Abstract
Spontaneous subarachnoid hemorrhage (SAH) is the most common cerebrovascular disease. The conventional treatment for SAH is usually associated with high mortality. The present study aims to assess the prognosis of microsurgical treatment for patients with poor-grade aneurysm (Hunt and Hess grades IV–V) associated with intracerebral hematoma. A total of 18 consecutive patients who were diagnosed with poor-grade aneurysm accompanied with intracerebral hematoma were retrospectively recruited. All patients underwent microsurgical treatment between April 2010 and June 2013 at The 101st Hospital of Chinese People's Liberation Army (Wuxi, China). Among them, 15 cases underwent microsurgery within 24 h of SAH, and 3 cases underwent microsurgery 24 h following SAH. All 18 cases were examined by computed tomography angiography (CTA). The outcome was assessed during a follow-up time of 6–36 months. According to the Glasgow Outcome Scale, 4 patients experienced a good recovery, 6 were dissatisfied with the outcome, 4 were in vegetative state and 4 succumbed to disease. Poor outcome occurred in patients with an aneurysm diameter >10 mm, exhibited >50 ml volume of intracerebral hematoma or presented cerebral hernia prior to the surgical operation. The outcome of ultra-early surgery (within 24 h of SAH) was improved, compared with that of surgery following 24 h of SAH (P=0.005). Among 7 patients who accepted extraventricular drainage, good outcomes were achieved in 4 of them, whereas dissatisfaction and mortality occurred in 2 and 1 patients, respectively. Therefore, ultra-early microsurgery (within 24 h of SAH) combined with extraventricular drainage may improve the prognosis of patients with poor-grade aneurysm.
Keywords: poor-grade aneurysm, intracerebral hematoma, microsurgery, outcome, ultra-early surgery
Introduction
Spontaneous subarachnoid hemorrhage (SAH) is the most common cerebral vascular disease, and 75% of SAHs are caused by rupture of an intracranial aneurysm (1–4). In patients with poor-grade aneurysm, 20–45% of SAHs are associated with high morbidity and mortality, although a few studies have reported fatality rates of ≤60–90% (1,2,5–8). The conventional approach to treat this condition suggests that surgery should be performed when the patients have recovered from the SAH or when their Hunt and Hess grade has reduced to level ≤III, since early surgery following SAH is considered to present a high risk, as is associated with brain swelling, bleeding and unstable vital signs (4). However, recent studies have observed that early rebleeding occurs mostly within 24 h of SAH, particularly in the first 6–12 h, when the risk of ultra-early rebleeding is highest (9–12). Intracerebral hematoma, rebleeding and severe cerebral vasospasm may lead to serious neurologic deficit in the early time subsequent to SAH (8,9). Therefore, the optimal time to operate and the strategy to select for poor-grade aneurysms remain controversial (9,11). The aim of the present study is to review the surgical management of poor-grade aneurysms by ultra-early microsurgery, and to investigate the prognosis of this strategy.
Materials and methods
Patient population
From April 2010 to June 2013, 18 cases of Hunt and Hess grade IV or V aneurysms accompanied with hematoma were treated by microsurgical clipping at the Department of Neurosurgery of The 101st Hospital of Chinese People's Liberation Army (Wuxi, China). The patients' characteristics are presented in Table I. Of 18 patients, 12 (66.7%) exhibited Hunt and Hess grade IV and 6 (33.3%) presented grade V (13). In total, 10 patients were men and 8 were women, and their mean age was 56.9±15.8 years (range, 31–75 years). Prior to the operation, 8 (44.4%) cases presented a cerebral hernia, the clinical features of which included sudden fall into a coma, acute vomiting and coma following headache for 1 h-3 days with Glasgow Coma Scale of 3–9. All surviving patients were followed-up for 6–36 months.
Table I.
Patients' characteristics.
| Variable | N (%) |
|---|---|
| Total | 18 (100.0) |
| Gender | |
| Male | 10 (55.6) |
| Female | 8 (44.4) |
| Age, years | |
| ≥60 | 8 (44.4) |
| <60 | 10 (55.6) |
| Hunt and Hess grade | |
| IV | 12 (66.7) |
| V | 6 (33.3) |
| Hypertension | |
| Yes | 8 (44.4) |
| No | 10 (55.6) |
| Cerebral hernia prior to operation | |
| Yes | 8 (44.4) |
| No | 10 (55.6) |
| Operation time following SAH | |
| <24 h | 15 (83.3) |
| >24 h | 3 (16.7) |
SAH, spontaneous subarachnoid hemorrhage.
