Key Points
Discrepancies exist between inhibitor titer and bleeding phenotype in anti-fVIII antibodies.
A subset of high-titer type 2 anti-A2 inhibitors can be treated with fVIII.
Abstract
The primary B-cell epitopes of factor VIII (fVIII) are in the A2 and C2 domains. Within the C2 domain, antibody epitope and kinetics are more important than inhibitor titer in predicting pathogenicity in a murine bleeding model. To investigate this within the A2 domain, the pathogenicity of a diverse panel of antihuman fVIII A2 domain monoclonal antibodies (MAbs) was tested in the murine model. MAbs were injected into hemophilia A mice, followed by injection of human B domain-deleted fVIII. Blood loss after a 4-mm tail snip was measured. The following anti-A2 MAbs were tested: high-titer type 1 inhibitors 4A4, 2-76, and 1D4; 2-54, a high-titer type 2 inhibitor; B94, a type 2 inhibitor; and noninhibitory MAbs GMA-012, 4C7, and B25. All high-titer type 1 MAbs produced blood loss that was significantly greater than control mice, whereas all non-inhibitory MAbs produced blood loss that was similar to control. The type 2 MAbs were not pathogenic despite 2-54 having an inhibitor titer of 34 000 BU/mg immunoglobulin G. In addition, a patient with a high-titer type 2 anti-A2 inhibitor who is responsive to fVIII is reported. The discrepancy between inhibitor titer and bleeding phenotype combined with similar findings in the C2 domain stress the importance of inhibitor properties not detected in the standard Bethesda assay in predicting response to fVIII therapy.
Introduction
The immune response to factor VIII (fVIII) currently is the most significant complication in the management of patients with hemophilia A. Approximately 30% of patients with severe hemophilia A develop detectable inhibitory anti-fVIII antibodies. In addition, autoimmune antibodies to fVIII can develop in persons without hemophilia, producing acquired hemophilia A, which frequently produces life- or limb-threatening bleeding.
fVIII contains a domain sequence designated A1-A2-B-ap-A3-C1-C2. It circulates as a heterodimer bound to von Willebrand factor (VWF). During the activation of fVIII by thrombin, the B domain and the light chain activation peptide, ap, are released, and cleavage between the A1 and A2 domains occurs, producing an A1/A2/A3-C1-C2 heterotrimer. The active heterotrimer, fVIIIa, binds to factor IXa (fIXa) in the membrane-bound intrinsic tenase complex (fIXa–fVIIIa).1,2 After thrombin activation, the A2 domain spontaneously dissociates, resulting in a loss of cofactor activity.3 In either congenital or acquired hemophilia A, the majority of inhibitory antibodies are directed at either the 40-kDa A2 or the 18-kDa C2 domains of fVIII.4 FVIII inhibitors can either inhibit fVIII completely or incompletely at saturating concentrations, corresponding to type 1 and type 2 behavior, respectively.5
We have characterized the diversity of a large panel of anti-A2 and anti-C2 monoclonal antibodies (MAbs) that were produced in a murine hemophilia A immunogenicity model.6-8 For the C2 antibodies, 5 groups of structural B-cell epitopes were defined on the basis of patterns of overlapping epitopes. Group A, AB, and B antibodies correspond to classical anti-C2 antibodies that inhibit the binding of fVIII and fVIIIa to phospholipid and VWF. Group BC antibodies are the most frequent and are type 2 inhibitors with inhibitor titers usually greater than 10 000 Bethesda units (BU) per milligram immunoglobulin G (IgG). These antibodies inhibit the activation of fVIII by thrombin and factor Xa in the presence and absence of VWF. Group C antibodies are represented by the well-characterized commercial MAb ESH8, which blocks the release of VWF from fVIII after thrombin activation.9 Nonclassical group BC/C antibodies are present in the plasmas of most human fVIII inhibitor patients.10 Group BC antibodies have inhibitor titers on an equimolar basis that are usually at least 10-fold higher than classical anti-C2 antibodies. However, at saturating concentrations, they produce residual fVIII levels of 20% to 40%. Given the distinct characteristics of the different antibodies, the pathogenicity of anti-C2 antibodies was assessed in a murine in vivo bleeding model. Type 1 and type 2 anti-C2 MAbs were pathogenic in this model. Higher doses of fVIII overcame the pathogenicity of the high-titer type 2 nonclassical antibodies. However, higher doses of fVIII did not overcome the lower-titer classical C2 antibodies or a single high-titer type 1 anti-A2 antibody, 4A4.11 Thus, within the C2 domain, it was shown that epitope specificity and inhibitor kinetics were more important than inhibitor titer in predicting response to high-dose fVIII therapy.
Anti-A2 antibodies were placed into groups A, AB, B, BCD, C, D, DE, and E on the basis of the pattern of overlap on the B-cell epitopes in a competition enzyme-linked immunosorbent assay (ELISA). Most group A MAbs recognize a previously described epitope bounded by Arg484-Ile508 in the N-terminal A2 subdomain, resulting in binding to activated fVIII and noncompetitive inhibition of the intrinsic tenase complex.12 Group B and C MAbs display little or no inhibitory activity. Group D and E MAbs recognize epitopes in the C-terminal A2 subdomain. A subset of group D MAbs inhibited the activation of fVIII by interfering with thrombin-catalyzed cleavage at Arg372 at the A1–A2 domain junction. Other Group D MAbs display indeterminate or no inhibitory activity, despite inhibiting cleavage at Arg740 at the A2–B domain junction. Group E MAbs inhibit fVIII light chain cleavage at Arg1689.8 In this study, we compared the pathogenicity of the various groups of anti-A2 MAbs in a murine in vivo bleeding model.
Materials and methods
Pooled citrated normal plasma (FACT) and fVIII-deficient plasma were obtained from George King Biomedical (Overland Park, KS). Isoflurane (VetOne, Boise, ID) and 0.9% sterile saline (Hospira, Lake Forest, IL) were obtained from the Emory University Department of Animal Resources. All other materials were reagent grade or are described in the cited literature.
