Abstract
Cortical reorganization subsequent to post-stroke motor rehabilitative training (RT) has been extensively examined in animal models and humans. However, similar studies focused on the effects of motor training after traumatic brain injury (TBI) are lacking. We previously reported that after a moderate/severe TBI in adult male rats, functional improvements in forelimb use were accomplished only with a combination of skilled forelimb reach training and aerobic exercise, with or without nonimpaired forelimb constraint. Thus, the current study was designed to examine the relationship between functional motor cortical map reorganization after experimental TBI and the behavioral improvements resulting from this combinatorial rehabilitative regime. Adult male rats were trained to proficiency on a skilled reaching task, received a unilateral controlled cortical impact (CCI) over the forelimb area of the caudal motor cortex (CMC). Three days post-CCI, animals began RT (n = 13) or no rehabilitative training (NoRT) control procedures (n = 13). The RT group participated in daily skilled reach training, voluntary aerobic exercise, and nonimpaired forelimb constraint. This RT regimen significantly improved impaired forelimb reaching success and normalized reaching strategies, consistent with previous findings. RT also enlarged the area of motor cortical wrist representation, derived by intracortical microstimulation, compared to NoRT. These findings indicate that sufficient RT can greatly improve motor function and improve the functional integrity of remaining motor cortex after a moderate/severe CCI. When compared with findings from stroke models, these findings also suggest that more intense RT may be needed to improve motor function and remodel the injured cortex after TBI.
Key words: : constraint-induced movement therapy, exercise, functional recovery, motor rehabilitation, neuroplasticity
Introduction
Traumatic brain injury (TBI) constitutes a devastating health concern in most developed countries; in high-income nations, it is one of the leading causes of death and disability among people under the age of 45.1 Deficits in cognition, memory, and mood are more commonly reported and studied post-TBI; however, motor deficits are also common, especially after moderate-to-severe injuries, but are currently understudied.2 Recommendations for physical therapy for TBI survivors are becoming more consistent; however, very little research has specifically examined post-TBI motor rehabilitative training (RT) and related functional recovery.3 In contrast, there has been extensive investigation of the effects of different types of motor experience in animal models of stroke, and the results of these studies have influenced the RT of human stroke patients.4
In animal models of stroke, there is compelling evidence that some forms of behavioral experience result in better motor recovery and neural plasticity.5–7 Recovery of motor function is related to neural restructuring and reorganization post-stroke and motor “relearning” and task-specific practice are important in driving this neural plasticity.5,8,9 Subsequent to unilateral ischemic damage of the forelimb representation area of the caudal motor cortex (CMC) in squirrel monkeys, rats, and mice, extensive practice in reaching with the impaired forelimb prevents the loss of movement representation in the remaining motor cortex (measured with intracortical microstimulation [ICMS] motor cortex mapping), resulting in enlarged forelimb motor maps compared to untrained controls.10–13
Direct extension of the above findings from stroke to TBI requires the assumption that mechanisms of behavioral responses and mechanisms of neuroplasticity are essentially similar after these two types of brain injuries. Two of our recent studies indicate that this assumption is flawed.14,15 After a unilateral controlled cortical impact (CCI) over the caudal motor cortex (CMC) in rats, there are long-term forelimb impairments, similar to what is found following other models of damage to the motor cortex; however, we found that neural remodeling was severely blunted post-CCI compared to similarly placed and sized ischemic lesions.14 Specifically, there is a decrease in dendritic density in the motor cortex (MC) surrounding the CCI and in the contralateral homotopic cortex, regions that undergo robust dendritic growth and plasticity after ischemic lesions placed in the same anatomical location and with similar cortical lesion volumes.14,16–18 Further, we also recently reported that motor treatments that have been found to improve forelimb function after ischemic stroke (reaching alone and forelimb constraint alone), were not effective post-CCI over the forelimb area of the CMC unless they were combined.15 Skilled forelimb reaching, voluntary aerobic exercise, and nonimpaired forelimb constraint individually have been shown to enhance recovery or enhance neural plasticity in animal models of stroke and focal lesions.8,10,17,19–21 However, after CCIs over the CMC, forelimb function on a skilled forelimb reaching task was improved only when animals received reaching practice and voluntary exercise, with or without nonimpaired forelimb constraint.15 Thus, our data support that the neuroplastic response to injury and the behavioral response to RT post-CCI are not the same as after ischemic damage, despite similar initial behavioral impairments and damage to similar areas of the brain.
Given that the behavioral and neural plastic response to motor experience are different between TBI-like damage and similar ischemic lesions, we investigated the relationship between motor behavioral improvements resulting from effective rehabilitative training and functional motor cortical organization post-CCI over the CMC in adult male rats compared to no intervention (CCI alone). Skilled forelimb reaching, nonimpaired forelimb constraint, and voluntary exercise were introduced at time points found to be effective after experimental stroke or TBI or at times demonstrated to reduce the potential for negative effects of early postinjury over use (e.g., previous works22–26). Additionally, this timing was previously found to be highly effective in enhancing motor recovery post-CCI.15 We thus examined the functional integrity of the remaining motor cortex using standard ICMS techniques. Future studies will examine motor map changes induced by potential additive and/or interaction effects of each therapy alone and in combination.
