
We used DTI to quantitatively assess white matter structural integrity in brain regions of interest within 10 mm of the location where the microdialysis catheters had been placed, as described in the ‘Materials and methods’ section. We performed identical analyses in spatially matched regions of interest in five age- and sex-matched controls for each patient. Our primary DTI measure was the normalized difference in fractional anisotropy between each patient and the controls, defined in units of standard deviations (z-scores). An individual region of interest was defined as abnormal if the DTI parameter was 2 SD above or below the mean for the matched controls. Using this criterion, nine patients had reduced fractional anisotropy in the peri-microdialysis catheter region of interest, whereas in six subjects, fractional anisotropy was not different from controls (Fig. 2). Fractional anisotropy in patients was never higher than controls. Fractional anisotropy reductions were the most consistently observed abnormalities, while the behaviour of the other metrics (e.g. mean diffusivity, axial diffusivity, radial diffusivity) was more variable (Fig. 2). By design, age was well matched between patients versus controls. Of the 11 patients scanned initially at 2–9 weeks after injury, seven were rescanned in the chronic phase 1–3 years after injury to explore the stability of the DTI abnormalities. All DTI parameters in the white matter regions around the microdialysis catheter insertion sites did not significantly differ in the chronic phase compared to the initial scans (Supplementary Table 2).
Figure 2.
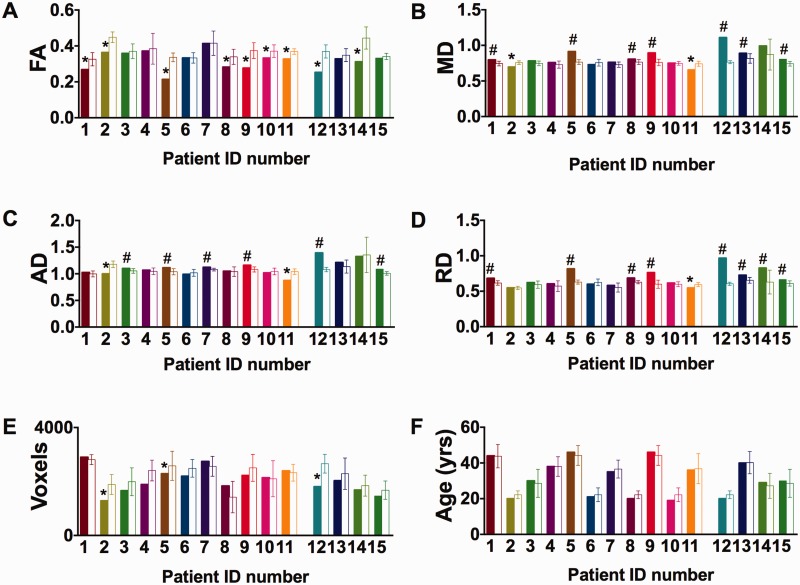
Abnormalities in DTI metrics in regions of interest around the locations where microdialysis catheters had been. (A) Differences in fractional anisotropy (FA) in patients (number 1 to 15) versus five age- and sex-matched, healthy controls for each patient (open bars). (B–D) Differences in mean diffusivity (MD), axial (AD), radial diffusivity (RD) in the same patients and controls. Fractional anisotropy reductions were the most consistently observed DTI abnormalities. Fractional anisotropy was reduced in 9 of 15 subjects. Mean diffusivity was increased in seven patients and decreased in two. Axial diffusivity was increased in six patients and decreased in two. Radial diffusivity was increased in eight patients and decreased in one. Interestingly, in the single patient with reduced radial diffusivity (Patient 11), axial diffusivity was reduced to an even greater extent and fractional anisotropy was also reduced. (E and F) Region of interest size (number of voxels) and age for the patients and controls. The regions of interest size tended to be smaller in patients versus controls, but in only three subjects was this reduction >2 SD below the controls. Age was not different in patients compared to controls. Differences are defined as variations of at least 2 SD above or below the means for healthy controls (five healthy control subjects for each patient). The last four patients on the right side (Patients 12–15) are those in whom DTI was performed in the chronic phase. Symbols indicate significant reductions (asterisk) or increases (hash). Error bars represent mean ± 2 SD for the healthy controls.
Correlation between cerebral microdialysis and DTI measures of axonal injury
Patients with white matter regions with reduced fractional anisotropy had higher tau levels than regions with normal anisotropy. The corresponding median tau levels over the first 24 h of microdialysis sampling were 13 747pg/ml (25th–75th percentile: 13 517–18 743 pg/ml) in the patients with reduced anisotropy (z-score < −2) and 4612 pg/ml (25th–75th percentile: 3942–5093 pg/ml) in the patients with normal (z-score ≥ −2) anisotropy (P = 0.036, Mann Whitney U-test). When analysing tau levels in the window between 13 and 36 h after injury, the results were similar; median tau levels were 13 441pg/ml (25th–75th percentile: 14 743–18 303 pg/ml) in the patients with reduced anisotropy (z-score < −2) and 5659 pg/ml (25th–75th percentile: 6344–5659 pg/ml) in the patients with normal (z-score ≥ −2) anisotropy (P = 0.036, Mann Whitney U-test).