Recombinant fVIII
A recombinant B domain deleted (rBDD) human fVIII was expressed from a baby hamster kidney-derived cell line and purified as previously described.13-15 Fractions were analyzed by 1-stage coagulation assay, by absorbance at 280 nm, and by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), as previously described.16 An extinction coefficient at 280 nm of 1.53 (mg/mL)−1⋅cm−1 was used to calculate the concentration of fVIII.17 The absorbance at 280 nm was used to estimate the concentration of fVIII. This concentration was used to calculate the specific activity in a 1-stage coagulation assay.18
Hemophilia A mice
Exon 16-disrupted hemophilia A mice in a C57BL/6 background were originally obtained from Leon Hoyer (American Red Cross, Holland Laboratory), and a breeding colony was established.19,20 In these experiments, 8- to 12-week-old male and female mice were used.
Anti-fVIII monoclonal antibodies from anti-fVIII hybridomas
Murine anti-fVIII A2 domain MAbs were isolated as previously described.6,7 The purity of the IgGs was judged to be greater than 90% by SDS-PAGE. IgG concentrations were calculated using an extinction coefficient at 280 nm of 1.37 (mg/mL)−1⋅cm−1. All inhibitor titers were calculated using the Bethesda assay with buffered pooled normal plasma as the human fVIII source.6,21 In vitro characteristics of all anti-A2 MAbs were previously described and are summarized in Table 1.8 MAbs 4A4 and 2-76 are high-titer type 1 group A inhibitors that block the activation of factor X by the intrinsic pathway factor X activation complex.11 Group E MAb, 1D4, is also a type 1 anti-A2 inhibitor with a titer of 7000 BU/mg IgG. B94 and 2-54 are both type 2 inhibitors that do not completely inhibit fVIII at saturating concentrations. The inhibitor titer for B94 cannot be determined because it does not reduce fVIII activity beyond 50% at saturating concentrations. MAb 2-54 has a high titer of 34 000 BU/mg IgG. GMA-012 and 4C7 are noninhibitory MAbs with titers less than 1 BU/mg IgG.8 B25 is a type 1 very low titer MAb with a titer of 100 BU/mg IgG. As dosed in this study, B25 has a titer of less than 1 BU/mL in plasma, and as such is considered to be noninhibitory.
Table 1.
Characteristics of anti-A2 MAbs
| Name | Domain | Group | Epitope | BU/mg IgG | BU/mL plasma* | Type |
|---|---|---|---|---|---|---|
| 2-76 | A2 | A | Arg484-Ile508 | 38 000 | 190 | I |
| 4A4 | A2 | A | Asp403-His444 | 40 000 | 200 | I |
| B94 | A2 | B | Arg541-Glu604 | Indeterminate† | Indeterminate | II |
| B25 | A2 | C | His444-Gln468 | 100 | <1 | I |
| 2-54 | A2 | D | Glu604-Arg740 | 34 000 | 170 | II |
| GMA-012 | A2 | D | Glu604-Arg740 | <1 | <1 | N/A |
| 4C7 | A2 | E | Unknown | <1 | <1 | N/A |
| 1D4 | A2 | E | Glu604-Arg740 | 7,000 | 35 | I |
Calculated using estimated plasma volume and antibody concentration.
Residual fVIII activity at saturating concentrations of MAb was higher than 50%.
Plasma from patient with hemophilia A and high-titer inhibitor
After approval by the institutional review board and written informed consent from patients, plasma samples were drawn and banked in accordance with Emory institutional review board protocol IRB00006290. All plasmas were heated to 56°C for 1 hour before use to denature fVIII and free antibody from immune complexes.
In vivo bleeding model
The bleeding model was performed as previously described, with changes as follows11: Mice received retro-orbital injections instead of tail vein injections, and both injections were set at a volume of 100 μL. Briefly, hemophilia A mice were weighed, and anesthesia was induced using 3% isoflurane at a flow of 1000 mL/min, using an RC2 Rodent Circuit Controller (VetEquip, Pleasanton, CA). Mice were then placed on a heating pad with 3% isoflurane at a flow rate of 500 mL/min, delivered via nose cone. Mice were injected with 100 μL saline or 0.5 mg/kg MAb diluted in sterile saline. Next, mice were injected with 100 μL of either 180 or 360 U/kg (about 0.02 and 0.04 mg/kg, respectively) human rBDD fVIII in sterile saline. The peak plasma concentration of MAb in vivo was approximately 13-fold higher than the peak concentration of the higher dose of fVIII (65 nM vs peak fVIII concentrations of 2.5 and 5 nM, respectively). Ten minutes before tail transection, mice were anesthetized and tails were warmed. At 120 minutes after fVIII injection, 4 mm of the distal tail was transected using a No. 15 blade scalpel, and the proximal tail was placed into a new, preweighed 15-mL conical weighing tube of normal saline. At the time of tail snip, isoflurane was reduced to 1.75% at a flow rate of 500 mL/min. Tails were removed from the weighing tubes at 40 minutes after tail transection, or at time of death. The amount of blood loss was calculated by measuring the change in tube weight in grams, with evaporative loss added back. This amount was recorded as milligrams of blood loss per gram of body weight.
In vivo recovery of fVIII
Cardiac puncture was performed on some mice instead of tail transection. At 120 minutes after fVIII injection, mice were killed using carbon dioxide, and a cardiac puncture was performed. Between 0.5 and 1.0 mL blood was collected to measure fVIII activity, using the 1-stage coagulation assay or a fVIII antigen ELISA.16 The fVIII activity levels were measured against a standard curve generated by adding rBDD human fVIII to pooled hemophilia mouse plasma at 1 U/mL. Values are reported as the mean of 4 independent measurements. For the ELISA, the anti-A1 domain MAb 2-116 was coated on a Costar half-area plate at a concentration of 6 μg/mL. Mouse plasma samples were serially diluted 2-fold, and 4 different dilutions were tested (1/16, 1/32, 1/64, 1/128). The presence of fVIII was detected using the biotinylated anti-C1 MAb 2A9, followed by alkaline phosphatase-conjugated streptavidin. The standard curve for the ELISA was created using rBDD human fVIII diluted into pooled human fVIII-deficient plasma. Mouse plasma dilutions that fell on the linear portion of the standard curve were used to calculate the concentration of fVIII.