Methods
Subjects
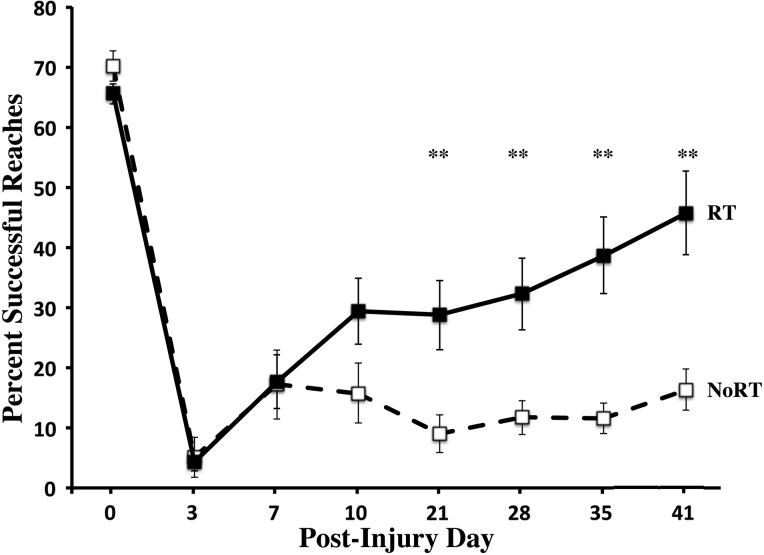
Twenty-six male Long Evans hooded rats (3.5-month-old; Harlan Laboratories, Indianapolis, IN) were housed in pairs at the Animal Resource Center at The University of Texas (Austin, TX) on a 12/12-h light/dark cycle. Rats were tamed by gentle handling and then were placed on scheduled feeding (15–17 g daily) of standard chow. Animals were trained to proficiency on the single-pellet reaching task with their preferred limb, and then a unilateral CCI was performed over the CMC opposite their preferred reaching limb. Based upon post-CCI behavioral performance and injury severity, animals were then assigned randomly to treatment condition, with the exception that they were carefully matched for initial severity of lesion-induced impairments. Equivalency in injury severity was based upon postinjury day 3 percent of successful retrievals on the single pellet retrieval task (Fig. 1) and subsequently validated with reaching abnormality scores (Fig. 2). There were no significant differences between groups in these two behaviors on day 3 (ps > 0.05). Further, there were also no significant differences in the size of the contusion produced by the injury in either of the two groups.
FIG. 1.
Success rate on the single-pellet reaching (SPR) task with the impaired forelimb. Post-CCI over the motor cortex, both groups are significantly impaired. The combination of reach training, aerobic exercise, and forelimb constraint significantly improved impaired forelimb reaching accuracy compared to NoRT. Data are means ± standard error of the mean; **p = 0.001. CCI, controlled cortical impact; NoRT, no rehabilitative training.
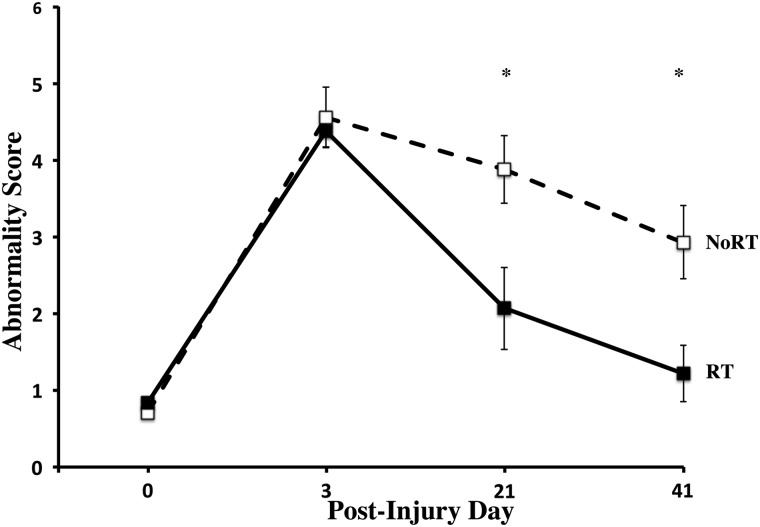
FIG. 2.
Abnormal reaching score on the single-pellet reaching (SPR) task. Post-CCI, both groups have an increase in the average number of abnormal reaching strategies during successful reaching on the SPR task. RT significantly reduces the abnormal reaching score over weeks of training compared to NoRT. Data are means ± standard error of the mean; *p = 0.05. CCI, controlled cortical impact; RT, rehabilitative training; NoRT, no rehabilitative training.
Animals either underwent combinatorial rehabilitative motor training (RT) or yoked treatment controls (no rehabilitative training; NoRT). All animal procedures were conducted in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved by the University of Texas, Austin Animal Care and Use Committee (Austin, TX).
Controlled cortical impact
All rats were anesthetized with a cocktail of ketamine and xylazine (90–100 and 10 mg/kg; intraperitoneally [i.p.]), a 4-mm craniotomy was created, centered over CMC (0.5 mm anterior and 4 mm lateral to bregma) opposite the preferred reaching limb, then a CCI was induced with a 3-mm diameter impact tip angled 18 degrees away from the vertical (Benchmark Stereotaxic Impactor; Leica, Buffalo Grove, IL), depressing the brain at 1.7 D.V, at 3.0 m/s for 300 ms. After the impact, the wound was covered with gel film and sutured. There was a fairly even split in preferred limb use, and thus side of injury, within and between groups (RT = 5 right, 8 left CCIs; NoRT = 6 right, 7 right CCIs).
