Abstract
Pancreatic cancer is a fatal human malignancy associated with an exceptionally poor prognosis. Novel therapeutic strategies are urgently required to treat this disease. In addition to immunosuppressive activity, triptolide possesses strong antitumor activity and synergistically enhances the antitumor activities of conventional chemotherapeutic drugs in preclinical models of pancreatic cancer. The present study investigated the antitumor effects of triptolide in pancreatic cancer cells, either in combination with gemcitabine, or alone. The pancreatic cancer BxPC-3 and PANC-1 cell lines were treated with triptolide, which resulted in time- and dose-dependent growth arrest. When incorporated into a sequential schedule, triptolide synergistically increased gemcitabine-induced cell growth inhibition and apoptosis, in addition to the cooperative regulation of B-cell lymphoma 2 family proteins and loss of mitochondrial membrane potential. Furthermore, triptolide enhanced gemcitabine-induced S phase arrest and DNA double-strand breaks, possibly through checkpoint kinase 1 suppression. The results of the present study suggest that triptolide has therapeutic potential for the treatment of pancreatic cancer, particularly when administered in combination with gemcitabine.
Keywords: triptolide, pancreatic cancer, gemcitabine, apoptosis, DNA damage
Introduction
Pancreatic cancer is an aggressive and highly fatal malignancy affecting a large number of individuals worldwide, and has a relative 5-year survival rate of <5% (1,2). It is the fourth leading cause of cancer-associated mortality in the United States, with ~46,420 new cases and 39,590 fatalities occurring as a result of the disease during 2014 (3). Surgical resection is currently the only treatment available that may cure pancreatic cancer (4); however, the majority of patients are diagnosed late in the natural course of the disease, with ~80% presenting with metastasis (5,6).
The drug 2′,2′-difluorodeoxycytidine, also known as gemcitabine, is the first-line chemotherapeutic agent for pancreatic cancer treatment that is approved by the Food and Drug Administration (7,8). It exerts antitumor activity depending on several inhibitory actions of DNA synthesis, functioning to impair DNA repair and induce apoptosis (9). Gemcitabine has been demonstrated to effectively inhibit pancreatic cancer cells that are insensitive to other drugs, including fluorouracil, doxorubicin and cisplatin (10). Although gemcitabine exhibits effective inhibition on pancreatic cancer cell growth in vitro, its clinical efficacy remains low (11). Thus, novel therapies are urgently required for the treatment of this extremely aggressive malignancy.
Triptolide is a diterpenoid triepoxide derived from the herb Tripterygium wilfordii, which has been utilized as a natural agent in China for centuries (12). Triptolide has been successfully applied as an immunosuppressant for clinical treatment of inflammation, autoimmune diseases and organ transplantations (13–15). In addition to immunosuppressive activity, triptolide has been demonstrated to possess potent antitumor activity and induce apoptosis in a variety of tumor types in vivo and in vitro (16–18). Furthermore, triptolide has been reported to synergistically increase the antitumor activities of conventional chemotherapies (19,20). Synergistic antitumor interactions between triptolide and other chemotherapeutic drugs, including cisplatin, artesunate and hydroxycamptothecin, have been previously observed in pancreatic cancer cells (21–23). In addition, triptolide is currently undergoing clinical trials for its antitumor and proapoptotic activities on primary cultures of human prostatic epithelial cells (24).
The present study hypothesized that triptolide synergistically enhances the antitumor activity of gemcitabine by inducing apoptosis in pancreatic cancer cells. The results demonstrate that triptolide inhibits pancreatic cell growth, and cooperatively augments gemcitabine-induced apoptosis by increasing gemcitabine-induced S phase arrest and DNA double-strand breaks (DSBs). This suggests that triptolide may possess therapeutic potential for the treatment of pancreatic cancer, particularly when administered in combination with gemcitabine.
Materials and methods
Chemicals
Triptolide was purchased from the National Institutes for Food and Drug Control (Beijing, China), and gemcitabine (GEM; Gemzar®) was purchased from Lilly (Fegersheim, France). All other chemicals were of analytical grade and obtained commercially.
Cell culture
The human pancreatic cancer BxPC-3 and PANC-1 cell lines were obtained from the Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences. (Shanghai, China). The cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), penicillin/streptomycin (100 U/ml each; Shijiazhuang No. 4 Pharmaceutical Co., Ltd., Shijiazhuang, China), sodium bicarbonate (2 g/l; Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and HEPES (2.4 g/l; Amresco, LLC, Solon, OH, USA), and were incubated at 37°C with 5% CO2.