FVIII inhibitor assays
FVIII inhibitor titers for the experiments involving patient plasma were measured using the Bethesda assay21 with the modifications previously described.22 Recombinant BDD human fVIII was added to human fVIII-deficient plasma at 1 and 3 U/mL and used as the source of fVIII activity. One BU per milliliter is defined as the dilution of inhibitor that produces 50% inhibition of fVIII activity. At least 8 MAb concentrations were tested in duplicate, and the resulting inhibition curve was fitted using the 4-parameter logistic equation to estimate the concentration of MAb producing 50% inhibition.
FVIII inhibitor titers were also measured with the Coatest SP FVIII kit (Chromogenix, Lexington, MA). Briefly, 25 µL buffered normal pooled human plasma was incubated for 2 hours at 37°C with 25 µL of MAb diluted in 0.15 M NaCl, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 0.05% (wt/vol) Tween 80 at pH 7.4 (HEPES buffered saline/Tween) or with 25 µL of HEPES buffered saline/Tween as the control. A standard curve based on serial dilutions of pooled normal plasma was used. fVIII levels were measured in triplicate for each sample, using the methods of the Coatest SP fVIII kit.
Effect of MAb 2-54 on thrombin catalyzed proteolytic cleavage of fVIII
rBDD human fVIII (100 nM) was incubated for 10 minutes at 37°C both with and without antibody (0, 500 nM) in a total volume of 100 µL, as previously reported.8 Thrombin was added (0 and 0.5 nM without antibody and 0, 0.5, 1, 2, 5, 7.5, and 10 nM in the presence of antibody), and the incubation continued for an additional 10 minutes. The reaction was stopped with the addition of 10 µL nonreducing SDS-PAGE sample buffer (Pierce 39001). The mixture was vortex-mixed and placed at 98°C for 5 minutes. After mixing and brief spinning, 40 µL was loaded onto a 4% to 15% SDS-PAGE Ready Gel (BioRad 161-1155), and electrophoresis was run at 150 constant volts for 40 minutes. The gels were stained overnight with GelCode Blue (Pierce) and destained with water. Imaging was done with an Odyssey Imaging System (Licor Biosciences). All dilutions were made with 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid/0.15 M NaCl/5 mM CaCl2/0.01% Tween-80 at pH 7.4, 0.2 µm filtered.
Statistics
Comparisons between groups were made using the Mann-Whitney U test and Prism 6.0 (GraphPad Software Inc., La Jolla, CA). A P value of less than .05 was considered statistically significant.
Approval
Approval for the use of animals in this study and approval of study methods was granted by the Emory University Institutional Animal Care and Use Committee. The Emory University School of Medicine Division of Animal Resources provided training for the proper handling and euthanasia of animals.
Results
High-titer type 1 anti-A2 antibodies are pathogenic in a murine bleeding model
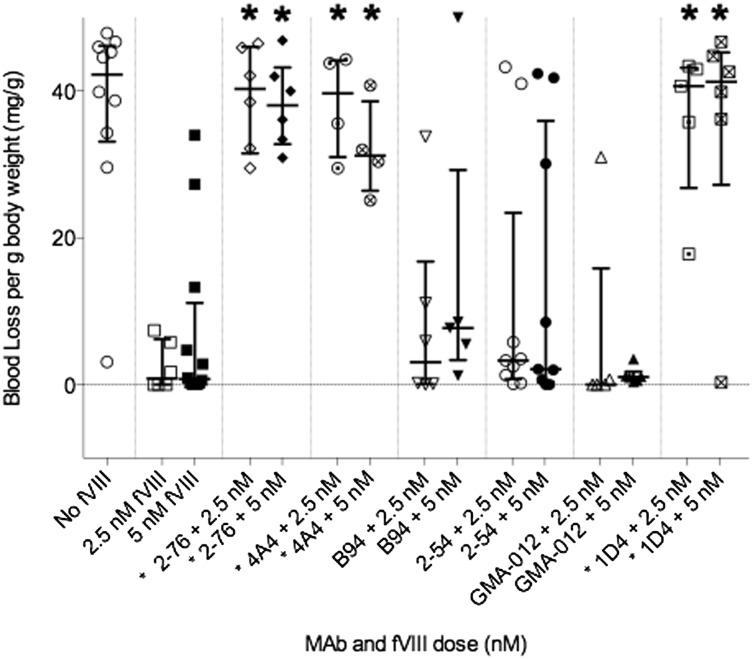
An in vivo bleeding model was established in which blood loss after a 4-mm tail-snip was used to determine the bleeding phenotype in hemophilia A mice injected with BDD human fVIII and various anti-fVIII MAbs. A dose of 180 U/kg fVIII was chosen based on preliminary experiments demonstrating that this dose prevented bleeding in the majority of control mice not receiving antibody (data not shown). In this model, retroorbital injections were used instead of the previously reported intravenous tail vein injections, with similar results in both the positive and negative control animals (data not shown).11 A total of 131 E16 hemophilia A mice aged between 8 and 12 weeks received retroorbital injections with anti-A2 MAb or normal saline, followed 15 minutes later by injection of fVIII or normal saline. A total of 11 mice were excluded from analysis because of missed injections or death resulting from tail snip. There were between 5 and 10 mice in each group. Median blood loss in saline control mice was 42.3 mg/g body weight. In contrast, mice that received no MAb and 180 U/kg fVIII displayed a median blood loss of 0.9 mg/g body weight (P = .001) (Table 2; Figure 1).
Table 2.
Antibody-dependent blood loss in hemophilia A mice treated with low- vs high-dose fVIII
| Antibody | Median blood loss (mg/g of body weight) | Mean blood loss (mg/g of body weight) | ||
|---|---|---|---|---|
| Low dose | High dose | Low dose | High dose | |
| No fVIII control | 42.3 | 37.6 | ||
| No MAb control | 0.9 | 0.8 | 2.5 | 7.0 |
| 2-76 | 40.3* | 38.1* | 39.1 | 38.2 |
| 4A4 | 39.7* | 31.2* | 38.2 | 32.1 |
| B94 | 3.1 | 7.7 | 8.5 | 14.6 |
| B25 | 0.6 | — | 1.4 | — |
| 2-54 | 3.3 | 2.1 | 11.2 | 14.2 |
| GMA-012 | 0.0 | 1.1 | 6.3 | 1.3 |
| 4C7 | 0.0 | — | 0.3 | — |
| 1D4 | 40.7* | 41.3* | 36.1 | 35.1 |
P < .05, MAb vs no MAb control, Mann-Whitney U test.