Single-pellet retrieval task
The single-pellet reaching (SPR) task is a sensitive test of forelimb skill and dexterity that can be used to reveal quantitative and qualitative injury-induced forelimb deficits and recovery of forelimb function.27–30 Before CCI surgery, animals were trained to reach with the preferred limb through a narrow window to retrieve a banana-flavored food pellet (45 mg; Bio-Serve, Frenchtown, NJ) from a well 1 cm from the opening. A wall was placed 0.5 cm from the window within the chamber and ipsilateral to the preferred limb to encourage reaching with only the preferred limb. The number of successes, failures, and drops were recorded for 30 trials, or 10 min (whichever came first). A success occurred when the animal reached through the window, grasped the pellet, brought the pellet into the cage, and ate it. Animals were pre-operatively trained approximately 18 days until they had a stable (5 consecutive days) reaching success rate above 60%.
Quantitative reaching performance was measured on post-CCI days 0, 3, 7, 10, 21, 28, 35, and 41 and reported as the percent of successful reaches out of the total number of reach attempts [(total successes/total reach attempts) *100] with the preferred/impaired forelimb. Data presented are the mean ± standard error of the mean (SEM) percent successful reaches. The experimenter was not blind to group assignment in the collection of the SPR data because the forelimb constraint condition was evident.
Abnormal reaching score
The reaching task was also qualitatively assessed using frame-by-frame analysis of successful reaches recorded on post-CCI days 0, 3, 21, and 41. The Abnormal Reaching Score was calculated based on the average number of abnormal reaching movements made during 5 successful reaches per time point. Movements were analyzed using an adaptation of a rating scale developed by Whishaw and colleagues, which is based upon Eshkol–Wachman movement notation.31–34 This movement analysis is sensitive to compensatory forelimb movements that reveal enduring impairments or compensatory strategies in reaching and grasping motor action patterns after brain injury.31–33,35
Briefly, the eight components of reaching movements that were analyzed included: 1) aim: the elbow is adducted while the digits are aligned with the mid-line of the reaching window and are oriented toward the food pellet; 2) advance: the limb is advanced directly through the reaching window, initially above and beyond the food pellet; 3) digits open: as the limb is advanced, the digits open and extend toward the pellet; 4) pronation: the wrist pronates over the pellet; 5) grasp: the pads of the palm or the digits touch the food and the food is grasped by closure of the digits around the pellet. 6) supination 1: the paw is dorsiflexed and supinated 90 degrees as the limb is withdrawn through the reaching window; 7) supination 2: the paw is supinated again by approximately 45 degrees to bring the pellet to the mouth; and 8) release: the digits are opened and the pellet is released into mouth. Each movement was rated with a score of 0 (normal), 0.5 (slightly abnormal), and 1 (absent or highly abnormal). Data were analyzed as the total abnormality score across all the reaching components (averaged over trials). One animal from the yoked control group was excluded because of poor video quality. Experimenters collecting these data were blind to group assignment.
Motor rehabilitative treatment
The rehabilitative treatment (RT) protocol for the treatment group (RT; n = 13) included tray reach training, voluntary exercise on a running wheel, and less-impaired (ipsilateral-to-CCI) forelimb constraint. Yoked controls (NoRT; n = 13) participated in control procedures at the same time as their matched pairs in the RT group.
Tray-reaching task
Animals in the RT group trained with their impaired forelimb on a tray-reaching task starting on post-op day 3. The tray-reaching task is different from the single-pellet task in that a greater variety of reaching distances and trajectories is required and grasping more than one banana pellet at a time is allowed. For tray-reach training, rats were placed in a clear Plexiglas reaching chamber (as described for SPR). Five days per week, CCI+RT rats (n = 13) reached through the 1-cm-wide window with their impaired limb for 100 banana-flavored pellets (45 mg; Bio-Serv) placed in a tray with a 25-degree incline outside of the reaching chamber, or for 20 min, whichever occurred first. The CCI+NoRT controls (n = 13) were placed in the reaching chamber and ate 100 pellets off of the chamber floor.
Voluntary exercise
Starting on day 14 post-surgery, animals in the RT group were individually placed in cages with access to a running wheel for 6 h/day (3 h light/3 h dark), 5 days/week until post-surgery day 41. Cages were connected to exercise wheels by wire-mesh tubes. During the period of time the rehabilitative group had access to running wheels, the CCI+NoRT controls were individually placed into cages attached to wheels, but their wheels were locked to prevent rotation. All animals had access to food (17 g) and water during the 6 h in their individual cages. After each session, rats were returned to their home cage with their cage mate and given their remaining food.
Forelimb constraint
On post-injury day 10, RT animals had the forelimb ipsilateral to the CCI constrained using a limb-restricting vest (soft customized two-holed jackets with athletic tape used to wrap around the forelimb; Lomir Biomedical, Malone, NY). The vests were worn continuously for 10 days and were gently removed on day 20 post-injury. The NoRT control group wore a two-holed vest only, which allowed movement of both limbs.