In vitro cytotoxicity assays
In vitro cytotoxicities of triptolide and gemcitabine, alone or in combination, in the pancreatic cancer cell lines were determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (25). Control groups were treated without gemcitabine and triptolide, and cultured for up 120 h. Briefly, BxPC-3 or PANC-1 cells were plated in 96-well plates (Corning Inc., Corning, NY, USA; 3×103 cells/well) and were treated with triptolide or gemcitabine at the indicated concentrations, alone (triptolide: 0, 6.25, 12.5, 25 and 50 nM; gemcitabine: 0, 5, 10, 20 and 40 nM or sequentially combined [gemcitabine treatment (0, 5, 10, 20 and 40 nM) for 48 h followed by triptolide treatment (0, 6.25, 12.5 and 25 nM) for 72 h], for up to 120 h. MTT (Amresco, LLC) was subsequently added to a final concentration of 1 mM. Following 4 h of incubation, the formazan crystals were dissolved by the addition of 100 µl 10% sodium dodecyl sulfate (SDS; Amresco, LLC) in 10 mM hydrogen chloride (Sinopharm Chemical Reagent Co., Ltd.). Optical densities were measured with a visible microplate reader (SpectraMax® M5; Molecular Devices, LLC, Sunnyvale, CA, USA) at 570 nm. The extent and direction of antitumor interactions between the two agents were determined by calculating the combination index (CI) using the CalcuSyn software version 2.1 (Biosoft, Cambridge, UK) (26). The CI indices were categorized as follows: CI <1, synergistic effect; CI=1, additive effect; and CI>1, antagonistic effect.
Cell cycle analysis
The effects of triptolide and gemcitabine treatment, alone or in combination, on cell cycle distribution in the pancreatic cancer cell lines were analyzed using propidium iodide staining (Sigma-Aldrich, St. Louis, MO, USA) and flow cytometry (Cytomics FC 500; Beckman Coulter, Inc., Brea, CA, USA). A total of 5×105 BxPC-3 and PANC-1 cells were seeded in each well, and were subsequently treated with 25 nM triptolide and 40 nM gemcitabine alone for 72 h. For the combined treatment, the cells were treated with gemcitabine for 24 h, followed by triptolide for up to 72 h. Cell medium and serum were the same as previously described in cell culture, and 70% alcohol (Sinopharm Chemical Reagent Co., Ltd.) was used as a fixative. Cells were treated with trypsin (Amresco, LCC) for 2 min, washed with phophate-buffered saline (PBS; 8 g sodium chloride, 0.2 g potassium chloride, 1.44 g disodium hydrogen phosphate and 0.24 g potassium dihydrogen phosphate, dissolved in 1 liter saline; Sinopharm Chemical Reagent Co., Ltd.) and DNA was stained with 50 µg/ml propidium iodide for 30 min in the dark. Cell cycle analysis was performed using the ModFit LT™ 3.0 DNA analysis software (Verity Software House, Inc., Topsham, ME, USA).
Assessment of baseline and drug-induced apoptosis
The effects of triptolide and gemcitabine treatment, alone or in combination, on apoptosis in the BxPC-3 or PANC-1 cell lines were analyzed using flow cytometry, as previously described (27). Briefly, the treated cells were harvested and apoptosis was determined using an Apoptosis Annexin V-FITC kit (Nanjing KeyGen Biotechnology Co. Ltd., Nanjing, China) and flow cytometric analysis. The results are presented as the percentage of Annexin V+ cells (mean ± standard error).
Western blot analysis
BxPC-3 or PANC-1 cells treated with triptolide and gemcitabine, alone or in combination, were washed three times with PBS (5 min each time; Sinopharm Chemical Reagent Co., Ltd.) and lysed in a lysis buffer [20 mM Tris (pH, 7.5; Amresco, LLC), 150 mM sodium chloride (Sinopharm Chemical Reagent Co., Ltd.), 1% Triton X-100 (Sigma-Aldrich), 1 mM ethylenediaminetetraacetic acid (Sinopharm Chemical Reagent Co., Ltd.), 1 mM sodium orthovanadate, 10 mM NaF, 1 mM phenylmethylsulfonyl fluoride (Amresco, LLC) and protease inhibitor cocktail (dilution, 1:100; Sigma-Aldrich)] for 20 min on ice. The protein lysates were clarified by centrifugation at 12,000 × g for 20 min at 4°C and quantified using a Pierce BCA Protein Assay kit (product no. 23227; Thermo Fisher Scientific Inc.). Protein was subsequently subjected to SDS-polyacrylamide gel electrophoresis and transferred onto an Immobilon®-P PVDF Membrane (EMD Millipore, Billerica, MA, USA), with at least 50 µg protein added to each lane. The membrane was immunoblotted with monoclonal rabbit anti-B-cell lymphoma 2 (Bcl-2; #2870), monoclonal rabbit anti-caspase 3 (#9665), monoclonal rabbit anti-phospho-H2AX (γH2AX; #9718), monoclonal rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (#2118) (dilutions, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), polyclonal rabbit anti-myeloid cell leukemia 1 (Mcl-1; #sc-819) or polyclonal rabbit anti-checkpoint kinase 1 (CHK1; #sc-7898) (dilutions, 1:100; Santa Cruz Biotechnology Inc., Dallas, TX, USA) antibodies. Immunoreactive proteins were visualized using the Odyssey® CLx Imaging system (Li-Cor, Lincoln, NE, USA).