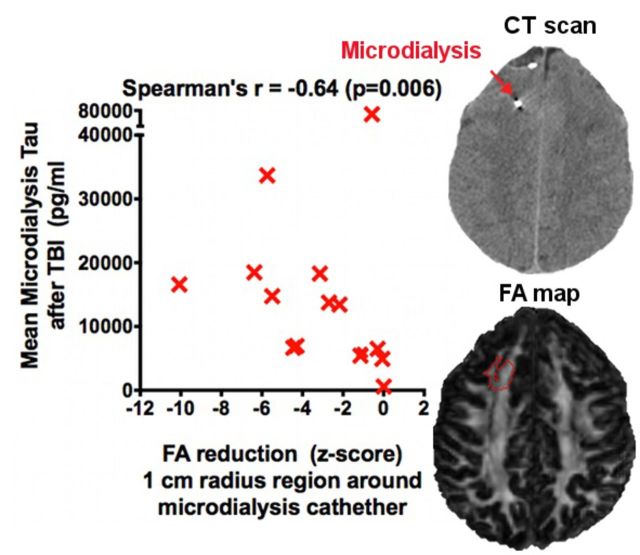
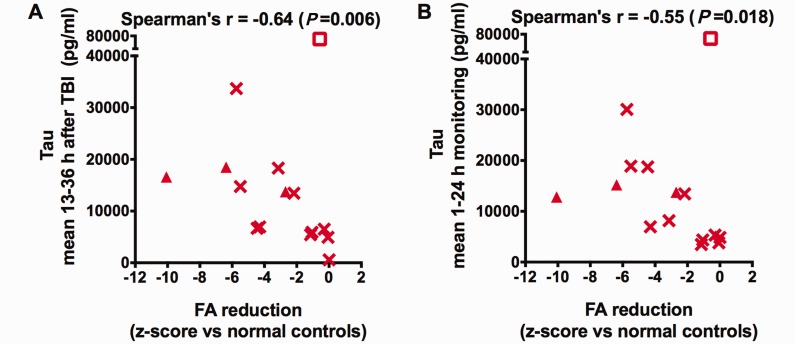
Most importantly, we found a significant inverse correlation between microdialysis measured levels of tau 13–36 h after injury and fractional anisotropy reductions measured by the z-score of fractional anisotropy between patients and their healthy controls (Spearman's r = −0.64, P = 0.006, Fig. 3A). The correlation was similar if we excluded the three patients with conventional MRI abnormalities in the region of microdialysis catheter insertion (Spearman's r = −0.62, P = 0.017). The correlation was preserved for tau analysed in the first 1–24 h (Fig. 3B) after catheter insertion and for tau measured at 24–48 and 48–72 h after catheter insertion (Supplementary Fig. 5), indicating that the exact time of tau assessment was not critical. Furthermore, a less strong, but still significant correlation (Supplementary Fig. 6; Spearman's r = −0.55, P = 0.04) was observed between tau and fractional anisotropy reductions measured 1–3 years after injury. The relationships would have been substantially stronger were it not for one notable outlier, Patient 3, in which tau levels were very high but fractional anisotropy was normal. To the best of our knowledge, both measures were performed correctly and we cannot exclude this patient on technical grounds (see ‘Discussion’ section).
Figure 3.
Correlation between fractional anisotropy reductions and microdialysis measured levels of tau. Fractional anisotropy (FA) was measured in patients (n = 15) and healthy volunteers, and the z-score of patients relative to controls was computed, which indicates the extent to which fractional anisotropy was below (negative score) or above (positive score) the mean. There were significant negative correlations (Spearman’s one-tailed correlation tests) between fractional anisotropy z-scores and (A) increased tau levels 13–36 h after initial injury as well as (B) increased tau levels over the first 24 h of microdialysis monitoring. The z-scores of fractional anisotropy were negative, indicating that anisotropy was globally reduced in patients compared to controls. Triangular symbols indicate patients with conventional MRI abnormalities in the region of microdialysis catheter insertion, compared to patients with normal-appearing regions of interest on conventional MRI (other symbols). The empty rectangular symbol corresponds to a possible outlier (Patient 3).