Intracortical microstimulation
Standard ICMS methods were used on all animals.13,36–39 On day 42 post-CCI, animals were anesthetized (120 mg/kg of ketamine and 60 mg/kg of xylazine, i.p.) and received a craniotomy and dura removal over the injured motor cortex. Supplemental doses of ketamine (0.05–0.2 mg) were given as needed. A digital image of the cortical surface was taken and a 500-μm grid was superimposed onto the image. Using the cortical image and grid as a guide, a glass microelectrode (controlled by a hydraulic microdrive) was used to make systematic penetrations across the caudal forelimb area of the injured motor cortex. We also collected motor maps in the cortex contralateral to the injury in a subset of animals (RT = 2; CCI = 3) for illustrative purposes. The electrode was lowered into cortical layer V (∼1550 μm below cortical surface) at each penetration site, and current pulses were delivered. Because of death during this second surgical procedure, mapping data from n = 9 animals in the RT group and n = 12 controls were analyzed.
The stimulation current consisted of 13,200-μs cathodal pulses delivered at 350 Hz. If motor movements were detected at ≤100 μA, they were recorded. Forelimb movements were labeled as either distal (wrist/digit) or proximal (elbow/shoulder), whereas nonforelimb movements were noted as jaw, whisker, hindlimb, or trunk. We denoted whether movements were elicited at either ≤60 or 100 μA. The site was deemed nonresponsive if no movement was detected at ≤100 μA. The motor maps were constructed by systematically bordering the forelimb and jaw/neck sites with other motor or nonresponsive sites
ICMS-derived movement representations in injured cortex were analyzed by first using skull landmarks (bregma, mid-line, lamda, and the plane of the interaueral line) to align maps from across animals relative to stereotaxic coordinates, as described previously.38 Movement representations were generated by filling in grid squares with colors coding for the response at the center and then overlaid with a fixed 0.5 by 0.5 mm increment grid of lines aligned with bregma and mid-line. Areas of movement representations per each grid square were then measured using ImageJ software (NIH, Bethesda, MD).40 Experimenters were blinded to the group assignment of the animals.
Euthanasia
Animals were euthanized immediately after the motor map was generated by an overdose of pentobarbital. Animals were transcardially perfused with 0.1 M phosphate buffer followed by 4% paraformaldehyde in the same buffer. Brains were removed, post-fixed in 4% paraformaldehyde, and then six sets of 50-μm coronal were collected and Nissl stained using Toluidine blue.
Contusion volume estimation
Nissl stained sections which contained the CCI were examined using a Leica Microscope (Leica Microsystems, Jena, Germany) and CCD camera at 2.5× magnification, and the images were observed and analyzed using Neurolucida (MBF Bioscience Inc., Williston, VT). For all brains, perimeter tracing was used to calculate the area of cortex in the ipsi- and contrainjury cortex. The sum of the area of nine sections was multiplied by the average distance between sections (600 μm) to obtain the estimate volume of cortex. Ipsi-injury cortical volume was subtracted from contrainjury cortical volume to obtain the estimated contusion size. Data are presented as mean ± SEM.
Statistical analyses
Behavioral data were analyzed using repeated-measures analyses of variance (ANOVAs) with SPSS software (SPSS, Inc., Chicago, IL). To assess the severity of injury-induced behavioral impairments, paired-sample t-tests were used to compare pre- and post-CCI performance on each behavioral task across groups. ICMS motor mapping data was analyzed using one-way ANOVAs. Results were considered significant at p ≤ 0.05. All statistics were performed using SPSS software (SPSS, Inc.).
Results
Single-pellet reaching task
As seen in Figure 1, there were no significant differences between the RT group and the yoked controls preoperatively or on post-op day 3 (ps > 0.05). There is evidence of a significant level of spontaneous recovery post-CMC injury, as observed with an increase in reaching accuracy between days 3 and 7 (p < 0.001), in both the RT and CCI alone groups (Fig. 1). There was very little improvement in the RT group between days 10 and 21, which coincides with initiation of the exercise and nonimpaired forelimb constraint. In fact, reaching success began to steadily improve in the RT group after removal of the cast and may reflect a greater synergistic effect of exercise and reach training. The CCI+NoRT group showed no improvement throughout this time period. CCI to the CMC resulted in major impairments in both groups, as assessed at day 3. Similar to our recently published findings,15 the combination RT regimen that included daily tray reaching, exercise, and forelimb constraint significantly improved forelimb function on the SPR task, compared to untrained controls, after CMC focused CCIs. There was a significant Group×Day interaction effect (F(7,168) = 9.72; p < 0.0001). There were also significant effects of day (F(7,168) = 75.53; p < 0.0001) and Group (F(1,24) = 7.04; p = 0.014). Post-hoc analysis revealed that RT improved reaching accuracy on post-op days 21, 28, 35, and 41 (ps < 0.01).
Abnormal reaching score
As seen in Figure 2, CCI's increased abnormal reaching strategies (Abnormality Score) compared to pre-CCI levels (t(1, 24) = 17.63; p < 0.001) in both groups. Similar to our previously reported findings,15 RT significantly reduced reaching abnormalities, compared to NoRT, over the days tested (Group×Day: F(3, 69) = 5.45, p = 0.004; Days: F(3,69) = 48.23; p < 0.001). There was also an overall significant difference between groups (F(1,23) = 6.79; p = 0.016). Post-hoc analysis revealed significant differences on post-op days 21 and 41 (ps < 0.05).
