Abstract
Irreversible hypofunction of salivary glands is common in head and neck cancer survivors treated with radiotherapy and can only be temporarily relieved with current treatments. We found in an inducible sonic hedgehog (Shh) transgenic mouse model that transient activation of the Hedgehog pathway after irradiation rescued salivary gland function in males by preserving salivary stem/progenitor cells and parasympathetic innervation. To translate these findings into feasible clinical application, we evaluated the effects of Shh gene transfer to salivary glands of wild-type mice on irradiation-induced hyposalivation. Shh or control GFP gene was delivered by noninvasive retrograde ductal instillation of corresponding adenoviral vectors. In both male and female mice, Shh gene delivery efficiently activated Hedgehog/Gli signaling, and significantly improved stimulated saliva secretion and preserved saliva-producing acinar cells after irradiation. In addition to preserving parasympathetic innervation through induction of neurotrophic factors, Shh gene delivery also alleviated the irradiation damage of the microvasculature, likely via inducing angiogenic factors, but did not expand the progeny of cells responsive to Hedgehog/Gli signaling. These data indicate that transient activation of the Hedgehog pathway by gene delivery is promising to rescue salivary function after irradiation in both sexes, and the Hedgehog/Gli pathway may function mainly in cell nonautonomous manners to achieve the rescue effect.
Introduction
Head and neck cancers (HNCs) including cancers in the oral cavity, pharynx, nasal cavity, sinuses, and larynx account for about 3.9% of all cancers in the United States, with 64,970 estimated new cases in 20151 and with about 342,550 estimated survivors in 2012.2 Radiotherapy is a common treatment for HNC, and nondiseased salivary glands are often exposed to irradiation (IR). Because of the exquisite radiosensitivity of salivary glands, irreversible hyposalivation or xerostomia is common (68.1–90.9%) in long-term HNC survivors treated by conventional radiotherapy.3 The reduction in saliva secretion correlates significantly with the mean irradiation dose received by salivary glands.4 Novel intensity-modulated radiotherapy significantly decreased irradiation dose to salivary glands and the incidence of xerostomia, but about 20% of patients treated with intensity-modulated radiotherapy still have long-term xerostomia and related weight loss.5 Hyposalivation exacerbates dental caries and periodontal disease and causes problems of mastication, swallowing, sleep, and speech, a burning sensation of the mouth, and dysgeusia, which severely impair the quality of life of patients. The irreversible hyposalivation is caused by the loss or impairment of saliva-producing acinar cells and their replacement by connective tissue and fibrosis, which has been attributed to the loss of functional glandular stem/progenitor cells6 and the impairment of parasympathetic innervation7 and microvessels.8 Current treatments for IR-induced xerostomia, such as artificial saliva and saliva secretion stimulators, can only temporarily relieve these symptoms. Gene therapies transferring growth factors or water channel protein Aquaporin-1 locally showed promise to restore salivary gland function by protection or regeneration of saliva-producing cells,9 but the rescue effect varies and needs to be improved.
Hedgehog (Hh) signaling is required for salivary branching morphogenesis10 and is activated during functional regeneration of adult salivary glands after duct ligation.11 Hh signaling is triggered by the binding of Hh ligands such as Sonic Hedgehog (Shh) with their receptor Patched (Ptch), which derepresses Smoothened (Smo) to activate Gli family zinc finger transcription factors and the consequent transcription of Hh target genes including Gli1 and Ptch1.12 In addition to the Gli-dependent canonical Hh pathway, the binding of Hh ligands with Ptch also activates several noncanonical pathways such as Rho small GTPases and phosphoinositide 3-kinase (PI3K)/Akt pathways.13,14 We have found that IR did not activate Hh/Gli signaling, whereas transient activation of the Hh pathway in salivary gland by inducible expression of Shh transgene in Keratin5+ epithelial cells rescued IR-induced hyposalivation.15 The underlying mechanisms of this rescue effect include the preservation of parasympathetic innervation and putative salivary stem/progenitor cells. In this article, to translate the above-described findings into feasible clinical application, we delivered adenoviral vectors carrying the Shh gene (Ad-Shh) noninvasively into irradiated submandibular glands (SMGs) of wild-type mice, and confirmed the rescue effect of Shh gene transfer on IR-induced hyposalivation in both male and female mice. The underlying mechanisms of such rescue effect include alleviation of IR damage to both the microvasculature and parasympathetic innervation through upregulation of angiogenic and neurotrophic factors, whereas cells responsive to the Hh/Gli signaling pathway appear not to contribute directly to regenerated tissues after IR.
Materials and Methods
Mice
Hh signaling activity was analyzed with B6;129-Ptch1tm1Mps/J (Ptch1-lacZ) mice, the fate of cells responding to Hh/Gli signaling was traced with Bmi1-CreER [B6;129-Bmi1tm1(cre/ERT)Mrc/J]/Rosa26R-lacZ [Gt(ROSA)26Sortm1Sor] mice, and all other experiments were performed in wild-type C57BL/6 mice. All of these mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Single-dose irradiation (15 Gy) of the head and neck region of mice and measurement of the stimulated whole saliva flow rate were as described previously.16 All animal procedures were approved by the Texas A&M Health Science Center (Temple, TX) and the Baylor Scott & White Hospital (Temple, TX) Institutional Animal Care and Use Committee.