Radiological features
All 18 patients underwent head computed tomography (CT; Lightspeed VCT; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) and CT angiography (CTA; Lightspeed VCT; GE Healthcare Bio-Sciences) prior to surgery. Bleeding was distributed in the anterior cerebral artery in 2 cases, in the middle cerebral artery in 6 cases, in the anterior communicating artery in 7 cases and in the posterior circulation in 3 cases. The location and diameter of the aneurysm, and the volume of intracerebral hematoma are presented in Table II.
Table II.
Radiological features of 18 cases of poor-grade aneurysm.
| Variable | Cases, n |
|---|---|
| Location of aneurysm | |
| Anterior circulation | 2 |
| Anterior communicating artery | 7 |
| Middle cerebral artery | 6 |
| Posterior circulation | 3 |
| Diameter of aneurysm, mm | |
| <5 | 7 |
| 5–10 | 9 |
| >10 | 2 |
| Volume of hematoma, ml | |
| <30 | 9 |
| 30–50 | 5 |
| >50 | 4 |
Procedure prior to surgery
In the emergency room, all patients received trachea intubation to preserve normal oxygen concentration, and ventilation to assist breathing. Intravenous access was established for all patients to ensure sufficient blood supply, while central venous catheter insertions were established in certain shocked patients. After the vital signs were stable, patients received radiographic examinations, including head CT and CTA. Prior to operation, all patients received hemostatic and anti-vasospasm agents such as a nimodipine injection (Bayer AG, Leverkusen, Germany). All patients with cerebral hernia or posttraumatic acute diffuse brain swelling also received 125 ml 20% mannitol (CR Double-Crane Pharmaceutical Co., Ltd., Beijing, China) via bolus intravenous injection to reduce intracranial pressure (ICP). The duration and frequency of the drugs were adjusted according to the seriousness of the disease.
Surgical procedure
In the operation room, all patients received urgent trachea intubation and general anesthesia. A total of 15 patients underwent microsurgery within 24 h of SAH, and 3 patients underwent emergency microsurgery 24 h later, since these patients were hospitalized 24 h after SAH. In total, 13 patients were operated by standard large trauma craniotomy with transsylvian fissure approach, while 2 patients with anterior cerebral artery and anterior interhemispheric hematoma were subjected to interhemispheric approach, and 3 patients with posterior circulation aneurysm were operated by posterior fossa craniotomy. A total of 7 patients received lateral ventricular drainage for 1 week. All 18 patients adopted bone flap decompression to reduce ICP and risk of cerebral infarction following surgery.
Post-surgical procedure
All patients received nimodipine following surgery to reduce cerebral vasospasm and improve blood circulation in the brain. Lumbar puncture was used to release hemorrhagic cerebrospinal fluid (CSF) as early as possible in patients who had not received post-surgery lateral ventricular drainage. All patients in coma were subjected to tracheotomy. Hyperbaric oxygenation and acupuncture were also used in certain patients as a later treatment if their clinical signs were stable. Hydrocephalus cases underwent ventriculoperitoneal shunting.
Statistical analysis and outcome assessment
Data analysis was performed using SPSS version 14.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference. Continuous variables were expressed as range and mean ± standard deviation. Independent samples tests were used for continuous variables. For categorical variables, χ2 test, rank-sum test or Fisher's exact test were used. The surgical curative effects were evaluated post-surgery based on the Glasgow Outcome Scale (GOS) (14): Favorable (grade 4-5), dissatisfied (grade 2-3) and deceased (grade 1). All patients were followed-up for 6–36 months.
The present study was approved by the Ethics Committee of Anhui Medical University (Wuxi, China), and signed informed consent was obtained from all patients.