Analysis of mitochondrial membrane potential (MMP)
MMP was assessed by retention of Rhodamine 123 within the pancreatic cancer cells (28). BxPC-3 and PANC-1 cells were treated with triptolide or gemcitabine, either alone or in combination, washed three times with PBS (5 min each time) and incubated with serum-free DMEM containing Rhodamine 123 (1 µM; Beyotime Biotechnology, Nantong, China) at 37°C for 30 min in the dark. The cells were subsequently washed three times with PBS (5 min each time), and the fluorescence was measured using a fluorescence spectrophotometer (SpectraMax® M5; Molecular Devices, LLC) with an excitation wavelength of 507 nm and an emission wavelength of 529 nm.
Statistical analysis
The differences between the two experimental groups were analyzed by Student's t-test, with the statistical tests performed using GraphPad Prism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Triptolide induces growth arrest and synergistically enhances the cytotoxicity of gemcitabine in pancreatic cancer cells
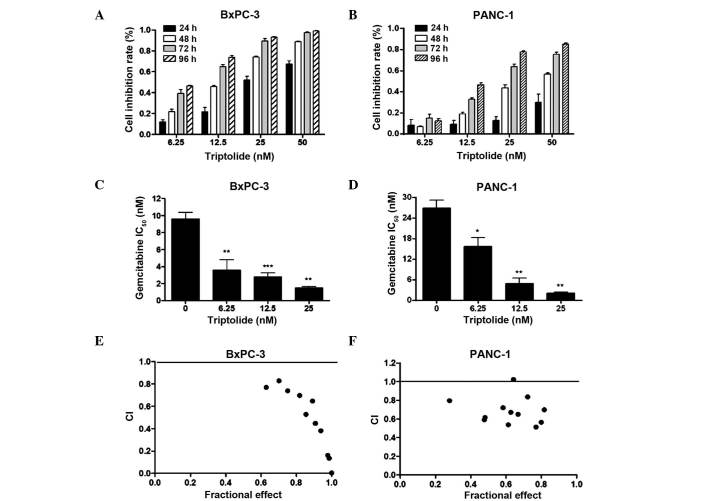
To investigate the antiproliferative activity of triptolide in pancreatic cancer cells, PANC-1 or BxPC-3 cells were treated with various concentrations of triptolide (0, 6.25, 12.5, 25 and 50 nM) for up to 96 h, and cell viability was analyzed by MTT assay. Treatment effects were determined as the percentage viability compared with untreated cells. Treatment with triptolide resulted in growth inhibition of the PANC-1 and BxPC-3 cell lines in a time- and dose-dependent manner compared with untreated control cells (Fig. 1A and B). Triptolide was observed to inhibit cell growth even at the lowest dose of 6.25 nM. Compared with control cells (0% cell inhibition), cell viability was significantly reduced by 99.2±0.3 and 85.4±0.7% when treated with 50 nM triptolide for 96 h in the BxPC-3 and PANC-1 cells, respectively. Triptolide has been demonstrated to possess antitumor activity and to synergistically enhance the anti-proliferative abilities of conventional chemotherapeutic drugs in pancreatic cancer cells (21–23). Therefore, it may be conceivable that triptolide is able to exert similar effects on gemcitabine in pancreatic cancer cells. To establish the effects of triptolide on gemcitabine cytotoxicity, the present study tested three drug administration schedules. Notably, pretreatment of the BxPC-3 and PANC-1 cell lines with gemcitabine for 48 h followed by triptolide treatment for 72 h was observed to be the optimal procedure for drug administration (data not shown). As presented in Fig. 1C and D, triptolide significantly increased gemcitabine-induced growth inhibition (2.7- to 6.5-fold in the BxPC-3 cells and 1.7- to 12.8-fold in the PANC-1 cells; P<0.05). The integrated effects of triptolide and gemcitabine were markedly synergistic, as determined by calculating a CI value of <1 for each of the drug combinations (Fig. 1E and F).