Figure 1.
Bleeding produced by anti-fVIII MAbs in a murine tail snip model. Hemophilia A mice were injected with 0.5 mg/kg MAb, corresponding to peak plasma concentrations of 65 nM, followed by either 180 or 360 U/kg rBDD fVIII, corresponding to peak plasma concentrations of 2.5 and 5.0 nM, respectively. Medians and interquartile ranges are shown. The asterisk indicates MAbs with significantly more bleeding than control mice treated with fVIII but no MAb. *P < .05, Mann-Whitney U test.
Experimental mice received MAb at saturating concentrations (peak plasma concentration, approximately 65 nM), followed by either 180 U/kg (low dose) or 360 U/kg (high dose) fVIII, corresponding to peak plasma concentrations of approximately 2.5 and 5 nM, respectively. The group A high-titer type 1 inhibitors 4A4 and 2-76 and the group E high-titer type 1 inhibitor 1D4 were pathogenic at low dose fVIII with median blood loss of 39.7, 40.3, and 40.7 mg/g body weight, respectively (P = .010, .002, .004, respectively). These antibodies produced similar levels of bleeding when mice were given the high dose of fVIII (Table 2, Figure 1).
Type 2 anti-A2 inhibitors are not pathogenic in a murine bleeding model
Group D MAb 2-54 and group B MAb B94 are both type 2 inhibitors. They produce maximum inhibition of 80% and 40%, respectively, at saturating concentrations, as measured by 1-stage coagulation assay. B94 produces less than 50% inhibition in this assay, and thus by definition, it cannot be assigned an inhibitor titer. MAb 2-54 has an inhibitor titer of 34 000 BU/mg IgG. Despite the high inhibitor titer, 2-54 did not produce significant bleeding at either low or high doses of fVIII. Mice treated with 2-54 and low-dose fVIII had median blood loss of 3.3 mg/g body weight (P = .23). Mice treated with 2-54 and high-dose fVIII had median blood loss of 2.1 mg/g body weight (P = .37). B94 also did not cause bleeding at either low or high doses of fVIII. Mice injected with B94 and low-dose fVIII had median blood loss of 3.1 mg/g body weight, and those with high-dose fVIII had median blood loss of 7.7 mg/g body weight (P = .38 and .098) (Table 2; Figure 1).
Noninhibitory antibodies are not pathogenic in the murine bleeding model
4C7 and GMA-012 are noninhibitory anti-A2 antibodies with titers less than 1 BU/mg IgG. B25 is a group C low-titer type 1 inhibitor (100 BU/mg IgG) with an estimated peak inhibitor titer lower than 1 BU/mL in plasmas of recipient at the dosage used. Mice treated with 4C7 and low-dose fVIII had a median blood loss of 0.0 mg/g (P = .27). MAb GMA-012 produced a median blood loss of 0.0 mg/g (P = .84) when administered with low-dose fVIII. When administered with high-dose fVIII, median blood loss was 1.1 mg/g (P = .77). Mice treated with B25 and low-dose fVIII had no significant bleeding, with median blood loss of 0.55 mg/g body weight (P = .71). Because B25 and 4C7 produced very little blood loss at low doses of fVIII, they were not tested at high doses of fVIII (Table 2).
Residual fVIII activity at the time of tail snip correlates with bleeding phenotype
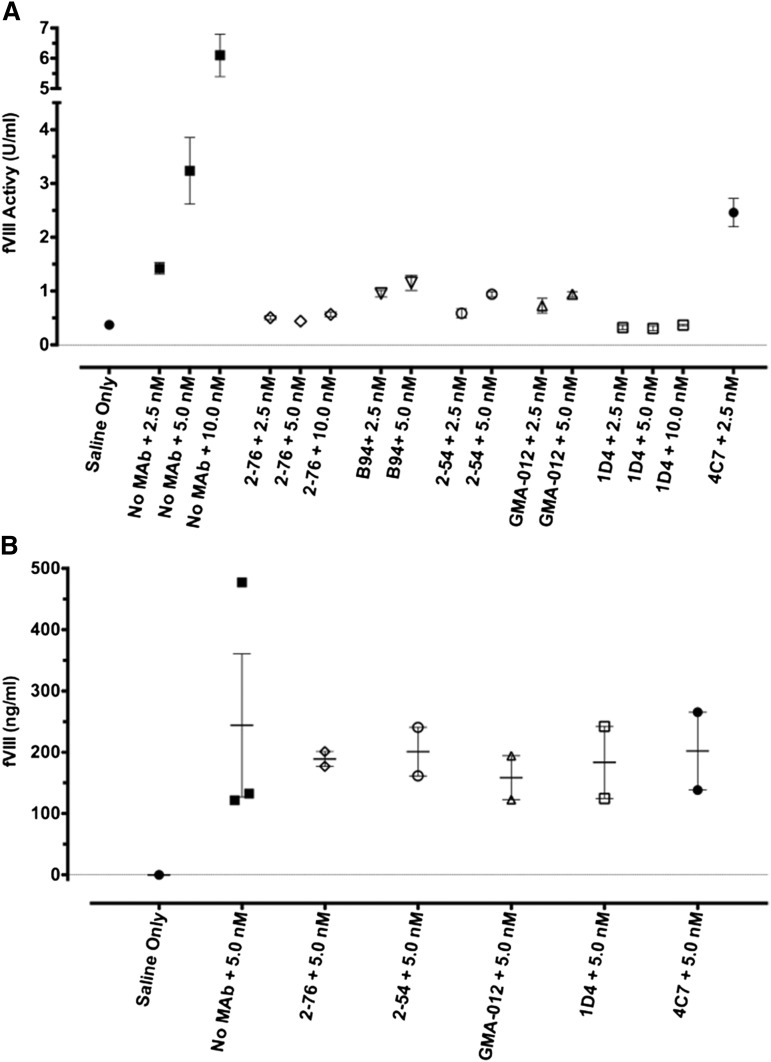
Residual fVIII levels were measured in plasmas obtained from hemophilia A mice injected with concentrations of MAb followed by fVIII at doses ranging from 0 to 720 U/kg, corresponding to peak plasma concentrations of 0-10 nM) (Figure 2). 2-76 and 1D4 completely inhibited fVIII activity throughout the dose range, as 4A4 did in a previous study.11 In contrast, mice treated with 2-54, B94, and GMA-012 all had residual fVIII levels of at least 0.4 U/mL.