Preparation and delivery of adenoviral vectors
Adenoviral vector encoding green fluorescent protein (GFP) or rat Shh (Ad-GFP or Ad-Shh; Applied Biological Materials, Richmond, BC, Canada) was expanded in 293A cells and purified by two rounds of ultracentrifugation through a cesium chloride gradient. The titers (particles/ml) of purified vectors were determined by qPCR, using an Adeno-X rapid titer kit (Clontech Laboratories, Mountain View, CA). Mice were anesthetized with ketamine (60 mg/kg) and xylazine (8 mg/kg) intraperitoneally. Vectors generally were given to both SMGs by retrograde ductal instillation as reported17 at a dose of 1 × 109 particles per SMG.
Quantitative RT-PCR and Western blot analysis
Quantitative RT-PCR (qRT-PCR) was done as reported.11 Primers for Gapdh, Gli1, Aqp5, Angpt1/2, and Vegfs were retrieved (PrimerBank; http://pga.mgh.harvard.edu/primerbank). For Western blotting, fresh SMG samples were homogenized with a 40-μl/mg concentration of T-PER (tissue protein extraction reagent) containing protease inhibitors (Thermo Fisher Scientific, Waltham, MA) followed by centrifugation at 10,000 × g for 5 min to collect supernatant. Western blotting was done as reported11 with antibodies (Abcam, Cambridge, UK) for Aquaporin 5 (Aqp5) (diluted 1:5000), Aqp1 (diluted 1:1000), and glial cell line-derived neurotrophic factor family receptor α2 (GFRα2) (diluted 1:1000).
Histology, immunofluorescence staining, and corresponding quantifications
Frozen SMG sections were subjected to immunofluorescence staining specific to acinar marker Aqp5 or endothelial markers CD31 or Aqp1. Sections were first incubated in a 1:100 dilution of either anti-Aqp5 (Abcam), anti-CD31 (BD Biosciences Pharmingen, San Diego, CA) or anti-Aqp1 (Abcam). Primary antibodies were detected with appropriate secondary antibodies labeled with Texas red (Vector Laboratories, Burlingame, CA), and nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Microvascular density (MVD) was determined by counting the number of CD31- or Aqp1-stained cells per field at ×200 magnification. Frozen SMG sections were also stained with an acetylcholinesterase (AChE) rapid staining kit (MBL International, Woburn, MA) in accordance with the manufacturer's instructions. The AChE+ areas were quantified with NIS-Elements AR (advanced research) software (Nikon Instruments, Melville, NY) and then normalized to that of the nontreated group. For all quantifications, three glands from three mice in each treatment group were studied, and 10 fields were counted per gland.
Lineage-tracing assays
For lineage tracing of progeny of Bmi1+ cells during functional regeneration, male Bmi1-CreER/Rosa26R-lacZ mice (6–8 weeks, n = 4) were anesthetized as mentioned previously, and the orifice of the main excretory duct of the left SMG inside the mouth was exposed and ligated with adjacent tissues, using a Synovis MicroClip (Synovis Micro Companies Alliance/Baxter, Birmingham, AL). Seven days later, the MicroClip was removed for deligation. These mice were given 4-hydroxytamoxifen (4-OHT, 0.1 mg/g body weight) intraperitoneally daily for 3 days after duct ligation. The gland on the right side served as the sham-operated internal control. Fourteen days after the deligation, SMGs were harvested and processed for 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining as described previously.11 For lineage tracing of these progeny after transient Hh activation after IR, male Bmi1-CreER/Rosa26R-lacZ mice (6–8 weeks, n = 8) were anesthetized and irradiated as mentioned previously, Ad-Shh was administered as described previously 3 days later in four mice, and then 4-OHT was administered as described previously in all mice. SMGs were collected 90 days after IR.
Statistics
All quantified data were analyzed by one-way analysis of variance followed by Tukey's multiple-comparison test. Statistical analysis and graphical generation of data were done with GraphPad Prism software (GraphPad, San Diego, CA). p < 0.05 was considered as significant.
Results
Retrograde ductal instillation of Ad-Shh transiently activated the Hh pathway in SMGs of both male and female mice.