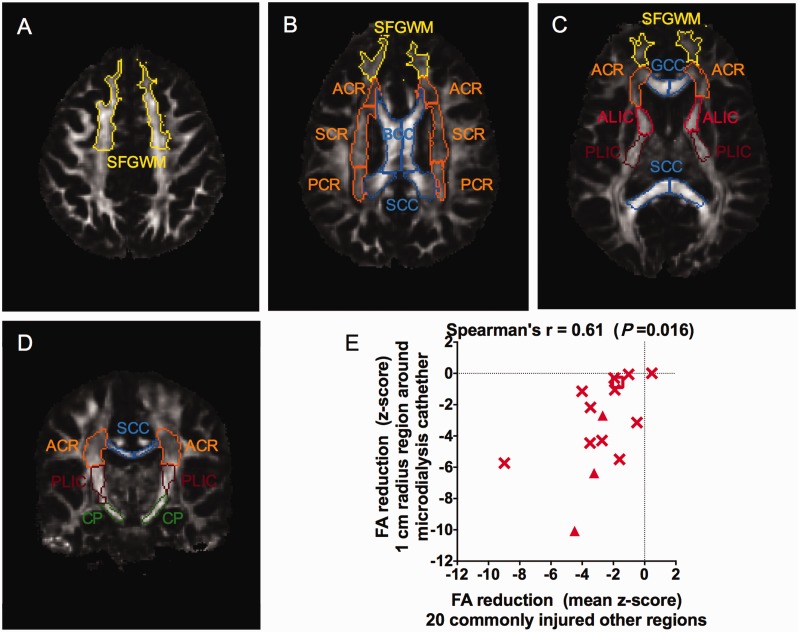
To address the possibility that tau and DTI abnormalities both arose from microdialysis catheter insertion trauma, we performed additional DTI analyses. Specifically, fractional anisotropy was analysed in 10 bilateral regions commonly affected by severe traumatic brain injury (Fig. 4A–D). Average z-scores compared to age-matched controls across the 20 regions analysed were strongly correlated (Spearman's r = 0.61, P = 0.016) with z-scores in the 1-cm radius region around the microdialysis catheter site (Fig. 4E). These findings are consistent with the hypothesis that these severe TBI patients had typically distributed traumatic axonal injury, and inconsistent with the hypothesis that the fractional anisotropy reductions observed surrounding the microdialysis catheters reflect local injury due to catheter insertion.
Figure 4.
Correlation between reduced fractional anisotropy in other commonly injured brain white matter regions and reduced fractional anisotropy in the 1-cm radius white matter regions around microdialysis catheter placement sites. Axial (A–C) and coronal (D) slices from DTI fractional anisotropy maps in a normal control indicating locations of regions of interest analysed using DTIStudio. (E) Correlation between mean fractional anisotropy reductions in other commonly injured regions versus fractional anisotropy reduction in the 1-cm radius region of interest around location where the microdialysis catheters had been. Data expressed as z-scores, defined as number of standard deviations away from the mean of five identically imaged normal control subjects age-matched to each patient. Triangles indicate three patients with conventional MRI abnormalities in the region of microdialysis catheter insertion (as in Fig. 3). Square indicates possible outlier in the tau versus fractional anisotropy measurement (as in Fig. 3). SFGWM = superior frontal gyrus white matter; ACR = anterior corona radiata; SCR = superior corona radiata; PCR = posterior corona radiata; BCC = body of the corpus callosum; SCC = splenium of the corpus callosum; GCC = genu of the corpus callosum; ALIC = anterior limb of the internal capsule; PLIC = posterior limb of the internal capsule; CP = cerebral peduncle.
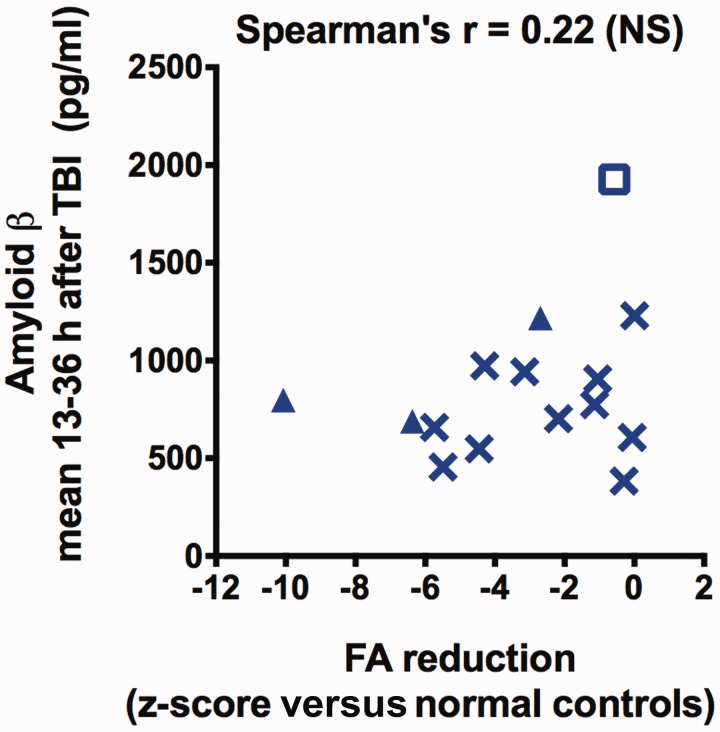
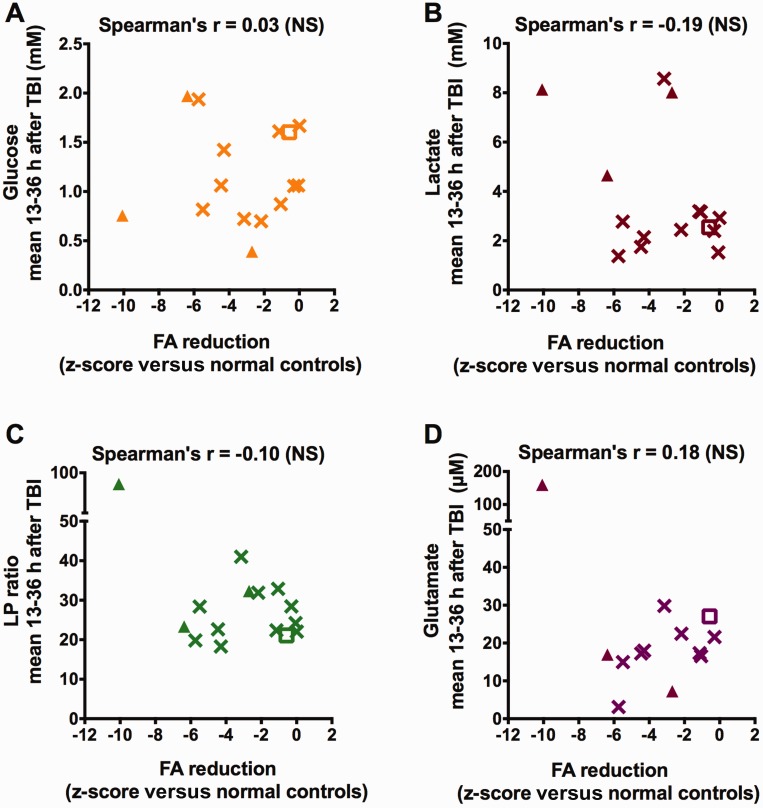
No correlation was found between fractional anisotropy and microdialysis measurements of amyloid-β (Fig. 5), metabolic markers and glutamate (Fig. 6). Likewise, no correlation was observed between other DTI parameters (mean diffusivity, axial diffusivity, radial diffusivity) and tau or amyloid-β (Supplementary Fig. 7).
Figure 5.
No correlation between fractional anisotropy reductions and microdialysis measured levels of amyloid-β. There was no correlation between fractional anisotropy (FA) z-scores and interstitial levels of amyloid-β 13–36 h after initial injury (Spearman’s one-tailed correlation test, n = 15). Z-scores are calculated as indicated in Fig. 3. Triangles indicate patients with conventional MRI abnormalities in the region of microdialysis catheter insertion and empty rectangular symbol corresponds to the outlier as in Fig. 3.
Figure 6.