Results
Clinical outcome
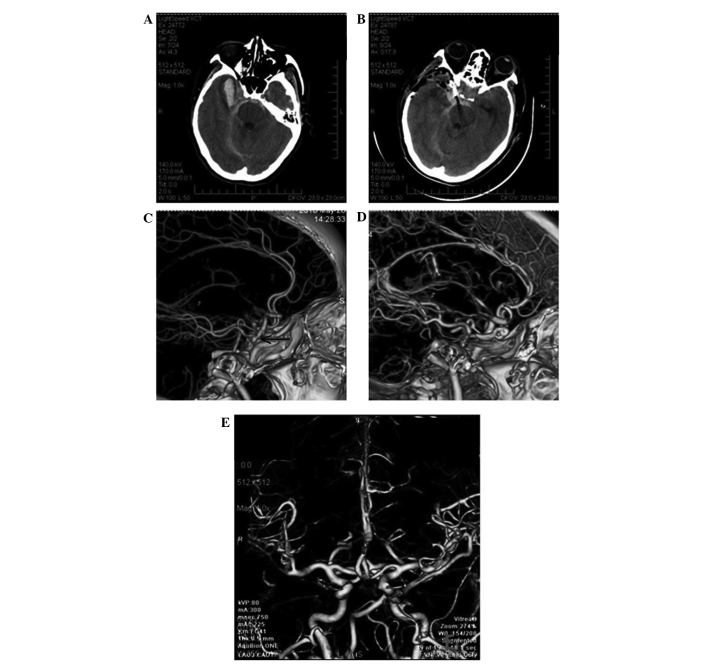
Follow-up CTA or angiography reexamination demonstrated that all aneurysms were completely occluded and none relapsed. All 18 patients received GOS assessment by follow-up for 6–36 months post-surgery. Of these, 4 (22.2%) cases presented favorable outcomes, 10 (55.5%) cases presented dissatisfied outcomes (including 4 cases who were severely disabled and 6 cases who were in a vegetative state), and 4 (22.2%) cases succumbed to the disease. Representative cases appear in Fig. 1. CT imaging prior to surgery demonstrated extensive SAH, particularly in the basal cistern. In addition, intracerebral hematoma was observed in the right temporal frontal lobes (Fig. 1A). Following surgery, the aneurysm was occluded and the hematoma was sufficiently removed (Fig. 1B). The right posterior communicating aneurysms are indicated by a red arrow in Fig. 1C. The immediate post-surgery CTA demonstrated perfect occlusion of the aneurysm (Fig. 1D). The follow-up CTA at 6 months post-surgery revealed no obvious intracranial vascular stenosis and good vascular morphology (Fig. 1E).
Figure 1.
Representative CT and CTA images of patients with poor-grade aneurysm, conducted prior to and subsequent to ultra-early microsurgical operation following spontaneous subarachnoid hemorrhage. (A) CT image prior to operation. (B) CT at follow-up subsequent to operation. (C) CTA prior to surgery. (D) CTA immediately post-surgery. (E) CTA performed 6 months following operation. CT, computed tomography; CTA, CT angiography.
Operation schedule
Patients who underwent surgery within 24 h of SAH (of 15 cases, 7 cases were operated within 6 h of SAH, while 8 cases were operated within 6–24 h) exhibited more favorable outcomes (favorable outcomes were observed in 4 patients), compared with 3 cases who were subjected to surgery after 24 h of SAH (of which, 2 patients were in vegetative state and 1 was deceased).
Vasospasm and cerebral infarction
Local vasospasm was identified by CTA and transcranial Doppler sonography (Pro Focus 2202; BK Medical, Herlev, Denmark) in 5 (27.8%) of 18 patients. These 5 patients received decompressive craniectomy, lumbar puncture and nimodipine therapy without technical complications. However, all of them had poor prognosis, including 3 patients who were dissatisfied (GOS grade 3) and 2 patients who succumbed to disease. Cerebral infarction in different parts of the brain was detected in 7 patients, including 4 cases with cerebral infarction in the basal ganglia region and 3 cases in the brain parenchyma.
Hunt and Hess grade and outcome
In the present study, 4 patients had favorable prognosis among the 12 cases whose Hunt and Hess grade was IV, while 6 patients whose Hunt and Hess grade was V had poor prognoses.
Individual factors and outcome
The prognosis factors of poor-grade aneurysm combined with intracerebral hematoma were the diameter of the aneurysm, volume of the hematoma and cerebral hernia and absence of operation. The association between these factors is indicated in Table III. The present study demonstrated that all patients with an aneurysm diameter >10 mm, intracerebral hematoma volume >50 ml and presence of cerebral hernia prior to operation had bad prognosis.
Table III.