Figure 2.
In vivo recovery of fVIII in the presence of saturating concentrations of anti-fVIII MAb. Blood was obtained by cardiac puncture 120 min after injection of rBDD fVIII to the indicated peak plasma concentrations in the presence of saturating plasma concentrations (65 nM) of the indicated MAb. fVIII recovery was determined by 1-stage coagulation assay (A) or fVIII antigen assay (B). Error bars represent SEM (each group n = 2 or 3).
No increased clearance of fVIII in complex with MAbs
To address the role of clearance of fVIII in complex with MAb to the pathogenicity of the MAb, the fVIII antigen level was assessed by ELISA 2 hours after injection of high-dose fVIII for a subset of both pathogenic and nonpathogenic MAbs. Compared with the no-antibody control, 2-76, 2-54, 4C7, 1D4, and GMA-012 did not affect the fVIII antigen level at 2 hours.
Discrepancies between 1-stage and chromogenic fVIII assays for MAb 2-54
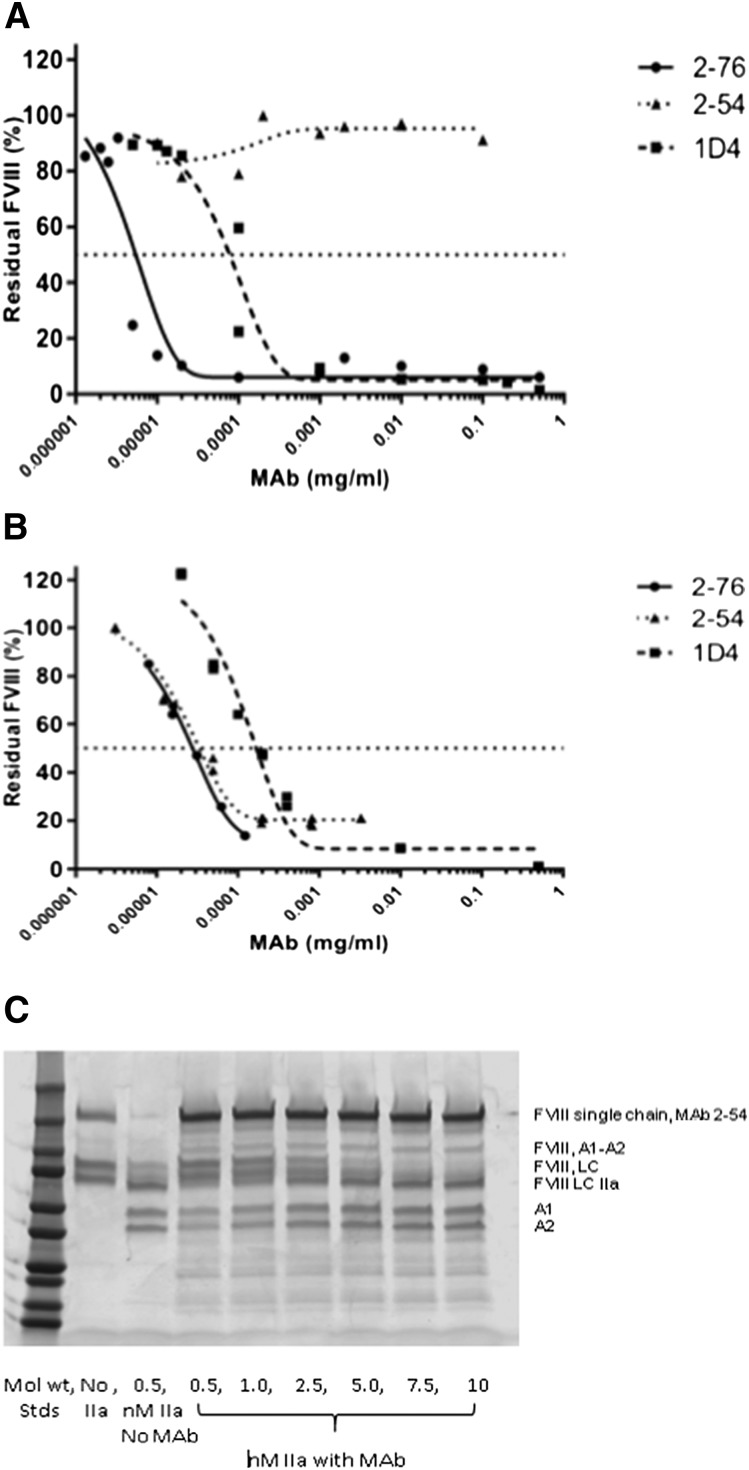
Given the known differences between 1-stage and chromogenic fVIII assays on some patients harboring point mutations in the A2 domain, we measured the inhibitor titer and residual fVIII activity for 2-54 using a chromogenic assay as well as the 1-stage fVIII assay.23-25 In the chromogenic assay, 2-54 produced no significant inhibition of the fVIII activity (Figure 3). In contrast, 2-76 and 1D4 were potent inhibitors in both the chromogenic and 1-stage assays, along with 4A4, which has been previously described.11 Given the higher level of thrombin used to activate fVIII in the chromogenic assay (1 NIH unit thrombin and 1 nM fVIII) and our previous data that 2-54 partially inhibits thrombin cleavage of fVIII at both arginine 372 and arginine 1689, we investigated the effect of higher levels of thrombin on the proteolytic cleavage of fVIII in the presence of 2-54. At thrombin concentrations of at least 2.5 nM (with 100 nM fVIII), the inhibition of fVIII cleavage by thrombin is overcome. Thus, the level of thrombin found in the chromogenic fVIII assay would be predicted to overcome any inhibition of fVIII cleavage by MAb 2-54.
Figure 3.