Figure 1.
Triptolide treatment results in growth arrest, and synergistically enhances the cytotoxicity of gemcitabine in pancreatic cancer cells. (A and B) BxPC-3 or PANC-1 cells were treated with vehicle control or triptolide at the indicated doses for up to 96 h. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The data are presented as the mean inhibition rate ± standard error from at least three independent experiments. (C and D) Gemcitabine IC50 of BxPC-3 or PANC-1 cells was determined in the absence or presence of sequential triptolide treatment (gemcitabine followed by triptolide) (presented as the mean ± standard error). (E and F) BxPC-3 or PANC-1 cells were treated with gemcitabine (4 varying concentrations: 5, 10, 20 and 40 nM) or triptolide (3 varying concentrations: 6.25, 12.5 and 25 nM) alone or combined sequentially (12 combined groups). CI values were calculated with CalcuSyn software (Biosoft, Cambridge, UK). *P<0.05, **P<0.01 and ***P<0.001 vs. vehicle control. IC50, half maximal inhibitory concentration; CI, combination index.
Triptolide augments gemcitabine-induced apoptosis in pancreatic cancer cells, accompanied by activation of caspase 3
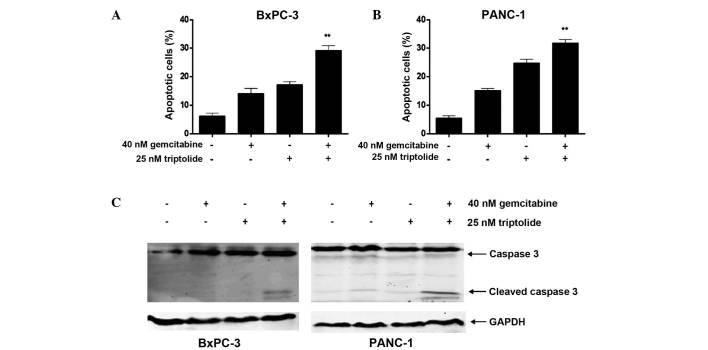
The present study investigated whether the synergistic antitumor effects of combined gemcitabine and triptolide treatment were a result of apoptosis induction. Flow cytometry was used to determine the apoptosis induced by triptolide combined with gemcitabine in pancreatic cancer cells, and the activation of caspase 3 was analyzed by immunoblotting. Following treatment with the two agents, triptolide significantly augmented the gemcitabine-induced apoptosis in the pancreatic cancer cell lines, particularly in the BxPC-3 cells (Fig. 2A and B). Although triptolide alone was observed to exert a modest apoptotic effect in the BxPC-3 cells at a concentration of 25 nM (17.3±0.9%), when combined with 40 nM of gemcitabine, it significantly enhanced gemcitabine-induced apoptosis (29.3±1.6%; (P<0.001). Similar results were additionally observed in the PANC-1 cells, although to a lesser extent (P<0.01). In addition, combined treatment cooperatively activated the cleavage of caspase 3 from 35 kDa to 17/19 kDa when compared to treatment with each drug individually (Fig. 2C).
Figure 2.