No correlation between fractional anisotropy reductions and microdialysis measured brain metabolic markers. There was no correlation between fractional anisotropy (FA) z-scores and interstitial levels of glucose (A), lactate (B), lactate to pyruvate ratio (C), and glutamate (D) 13–36 h after initial injury (Spearman’s one-tailed correlation tests, n = 15). Z-scores are calculated as indicated in Fig. 3. Triangles indicate patients with conventional MRI abnormalities in the region of microdialysis catheter insertion and open squares correspond to the outlier as in Fig. 3.
Discussion
In summary, we have found that microdialysis measurements of the axonal cytoskeletal protein tau in the brain extracellular space correlate well with diffusion tensor MRI-based measurements of reduced brain white matter integrity in the region near the microdialysis catheters. The most likely interpretation of this result is that both methods accurately reflect the same pathophysiological process: traumatic axonal injury.
These results reinforce and complement previous findings on the topic of microdialysis-based measurement of tau levels in the brain extracellular space to assess axonal injury in the intensive care unit following severe TBI (Magnoni et al., 2012). Most importantly, these findings strongly support the hypothesis that brain interstitial levels of tau can be used as an accurate method for the in vivo assessment of the severity of traumatic axonal damage. In contrast, amyloid-β and other metabolic markers including glucose, lactate, lactate/pyruvate ratio and glutamate were not useful in this regard.
With this work we have demonstrated the feasibility of combining microdialysis and advanced MRI. By direct analogy, microdialysis and PET have been used before, in combination, to study brain metabolic disturbances following severe TBI and cross-validate the respective methods (Hutchinson et al., 2002, 2009; Vespa et al., 2005). There is only one study of which we are aware comparing microdialysis and MRI in the same cohort of TBI patients (Marcoux et al., 2008). In this previous study, the authors described a positive correlation between persistent metabolic dysfunction (i.e. elevated lactate/pyruvate ratio) measured with microdialysis in normal-appearing pericontusional frontal lobe tissue of TBI patients and long-term atrophy measured by MRI. However, important differences between the two studies need to be considered. The metabolic disturbances described by Marcoux et al. (2008) were persistent elevations of the lactate/pyruvate ratio values above 40. On the contrary, our patients’ lactate to pyruvate ratio increases were generally not as severe. Furthermore, most of our patients had diffuse injury, and none had the microdialysis catheters located in pericontusional regions. Therefore, the two data sets are not directly comparable; it appears that our study represents assessments of less severely injured and less metabolically compromised tissue, closer to ‘pure’ axonal injury. Finally, even though cerebral atrophy is considered the signature pathology of acute diffuse axonal injury in TBI, the contribution of white matter damage, as measured by DTI, to brain atrophy is still a relatively unexplored topic (Palacios et al., 2013).