In vitro inhibition of fVIII by MAbs 2-76, 2-54, and 1D4. fVIII was reconstituted into fVIII-deficient plasma at 1 U/mL, and the inhibition of fVIII by MAbs 2-76, 2-54, and 1D4 was measured by (A) chromogenic assay and (B) 1-stage coagulation assay. The curves represent 4-parameter logistic fits to the data. (C) The effect of high-dose thrombin on the cleavage of fVIII in the presence of MAb 2-54. Standards represent 250, 150, 100, 75, 50, 37, 25, 20, 15, and 10 kD. LC, light chain; LC IIA, thrombin-activated LC.
Hemophilia A patient with a high-titer type 2 anti-A2 inhibitor successfully treated with fVIII
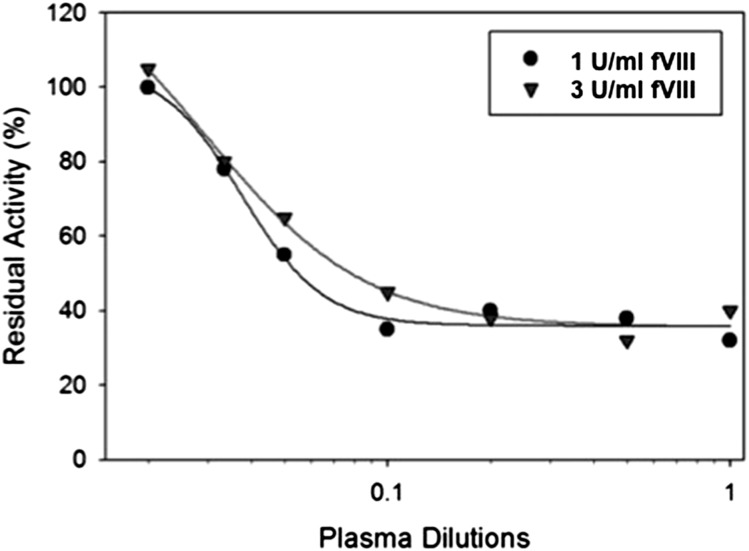
Patient IB001 is a patient with moderate hemophilia A (baseline fVIII activity 1.7%) secondary to a glycine to valine change at position 420 within the A2 domain. He initially developed an inhibitor 20 years before the sample that was evaluated. His inhibitor peaked at a titer of 39 BU/mL. The patient’s endogenous fVIII was unaffected by the presence of the inhibitor, indicating that the inhibitor response to wild-type, exogenous fVIII was not cross-reactive with his endogenous fVIII. At the time of our study, he had a persistent high-titer inhibitor despite a prior attempt at immune tolerance induction with 100 IU/kg fVIII 3 times per week. The patient and his physician reported good clinical bleeding response to fVIII, and he was transitioned from immune tolerance to on-demand therapy with 100 IU/kg. His inhibitor titer on the day of sample collection was 22 BU/mL (Figure 4) with type 2 inhibition and residual activity of ∼35% (0.35 U/mL) at saturating levels of antibody. Increasing the fVIII in the assay to 3 U/mL resulted in a shift of the curve to the right, but a similar residual activity (∼35% or 1.15 U/mL) at saturating amounts of antibody. A limited pharmacokinetic study was done that showed a recovery of 0.37 IU/kg per IU/dL with a normal half-life. The patient was able to undergo a laparoscopic mitral valve repair, using the da Vinci Surgical Robotic System, with good bleeding control with a bolus dose followed by a continuous infusion of fVIII at 4 to 8 IU/kg/h.26
Figure 4.
In vitro inhibition of fVIII by plasma from a patient with congenital hemophilia A and high-titer inhibitor. fVIII was reconstituted into hemophilia A plasma at the indicated concentrations, and the inhibition of fVIII by patient plasma was measured by a 1-stage coagulation assay. The curves represent 4-parameter logistic fits to the data.
Discussion
In this study, we found that within the A2 domain of fVIII, only the high-titer type 1 inhibitors 4A4, 2-76, and 1D4 (35, 190, and 200 BU/mL) were pathogenic in the murine bleeding model, as evidenced by increased bleeding and a failure to respond to infusions of fVIII. As with the type 1 classical C2 inhibitors, these inhibitors were pathogenic even when high doses of fVIII were given. These results were consistent with the residual fVIII activity at time of injury. As expected, the noninhibitory MAbs did not cause bleeding.
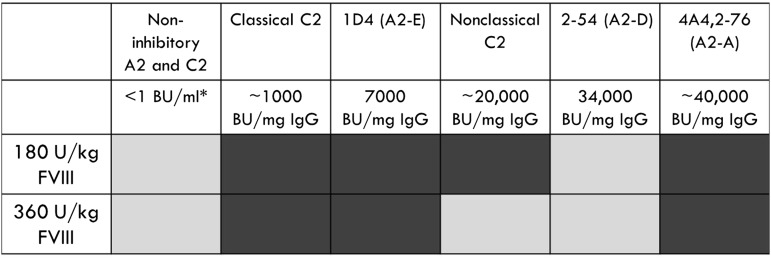
The type 2 anti-A2 MAbs, B94 and 2-54, were not pathogenic in the murine bleeding model. B94 has a residual fVIII level at saturating concentration that is greater than 50%, which results in no definable inhibitor titer in the Bethesda assay. Given that 50% fVIII levels are considered normal, it is not surprising that this MAb does not cause bleeding. In contrast, 2-54 is a type 2 inhibitor with a titer of 34 000 BU/mg IgG and was dosed to 170 BU/mL in our study. It produces maximum inhibition of 80% (residual fVIII activity of 20%) in the Bethesda assay. The lack of pathogenicity of this anti-A2 MAb is in stark contrast to the 3 type 1 anti-A2 MAbs tested, which were pathogenic at similar or lower inhibitor titers (Figure 5).
Figure 5.
Summary of bleeding phenotype produced by a panel of anti-A2 and anti-C2 MAbs. Light gray, no bleeding phenotype; dark gray, bleeding phenotype. *Inhibitor titer at experimental dose of MAb.
Given the potential effect of clearance of fVIII/antibody complexes in the pathogenicity, fVIII antigen levels were measured for 2-76, 1D4, 2-54, 4C7, and GMA-012 2 hours after fVIII infusion, and none had any effect on fVIII antigen levels; thus, the pathogenicity is related to the inhibition of fVIII function. Although we cannot rule out a shortened half-life of fVIII by looking at a single time, the presence of fVIII antigen level similar to control at 2 hours suggests that the half-life of fVIII in the presence of these antibodies is long enough to allow the use of fVIII for treatment in the presence of these MAbs.