Intracortical microstimulation motor map
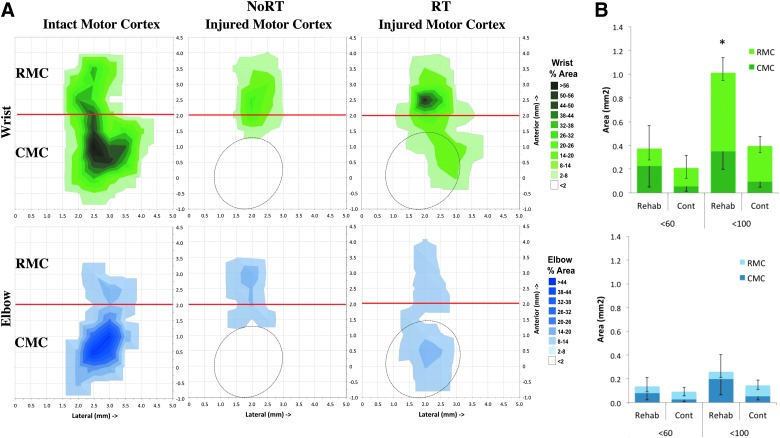
Previous studies have demonstrated that reach training in naïve and retraining after unilateral CMC ischemia in rats and monkeys expand wrist representation.10,13,36,37,39,41 As seen in Figure 3, we found that motor RT significantly increased the total area of wrist representation in the injured cortex elicited with ≤100 μA, inclusive of RMC and CMC (F(1,20) = 6.193; p = 0.02), compared to NoRT. The overall increase in wrist representation was mainly driven by a significant expansion of wrist in the RMC (Fig 3B; F(1,20) = 7.69; p = 0.012), although there was an nonsignificant enlargement of wrist representation in CMC, as well (F(1,20) = 2.913; p = 0.104). Neither elbow representation nor total number of motor movements (wrist/digit, elbow, shoulder, neck, jaw, or trunk) were different between groups (ps > 0.05). There were also no significant differences in mean threshold for wrist and elbow within or between groups (ps > 0.05).
FIG. 3.
ICMS mapping of forelimb representations in the injured cortex. (A) Surface plots of the percent area of wrist (green, top panel) and elbow (blue, bottom panel) representations in the remaining MC. Data are the mean percent area per 0.5 by 0.5 mm grid square. Oval outlines indicate average placement of CCI injury. (B) RT significantly expanded the area of rostral motor cortex (RMC) wrist representation, but not in remaining caudal motor cortex (CMC), compared to NoRT (top panel). There were no significant differences in the area of elbow representation between groups in RMC or CMC (bottom panel). Data are means ± standard error of the mean; *p = 0.05. ICMS, intracortical microstimulation; MC, motor cortex; CCI, controlled cortical impact; RT, rehabilitative training; NoRT, no rehabilitative training. Color image is available online at www.liebertpub.com/neu
Contusion volume estimation
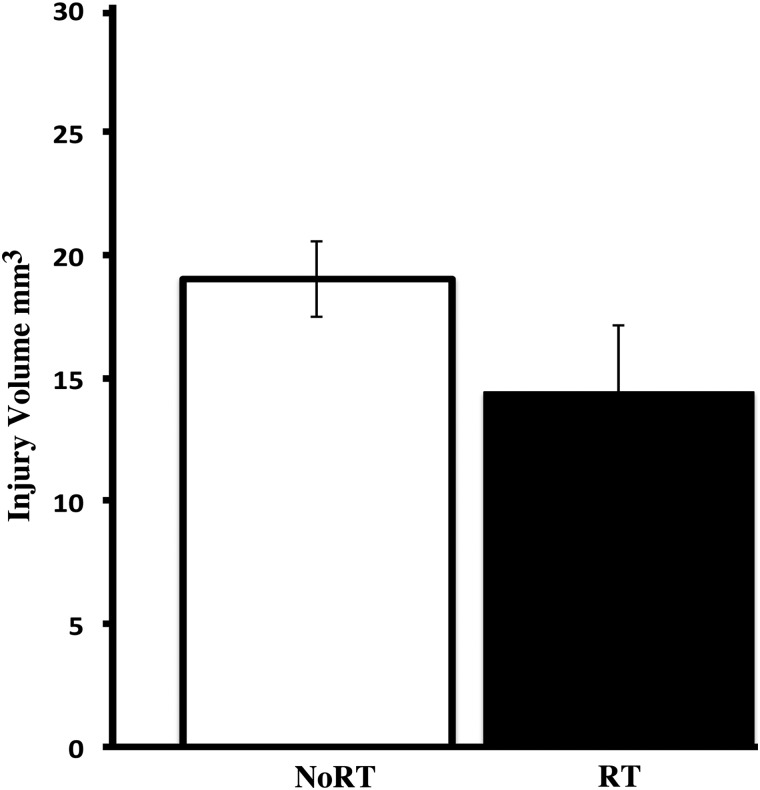
There were no significant differences in the estimated size of the contusion between groups (See Fig. 4A; F(1,24) = 2.42; p = 0.129); however, there is a tendency for the RT group to have smaller injuries. This is similar to our previous study in which we found that the combination of these three behavioral treatments nonsignificantly tended to decrease injury volume compared to no-treatment CCI controls.15
FIG. 4.