Effects of gemcitabine and triptolide alone, or sequentially combined, on pancreatic cancer cell apoptosis. (A and B) BxPC-3 or PANC-1 cells were treated initially with gemcitabine for 24 h, followed by treatment with triptolide for 48 h without washing off the gemcitabine. Apoptosis analysis was performed as described in the Materials and methods section. Results are presented as the mean percentage of Annexin V+ cells of triplicates from one representative experiment (error bars represent the three replicates). (C) Cleavage of caspase 3 was detected by immunoblotting analysis. **P<0.01 refers to combined treatment vs. treatment with gemcitabine and triptolide alone. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Triptolide and gemcitabine cooperatively induce the loss of MMP in pancreatic cancer cells
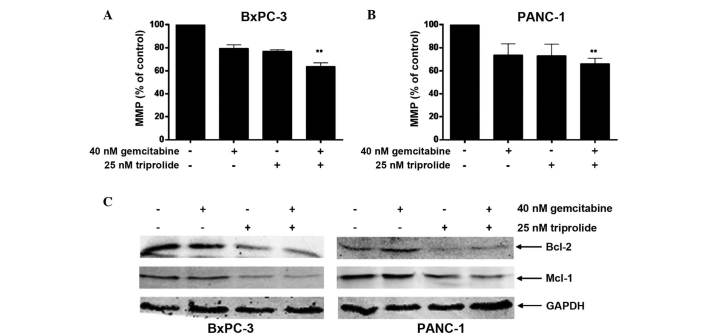
Mitochondria serve a crucial function in the regulation of apoptosis, with apoptosis often associated with a decrease in MMP (29). To establish the effects of gemcitabine, triptolide and the drugs in combination on mitochondrial function, the present study determined the MMP induced by each treatment using the Rhodamine 123 staining method. The expression levels of Bcl-2 family proteins were analyzed by immunoblot analysis. Treatment with gemcitabine or triptolide alone resulted in a reduction in MMP in each cell line (P<0.05; Fig. 3A and B). Consistent with the results of apoptosis analysis (Fig. 2A and B), the combined treatment of the cell lines with triptolide and gemcitabine resulted in an additional reduction in MMP when compared with gemcitabine or triptolide treatment alone (Fig. 3A and B). As presented in Fig. 3C, combined treatment cooperatively downregulated the expression of Mcl-1 and Bcl-2. These results indicate that gemcitabine and triptolide cooperatively regulate the expression of Bcl-2 family proteins, resulting in MMP reduction and apoptosis in pancreatic cancer cells.
Figure 3.
Triptolide and gemcitabine cooperatively induce loss of MMP in pancreatic cancer cells. (A and B) BxPC-3 or PANC-1 cells were treated initially with gemcitabine for 24 h, followed by treatment with triptolide for 48 h without washing off the gemcitabine. MMP was determined by Rhodamine 123 staining. (C) Whole cell lysates from the BxPC-3 or PANC-1 cells used in A and B were subjected to immunoblotting to determine protein levels for the Bcl-2 family members, Bcl-2 and Mcl-1. **P<0.01 refers to combined treatment vs. treatment with gemcitabine and triptolide alone. MMP, mitochondrial membrane potential; Bcl-2, B-cell lymphoma 2; Mcl-1, myeloid cell leukemia 1; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Triptolide synergistically enhances gemcitabine-induced cell cycle arrest and DNA DSBs by suppressing CHK1 expression in pancreatic cancer cells
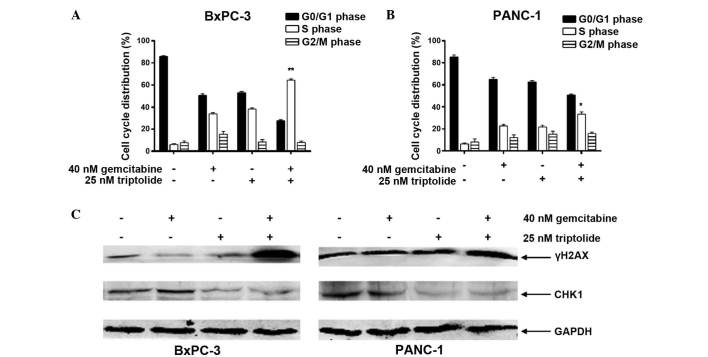
Cell cycle arrest may additionally contribute to the synergistic antitumor activity of gemcitabine and triptolide in pancreatic cancer cell lines. BxPC-3 and PANC-1 cells treated with gemcitabine or triptolide alone demonstrated S phase arrest. Notably, the combined treatment of the cell lines with the two agents resulted in cooperative induction of S phase arrest, with a larger number of cells remaining in S phase (P<0.05; Fig. 4A and B). As a deoxycytidine analogue, the anticancer activity of gemcitabine is exerted to inhibit DNA synthesis and induce DNA damage (9). The cooperative induction of S phase arrest suggests that triptolide may increase gemcitabine-induced DNA damage and promote apoptosis in pancreatic cancer cells. As presented in Fig. 4C, triptolide significantly augmented DNA DSBs induced by gemcitabine represented by the induction of γH2AX. Notably, triptolide inhibited the expression of CHK1, a vital protein in DNA DSB repair and cell cycle checkpoint pathways (30), which was induced following gemcitabine treatment (Fig. 4C). These results indicate that triptolide cooperates with gemcitabine in pancreatic cancer cells to induce DNA DSBs by repressing the expression of CHK1.
Figure 4.