The volume of brain extracellular space sampled by the microdialysis catheters is not entirely clear. Thus, methodologically our choice of 10 mm radius regions of interest, as used by Hutchinson et al. (2002), was somewhat arbitrary and based primarily on practical considerations: 5-mm radius regions of interest were difficult to consistently assess with 2 mm voxel spatial resolution DTI, and 20 mm regions of interest yielded substantial amounts of grey matter and CSF. In the future, as the spatial resolution of clinically feasible DTI methods improves (Van Essen et al., 2014), various region of interest sizes could be assessed and global measurements based on diffusion modelling could be performed.
The relationship between tau and fractional anisotropy would have been even stronger if it was not for one possible outlier (Patient 3) with exceptionally high levels of tau. Unexpectedly, in this patient elevated tau was associated with normal metabolic markers, elevated amyloid-β (not reduced, as expected from our previous data; Magnoni et al., 2012), and normal anisotropy. Unfortunately, we could not obtain additional samples to repeat the tau measurements; all of the samples had been used for various assays. It appeared that the catheter was functioning normally, as urea concentrations in the same samples were in the normal range. However, the integrity of the catheter’s membrane was not verified directly (e.g. by electron microscopy) and so the possibility of changes in membrane permeability to high molecular weight proteins such as tau cannot be excluded. Another possibility was that sufficient tau was released due to the insertion of the intracranial pressure monitor ∼1.2 cm away (see Supplementary Fig. 1, Patient 3) to elevate microdialysis-sampled extracellular tau. The tract of the intracranial pressure monitor was outside of the 1 cm radius DTI region of interest, and so would not have affected the measured fractional anisotropy. However, there are several other patients in whom the intracranial pressure monitor was placed close to the microdialysis catheter that did not appear to be outliers. We have not quantitatively determined the extent of any intracranial pressure monitor insertion-related injury. Furthermore, the distance tau diffuses in the extracellular space is not known, and therefore the effect of the proximity of the two inserted probes cannot be determined. The DTI of both the patient and the matched controls were of good quality, and the regions of interest were well segmented and homogeneous in size. The patient’s chronic phase scan demonstrated almost identical fractional anisotropy values in the region corresponding to the microdialysis catheter compared to the initial scan, indicating that the DTI data are most likely reliable. However, we cannot fully rule out co-registration errors or coincidental anomalies in the control DTI scans. It is not clear whether this result represents a rare anomaly, or whether it has clinically significant implications with regard to the utility of tau microdialysis as a prognostic or pharmacodynamic biomarker. Ultimately, larger studies will be required.
There are several limitations of this work. First the sample size is small, which may affect the reproducibility of the data (Button et al., 2013). Larger sample sizes will be required to address questions regarding the relationships between categorical measures (e.g. Marshall CT scan, conventional MRI-based axonal injury grade) versus quantitative measures of axonal injury by DTI or microdialysis. Likewise, the question of whether DTI, microdialysis, or a combination of both measures best predicts long-term clinical outcomes will require sufficient sample size for multivariate analyses (Maas et al., 2007).
Second, catheter placement may cause some local injury, and tau elevation and fractional anisotropy reduction could both, in theory, reflect catheter insertion damage. By careful inspection of all the MRI conventional images, we found small non-haemorrhagic abnormalities in the regions corresponding to the insertion of microdialysis catheters in three patients, similar to the descriptions by others (Marcoux et al., 2008). These minor alterations in the regions of the microdialysis catheters have been interpreted as local tissue oedema. Nonetheless, after excluding the three patients with conventional MRI abnormalities in the regions of microdialysis catheter, the correlation between microdialysis measured levels of tau and fractional anisotropy reductions was still significant. As discussed previously, a stereotyped procedure like catheter insertion is not likely to explain the variability of tau measures across subjects (Magnoni et al., 2012). Along these lines, recent tau microdialysis experiments in uninjured mice indicate that catheter insertion has little effect on tau levels (Yamada et al., 2014).
Further studies will be required to determine the true baseline levels of extracellular tau in the uninjured human brain. To our knowledge, tau has been measured only in one uninjured patient by microdialysis. In this patient with an unruptured aneurysm, initial tau levels were 7210 pg/ml, and fell to 1554 pg/ml by 12 h. Amyloid-β microdialysis data from this patient were reported previously (Brody et al., 2008). Longer duration microdialysis measurements of tau in uninjured patients undergoing invasive intracranial procedures for other indications will be necessary to determine the normal extracellular levels of tau in the human brain.