Volume of CCI-induced injury to the motor cortex. There were no significant differences in contusion size between, although RT tended (p = 0.129) to reduce injury volume compared to NoRT. CCI, controlled cortical impact; RT, rehabilitative training; NoRT, no rehabilitative training.
Discussion
We previously found that unilateral CCIs over CMC induce profound impairments in the limb opposite the contusion that can be improved by a combination of three behavioral interventions (reach training, aerobic exercise, and nonimpaired forelimb constraint), compared to single treatments alone, which are ineffective alone post-CCI.15 In this study, we sought to investigate whether this effective combinatorial RT regime would increase the area of remaining motor cortex compared to CCI alone, as revealed by standard high-resolution ICMS. These data support our previous findings in that the combination of task-specific practice on a reaching task, exercise, and constraint therapy (RT group) produced a highly significant improvement in reaching accuracy and normalized reaching movements compared to CCI controls (NoRT). We also found that combined RT significantly increased the area of wrist, but not elbow, representations in the remaining motor cortex of the injured hemisphere. This type of peri-injury reorganization has been associated with motor improvements in both spontaneous recovery of function42,43 and with the promotion of behavioral improvements by skilled reaching RT in experimental stroke models.12,28,44,45
The CMC region contains primary motor cortex, and the RMC is analogous to the premotor and supplementary motor cortex of primates.46–48 In rats, CMC and RMC have many reciprocal connections47 and are tightly coupled in driving movement production.49 RMC has strong modulatory effects on CMC50 and is likely to have a key role in the resolution of upper-extremity impairments, as has been found in other injury models10,41 and in human and primate studies.51–53 In standard ICMS studies, forelimb movements can be evoked at ≤60 uA.37–39,54–56 However, we and others have previously found that post-CCI of the CMS requires higher current to drive ICMS-evoked movements.57,58 The fact that wrist and elbow movements are primarily elicited above 60 uA indicates that, even 41 days post-CCI, the motor cortex is highly dysfunctional, further supporting our previous findings that dendrites in peri-injury remaining MC are sparse, and NOGO is upregulated compared to control animals.14 Nishibe and colleagues58 found that wrist movements in the RMC post-CCI over the CMC required, on average, 11–20 uA more current compared to non-CCI controls. It is also possible that intracortical and corticospinal white matter damage, ongoing neuroinflammation, or other inhibitory mechanisms underlie the reduced motor cortex representation and higher movement thresholds post-CCI. Further studies are needed to determine the mechanisms involved in the blunted neural output of the remaining RMC post-CCI. Additional future studies are needed to determine the possible contribution of each behavior treatment alone and whether the treatments are additive or synergistic with or without CCI.
Conclusion
In summary, the brain has the capability of regenerating post-TBI; however, brain plasticity and reorganization of peri-injury cortex are limited. Although it has been shown that neuroplasticity can be driven by some behaviors, it requires an extremely focused and intense RT regime in order to drive brain remodeling and functional recovery. An intense RT that incorporated exercise (on a running wheel), forelimb constraint therapy, and skilled reach training (similar to skilled motor learning in humans) provides an effective means for improving motor function post-TBI and results in the expansion of the distal forelimb representation, in remaining RMC.
Acknowledgments
This study was supported by the Department of Defense (#W81XWH-08-1-0624; to D.A.K., D.L.A., and T.A.J.). Further support was provided, in part, by the National Institute of Neurological Disorders and Stroke (NS065866) and by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM109040 (to D.L.A.). The authors are grateful for assistance with behavioral assessments and testing provided by Katya Boucek, Amanda Dodson, and Unekwu Yakubu and for Nicole Donlan's invaluable help with organization and aid with histology.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Maas A.I., Stocchetti N., and Bullock R. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 [DOI] [PubMed] [Google Scholar]
- 2.Kozlowski D.A., Leasure J.L., and Schallert T. (2013). The control of movement following traumatic brain injury. Compr. Physiol. 3, 121–139 [DOI] [PubMed] [Google Scholar]
- 3.Weightman M.M., Bolgla R., McCulloch K.L., and Peterson M.D. (2010). Physical therapy recommendations for service members with mild traumatic brain injury. J. Head Trauma Rehabil. 25, 206–218 [DOI] [PubMed] [Google Scholar]
- 4.Jain K.K. (2008). Neuroprotection in traumatic brain injury. Drug Discov. Today 13, 1082–1089 [DOI] [PubMed] [Google Scholar]
- 5.Jones T.A., and Adkins D.L. (2010). Behavioral influences on neuronal events after stroke. In: Brain Repair after Stroke. Nudo R.J. (ed). Cambridge University Press: Cambridge, UK [Google Scholar]
- 6.Allred R.P., Kim S.Y., and Jones T.A. (2014). Use it and/or lose it—experience effects on brain remodeling across time after stroke. Front. Hum. Neurosci. 8, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleim J.A., and Jones T.A. (2008). Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 51, S225–S239 [DOI] [PubMed] [Google Scholar]