Individual factors and outcome for 18 cases of poor-grade aneurysm.
| Cases, n | ||||
|---|---|---|---|---|
| Variable | Fav | Dis | Dec | P-value |
| Diameter of aneurysm, mm | 0.035 | |||
| <5 | 1 | 4 | 2 | |
| 5–10 | 3 | 6 | 0 | |
| >10 | 0 | 0 | 2 | |
| Volume of hematoma, ml | 0.046 | |||
| <30 | 3 | 5 | 1 | |
| 30–50 | 1 | 4 | 0 | |
| >50 | 0 | 1 | 3 | |
| Cerebral hernia | 0.038 | |||
| Yes | 0 | 5 | 3 | |
| No | 4 | 5 | 1 | |
Fav, favorable; Dis, dissatisfied; Dec, deceased.
Extraventricular drainage and outcome
In the present study, 7 patients underwent extraventricular drainage following operation. Of them, 4 had favorable outcomes, whereas the others had bad prognoses. In addition, the 11 cases who did not accept extraventricular drainage following operation had unfavorable outcomes (P=0.034 vs. extraventricular drainage group; Table IV).
Table IV.
Extraventricular drainage and outcome of patients with poor-grade aneurysm.
| Cases, n (%) | ||||
|---|---|---|---|---|
| Variable | Favorable | Dissatisfied | Deceased | P-value |
| Extraventricular drainage | ||||
| Yes | 4 (57.1%) | 2 (28.6%) | 1 (14.3%) | 0.034 |
| No | 0 (0.0%) | 8 (72.7%) | 3 (27.3%) | – |
Discussion
Conventionally, conservative treatment has been initially employed for poor-grade aneurysms, followed by operation when patients recovered or their Hunt and Hess grade reduced to level ≤III (4). However, the majority of patients would succumb to rebleeding and cerebral vasospasm prior to surgery (1–3). Recent studies have suggested that ultra-early microsurgery within 24 h of SAH may improve the prognosis of patients with poor-grade aneurysm, since it may prevent rebleeding and may delay the release of toxic substances during cerebral vasospasm, thus reducing damage to the brain (15–18).
It was previously considered that early surgery or delayed surgery was meaningless to improve the prognosis of patients with poor-grade aneurysm following SAH (4). To date, the selection of treatment time in patients with Hunt and Hess grades IV and V remains controversial (19,20). Certain authors disagree with the use of ultra-early surgery due to the following reasons: i) Difficulty of ultra-early diagnosis; ii) the bleeding of the ruptured aneurysm may be not stopped, as time is limited; iii) the aneurysm may rupture easily during the operation; iv) temporary occlusion of the parent artery during microsurgery may lead to cerebral infarction; and iv) surgery on the exposed aneurysm and parent artery is difficult and has a high risk associated (4). By contrast, other authors agree with the use of ultra-early surgery, due to the following benefits: i) Ultra-early surgery may reduce the risk of rebleeding and the occurrence of cerebral vasospasm; ii) ultra-early surgery may clip the aneurysm and also remove the intracerebral hematoma, which may lead to injury of the brain tissue; and iii) ultra-early decompressive craniectomy may improve cerebral hernia by decreasing ICP (9,15,16,18). In the present study, 15 of 18 cases who underwent ultra-early surgery had favorable outcomes, while 3 cases who underwent surgery after 24 h of SAH had poor prognosis. Therefore, ultra-early microsurgery may improve the prognosis of patients with poor-grade aneurysm and intracerebral hematoma.
The management of patients with poor-grade aneurysm combined with intracerebral hematoma remains controversial (21–23), due to the cerebral vasospasm and intracerebral hematoma space-occupying effect as a consequence of elevated ICP (21–23). Therefore, it is difficult to solve the problem by simple microsurgical clipping and endovascular coiling. Certain authors prefer to use endovascular coiling, since it is considered to present several advantages, including less trauma and shorter preoperative preparation or operative time when compared to general surgery (17). However, this procedure is generally ineffective or even aggravating for removing the intracranial hematoma and resolving the vasospasm (24,25). Prat and Galeano (26) reported 12 cases of mild cerebral artery aneurysm with intracerebral hematoma who demonstrated good outcomes following early surgery. Sasaki et al (27) also observed that early surgery clipped the aneurysm and removed the subarachnoid hematocele and intracerebral hematoma. In addition, for the 26 patients who accepted conservative treatment, 20 succumbed to disease and 6 suffered severe disabilities (28). These findings were significant (P<0.01), compared with the ultra-early surgery group (28). Whether poor-grade aneurysms combined with intracerebral hematoma are more suitable for ultra-early surgery remains to be determined. Ultra-early surgery may achieve four major goals: i) To remove the hematoma, thus relieving the space-occupying effect and reducing the secondary injury of perihematomal tissues; ii) intraoperative continuous irrigation of papaverine or nimodipine may relieve cerebral vasospasm; iii) decompressive craniectomy may reduce the ICP and release more space to aid patients during the period of edema; and iv) it may reduce the risks of delayed cerebral vasospasm (15–17). In the present study, of the 18 patients that accepted early surgical treatment, 4 (22.2%) cases had favorable outcomes, 11 (61.1%) cases had dissatisfied outcomes, and 3 (16.7%) cases were deceased during a follow-up of 1–2 years.