On the DTI side, the wide spectrum of observed anisotropy changes, ranging from normal to substantially reduced fractional anisotropy values is more consistent with traumatic axonal injury than iatrogenic trauma caused by relatively stereotyped catheter insertion. The strong correlation between fractional anisotropy abnormalities near the microdialysis catheter insertion site with fractional anisotropy abnormalities in 20 other regions is consistent with reductions in fractional anisotropy caused by widespread multifocal traumatic axonal injury and not consistent with local trauma caused by catheter insertion.
A third caveat is that the location of microdialysis catheter placement was not standardized, resulting in different anatomical locations of the catheters and, ultimately, in increased variability of the DTI measures. The true relationship between DTI-based metrics of axonal injury and microdialysis-based metrics would likely be even stronger were it not for this heterogeneity. Thus, the relationship we observed should be considered a lower bound. Another important source of variability may have arisen from errors in the exact region of interest matching. We used a well-established automated registration algorithm (Shimony et al., 2009; Mac Donald et al., 2011). In addition, we carried out a visual inspection for the anatomical correspondence of the regions of interest between patients and controls, for every subject, and if necessary we carried out manual correction. To further minimize registration problems, we used matched controls to account for anatomical differences between sexes and with age. With this approach, the controls had acceptable levels of variability of the DTI measures. While it is possible that imperfect spatial normalization may have affected the results, we consider it unlikely that such effects would give rise to the systematic relationships observed. For white matter segmentation of the regions of interest, we used a highly reproducible threshold method, which resulted in no substantial differences between region of interest size in patients versus controls.
Fourth, inherent limitations in the DTI methodology, which includes partial volume effects, may have biased the DTI measures and influenced the results. In regions with complex architecture and crossing fibres such as the microdialysis regions of interest, fractional anisotropy measures may not be as accurate as in more structured white matter regions such as in the corpus callosum (Le Bihan et al., 2001; Assaf and Pasternak, 2008). However, this caveat holds for both patients and controls and there is no reason to believe that it could result in the correlations with tau levels as measured by microdialysis. The use of more advanced diffusion imaging analyses and higher spatial resolution methods will allow more accurate measures, especially in regions with complex fibre architecture in future investigations (Seunarine and Alexander, 2014).
These results have important implications for future studies. A major goal will be to standardize the approach to microdialysis and DTI for multicentre assessment of prognostic accuracy when combined with clinical and conventional imaging predictors (Yuh et al., 2013, 2014). The development of rapid point-of-care quantitative tau measurements will be required to implement microdialysis-based real-time decision-making in the intensive care unit (Das et al., 2014). Similarly, establishing the safety and logistical feasibility of earlier and more automated DTI measurements will aid clinical translation of these findings. We envision future use of both microdialysis and DTI methods to stratify TBI patients for multicentre clinical trials and to perform pharmacodynamic assessments of candidate therapeutics for traumatic axonal injury.
In conclusion, this study demonstrates that two distinct methods, DTI MRI and cerebral microdialysis, can be applied in conjunction in patients with severe TBI to quantitatively assess post-traumatic white matter pathology. Most importantly, the cross-validation of these two independent methods greatly increases the confidence of interpretation of both methods, as it is very unlikely that two entirely distinct sets of technical caveats and noise sources would give such concordant results. Our work should therefore aid in the interpretation of DTI findings in the injured CNS and will therefore have important implications for patient management and prognosis.
Supplementary Material
Acknowledgements
We would like to especially thank the participants and their families for their invaluable contributions. We also want to thank the healthy volunteers. We appreciate the assistance of the Ca` Granda Ospedale Maggiore Policlinico Neurointensive care team. Valuable discussions with Drs. R. Bateman, R. Bullock, J. Cirrito, R. Dacey, M. Diringer, L. Hillered, D. Holtzman, P. Hutchinson, J. Ladensen, W. Powers, N. Temkin, and G. Zipfel are acknowledged.
Glossary
Abbreviations
- DTI
diffusion tensor imaging
- TBI
traumatic brain injury
Funding
This work was supported by funds from a Burroughs Wellcome Career Award in the Biomedical Sciences to D.L.B.; National Institutes of Health [grant numbers K08 NS049237 to D.L.B. and R01 NS065069 to D.L.B.; and departmental fund of the Fondazione IRCCS Cà Granda-Ospedale Maggiore Policlinico.
Supplementary material
Supplementary material is available at Brain online.