- 8.Johansson B.B. (2000). Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke 31, 223–230 [DOI] [PubMed] [Google Scholar]
- 9.Kleim J.A., Jones T.A., and Schallert T. (2003). Motor enrichment and the induction of plasticity before or after brain injury. Neurochem. Res. 28, 1757–1769 [DOI] [PubMed] [Google Scholar]
- 10.Nudo R.J., Wise B.M., SiFuentes F., and Milliken G.W. (1996). Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 272, 1791–1794 [DOI] [PubMed] [Google Scholar]
- 11.Conner J.M., Chiba A.A., and Tuszynski M.H. (2005). The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 46, 173–179 [DOI] [PubMed] [Google Scholar]
- 12.Tennant K.A., Kerr A.L., Adkins D.L., Donlan N., Thomas N., Kleim J.A., and Jones T.A. (2015). Age-dependent reorganization of peri-infarct “premotor” cortex with task-specific rehabilitative training in mice. Neurorehabil. Neural Repair 29, 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleim J.A., Hogg T.M., VandenBerg P.M., Cooper N.R., Bruneau R., and Remple M. (2004). Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J. Neurosci. 24, 628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones T.A., Liput D.J., Maresh E.L., Donlan N., Parikh T.J., Marlowe D., and Kozlowski D.A. (2012). Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J. Neurotrauma 29, 1455–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adkins D.L., Ferguson L., Lance S., Pevtsov A., McDonough K., Stamschror J., Jones T.A., and Kozlowski D.A. (2015). Combining multiple types of motor rehabilitation enhances skilled forelimb use following experimental traumatic brain injury in rats. Neurorehabil. Neural Repair 989–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozlowski D.A., Leasure J.L., and Schallert T. (2013). The control of movement following traumatic brain injury. Compr. Physiol. 3, 121–139 [DOI] [PubMed] [Google Scholar]
- 17.Jones T.A., and Schallert T. (1994). Use-dependent growth of pyramidal neurons after neocortical damage. J. Neurosci. 14, 2140–2152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adkins D.L., Voorhies A.C., and Jones T.A. (2004). Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience 128, 473–486 [DOI] [PubMed] [Google Scholar]
- 19.Hsu J.E., and Jones T.A. (2005). Time-sensitive enhancement of motor learning with the less-affected forelimb after unilateral sensorimotor cortex lesions in rats. Eur. J. Neurosci. 22, 2069–2080 [DOI] [PubMed] [Google Scholar]
- 20.Maldonado M.A., Allred R.P., Felthauser E.L., and Jones T.A. (2008). Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil. Neural Repair 22, 250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones T.A., and Adkins D.L. (2010). Behavioral influences on neuronal events after stroke, in: Brain Repair after Stroke. Nudo R.J. (ed). Cambridge University Press: Cambridge, UK [Google Scholar]
- 22.Kozlowski D.A., James D.C., and Schallert T. (1996). Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J. Neurosci. 16, 4776–4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldonado M.A., Allred R.P., Felthauser E.L., and Jones T.A. (2008). Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil. Neural Repair 22, 250–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griesbach G.S., Gomez-Pinilla F., and Hovda D.A. (2007). Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J. Neurotrauma 24, 1161–1171 [DOI] [PubMed] [Google Scholar]
- 25.Griesbach G.S. (2011). Exercise after traumatic brain injury: is it a double-edged sword? PM R. 3, 6 Suppl. 1, S64–S72 [DOI] [PubMed] [Google Scholar]
- 26.Humm J.L., Kozlowski D.A., James D.C., Gotts J.E., and Schallert T. (1998). Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 783, 286–292 [DOI] [PubMed] [Google Scholar]
- 27.Adkins D.L., Boychuk J., Remple M.S., and Kleim J.A. (2006). Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J. Appl. Physiol. (1985) 101, 1776–1782 [DOI] [PubMed] [Google Scholar]
- 28.Adkins D.L., Hsu J.E., and Jones T.A. (2008). Motor cortical stimulation promotes synaptic plasticity and behavioral improvements following sensorimotor cortex lesions. Exp. Neurol. 212, 14–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adkins D.L., and Jones T.A. (2005). D-amphetamine enhances skilled reaching after ischemic cortical lesions in rats. Neurosci. Lett. 380, 214–218 [DOI] [PubMed] [Google Scholar]
- 30.O'Bryant A.J., Adkins D.L., Sitko A.A., Combs H.L., Nordquist S.K. and Jones T.A. (2014). Enduring poststroke motor functional improvements by a well-timed combination of motor rehabilitative training and cortical stimulation in rats. Neurorehabil. Neural Repair December 19 pii: . [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whishaw I.Q. (1993). Activation, travel distance, and environmental change influence food carrying in rats with hippocampal, medial thalamic and septal lesions: implications for studies on hoarding and theories of hippocampal function. Hippocampus 3, 373–385 [DOI] [PubMed] [Google Scholar]
- 32.Whishaw I.Q., Pellis S.M., Gorny B., Kolb B., and Tetzlaff W. (1993). Proximal and distal impairments in rat forelimb use in reaching follow unilateral pyramidal tract lesions. Behav. Brain Res. 56, 59–76 [DOI] [PubMed] [Google Scholar]
- 33.Metz G.A., and Whishaw I.Q. (2000). Skilled reaching an action pattern: stability in rat (Rattus norvegicus) grasping movements as a function of changing food pellet size. Behav. Brain Res. 116, 111–122 [DOI] [PubMed] [Google Scholar]