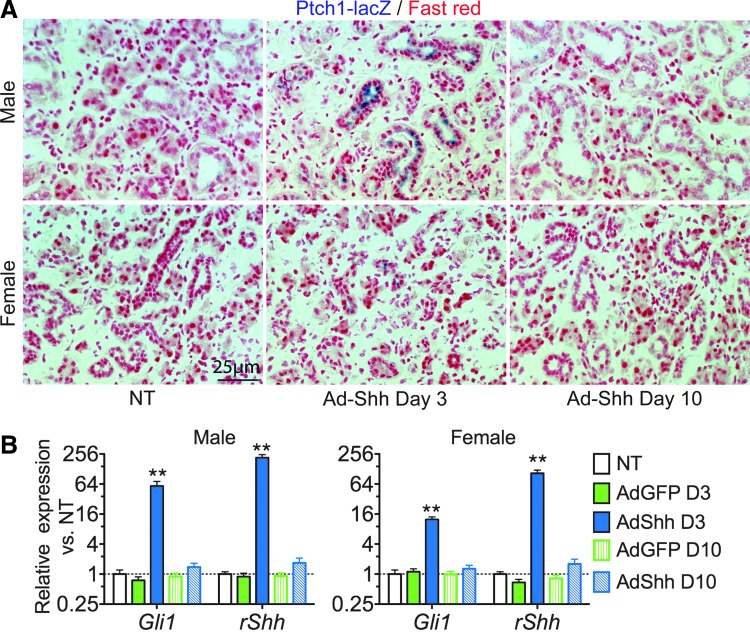
We found previously that in Keratin5-rtTA/tetO-Shh double-transgenic mice, the induction of Shh transgene expression with doxycycline for 7 days efficiently activated the Hh/Gli signaling pathway in male SMGs but not in female SMGs.15 To determine the effect of adenovirus carrying rat Shh gene (Ad-Shh) on the activation of Hh/Gli signaling in SMGs of both male and female mice, 1 × 109 particles per gland of Ad-Shh or control adenoviral vector carrying the GFP gene (Ad-GFP) were delivered via retrograde instillation through duct cannulation into SMGs of Ptch1-lacZ Hh/Gli signaling reporter mice. In SMGs of both sexes collected 3 days after adenovirus (Ad) instillation, the activity of lacZ reporter and the expression of rat Shh (rShh) and mouse Gli1, an endogenous target gene of the Hh/Gli pathway, were significantly increased in the Ad-Shh group but not in the Ad-GFP group in comparison with nontreated controls, as indicated by X-Gal staining and qRT-PCR analysis, respectively (p < 0.01, n = 3; Fig. 1). lacZ reporter activity was found mainly in the ductal epithelial cells 3 days after Ad-Shh instillation (Fig. 1A). In SMGs of both sexes collected 10 days after Ad instillation, these indexes were not significantly affected in either group (p > 0.05; Fig. 1). These data indicated that Shh gene delivery transiently and efficiently activated the Hh/Gli pathway in mouse SMGs of both sexes.
Figure 1.
Transient activation of the Hh/Gli pathway in SMGs by retrograde ductal instillation of Ad-Shh. Ad-GFP or Ad-Shh was delivered into SMGs of male and female Ptch1-lacZ mice by retrograde ductal instillation. SMGs were collected 3 or 10 days after Ad delivery and subjected to X-Gal staining of sections (A) or qRT-PCR analysis of the expression of rat Shh (rShh) transgene or Hh target gene Gli1 (B). **p < 0.01 versus the nontreated (NT) group.
Delivery of Shh gene into SMGs partially rescues irradiation-induced hyposalivation
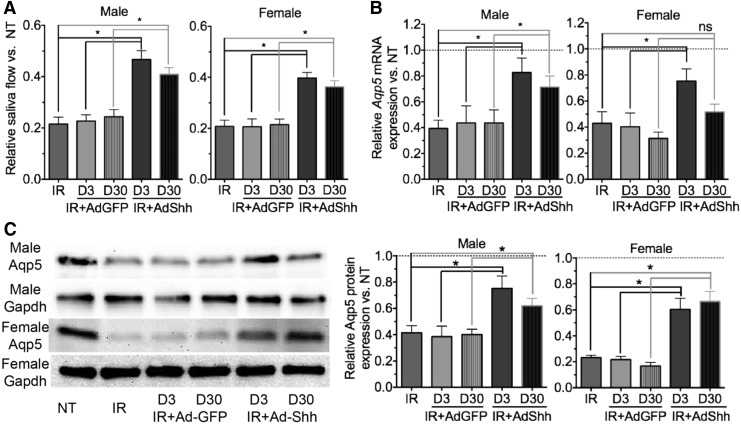
To examine the effect of Ad-Shh delivery on IR-induced hyposalivation, male and female wild-type C57BL/6 mice were not treated (NT) or were subjected to 15-Gy single-dose IR of the SMG region (IR), retrograde ductal instillation of 1 × 109 particles of Ad-GFP or Ad-Shh per SMG 3 or 30 days after IR (IR+Ad-GFP or IR+Ad-Shh, D3 or D30). IR significantly reduced the stimulated whole saliva flow rate in 90 days as expected (Fig. 2A). Shh gene delivery 3 or 30 days after IR both significantly improved the stimulated whole saliva flow rate on day 90 compared with IR or IR+Ad-GFP treatment in both male and female mice (n = 5; Fig. 2A). Consistently, the expression of mRNA and/or protein of acinar marker Aquaporin 5 (Aqp5) in SMGs 90 days after IR was significantly downregulated by IR on day 90 as reported,15,16,18 but improved by Shh gene delivery 3 or 30 days after IR in both sexes as indicated by qRT-PCR and Western blot assays (n = 5; Fig. 2B and C). Notably, although the differences are not statistically significant, earlier delivery of the Shh gene appears to result in better preservation of saliva secretion and Aqp5 expression than later delivery in both sexes. The preservation of Aqp5 by Shh gene delivery was further confirmed by immunofluorescence (IF) staining of frozen sections of SMGs 90 days after IR (Supplementary Fig. S1; supplementary data are available online at www.liebertpub.com/hum). These data indicate that single Shh gene delivery into SMGs partially rescued the salivary gland function impaired by IR.