The discrepancies seen in this bleeding model between inhibitor titer and bleeding phenotype are also seen in patients with inhibitors. The Bethesda assay, as originally described, is based on the 1-stage coagulation assay and is a sensitive and specific test in the diagnosis of clinically significant fVIII inhibitors.21 Although the Bethesda assay is useful for the detection of clinically significant fVIII inhibitors, the inhibitor titer and the residual fVIII activity in the plasma are not necessarily good predictors of clinical severity.27 Patients with acquired hemophilia A often have type 2 inhibitors, and in a recent observational study, fVIII levels and inhibitor titers at presentation were not predictive of the severity of bleeding events.28 In that study, the median fVIII level and inhibitor titers were nearly identical for patients with fatal bleeding events compared with those who did not require treatment of their bleeding symptoms. In addition, it has also been reported that patients with high-titer inhibitors have had a clinical response to high doses of fVIII, despite being given significantly less fVIII than would be needed to bind all circulating anti-fVIII antibody.29,30 An example of 1 of these patients is IB001, who was described above. This patient’s in vivo recovery of fVIII corresponded to the residual activity produced by his type 2 inhibitor in vitro with a normal half-life. Increasing the dose of fVIII to 100 U/kg resulted in hemostatic levels of fVIII, despite an inhibitor titer of 22 BU/mL. Given his normal half-life, he has been successfully treated with higher doses of fVIII at standard intervals used in noninhibitor patients for more than a decade.
Comparing this study with our previous study of anti-C2 antibodies, not only did the bleeding phenotype between type 1 and type 2 inhibitors differ, but the bleeding phenotype also differed among the type 2 inhibitors based on whether the MAb was directed to the A2 or the C2 domain. Type 2 anti-A2 group D MAb 2-54 has similar inhibitor titer in the 1-stage coagulation assay as the nonclassical C2 MAb 2-77 (25 000 BU/mg IgG). These anti-fVIII MAbs inhibit fVIII by different mechanisms. The anti-A2 MAb, 2-54, inhibits thrombin cleavage of both the heavy chain and light chain of fVIII, whereas the anti-C2 MAb, 2-77, does not inhibit thrombin cleavage of fVIII.6,8,11 In addition, both 2-54 and 2-77 have only minimal inhibition in another in vitro assay, the thrombin generation assay.31 However in the murine bleeding model, 2-54 does not cause bleeding, whereas 2-77 causes bleeding at 180 U/kg fVIII. However, this bleeding phenotype is overcome by giving a higher dose of fVIII. In contrast, classical anti-C2 antibodies are pathogenic at both low and high doses of fVIII, despite having inhibitor titers that are 10- to 20-fold lower than nonclassical C2 antibodies and that are at least 4-fold lower than the lowest pathogenic A2 antibody.
Our results clearly demonstrate that antibody binding to specific epitopes on the A2 and C2 domains can result in type 2 inhibitory activity, in which the inhibitor titers are a poor indicator of bleeding phenotype. Antibody epitope differences may help explain why clinical studies have shown that response to fVIII therapy and bleeding phenotype do not necessarily correlate with the degree of inhibition measured in the Bethesda assay. These discrepancies stress the importance of inhibitor properties that are not measured by current in vitro assays. The differences in mechanism of action of inhibitors to different fVIII epitopes such as nonclassical C2 MAbs and anti-A2 MAb, 2-54, may not be detected in the classic in vitro assays but are important to the in vivo bleeding phenotype. Fine mapping of inhibitor epitopes may help better predict which high titer inhibitor patients will benefit from fVIII therapy.
Acknowledgments
This work was supported by grants from the National Institutes of Health, National Heart, Lung, and Blood Institute (K23HL105785 to C.L.K., U54HL112309 and K08HL102262 to S.L.M.) and Hemophilia of Georgia (S.L.M.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.E. and S.L.M. designed research, performed research, analyzed data, and wrote the manuscript; W.H.B., R.M., E.T.P., and C.C. performed research and analyzed data; and C.L.K. collected and analyzed patient data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shannon L. Meeks, Emory Children's Center, Room 442, 2015 Uppergate Dr, Atlanta, GA 30322; e-mail: shannon.meeks@choa.org.
References
- 1.Fay PJ, Koshibu K. The A2 subunit of factor VIIIa modulates the active site of factor IXa. J Biol Chem. 1998;273(30):19049–19054. doi: 10.1074/jbc.273.30.19049. [DOI] [PubMed] [Google Scholar]
- 2.Jenkins PV, Dill JL, Zhou Q, Fay PJ. Contribution of factor VIIIa A2 and A3-C1-C2 subunits to the affinity for factor IXa in factor Xase. Biochemistry. 2004;43(17):5094–5101. doi: 10.1021/bi036289p. [DOI] [PubMed] [Google Scholar]
- 3.Lollar P, Parker ET. Structural basis for the decreased procoagulant activity of human factor VIII compared to the porcine homolog. J Biol Chem. 1991;266(19):12481–12486. [PubMed] [Google Scholar]
- 4.Prescott R, Nakai H, Saenko EL, et al. Recombinate and Kogenate Study Groups. The inhibitor antibody response is more complex in hemophilia A patients than in most nonhemophiliacs with factor VIII autoantibodies. Blood. 1997;89(10):3663–3671. [PubMed] [Google Scholar]
- 5.Gawryl MS, Hoyer LW. Inactivation of factor VIII coagulant activity by two different types of human antibodies. Blood. 1982;60(5):1103–1109. [PubMed] [Google Scholar]
