Abstract
We have developed and implemented a noninvasive, objective neurofunctional assessment for evaluating the sustained effects of traumatic brain injury (TBI) in piglets with both diffuse and focal injury types. Derived from commercial actigraphy methods in humans, this assessment continuously monitors the day/night activity of piglets using close-fitting jackets equipped with tri-axial accelerometers to monitor movements of the thorax. Acceleration metrics were correlated (N = 7 naïve piglets) with video images to define values associated with a range of activities, from recumbancy (rest) to running. Both focal (N = 8) and diffuse brain injury (N = 9) produced alterations in activity that were significant 4 days post-TBI. Compared to shams (N = 6) who acclimated to the animal facility 4 days after an anesthesia experience by blurring the distinction between day and night activity, post-TBI time-matched animals had larger fractions of inactive periods during the daytime than nighttime, and larger fractions of active time in the night were spent in high activity (e.g., constant walking, intermittent running) than during the day. These persistent disturbances in rest and activity are similar to those observed in human adults and children post-TBI, establishing actigraphy as a translational metric, used in both humans and large animals, for assessment of injury severity, progressions, and intervention.
Keywords: : behavior, pediatric, sleep, symptoms, TBI
Introduction
Concussion and other mild traumatic brain injuries (TBIs) typically produce signs and symptoms spanning the physical, cognitive, emotional, and sleep domains.1 Sleep disturbances include sleeping more or less than usual, trouble falling asleep, and daytime drowsiness, and are typically assessed by administration of a multi-modal survey instrument.2–4 The reference standard for evaluating sleep disturbances in humans is polysomnography (PSG) with a multi-channel electroencephalography system,5 typically performed in a sleep center or, increasingly, at home. More recently, to provide a familiar sleep setting, as well as long-term day and night monitoring, researchers have used actigraphy measurements made by wearable accelerometers that reduce data collection cost and do not restrict patient movement. The patient wears the accelerometer on his or her wrist, ankle, or head, and the recorded data are then interpreted to determine features such as the patient's sleep onset time, sleep interval durations, total sleep time, and consistency of sleep/disturbance patterns. Many commercially available products, such as FitBit and Jawbone, use accelerometers in a similar way to allow people to track their own day- and nighttime activity.6 Several commercial algorithms have been developed that translate human acceleration data into useful activity-level and wake-rest information after verification with PSG data.7,8
Recent patient studies have used accelerometer data from traumatic brain injury (TBI) patients to document persistent sleep disturbances days and years after mild-to-moderate TBI, with longer 24-h sleep durations, increased nighttime sleep interruptions, and more daytime drowsiness.9–14 Although animal activity is typically captured from video analysis, there is an emerging science of acceleration-based actigraphy measurements for animal (rats, nonhuman primates, and marmots)15 species, but none for pigs, a large animal used as a model for studying neurological diseases.16,17 Given the value of large animal models in understanding the mechanisms and responses to TBI for the development of interventions and therapies,17 and the similarity of the pig to humans,16,18 we have adapted this technology and its analysis metrics for use in piglets with TBI.
Methods
Female bred-for-research farm piglets (N = 30) were delivered to the university at age 21–25 days and pair-housed during a 3- to 7-day acclimation and quarantine period. After acclimation, a tri-axial accelerometer (model X6-1A; Gulf Coast Data Concepts, LLC, Waveland, MS) was zip-tied to a close-fitting pet harness or jacket (Lomir Biomedical Inc., Notre-Dame-de-l'Île-Perrot, Quebec, Canada) and positioned directly above and parallel to the mid-thoracic spine of the piglets. Piglets wore the harness or jacket for the remainder of the study. The back position is ideal for measuring normal range of piglet movement at its center of gravity, while preventing device disturbance/removal by limb, hock, or mouth contact. Because the piglets had a tendency to chew on one another's harness or jacket, the piglets were housed for the remainder of the study in separate, adjacent cages, within site, sound, and smell of one another. Each accelerometer required one AA battery. Battery life was variable, typically lasting 3–4 days. To prevent inadvertent data loss, batteries were replaced every 2–3 days.