Figure 2.
Delivery of Shh gene into SMGs partially rescued irradiation-induced hyposalivation. Male and female C57BL/6 mice were subjected to 15 Gy of irradiation in the neck region and retrograde ductal instillation of Ad-GFP or Ad-Shh 3 or 30 days later. The stimulated whole saliva flow rate was measured on day 90 after IR and normalized with body weight and then with saliva flow rate of age-matched NT mice (A). SMGs were collected 90 days after IR and analyzed by qRT-PCR (B) or Western blotting (C) for the expression of acinar marker Aqp5. *p < 0.05. IR, irradiation.
Intragland Shh gene delivery rescues microvascular damaged by IR
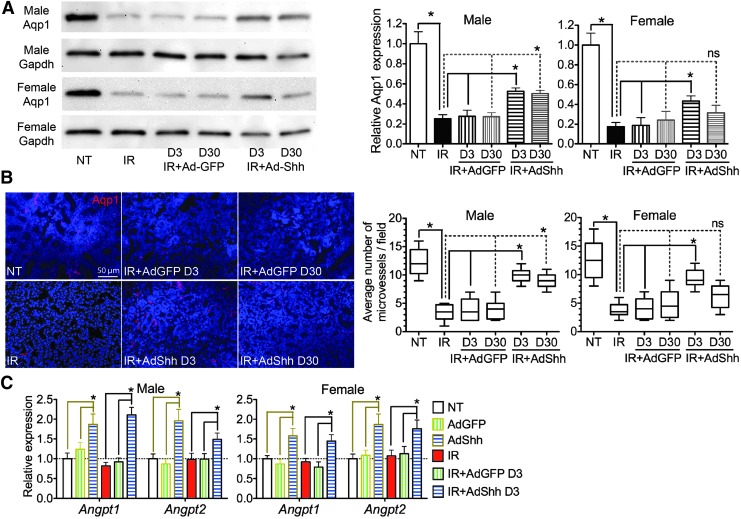
The microvascular damage by IR is a major cause of hyposalivation after IR.8,19 SMGs collected 90 days after IR were examined for microvascular density (MVD) by IF against endothelial markers Aqp1 or CD31 as reported,8,20 and the expression of Aqp1 protein was further quantified by Western blotting. IR significantly decreased Aqp1+ MVD and the expression of Aqp1 protein, whereas Ad-Shh instillation on day 3 after IR significantly restored both indexes in SMGs of both sexes, and that on day 30 significantly preserved both indexes in males to a lesser extent but not significantly in females (Fig. 3A and B). Similar results were obtained by CD31 IF and corresponding MVD quantification (Supplementary Fig. S2). In other organs, Hh/Gli signaling promotes angiogenesis during tissue repair and/or regeneration by inducing the secretion of proangiogenic factors.21 In SMGs collected 7 days after Ad instillation or 10 days after IR, the expression of mRNAs for Angiopoietin-1/2 (Angpt1/2) was significantly upregulated by Ad-Shh instillation alone or 3 days after IR compared with that in NT/Ad-GFP or IR/IR+Ad-GFP groups, respectively (Fig. 3C), whereas the expression of vascular endothelial growth factor (Vegf)-a, -b, and -c was not significantly affected (data not shown). These data indicate that transient Hh activation alleviates the IR damage to microvascular endothelial cells, likely through the upregulation of proangiogenic factors.
Figure 3.
Transient Hh activation alleviated IR damage to microvascular endothelial cells by upregulating angiogenic factors. (A) Western blot of Aqp1 in SMGs 90 days after IR and quantitative analysis of relative Aqp1 protein level normalized with GAPDH protein level (means ± SEM, n = 3). (B) Representative immunofluorescence staining of the endothelial marker Aqp1 in male SMGs and quantification of Aqp1+ microvessel density in SMGs 90 days after IR (95% confidence intervals shown in a box plot; the upper and lower boundaries of the box represent the 75th and 25th percentiles of the number of Aqp1+ microvessels per field per mouse, and the horizontal line within the box represents the median value). (C) qRT-PCR analysis of the expression of genes related to angiogenesis in SMGs collected 7 days after Ad instillation or 10 days after IR (n = 3). *p < 0.05. Ad, adenovirus.