- 6.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Antihuman factor VIII C2 domain antibodies in hemophilia A mice recognize a functionally complex continuous spectrum of epitopes dominated by inhibitors of factor VIII activation. Blood. 2007;110(13):4234–4242. doi: 10.1182/blood-2007-06-096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Healey JF, Parker ET, Barrow RT, Langley TJ, Church WR, Lollar P. The humoral response to human factor VIII in hemophilia A mice. J Thromb Haemost. 2007;5(3):512–519. doi: 10.1111/j.1538-7836.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- 8.Markovitz RC, Healey JF, Parker ET, Meeks SL, Lollar P. The diversity of the immune response to the A2 domain of human factor VIII. Blood. 2013;121(14):2785–2795. doi: 10.1182/blood-2012-09-456582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saenko EL, Shima M, Gilbert GE, Scandella D. Slowed release of thrombin-cleaved factor VIII from von Willebrand factor by a monoclonal and a human antibody is a novel mechanism for factor VIII inhibition. J Biol Chem. 1996;271(44):27424–27431. doi: 10.1074/jbc.271.44.27424. [DOI] [PubMed] [Google Scholar]
- 10.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Nonclassical anti-C2 domain antibodies are present in patients with factor VIII inhibitors. Blood. 2008;112(4):1151–1153. doi: 10.1182/blood-2008-01-132639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meeks SL, Healey JF, Parker ET, Barrow RT, Lollar P. Non-classical anti-factor VIII C2 domain antibodies are pathogenic in a murine in vivo bleeding model. J Thromb Haemost. 2009;7(4):658–664. doi: 10.1111/j.1538-7836.2009.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Healey JF, Lubin IM, Nakai H, et al. Residues 484-508 contain a major determinant of the inhibitory epitope in the A2 domain of human factor VIII. J Biol Chem. 1995;270(24):14505–14509. doi: 10.1074/jbc.270.24.14505. [DOI] [PubMed] [Google Scholar]
- 13.Barrow RT, Healey JF, Gailani D, Scandella D, Lollar P. Reduction of the antigenicity of factor VIII toward complex inhibitory antibody plasmas using multiply-substituted hybrid human/porcine factor VIII molecules. Blood. 2000;95(2):564–568. [PubMed] [Google Scholar]
- 14.Healey JF, Barrow RT, Tamim HM, et al. Residues Glu2181-Val2243 contain a major determinant of the inhibitory epitope in the C2 domain of human factor VIII. Blood. 1998;92(10):3701–3709. [PubMed] [Google Scholar]
- 15.Lollar P, Fay PJ, Fass DN. Factor VIII and factor VIIIa. Methods Enzymol. 1993;222:128–143. doi: 10.1016/0076-6879(93)22010-d. [DOI] [PubMed] [Google Scholar]
- 16.Doering CB, Healey JF, Parker ET, Barrow RT, Lollar P. High level expression of recombinant porcine coagulation factor VIII. J Biol Chem. 2002;277(41):38345–38349. doi: 10.1074/jbc.M206959200. [DOI] [PubMed] [Google Scholar]
- 17.Lollar P, Parker CG, Tracy RP. Molecular characterization of commercial porcine factor VIII concentrate. Blood. 1988;71(1):137–143. [PubMed] [Google Scholar]
- 18.Pace CN, Vajdos F, Fee L, Grimsley G, Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4(11):2411-2423. [DOI] [PMC free article] [PubMed]
- 19.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10(1):119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 20.Bi L, Sarkar R, Naas T, et al. Further characterization of factor VIII-deficient mice created by gene targeting: RNA and protein studies. Blood. 1996;88(9):3446–3450. [PubMed] [Google Scholar]
- 21.Kasper CK, Aledort L, Aronson D, et al. Proceedings: A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975;34(2):612. [PubMed] [Google Scholar]
- 22.Barrow RT, Lollar P. Neutralization of antifactor VIII inhibitors by recombinant porcine factor VIII. J Thromb Haemost. 2006;4(10):2223–2229. doi: 10.1111/j.1538-7836.2006.02135.x. [DOI] [PubMed] [Google Scholar]
- 23.Cid AR, Calabuig M, Cortina V, et al. One-stage and chromogenic FVIII:C assay discrepancy in mild haemophilia A and the relationship with themutation and bleeding phenotype. Haemophilia. 2008;14(5):1049-1054. [DOI] [PubMed]
- 24.Duncan EM, Duncan BM, Tunbridge LJ, Lloyd JV. Familial discrepancy between the one-stage and two-stage factor VIII methods in a subgroup of patients with haemophilia A. Br J Haematol. 1994;87(4):846–848. doi: 10.1111/j.1365-2141.1994.tb06749.x. [DOI] [PubMed] [Google Scholar]
- 25.Oldenburg J, Pavlova A. Discrepancy between one-stage and chromogenic factor VIII activity assay results can lead to misdiagnosis of haemophilia A phenotype. Hamostaseologie. 2010;30(4):207–211. [PubMed] [Google Scholar]
- 26.Tran DQ, Moss E, Murphy DA, Kempton CL. Mitral valve repair in a Jehovah's witness with haemophilia A with high-titre inhibitor. Haemophilia. 2015;21(6):e523-e525. [DOI] [PubMed]
- 27.Hay CR. Acquired haemophilia. Baillieres Clin Haematol. 1998;11(2):287–303. doi: 10.1016/s0950-3536(98)80049-8. [DOI] [PubMed] [Google Scholar]
- 28.Collins PW, Hirsch S, Baglin TP, et al. UK Haemophilia Centre Doctors’ Organisation. Acquired hemophilia A in the United Kingdom: a 2-year national surveillance study by the United Kingdom Haemophilia Centre Doctors’ Organisation. Blood. 2007;109(5):1870–1877. doi: 10.1182/blood-2006-06-029850. [DOI] [PubMed] [Google Scholar]
- 29.Negrier C. Inhibitors to factor VIII: treatment of acute bleeds. In: Lee CA, Berntorp EE, Hoots WK, eds. Textbook of Hemophilia. Oxford, United Kingdom: Blackwell Publishing Ltd.; 2005:80-85. [Google Scholar]
- 30.Luna-Záizar H, Esparza-Flores MA, López-Guido B, Aguilar-López LB, Cortés Alvarez CR, Jaloma-Cruz AR. Kinetics of factor VIII:C inhibitors and treatment response in severe hemophilia A patients. Int J Lab Hematol. 2009;31(6):673–682. doi: 10.1111/j.1751-553X.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 31.Doshi BS, Gangadharan B, Doering CB, Meeks SL. Potentiation of thrombin generation in hemophilia A plasma by coagulation factor VIII and characterization of antibody-specific inhibition. PLoS One. 2012;7(10):e48172. doi: 10.1371/journal.pone.0048172. [DOI] [PMC free article] [PubMed] [Google Scholar]