References
- Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology 1989; 15: 49–59. [DOI] [PubMed] [Google Scholar]
- Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 1982; 12: 557–63. [DOI] [PubMed] [Google Scholar]
- Adams JH, Jennett B, Murray LS, Teasdale GM, Gennarelli TA, Graham DI. Neuropathological findings in disabled survivors of a head injury. J Neurotrauma 2011; 28: 701–9. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol 2002; 23: 794–802. [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008; 34: 51–61. [DOI] [PubMed] [Google Scholar]
- Bennett RE, Mac Donald CL, Brody DL. Diffusion tensor imaging detects axonal injury in a mouse model of repetitive closed-skull traumatic brain injury. Neurosci Lett 2012; 513: 160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma 1995; 12: 565–72. [DOI] [PubMed] [Google Scholar]
- Brody DL, Magnoni S, Schwetye KE, Spinner ML, Esparza TJ, Stocchetti N, et al. Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 2008; 321: 1221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büki A, Povlishock JT. All roads lead to disconnection?–Traumatic axonal injury revisited. Acta Neurochir 2006; 148: 181–93. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 2013; 14: 365–76. [DOI] [PubMed] [Google Scholar]
- Coronado VG, McGuire LC, Sarmiento K, Bell J, Lionbarger MR, Jones CD, et al. Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995-2009. J Safety Res 2012; 43: 299–307. [DOI] [PubMed] [Google Scholar]
- Das C, Agarwal A, Nguyen C, Wang G, Ledden B, Kumar S. Development of a portable cerebral microdialysis platform for automated inline multianalyte detection system. In: Abstracts from The 32nd Annual National Neurotrauma Symposium June 29–July 2, 2014 San Francisco, California. J Neurotrauma 2014; 31: A1-05. [Google Scholar]
- Galanaud D, Perlbarg V, Gupta R, Stevens RD, Sanchez P, Tollard E, et al. Assessment of white matter injury and outcome in severe brain trauma: a prospective multicenter cohort. Anesthesiology 2012; 117: 1300–10. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol 1982; 12: 564–74. [DOI] [PubMed] [Google Scholar]
- Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol 1988; 150: 663–72. [DOI] [PubMed] [Google Scholar]
- Hillered L, Vespa PM, Hovda DA. Translational neurochemical research in acute human brain injury: the current status and potential future for cerebral microdialysis. J Neurotrauma 2005; 22: 3–41. [DOI] [PubMed] [Google Scholar]
- Hillered L, Persson L, Nilsson P, Ronne-Engstrom E, Enblad P. Continuous monitoring of cerebral metabolism in traumatic brain injury: a focus on cerebral microdialysis. Curr Opin Crit Care 2006; 12: 112–8. [DOI] [PubMed] [Google Scholar]
- Hulkower MB, Poliak DB, Rosenbaum SB, Zimmerman ME, Lipton ML. A decade of DTI in traumatic brain injury: 10 years and 100 articles later. AJNR Am J Neuroradiol 2013; 34: 2064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson PJ, Gupta AK, Fryer TF, Al-Rawi PG, Chatfield DA, Coles JP, et al. Correlation between cerebral blood flow, substrate delivery, and metabolism in head injury: a combined microdialysis and triple oxygen positron emission tomography study. J Cereb Blood Flow Metab 2002; 22: 735–45. [DOI] [PubMed] [Google Scholar]
- Hutchinson PJ, O’Connell MT, Seal A, Nortje J, Timofeev I, Al-Rawi PG, et al. A combined microdialysis and FDG-PET study of glucose metabolism in head injury. Acta Neurochir 2009; 151: 51–61. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg 2005; 103: 298–303. [DOI] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain 2011; 134: 449–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007; 130: 2508–19. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 2003; 4: 469–80. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 2001; 13: 534–46. [DOI] [PubMed] [Google Scholar]
- Li J, Li XY, Feng DF, Gu L. Quantitative evaluation of microscopic injury with diffusion tensor imaging in a rat model of diffuse axonal injury. Eur J Neurosci 2011; 33: 933–45. [DOI] [PubMed] [Google Scholar]
- Li S, Sun Y, Shan D, Feng B, Xing J, Duan Y, et al. Temporal profiles of axonal injury following impact acceleration traumatic brain injury in rats–a comparative study with diffusion tensor imaging and morphological analysis. Int J Legal Med 2013; 127: 159–67. [DOI] [PubMed] [Google Scholar]
- Maas AIR, Marmarou A, Murray GD, Teasdale SGM, Steyerberg EW. Prognosis and clinical trial design in traumatic brain injury: the IMPACT study. J Neurotrauma 2007; 24: 232–8. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury TAI and indicates approximate time of injury. J Neurosci 2007a; 27: 11869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Song SK, Bayly P V, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp. Neurol 2007b; 205: 116–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med 2011; 364: 2091–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnoni S, Esparza TJ, Conte V, Carbonara M, Carrabba G, Holtzman DM, et al. Tau elevations in the brain extracellular space correlate with reduced amyloid-β levels and predict adverse clinical outcomes after severe traumatic brain injury. Brain 2012; 135: 1268–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcoux J, McArthur DA, Miller C, Glenn TC, Villablanca P, Martin NA, et al. Persistent metabolic crisis as measured by elevated cerebral microdialysis lactate-pyruvate ratio predicts chronic frontal lobe brain atrophy after traumatic brain injury. Crit Care Med 2008; 36: 2871–7. [DOI] [PubMed] [Google Scholar]