Intragland Shh gene delivery rescues parasympathetic innervation of SMGs
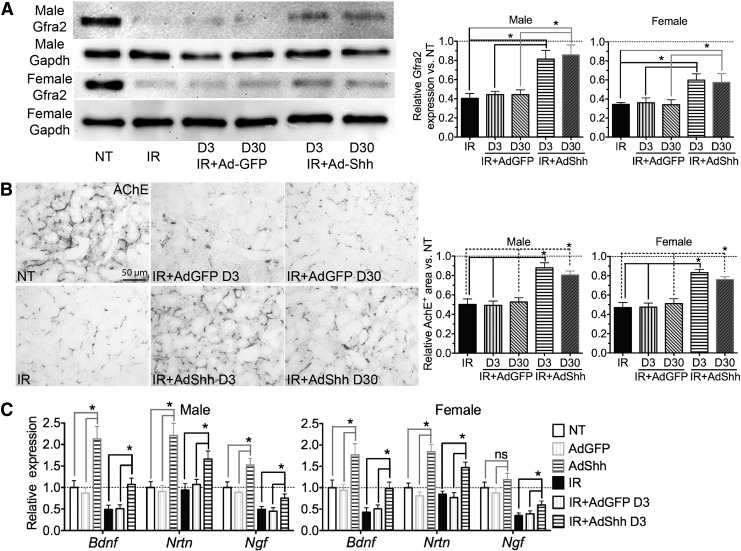
Parasympathetic stimulation improves regeneration of mouse embryonic salivary gland after IR, and adult human salivary glands damaged by IR have reduced parasympathetic innervation.7 We found previously that IR impaired parasympathetic innervation similarly in adult mouse SMGs, whereas transient overexpression of Shh transgene in Keratin5+ epithelial cells rescued such damage in male mice by increasing production of neurotrophic factors such as brain-derived neurotrophic factor (Bdnf), nerve growth factor (Ngf), and Neurturin (Nrtn).15 By examining the expression of glial cell line-derived neurotrophic factor family receptor α2 (GFRα2), a marker of parasympathetic nerve,22 we confirmed that in SMGs 90 days after IR the impairment of GFRα2 expression by IR was significantly ameliorated by Ad-Shh instillation 3 or 30 days after IR in both male and female mice (n = 3, p < 0.05; Fig. 4A). Consistently, the activity of acetylcholinesterase (AChE), another marker of parasympathetic nerves, was similarly decreased by IR but preserved by Shh gene transfer 3 or 30 days after IR in both male and female SMGs (Fig. 4B). Consistent with the previous finding,15 in SMGs collected 7 days after Ad instillation or 10 days after IR the expression of Bdnf, Neurturin, and Ngf mRNAs was significantly increased or preserved by Ad-Shh instillation alone or 3 days after IR in both male and female mice (Fig. 4C: n = 3, p < 0.05 for all comparisons except that of Ngf expression in nonirradiated female SMGs). We also examined the expression of glial cell line-derived neurotrophic factor (Gdnf), a neurotrophic factor highly expressed in putative salivary gland stem cells and capable of promoting the restoration of salivary gland function after IR,23 but the expression level of Gdnf mRNA in all of our SMG samples was below the detection threshold of our qRT-PCR assay (Ct >35 in reactions with 40 cycles of amplification). These data indicated that Shh gene transfer after IR preserved parasympathetic innervation, likely through the preservation or upregulation of neurotrophic factors including Bdnf, Ngf, and Neurturin.
Figure 4.
Intragland Shh gene delivery rescued parasympathetic innervation of SMGs. (A) SMGs collected 90 days after IR were analyzed with Western blot for expression of Gfra2, a makers of parasympathetic innervation. (B) Representative staining of acetylcholinesterase (AChE) activities on male SMG sections 90 days after IR and the quantification of relative AChE+ areas normalized to NT. (C) SMGs collected 10 days after IR or 7 days after Ad instillation were analyzed with qRT-PCR assay for the mRNA expression of neurotrophic factors (n = 3). *p < 0.05.