Triptolide synergistically enhances gemcitabine-induced cell cycle arrest and DNA double-strand breaks by suppressing CHK1 expression in pancreatic cancer cells. (A and B) BxPC-3 or PANC-1 cells were treated initally with gemcitabine for 24 h, followed by treatment with triptolide for 48 h without washing off the gemcitabine. Cell cycle distribution was determined by propidium iodide staining and flow cytometric analysis. (C) BxPC-3 or PANC-1 cells were treated initially with gemcitabine for 24 h, followed by treatment with triptolide for 48 h without washing off the gemcitabine. Cells were harvested and whole cell lysates were prepared and subjected to immunoblot analysis of γH2AX, CHK1 and GAPDH. CHK1, checkpoint kinase 1; γH2AX, phospho-H2AX; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. *P<0.05, **P<0.01, combined treatment vs. treatment with gemcitabine and triptolide.
Discussion
As an immunosuppressant, triptolide has been successfully applied for the clinical treatment of inflammation, autoimmune diseases and organ transplantation (13–15,31). Previous research has demonstrated that the drug additionally possesses potent antitumor activity, and is able to synergistically enhance the antitumor activities of conventional chemotherapeutic drugs (19,20). In the present study, the cooperative antitumor activities of triptolide and gemcitabine were investigated in vitro in pancreatic cancer cells. The results demonstrated that triptolide treatment initiated growth inhibition in BxPC-3 and PANC-1 cell lines and synergistically enhanced gemcitabine-induced apoptosis. This was accompanied by the cooperative induction of DNA DSBs when the two agents were combined, and suppression of CHK1 when treated with triptolide alone. These results suggest that a shared mechanism may underlie the synergistic antitumor interactions between triptolide and DNA damaging agents in cancer cells. Triptolide may overcome the chemoresistance to gemcitabine which the first-line chemotherapy drugs for pancreatic cancer and improve the clinical therapy.
Antiproliferation assays demonstrated that triptolide treatment inhibited pancreatic cell growth in a time- and dose-dependent manner. When applied in combination with gemcitabine, triptolide synergistically increased the cytotoxicity of gemcitabine in the BxPC-3 and PANC-1 cell lines. It may be assumed that this synergistic cytotoxicity occurs as a result of cell death as the cooperative induction of apoptosis by the two agents was detected. Triptolide has previously been demonstrated to induce apoptosis in cancer cells by regulating members of the Bcl-2 family, resulting in MMP loss and activation of caspase-3 (12,32). Notably, combined treatment with triptolide and gemcitabine resulted in cooperative MMP loss in the present study, as well as downregulation of Mcl-1 and Bcl-2. These results strongly suggest that gemcitabine and triptolide cooperatively regulate the expression of Bcl-2 family proteins, which leads to a reduction in MMP and induction of apoptosis.
Triptolide has been demonstrated to increase S phase arrest of cancer cells and sensitize cancer cells to conventional anti-cancer drugs (12,33). Similarly, in the present study, triptolide and gemcitabine collectively induced S phase arrest, indicating that triptolide may increase the DNA damage induced by gemcitabine. Triptolide and gemcitabine cooperatively induced DNA DSBs, represented by an increase in γH2AX in the PANC-1 and BxPC-3 cell lines, which may serve as an explanation for the collective induction of S phase arrest. The results of the present study indicate that triptolide increases gemcitabine-induced DNA DSBs, possibly resulting in S phase arrest, regulation of the expression of Bcl-2 family proteins, and subsequent MMP loss and apoptosis.
Triptolide has been demonstrated to repress DNA repair genes (including ataxia telangiectasia mutated, ataxia telangiectasia and Rad3 related, breast cancer 1 and DNA-dependent protein kinase), subsequently increasing radiation sensitivity in human malignant melanoma cells (34). It is possible that triptolide may additionally downregulate DNA repair genes in pancreatic cancer cells and enhance gemcitabine-induced DNA DSBs. Triptolide significantly suppressed CHK1, which is induced by gemcitabine and has a significant role in the repair of DNA DSBs and cell cycle checkpoint pathways (30).
In conclusion, the current study demonstrates that triptolide promotes growth arrest of pancreatic cancer cells, and enhances gemcitabine-induced DNA DSBs and apoptosis. Such results indicate that triptolide possesses antitumor potential against pancreatic cancer cells, particularly when administered in combination with gemcitabine. These in vitro findings require follow-up in vivo studies for validation, and the data from the present study suggests that the co-treatment of pancreatic cancer with gemcitabine and triptolide may provide additional clinical benefits.
Acknowledgements
The present study was supported by the National Scientific and Technological Major Project for ‘Significant New Drugs Development’, Beijing, China (grant no. 2011ZXJ09302) and China's 12th Five Year Plan, Beijing, China (grant no. 2012ZX10001003).