- Marklund N, Blennow K, Zetterberg H, Ronne-Engström E, Enblad P, Hillered L. Monitoring of brain interstitial total tau and beta amyloid proteins by microdialysis in patients with traumatic brain injury. J Neurosurg 2009; 110: 1227–37. [DOI] [PubMed] [Google Scholar]
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain 2003; 126: 515–30. [DOI] [PubMed] [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Yasokawa Y, Miwa K, Yoshimura S, et al. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry 2006; 77: 850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcombe VFJ, Williams GB, Nortje J, Bradley PG, Harding SG, Smielewski P, et al. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br J Neurosurg 2007; 21: 340–8. [DOI] [PubMed] [Google Scholar]
- Newcombe VF, Correia MM, Ledig C, Abate MG, Outtrim JG, Chatfield D, et al. Dynamic changes in white matter abnormalities correlate with late improvement and deterioration following TBI: a diffusion tensor imaging study. Neurorehabil Neural Repair 2015. Apr 28. doi:10.1177/1545968315584004. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J Head Trauma Rehabil 2010; 25: 241–55. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR. Am J Neuroradiol 2008; 29: 967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage 2008; 43: 447–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios EM, Sala-Llonch R, Junque C, Fernandez-Espejo D, Roig T, Tormos JM, et al. Long-term declarative memory deficits in diffuse TBI: Correlations with cortical thickness, white matter integrity and hippocampal volume. Cortex 2013: 49: 646–57. [DOI] [PubMed] [Google Scholar]
- Perlbarg V, Puybasset L, Tollard E, Lehéricy S, Benali H, Galanaud D. Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: a diffusion tensor imaging study using voxel-based approaches. Hum Brain Mapp 2009; 30: 3924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A, Tisdall MM, Girbes AR, Martinian L, Thom M, Kitchen N, et al. In vivo monitoring of neuronal loss in traumatic brain injury: a microdialysis study. Brain 2011; 134: 464–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinstrup P, Ståhl N, Mellergård P, Uski T, Ungerstedt U, Nordström CH. Intracerebral microdialysis in clinical practice: baseline values for chemical markers during wakefulness, anesthesia, and neurosurgery. Neurosurgery 2000; 47: 701–9. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage 2006; 29: 117–24. [DOI] [PubMed] [Google Scholar]
- Seunarine KK, Alexander DC. Multiple fibers: beyond the diffusion tensor. In: Johansen-Berg H, Behrens TEJ, editors. Diffusion MRI. Amsterdam: Elsevier; 2014. p. 105–23. [Google Scholar]
- Shimony JS, Sheline YI, D’Angelo G, Epstein AA, Benzinger TLS, Mintun MA, et al. Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry 2009; 66: 245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 2008; 131: 559–72. [DOI] [PubMed] [Google Scholar]
- Siman R, McIntosh TK, Soltesz KM, Chen Z, Neumar RW, Roberts VL. Proteins released from degenerating neurons are surrogate markers for acute brain damage. Neurobiol Dis 2004; 16: 311–20. [DOI] [PubMed] [Google Scholar]
- Skandsen T, Kvistad KA, Solheim O, Strand IH, Folvik M, Vik A. Prevalence and impact of diffuse axonal injury in patients with moderate and severe head injury: a cohort study of early magnetic resonance imaging findings and 1-year outcome. J Neurosurg 2010; 113: 556–563. [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil 2003; 18: 307–16. [DOI] [PubMed] [Google Scholar]
- Smith DH, Nonaka M, Miller R, Leoni M, Chen XH, Alsop D, et al. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J Neurosurg 2000; 93: 315–22. [DOI] [PubMed] [Google Scholar]
- Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry 1956; 19: 163–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Jbabdi S, Sotiropoulos SN, Chen C, Dikranian K, Coalson T, et al. Mapping connections in humans and non-human primates: aspirations and challenges for diffusion imaging. In: Johansen-Berg H, Behrens TEJ, editors. Diffusion MRI. Amsterdam: Elsevier; 2014. p. 337–58. [Google Scholar]
- Vespa P, Bergsneider M, Hattori N, Wu HM, Huang SC, Martin NA, et al. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J Cereb Blood Flow Metab 2005; 25: 763–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa PM, O’Phelan K, McArthur D, Miller C, Eliseo M, Hirt D, et al. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit Care Med 2007; 35: 1153–60. [DOI] [PubMed] [Google Scholar]
- Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med 2014; 211: 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh EL, Cooper SR, Mukherjee P, Yue JK, Lingsma HF, Gordon WA, et al. Diffusion Tensor Imaging for Outcome Prediction in Mild Traumatic Brain Injury: A TRACK-TBI Study. J Neurotrauma 2014; 31: 1457-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh EL, Mukherjee P, Lingsma HF, Yue JK, Ferguson AR, Gordon WA, et al. Magnetic resonance imaging improves 3-month outcome prediction in mild traumatic brain injury. Ann Neurol 2013; 73: 224–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.