Hh-responsive cells do not directly contribute to regenerated SMG tissues after IR
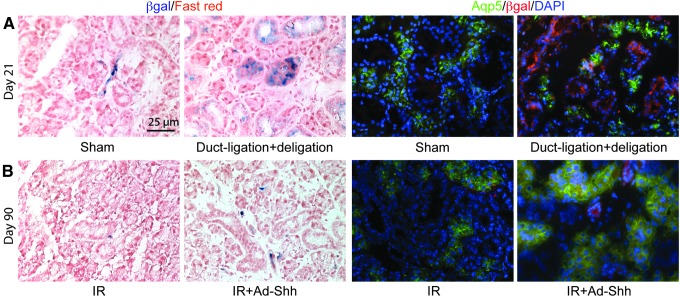
Bmi1 is a direct target gene of the Hh/Gli pathway and a putative marker of stem/progenitor cells in other organs,24–26 and transient Hh activation preserved Bmi1 expression and the Bmi1+ cell population in irradiated mouse SMGs.15 To determine whether Bmi1+ cells give rise to regenerated salivary gland tissues after functional regeneration caused by obstuctive damage or after transient Hh activation after IR, we traced the progeny of Bmi1+ cells in Bmi1-CreER/Rosa26R-lacZ mice. Twenty-one days after Cre recombinase (CreER) activation with 4-hydroxytamoxifen, as indicated by X-Gal staining of β-galactosidase (β-Gal) activity from the recombined Rosa26R-lacZ reporter cassette in the presence of actived CreER, progeny of Bmi1+ cells were occasionally found in a few stromal or ductal cells in mice that underwent sham surgery, but were present in many ductal cells and some cells in large acini-like structures in mice that underwent ligation and deligation of the main excretory duct of SMGs right before 4-hydroxytamoxifen treatment (Fig. 5A); double-immunofluorescence staining of Aqp5 and β-Gal indicated that the β-Gal+ progeny of Bmi1+ cells did not express Aqp5, suggesting that β-Gal+ cells adjacent to Aqp5+ acini are likely myoepithelial cells (Fig. 5A). These data indicated that after obstruction-induced regeneration Bmi1+ cells gave rise to regenerated ductal structures but did not significantly contribute to acinar structures, which is consistent with the finding that duplication of surviving acinar cells contributes to salivary gland regeneration.27 Ninety days after IR and after CreER activation, the β-Gal+ progeny of Bmi1+ cells were rarely found in SMGs, and transient Hh activation by Ad-Shh instillation did not significantly increase the number of β-Gal+ progeny of Bmi1+ cells as indicated by both X-Gal staining and β-Gal IF (Fig. 5B). These data suggest that transient activation of the Hh/Gli pathway might rescue IR-damaged salivary function, mainly in a cell-nonautonomous manner, such as by upregulation of paracrine factors.
Figure 5.
Distribution of progeny of Bmi1+ cells during functional regeneration or after IR with or without transient Hh activation. SMGs were collected 21 days after duct ligation (A) or 90 days after IR (B) from Bmi1-CreER/Rosa26R-lacZ mice treated with 4-OHT after duct ligation or Ad-Shh distillation. Frozen sections of these SMG samples were analyzed by X-Gal staining for activity of the lacZ/β-Gal reporter or by double-immunofluorescence staining for the expression of Aqp5 and β-Gal reporter.
Discussion
In our previous study, the transient inducible expression of the Shh transgene, controlled by Keratin5-rtTA (reverse tetracycline-controlled transactivator) and the tetO (tetracycline operator) promoter, efficiently activated the Hh/Gli pathway and consequently rescued IR-induced hyposalivation only in male mice, not female mice, likely due to much lower expression of Keratin5 and Hh receptors Patched in female mouse salivary glands.15 However, effects of IR and transient Hh activation with a small-molecule Hh agonist SAG (Smoothened Agonist) in SMGs of female mice are comparable to those in males.15 Expression of the Shh transgene as describe in the current article is controlled by the ubiquitous cytomegalovirus promoter in the Ad-Shh vector, and most SMG parenchymal cells are infected by adenovirus delivered via duct cannulation.28 These factors may lead to the efficient activation of Hh signaling in female SMGs by Ad-Shh, although the expression of target genes of the Hh/Gli pathway in female SMGs was still relatively lower than that in males. Consistently, Ad-Shh instillation rescued hypofunction of salivary glands after IR in both male and female mice, indicating that there is no significant sex difference in rescue effects mediated by efficient transient Hh activation.
Protecting salivary gland endothelial cells before IR with vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF/FGF2), or keratinocyte growth factor (KGF/FGF7) significantly prevented the decline of salivary flow after IR in mouse and miniature pig models.8,17,29 However, after IR KGF treatment did not significantly rescue IR-induced hyposalivation, whereas mobilizing bone marrow-derived cells including endothelial progenitors ameliorated vascular damage and facilitated functional restoration of salivary glands.30,31 Hh signaling promotes homeostatic angiogenesis during tissue repair and/or regeneration via multiple pathways. In the adult heart and skeletal muscle, ischemic episodes lead to an upregulation of Shh expression by perivascular and fibroblast-like stromal cells, and paracrine effects of Shh on stromal fibroblasts induce the secretion of proangiogenic factors VEGFs and Angiopoietin-1/2 in a Gli-dependent transcriptional manner, which in turn act on endothelial cells to promote angiogenesis.21 We found that the expression of Angiopoietin-1/2 in irradiated SMGs was upregulated by Shh gene transfer, which may contribute to the alleviation of IR damage to the microvasculature. Earlier delivery of the Shh gene after IR appears to result in better amelioration of the vascular damage that starts shortly after IR and persists for a long period, which may contribute to the better rescue effects of early Shh delivery in both sexes.
Aberrant activation of Hh signaling is associated with tumorigenesis and radioresistance of head and neck cancer.32,33 However, transient Hh activation in salivary glands, such as overexpression of Gli1 transgene for 15 weeks and overexpression of Shh transgene for 1 week, did not result in any detectable tumor in mice.15,34 Gene therapy for salivary gland hypofunction has several unique advantages, including easy access to most parenchymal cells via duct cannulation, the capacity to produce high amounts of protein, and tight encapsulation that minimizes concern about transgene spread.35 We reported earlier that in wild-type mice with subcutaneous head and neck squamous cell carcinoma, the delivery of Ad-Shh to SMGs by retrograde ductal instillation efficiently activated the Hh/Gli pathway in SMGs, but did not activate the Hh/Gli pathway in tumors and did not affect the growth and response to radiotherapy of tumors,15 indicating that local Hh activation in salivary glands will not promote the growth and radioresistance of preexisting tumors outside salivary glands.