In total, 13 patients were operated by standard large trauma craniotomy transsylvian fissure approach, 2 patients with anterior cerebral artery were operated with interhemispheric approach and 3 patients with posterior circulation aneurysms were operated with posterior fossa craniotomy in the present study. During surgery, it is important to fully remove the sphenoid ridge upon removing the bone flap to facilitate the vision of the operation (26). It is possible to identify the majority of aneurysms from the sylvian fissure, while a small number of anterior communicating artery aneurysms or distal anterior cerebral artery aneurysms may be identified from longitudinal observation (26). The major difficulties of poor-grade aneurysm combined with intracerebral hematoma are acute diffuse brain swelling, high ICP, intraoperative rupture of the aneurysm and hematoma surrounding the blood vessels, which results in a complicated local anatomic structure (27). Mannitol may be used as a dehydration therapy to partially remove the CSF and reduce the ICP, but the hematoma should not be completely removed prior to the occlusion of the aneurysm, since otherwise the aneurysm would easily rupture during the operation. Prior to clipping the aneurysm, the parent artery must be fully exposed to enable adaptation to the temporary arterial occlusion (23). However, temporary arterial occlusion must last <15 min, since otherwise it may lead to ischemia and anoxia of the brain tissue. The hematoma should be extensively removed once the aneurysm is occluded (18). Meanwhile, continuous irrigation of papaverine or nimodipine intraoperative aids to relieve the cerebral vasospasm (29).
Extraventricular drainage has been widely used for treating poor-grade aneurysm, particularly when it is combined with ventricle hematocele or obstruction of the fourth ventricle (15). Occasionally, patients who have received conservative treatments or delayed operation treatments may adopt extraventricular drainage at the earliest opportunity, in order to control acute ICP and improve the outcome (30). In the present study, a high risk of rebleeding was observed when patients underwent extraventricular drainage prior to occlusion of the aneurysm. Of 7 patients with poor-grade aneurysms that received extraventricular drainage, 4 patients succumbed to rebleeding within 48 h. These findings are in agreement with those from previous studies on extraventricular drainage prior to operation (15,16). In the present study, the effect of extraventricular drainage following the occlusion of the aneurysm was evaluated. Of the 18 patients enrolled in the study, 7 underwent this treatment, and good recovery occurred in 4 (57.1%) patients, while severe disabilities occurred in 2 (28.6%) patients and vegetative state occurred in 1 (14.3%) patient. No rebleeding occurred subsequent to the procedure. The prognosis of patients treated with extraventricular drainage following the occlusion of the aneurysm was improved, compared with those who did not undergo this treatment (P<0.05).
The mortality and disability rates for patients with poor-grade aneurysms remain high, particularly for those that are accompanied by hematoma (18,23,26). The diameter of the aneurysm, the volume of the hematoma and the presence of cerebral hernia or absence of previous operation are important prognosis factors for these patients (4). Early diagnosis, followed by ultra-early surgery or emergency surgery to clip the aneurysm and remove the intracerebral hematoma, contribute to a good prognosis. Furthermore, the outcome improves if surgery is combined with extraventricular drainage and decompressive craniectomy (4).
Although the current study is not a randomized controlled trial, the present preliminary results suggest a favorable outcome for patients with poor-grade aneurysm subjected to ultra-early surgery following SAH, and constitute the basis for future studies. In conclusion, ultra-early surgery or emergency surgery may be beneficial for patients with severe SAH.