References
- 1.Shaib YH, Davila JA, El-Serag B. The epidemiology of pancreatic cancer in the United States: Changes below the surface. Aliment Pharmacol Ther. 2006;24:87–94. doi: 10.1111/j.1365-2036.2006.02961.x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg L. Pancreatic cancer: A review of emerging therapies. Drugs. 2000;59:1071–1089. doi: 10.2165/00003495-200059050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Freelove R, Walling AD. Pancreatic cancer: Diagnosis and management. Am Fam Physician. 2006;73:485–492. [PubMed] [Google Scholar]
- 5.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Surg. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuckerman DS, Ryan DP. Adjuvant therapy for pancreatic cancer: A review. Cancer. 2008;112:243–249. doi: 10.1002/cncr.23174. [DOI] [PubMed] [Google Scholar]
- 7.Rothenberg ML, Moore MJ, Cripps MC, Andersen JS, Portenoy RK, Burris HA, III, Green MR, Tarassoff PG, Brown TD, Casper ES, et al. A phase II trial of gemcitabine in patients with 5-FU-refractory pancreas cancer. Ann Oncol. 1996;7:347–353. doi: 10.1093/oxfordjournals.annonc.a010600. [DOI] [PubMed] [Google Scholar]
- 8.Burris HA, III, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 9.Mini E, Nobili S, Caciagli B, Landini I, Mazzei T. Cellular pharmacology of gemcitabine. Ann Oncol. 2006;17(Suppl 5):v7–v12. doi: 10.1093/annonc/mdj941. [DOI] [PubMed] [Google Scholar]
- 10.Schultz RM, Merriman RL, Toth JE, Zimmermann JE, Hertel LW, Andis SL, Dudley DE, Rutherford PG, Tanzer LR, Grindey GB. Evaluation of new anticancer agents against the MIA PaCa-2 and PANC-1 human pancreatic carcinoma xenografts. Oncol Res. 1993;5:223–228. [PubMed] [Google Scholar]
- 11.Nakano Y, Tanno S, Koizumi K, Nishikawa T, Nakamura K, Minoguchi M, Izawa T, Mizukami Y, Okumura T, Kohgo Y. Gemcitabine chemoresistance and molecular markers associated with gemcitabine transport and metabolism in human pancreatic cancer cells. Br J Cancer. 2007;96:457–463. doi: 10.1038/sj.bjc.6603559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu B, Guo X, Mathew S, Armesilla AL, Cassidy J, Darling JL, Wang W. Triptolide simultaneously induces reactive oxygen species, inhibits NF-kappaB activity and sensitizes 5-fluorouracil in colorectal cancer cell lines. Cancer Lett. 2010;291:200–208. doi: 10.1016/j.canlet.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 14.Liu Q. Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol. 2011;11:377–383. doi: 10.1016/j.intimp.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Brinker AM, Ma J, Lipsky PE, Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae) Phytochemistry. 2007;68:732–766. doi: 10.1016/j.phytochem.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, He T, Chai C, Yang Y, Zheng Y, Zhou P, Qiao X, Zhang B, Liu Z, Wang J, et al. Triptolide inhibits the proliferation of prostate cancer cells and down-regulates SUMO-specific protease 1 expression. PLoS One. 2012;7:e37693. doi: 10.1371/journal.pone.0037693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillips PA, Dudeja V, McCarroll JA, Borja-Cacho D, Dawra RK, Grizzle WE, Vickers SM, Saluja AK. Triptolide induces pancreatic cancer cell death via inhibition of heat shock protein 70. Cancer Res. 2007;67:9407–9416. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 18.Shamon LA, Pezzuto JM, Graves JM, Mehta RR, Wangcharoentrakul S, Sangsuwan R, Chaichana S, Tuchinda P, Cleason P, Reutrakul V. Evaluation of the mutagenic, cytotoxic and antitumor potential of triptolide, a highly oxygenated diterpene isolated from Tripterygium wilfordii. Cancer Lett. 1997;112:113–117. doi: 10.1016/S0304-3835(96)04554-5. [DOI] [PubMed] [Google Scholar]
- 19.Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF, Yeh JI, Hsu HY. Synergistic anticancer activity of triptolide combined with cisplatin enhances apoptosis in gastric cancer in vitro and in vivo. Cancer Lett. 2012;319:203–213. doi: 10.1016/j.canlet.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Chen YW, Lin GJ, Chuang YP, Chia WT, Hueng DY, Lin CK, Nieh S, Sytwu HK. Triptolide circumvents drug-resistant effect and enhances 5-fluorouracil antitumor effect on KB cells. Anticancer Drugs. 2010;21:502–513. doi: 10.1097/CAD.0b013e328337337c. [DOI] [PubMed] [Google Scholar]