Four groups of piglets were studied, as described later in this section: naïve piglets (N = 7) to develop the actigraphy thresholds; piglets before and after focal brain injury by controlled cortical impact (CCI) with a rigid indenter (N = 8); piglets before and after diffuse brain injury from a rapid nonimpact head rotation (RNR; N = 9); and sham piglets before and after anesthesia experience matched to the TBI piglets (N = 6). All animal study protocols were approved by the University of Pennsylvania Institutional Care and Use Committee (Philadelphia, PA).
Resultant acceleration data were obtained continuously (25 Hz), stored on the reusable USB memory of the sensor, uploaded to computer, and segmented into dark (7 pm to 7 am, defined as nighttime, NT) and light (from 4 pm to 7 pm, defined as daytime, DT) periods maintained in the animal husbandry unit. Although the housing unit was light from 7 am to 7 pm, we defined our daytime period to a reliable 4 pm to 7 pm portion of the lights-on epoch when animals were consistently in their cages after the end of the husbandry staff shift, to minimize human interaction and distraction. Animals were not sleep-deprived during the study. Animals were fed normally and could move freely, and were allowed to stay awake or fall asleep at will.
Development of porcine actigraphy algorithm
The Actigraph Data Analysis Software (ADAS) algorithm19 was developed for differentiating periods of sleep from wake by correlating human PSG with actigraphy data measured on the wrist. We adapted the human ADAS algorithm to fit the piglet actigraphy data measured on the torso using limited data available for sleeping patterns in pigs20,21 and other mammals.22 Briefly, acceleration time history is segmented into minute-long epochs and the human ADAS algorithm differentiates epochs as sleep or wake based on a threshold-crossing approach. If the number of times the magnitude of the acceleration exceeds 0.1 g is 10 or fewer “crossings” in a minute-long epoch, that epoch is defined as “sleep.” If more than 10 crossings occur in the epoch, the epoch is categorized as a minute of “wake.” To account for wake-interval post-arousal and false positives from wrist movements during sleep, if fewer than four “wake” epochs occur consecutively, then these “wake” epochs are recategorized as minutes of “sleep.”
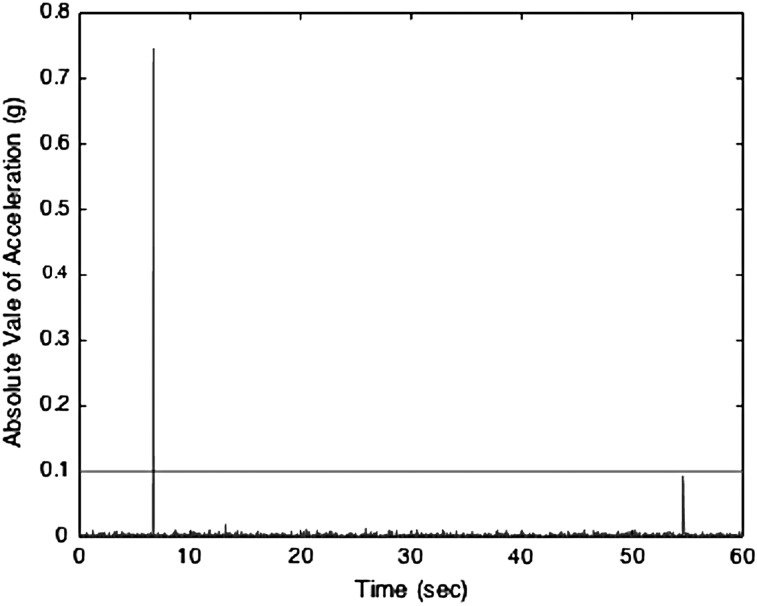
We recorded actigraphic acceleration data as described above from naïve piglets (N = 7) over a 24-h period after acclimation to the husbandry unit. After uploading to the computer, the absolute value of the resultant acceleration was determined, and 1 g was subtracted to account for acceleration resulting from gravity. The data were segmented into minute-long epochs, and the number of 0.1-g crossings in each minute were determined. We found that the human acceleration threshold of 0.1 g was low enough to capture torso movements, yet high enough to exclude any noise or chest wall movement resulting from respiration (Fig. 1).
FIG. 1.
Representative 1-min epoch of actigraphy acceleration (g) in a naïve piglet. A 1-min-long epoch is shown and the horizontal line shows the threshold of 0.1 g. With only one crossing in this minute-long epoch, this epoch is defined as inactive.