It was widely believed that acinar cells of the salivary gland are replaced through stem cell differentiation,36 but one study has demonstrated that acinar cell proliferation accounts for postnatal growth and expansion of the salivary gland, as well as for maintenance and regeneration of the adult organ.27 Similar mechanisms have been reported for the growth and maintenance of other glands in the digestive tract including liver and pancreas.37–39 Our data from the lineage-tracing assay of cells expressing Bmi1, a putative marker of salivary gland stem/progenitor cells and a direct target of Hh/Gli signaling, are consistent with the acinar cell self-duplication model, and suggest that transient activation of Hh/Gli signaling rescues IR-induced hyposalivation mainly in a non-cell-autonomous manner.
Taken together, our data indicate that Shh gene transfer is a feasible approach to restore salivary gland function after radiotherapy, which functions through ameliorations of IR damage to the microvasculature and parasympathetic innervation by upregulation of paracrine factors. These paracrine factors downstream of Shh signaling interact with receptors on endothelial cells40 and parasympathetic neurons and their projections7,41 to preserve the microvasculature and parasympathetic innervation, which leads to the restoration of salivary gland function likely by improving blood supply and activating the cholinergic receptor muscarinic 1 (Chrm1)/heparin-binding EGF-like growth factor (HB-EGF) pathway with acetylcholine to promote proliferation of Chrm1+ epithelial cells in both acini and ducts of adult salivary glands.42,43 Further investigation of other responses triggered by transient Hh activation, beneficial or harmful to restoration of the salivary gland, will improve the efficacy of this approach and facilitate its potential clinical application.
Supplementary Material
Acknowledgments
This study was supported by NIH/NIDCR 1R01DE022975-01 (F.L.). The study was conceived and supervised by F.L.; B.H. performed most of the experiments; Q.Z. and L.Q. contributed to mouse breeding and genotyping, lineage tracing, and Western blotting; V.R.G. and D.R. performed and supervised IR; F.L. wrote the manuscript. The authors thank Christina Du and Angie Hitt (Department of Comparative Medicine, Baylor Scott & White Hospital) for help in animal work. This work was done in Temple, Texas.
Author Disclosure
The authors declare that no competing financial interests exist.
References
- 1.Siegel RL, Miller KD, and Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5–29 [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220–241 [DOI] [PubMed] [Google Scholar]
- 3.Jensen SB, Pedersen AM, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer 2010;18:1061–1079 [DOI] [PubMed] [Google Scholar]
- 4.Roesink JM, Moerland MA, Hoekstra A, et al. Scintigraphic assessment of early and late parotid gland function after radiotherapy for head-and-neck cancer: a prospective study of dose–volume response relationships. Int J Radiat Oncol Biol Phys 2004;58:1451–1460 [DOI] [PubMed] [Google Scholar]
- 5.Ghosh-Laskar S, Yathiraj PH, Dutta D, et al. Prospective randomized controlled trial to compare 3-dimensional conformal radiotherapy to intensity-modulated radiotherapy in head and neck squamous cell carcinoma: long-term results. Head Neck 2015. November 11 [Epub ahead of print] DOI: 10.1002/hed.24263 [DOI] [PubMed] [Google Scholar]
- 6.Konings AW, Coppes RP, and Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys 2005;62:1187–1194 [DOI] [PubMed] [Google Scholar]
- 7.Knox SM, Lombaert IM, Haddox CL, et al. Parasympathetic stimulation improves epithelial organ regeneration. Nat Commun 2013;4:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotrim AP, Sowers A, Mitchell JB, et al. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther 2007;15:2101–2106 [DOI] [PubMed] [Google Scholar]
- 9.Vissink A, Mitchell JB, Baum BJ, et al. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys 2010;78:983–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haara O, Fujimori S, Schmidt-Ullrich R, et al. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development 2011;138:2681–2691 [DOI] [PubMed] [Google Scholar]
- 11.Hai B, Yang Z, Millar SE, et al. Wnt/β-catenin signaling regulates postnatal development and regeneration of the salivary gland. Stem Cells Dev 2010;19:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osterlund T, and Kogerman P. Hedgehog signalling: how to get from Smo to Ci and Gli. Trends Cell Biol 2006;16:176–180 [DOI] [PubMed] [Google Scholar]
- 13.Chinchilla P, Xiao L, Kazanietz MG, et al. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle 2010;9:570–579 [DOI] [PubMed] [Google Scholar]
- 14.Kanda S, Mochizuki Y, Suematsu T, et al. Sonic hedgehog induces capillary morphogenesis by endothelial cells through phosphoinositide 3-kinase. J Biol Chem 2003;278:8244–8249 [DOI] [PubMed] [Google Scholar]