- 21.Zhu W, Li J, Wu S, Li S, Le L, Su X, Qiu P, Hu H, Yan G. Triptolide cooperates with Cisplatin to induce apoptosis in gemcitabine-resistant pancreatic cancer. Pancreas. 2012;41:1029–1038. doi: 10.1097/MPA.0b013e31824abdc0. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Cui YF. Synergism of cytotoxicity effects of triptolide and artesunate combination treatment in pancreatic cancer cell lines. Asian Pac J Cancer Prev. 2013;14:5243–5248. doi: 10.7314/APJCP.2013.14.9.5243. [DOI] [PubMed] [Google Scholar]
- 23.Yang SW, Wang W, Xie XY, Zhu WP, Li FQ. In vitro synergistic cytotoxic effect of triptolide combined with hydroxycamptothecin on pancreatic cancer cells. Am J Chin Med. 2011;39:121–134. doi: 10.1142/S0192415X11008695. [DOI] [PubMed] [Google Scholar]
- 24.Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and proapoptotic activities of triptolide (PG490), a natural product entering clinical trials, on primary cultures of human prostatic epithelial cells. Clin Cancer Res. 2002;8:2666–2674. [PubMed] [Google Scholar]
- 25.Qiao Z, Ren S, Li W, Wang X, He M, Guo Y, Sun L, He Y, Ge Y, Yu Q. Chidamide, a novel histone deacetylase inhibitor, synergistically enhances gemcitabine cytotoxicity in pancreatic cancer cells. Biochem Biophys Res Commun. 2013;434:95–101. doi: 10.1016/j.bbrc.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 26.Donadelli M, Costanzo C, Beghelli S, Scupoli MT, Dandrea M, Bonora A, Piacentini P, Budillon A, Caraglia M, Scarpa A, Palmieri M. Synergistic inhibition of pancreatic adenocarcinoma cell growth by trichostatin A and gemcitabine. Biochim Biophys Acta. 2007;1773:1095–1106. doi: 10.1016/j.bbamcr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Xie C, Edwards H, Xu X, Zhou H, Buck SA, Stout ML, Yu Q, Rubnitz JE, Matherly LH, Taub JW, Ge Y. Mechanisms of synergistic antileukemic interactions between valproic acid and cytarabine in pediatric acute myeloid leukemia. Clin Cancer Res. 2010;16:5499–5510. doi: 10.1158/1078-0432.CCR-10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiang M, Qian ZY, Zhou CH, Liu J, Li WN. Crocetin inhibits leukocyte adherence to vascular endothelial cells induced by AGEs. J Ethnopharmacol. 2006;107:25–31. doi: 10.1016/j.jep.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Kinnally KW, Antonsson B. A tale of two mitochondrial channels, MAC and PTP, in apoptosis. Apoptosis. 2007;12:857–868. doi: 10.1007/s10495-007-0722-z. [DOI] [PubMed] [Google Scholar]
- 30.Dai Y, Grant S. New insights into checkpoint kinase 1 in the DNA damage response signaling network. Clin Cancer Res. 2010;16:376–383. doi: 10.1158/1078-0432.CCR-09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kupchan SM, Court WA, Dailey RG, Jr., Gilmore CJ, Bryan RF. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94:7194–7195. doi: 10.1021/ja00775a078. [DOI] [PubMed] [Google Scholar]
- 32.Hu YP, Tan ZJ, Wu XS, Liu TY, Jiang L, Bao RF, Shu YJ, Li ML, Weng H, Ding Q, Tao F, Liu YB. Triptolide induces s phase arrest and apoptosis in gallbladder cancer cells. Molecules. 2014;19(2):2612–2628. doi: 10.3390/molecules19022612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ho JN, Byun SS, Lee S, Oh JJ, Hong SK, Lee SE, Yeon JS. Synergistic antitumor effect of triptolide and cisplatin in cisplatin resistant human bladder cancer cells. The Journal of urology. 2015;193(3):1016–1022. doi: 10.1016/j.juro.2014.09.007. 14. [DOI] [PubMed] [Google Scholar]
- 34.Chueh FS, Chen YL, Hsu SC, Yang JS, Hsueh SC, Ji BC, Lu HF, Chung JG. Triptolide induced DNA damage in A375.S2 human malignant melanoma cells is mediated via reduction of DNA repair genes. Oncol Rep. 2013;29:613–618. doi: 10.3892/or.2012.2170. [DOI] [PubMed] [Google Scholar]