References
- 1.Rosengart AJ, Schultheiss KE, Tolentino J, Macdonald RL. Prognostic factors for outcome in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2315–2321. doi: 10.1161/STROKEAHA.107.484360. [DOI] [PubMed] [Google Scholar]
- 2.Komotar RJ, Schmidt JM, Starke RM, Claassen J, Wartenberg KE, Lee K, Badjatia N, Connolly ES, Jr, Mayer SA. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009;64:397–411. doi: 10.1227/01.NEU.0000338946.42939.C7. [DOI] [PubMed] [Google Scholar]
- 3.Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European Stroke Organization: European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. 2013;35:93–112. doi: 10.1159/000346087. [DOI] [PubMed] [Google Scholar]
- 4.Yasargil MG, Feng L. Microneurosurgical. 1st. Vol. 3. Beijing Science and Technology Press; Beijing: 2005. [Google Scholar]
- 5.Longstreth WT, Jr, Nelson LM, Koepsell TD, van Belle G. Clinical course of spontaneous subarachnoid hemorrhage: A population-based study in King County, Washington. Neurology. 1993;43:712–718. doi: 10.1212/WNL.43.4.712. [DOI] [PubMed] [Google Scholar]
- 6.Nowak G, Schwachenwald R, Arnold H. Early management in poor grade aneurysm patients. Acta Neurochir (Wien) 1994;126:33–37. doi: 10.1007/BF01476491. [DOI] [PubMed] [Google Scholar]
- 7.Rordorf G, Ogilvy CS, Gress DR, Crowell RM, Choi IS. Patients in poor neurological condition after subarachnoid hemorrhage: Early management and long-term outcome. Acta Neurochir (Wien) 1997;139:1143–1151. doi: 10.1007/BF01410974. [DOI] [PubMed] [Google Scholar]
- 8.Le Roux PD, Elliott JP, Newell DW, Grady MS, Winn HR. Predicting outcome in poor-grade patients with subarachnoid hemorrhage: A retrospective review of 159 aggressively managed cases. J Neurosurg. 1996;85:39–49. doi: 10.3171/jns.1996.85.1.0039. [DOI] [PubMed] [Google Scholar]
- 9.Jartti P, Isokangas JM, Karttunen A, Jartti A, Haapea M, Koskelainen T, Tervonen O. Early rebleeding after coiling of ruptured intracranial aneurysms. Acta Radiol. 2010;51:1043–1049. doi: 10.3109/02841851.2010.508172. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson PJ, Power DM, Tripathi P, Kirkpatrick PJ. Outcome from poor grade aneurysmal subarachnoid haemorrhage - which poor grade subarachnoid haemorrhage patients benefit from aneurysm clipping? Br J Neurosurg. 2000;14:105–109. doi: 10.1080/02688690050004516. [DOI] [PubMed] [Google Scholar]
- 11.van den Berg R, Rinkel GJ, Vandertop WP. Treatment of ruptured intracranial aneurysms: Implications of the ISAT on clipping versus coiling. Eur J Radiol. 2003;46:172–177. doi: 10.1016/S0720-048X(03)00089-5. [DOI] [PubMed] [Google Scholar]
- 12.Laidlaw JD, Siu KH. Ultra-early surgery for aneurysmal subarachnoid hemorrhage: Outcomes for a consecutive series of 391 patients not selected by grade or age. J Neurosurg. 2002;97:250–258. doi: 10.3171/jns.2002.97.2.0250. [DOI] [PubMed] [Google Scholar]
- 13.Botterell EH, Cannell DE. Subarachnoid hemorrhage and pregnancy. Am J Obstet Gynecol. 1956;72:844–855. doi: 10.1016/0002-9378(56)90176-4. [DOI] [PubMed] [Google Scholar]
- 14.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/S0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 15.Hosoda K, Fujita S, Kawaguchi T, Shose Y, Hamano S, Iwakura M. Effect of clot removal and surgical manipulation on regional cerebral blood flow and delayed vasospasm in early aneurysm surgery for subarachnoid hemorrhage. Surg Neurol. 1999;51:81–88. doi: 10.1016/S0090-3019(97)00508-9. [DOI] [PubMed] [Google Scholar]
- 16.Fujii Y, Takeuchi S, Sasaki O, Minakawa T, Koike T, Tanaka R. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. J Neurosurg. 1996;84:35–42. doi: 10.3171/jns.1996.84.1.0035. [DOI] [PubMed] [Google Scholar]