- 34.Eshkol N., and Wachmann A. (1958). Movement Notation. Weidenfeld and Nicolson: London [Google Scholar]
- 35.Gharbawie O.A., Gonzalez C.L., and Whishaw I.Q. (2005). Skilled reaching impairments from the lateral frontal cortex component of middle cerebral artery stroke: a qualitative and quantitative comparison to focal motor cortex lesions in rats. Behav. Brain Res. 156, 125–137 [DOI] [PubMed] [Google Scholar]
- 36.Kleim J.A., Barbay S., and Nudo R.J. (1998). Functional reorganization of the rat motor cortex following motor skill learning. J. Neurophysiol. 80, 3321–3325 [DOI] [PubMed] [Google Scholar]
- 37.Boychuk J.A., Adkins D.L., and Kleim J.A. (2011). Distributed versus focal cortical stimulation to enhance motor function and motor map plasticity in a rodent model of ischemia. Neurorehabil. Neural Repair 25, 88–97 [DOI] [PubMed] [Google Scholar]
- 38.Tennant K.A., Adkins D.L., Donlan N.A., Asay A.L., Thomas N., Kleim J.A., and Jones T.A. (2011). The organization of the forelimb representation of the C57BL/6 mouse motor cortex as defined by intracortical microstimulation and cytoarchitecture. Cereb. Cortex 21, 865–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teskey G.C., Monfils M.H., VandenBerg P.M., and Kleim J.A. (2002). Motor map expansion following repeated cortical and limbic seizures is related to synaptic potentiation. Cereb. Cortex 12, 98–105 [DOI] [PubMed] [Google Scholar]
- 40.Schneider C.A., Rasband W.S., and Eliceiri K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nudo R.J., and Milliken G.W. (1996). Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J. Neurophysiol. 75, 2144–2149 [DOI] [PubMed] [Google Scholar]
- 42.Carmichael S.T. (2012). Brain excitability in stroke: the yin and yang of stroke progression. Arch. Neurol. 69, 161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward N.S., Newton J.M., Swayne O.B., Lee L., Thompson A.J., Greenwood R.J., Rothwell J.C., and Frackowiak R.S. (2006). Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain 129, 809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramanathan D., Conner J.M., and Tuszynski M.H. (2006). A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proc. Natl. Acad. Sci. U. S. A. 103, 11370–11375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nudo R.J. (2013). Recovery after brain injury: mechanisms and principles. Front. Hum. Neurosci. 7, 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neafsey E.J., Bold E.L., Haas G., Hurley-Gius K.M., Quirk G., Sievert C.F., and Terreberry R.R. (1986). The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 396, 77–96 [DOI] [PubMed] [Google Scholar]
- 47.Rouiller E.M., Moret V., and Liang F. (1993). Comparison of the connectional properties of the two forelimb areas of the rat sensorimotor cortex: support for the presence of a premotor or supplementary motor cortical area. Somatosens. Mot. Res. 10, 269–289 [DOI] [PubMed] [Google Scholar]
- 48.Wise S.P., Murray E.A., and Coulter J.D. (1979). Somatotopic organization of corticospinal and corticotrigeminal neurons in the rat. Neuroscience 4, 65–78 [DOI] [PubMed] [Google Scholar]
- 49.Hyland B. (1998). Neural activity related to reaching and grasping in rostral and caudal regions of rat motor cortex. Behav. Brain Res. 94, 255–269 [DOI] [PubMed] [Google Scholar]
- 50.Deffeyes J.E., Touvykine B., Quessy S., and Dancause N. (2015). Interactions between rostral and caudal cortical motor areas in the rat. J. Neurophysiol. 113, 3893–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fridman E.A., Hanakawa T., Chung M., Hummel F., Leiguarda R.C., and Cohen L.G. (2004). Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127, 747–758 [DOI] [PubMed] [Google Scholar]
- 52.Seitz R.J., Hoflich P., Binkofski F., Tellmann L., Herzog H., and Freund H.J. (1998). Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch. Neurol. 55, 1081–1088 [DOI] [PubMed] [Google Scholar]
- 53.Ward N.S., Newton J.M., Swayne O.B., Lee L., Frackowiak R.S., Thompson A.J., Greenwood R.J., and Rothwell J.C. (2007). The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur. J. Neurosci. 25, 1865–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piecharka D.M., Kleim J.A., and Whishaw I.Q. (2005). Limits on recovery in the corticospinal tract of the rat: partial lesions impair skilled reaching and the topographic representation of the forelimb in motor cortex. Brain Res. Bull. 66, 203–211 [DOI] [PubMed] [Google Scholar]
- 55.Gharbawie O.A., Gonzalez C.L., Williams P.T., Kleim J.A., and Whishaw I.Q. (2005). Middle cerebral artery (MCA) stroke produces dysfunction in adjacent motor cortex as detected by intracortical microstimulation in rats. Neuroscience 130, 601–610 [DOI] [PubMed] [Google Scholar]
- 56.Kleim J.A., Bruneau R., VandenBerg P., MacDonald E., Mulrooney R., and Pocock D. (2003). Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol. Res. 25, 789–793 [DOI] [PubMed] [Google Scholar]
- 57.Jefferson S.C., Clayton E.R., Donlan N., Kozlowski D.A., Jones D.A., and Adkins D.L. (2015). Cortical stimulation concurrent with skilled motor training improves behavioral function and enhances motor cortical reorganization following controlled cortical impact. Neurorehabil. Neural Repair August 5 pii: . [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishibe M., Barbay S., Guggenmos D., and Nudo R.J. (2010). Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J. Neurotrauma 27, 2221–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]