- 15.Hai B, Qin L, Yang Z, et al. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin Cancer Res 2014;20:140–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hai B, Yang Z, Shangguan L, et al. Concurrent transient activation of Wnt/β-catenin pathway prevents radiation damage to salivary glands. Int J Radiat Oncol Biol Phys 2012;83:e109–e116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng C, Cotrim AP, Rowzee A, et al. Prevention of radiation-induced salivary hypofunction following hKGF gene delivery to murine submandibular glands. Clin Cancer Res 2011;17:2842–2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takagi K, Yamaguchi K, Sakurai T, et al. Secretion of saliva in X-irradiated rat submandibular glands. Radiat Res 2003;159:351–360 [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Yan X, Gao R, et al. Effect of irradiation on microvascular endothelial cells of parotid glands in the miniature pig. Int J Radiat Oncol Biol Phys 2010;78:897–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delporte C, O'Connell BC, He X, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci U S A 1997;94:3268–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pola R, Ling LE, Silver M, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 2001;7:706–711 [DOI] [PubMed] [Google Scholar]
- 22.Rossi J, Luukko K, Poteryaev D, et al. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR α2, a functional neurturin receptor. Neuron 1999;22:243–252 [DOI] [PubMed] [Google Scholar]
- 23.Xiao N, Lin Y, Cao H, et al. Neurotrophic factor GDNF promotes survival of salivary stem cells. J Clin Invest 2014;124:3364–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res 2006;66:6063–6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subkhankulova T, Zhang X, Leung C, et al. Bmi1 directly represses p21Waf1/Cip1 in Shh-induced proliferation of cerebellar granule cell progenitors. Mol Cell Neurosci 2010;45:151–162 [DOI] [PubMed] [Google Scholar]
- 26.Yadirgi G, Leinster V, Acquati S, et al. Conditional activation of Bmi1 expression regulates self-renewal, apoptosis, and differentiation of neural stem/progenitor cells in vitro and in vivo. Stem Cells 2011;29:700–712 [DOI] [PubMed] [Google Scholar]
- 27.Aure MH, Konieczny SF, and Ovitt CE. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell 2015;33:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delporte C, Redman RS, and Baum BJ. Relationship between the cellular distribution of the αvβ3/5 integrins and adenoviral infection in salivary glands. Lab Invest 1997;77:167–173 [PubMed] [Google Scholar]
- 29.Guo L, Gao R, Xu J, et al. AdLTR2EF1α–FGF2-mediated prevention of fractionated irradiation-induced salivary hypofunction in swine. Gene Ther 2014;21:866–873 [DOI] [PubMed] [Google Scholar]
- 30.Lombaert IM, Brunsting JF, Wierenga PK, et al. Keratinocyte growth factor prevents radiation damage to salivary glands by expansion of the stem/progenitor pool. Stem Cells 2008;26:2595–2601 [DOI] [PubMed] [Google Scholar]
- 31.Lombaert IM, Brunsting JF, Wierenga PK, et al. Cytokine treatment improves parenchymal and vascular damage of salivary glands after irradiation. Clin Cancer Res 2008;14:7741–7750 [DOI] [PubMed] [Google Scholar]
- 32.Wang YF, Chang CJ, Lin CP, et al. Expression of hedgehog signaling molecules as a prognostic indicator of oral squamous cell carcinoma. Head Neck 2012;34:1556–1561 [DOI] [PubMed] [Google Scholar]
- 33.Gan GN, Eagles J, Keysar SB, et al. Hedgehog signaling drives radioresistance and stroma-driven tumor repopulation in head and neck squamous cancers. Cancer Res 2014;74:7024–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiaschi M, Kolterud A, Nilsson M, et al. Targeted expression of GLI1 in the salivary glands results in an altered differentiation program and hyperplasia. Am J Pathol 2011;179:2569–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simmons RK, and Baum BJ. Transferring genes to salivary glands. J Dent Educ 2001;65:907–910 [PubMed] [Google Scholar]
- 36.Pringle S, Van Os R, and Coppes RP. Concise review: adult salivary gland stem cells and a potential therapy for xerostomia. Stem Cells 2013;31:613–619 [DOI] [PubMed] [Google Scholar]
- 37.Dor Y, Brown J, Martinez OI, et al. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41–46 [DOI] [PubMed] [Google Scholar]
- 38.Teta M, Rankin MM, Long SY, et al. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817–826 [DOI] [PubMed] [Google Scholar]
- 39.Yanger K, Knigin D, Zong Y, et al. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell 2014;15:340–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Partanen J, Armstrong E, Makela TP, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol 1992;12:1698–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi J, Tomac A, Saarma M, et al. Distinct roles for GFRα1 and GFRα2 signalling in different cranial parasympathetic ganglia in vivo. Eur J Neurosci 2000;12:3944–3952 [DOI] [PubMed] [Google Scholar]
- 42.Knox SM, Lombaert IM, Reed X, et al. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science 2010;329:1645–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Proctor GB, and Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci 2007;133:3–18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.