- 17.Laidlaw JD, Siu KH. Poor-grade aneurysmal subarachnoid hemorrhage: Outcome after treatment with urgent surgery. Neurosurgery. 2003;53:1275–1282. doi: 10.1227/01.NEU.0000093199.74960.FF. [DOI] [PubMed] [Google Scholar]
- 18.Wong GK, Boet R, Ng SC, Chan M, Gin T, Zee B, Poon WS. Ultra-early (within 24 hours) aneurysm treatment after subarachnoid hemorrhage. World Neurosurg. 2012;77:311–315. doi: 10.1016/j.wneu.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 19.Haug T, Sorteberg A, Finset A, Lindegaard KF, Lundar T, Sorteberg W. Cognitive functioning and health-related quality of life 1 year after aneurysmal subarachnoid hemorrhage in preoperative comatose patients (Hunt and Hess grade V patients) Neurosurgery. 2010;66:475–485. doi: 10.1227/01.NEU.0000365364.87303.AC. [DOI] [PubMed] [Google Scholar]
- 20.Nieuwkamp DJ, de Gans K, Algra A, Albrecht KW, Boomstra S, Brouwers PJ, Groen RJ, Metzemaekers JD, Nijssen PC, Roos YB, et al. Timing of aneurysm surgery in subarachnoid haemorrhage - an observational study in The Netherlands. Acta Neurochir (Wien) 2005;147:815–821. doi: 10.1007/s00701-005-0536-0. [DOI] [PubMed] [Google Scholar]
- 21.Natarajan SK, Sekhar LN, Ghodke B, Britz GW, Bhagawati D, Temkin N. Outcomes of ruptured intracranial aneurysms treated by microsurgical clipping and endovascular coiling in a high-volume center. AJNR Am J Neuroradiol. 2008;29:753–759. doi: 10.3174/ajnr.A0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, Rischmiller J. ISAT Collaborators: Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): Long-term follow-up. Lancet Neurol. 2009;8:427–433. doi: 10.1016/S1474-4422(09)70080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohashi Y, Horikoshi T, Sugita M, Yagishita T, Nukui H. Size of cerebral aneurysms and related factors in patients with subarachnoid hemorrhage. Surg Neurol. 2004;61:239–247. doi: 10.1016/S0090-3019(03)00427-0. [DOI] [PubMed] [Google Scholar]
- 24.Murayama Y, Malisch T, Guglielmi G, Mawad ME, Viñuela F, Duckwiler GR, Gobin YP, Klucznick RP, Martin NA, Frazee J. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms: Report on 69 cases. J Neurosurg. 1997;87:830–835. doi: 10.3171/jns.1997.87.6.0830. [DOI] [PubMed] [Google Scholar]
- 25.Abbed KM, Ogilvy CS. Intracerebral hematoma from aneurysm rupture. Neurosurg Focus. 2003;15:E4. doi: 10.3171/foc.2003.15.4.4. [DOI] [PubMed] [Google Scholar]
- 26.Prat R, Galeano I. Early surgical treatment of middle cerebral artery aneurysms associated with intracerebral haematoma. Clin Neurol Neurosurg. 2007;109:431–435. doi: 10.1016/j.clineuro.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki T, Sato M, Oinuma M, Sakuma J, Suzuki K, Matsumoto M, Kodama N. Management of poor-grade patients with aneurysmal subarachnoid hemorrhage in the acute stage: Importance of close monitoring for neurological grade changes. Surg Neurol. 2004;62:531–537. doi: 10.1016/j.surneu.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 28.Chen JH, Wang YH, Yang LK, Shi ZH, Cai S, Zhou JX, Feng Y. Analysis the prognosis factors of microsurgical treatment for poor-grade aneurysms associated with hematoma. Zhong Hua Shen Jing Wai Ke Za Zhi. 2015;31:158–160. (In Chinese) [Google Scholar]
- 29.Yin YH, Wang F, Pan YH, Wang Y, Wang Y, Luo QZ, Jiang JY. Effect of dose-response of topical administration of nimodipine on cerebral vasospasm following subarachnoid hemmorrhage in rabbits. Am J Med Sci. 2009;337:123–125. doi: 10.1097/MAJ.0b013e31817d1ca1. [DOI] [PubMed] [Google Scholar]
- 30.Shirao S, Yoneda H, Kunitsugu I, Ishihara H, Koizumi H, Suehiro E, Nomura S, Kato S, Fujisawa H, Suzuki M. Preoperation prediction of outcome in 283 poor-grade patients with subarachnoid hemorrhage: A project of the Chugoku-Shikoku Division of the Japan Neurosurgical Society. Cerebrovasc Dis. 2010;30:105–113. doi: 10.1159/000314713. [DOI] [PubMed] [Google Scholar]