To determine how many threshold crossings/min would distinguish an inactive (or rest) epoch from an active epoch, the inactive efficiency (percent time inactive) per 24-h period for the naïve piglets was calculated for a variety of crossing/min limits. The human ADAS algorithm was applied to the porcine data such that the “inactive” threshold was ≤10 crossings. If 3 or fewer consecutive “active” minutes occurred, they were reclassified as “inactive” minutes. This method overestimated the inactive efficiency for the pig. This overestimation is likely attributable to the more deliberate manner of torso movement compared to wrist movement, resulting in fewer false positives of “active” and necessitating a more sensitive threshold to define “active.” Reducing the threshold for an inactive or rest epoch as a minute with ≤3 threshold crossings and eliminating the requirement for any number of consecutive active periods resulted in an average inactive efficiency that most closely approximated Robert and Dallaire's finding that naïve 4-week-old piglets sleep approximately 55% of a 24-h period.21
Importantly, in the absence of PSG data, the piglet actigraphy algorithm cannot identify sleep; rather, the algorithm distinguishes between activity and inactivity. The piglet threshold for inactivity was validated, and three levels of activity (low, medium, and high) were defined by aligning 30-min long video segments of the behavior of two piglets wearing actigraphs with their time-synced acceleration data. During epochs classified as inactive by the algorithm (≤ 3 crossings over 0.1 g per minute), the piglet indeed appeared to be at rest—lying down, with few, if any, slight movements to shift position. Also, the analysis revealed that the piglet appeared active during epochs classified as active by the algorithm (>3 crossings over 0.1 g/min). This analysis led to the following definitions: inactive epoch (≤ 3 crossings/min); low-activity epoch (4–9 crossings/min); moderate-activity epoch (10–99 crossings/min); and high-activity epoch (≥ 100 crossings/min). Low activity was characterized by recumbancy with some movement (e.g., chewing on a toy) or standing stationary (no walking) for a full minute. Moderate activity was characterized by intermittent walking and standing with only brief, if any, intervals of lying down. High activity was defined as constant walking with possibly intermittent running during the epoch. In summary, for the piglet actigraphy algorithm, the data were segmented into minute-long epochs and marked as inactive, low activity, moderate activity, or high activity based on the number of times the absolute value of the acceleration crossed a threshold of 0.1 g.
Actigraphy study design
Female 4-week-old piglets were housed and acclimated as described above and designated to receive a focal CCI TBI (N = 8), diffuse white matter TBI by a rapid head rotation in the sagittal direction (N = 9; mean velocity and standard deviation [SD] of 124.9 ± 1.75 rad/s), or served as an anesthetized sham (N = 6). Importantly, a “piglet week” is developmentally compared to a “human child month.”23 Each pig wore an instrumented harness for 1–2 days before injury or anesthesia to determine a baseline activity and then actigraphy was measured for 4–6 days post-injury to account for sustained effects of brain injury. Levels of activity were determined by interpreting data collected from accelerometers strapped to their backs. After sacrifice, 8 days post-TBI, brains (N = 8 CCI, N = 6 RNR) were fixed, sectioned into 3-mm-thick coronal blocks, and 6-μm slices from each section were stained with beta-amyloid precursor protein (β-APP) and hemoxylin and eosin (H&E) for traumatic injury. Brains from other subjects were harvested for fresh tissue studies. Briefly, the neuropathologist on our team (C.S., blinded to experimental group) examined every 6-μm-thick slice from each 3-mm coronal section for gross pathology and documented any hemorrhages, established infarcts (changes in staining intensity), and ischemic neurons (cell shrinkage and eosinophilia) from the H&E slides, and traumatic and ischemic axonal injury from the β-APP slides. Distribution of axonal and neuronal injury were annotated on the digital photographs for each animal, and regions of white matter damage, infarct, and ischemia were traced in each slice and normalized by slice area. Lesion volume (Vinj) was defined as total injured area (axonal injury plus infarct/ischemic lesions) divided by total slice area. All details regarding anesthesia, injury methods, and immunohistochemistry quantification have been described previously.17,24
The nighttime (NT, 7 pm to 7 am) and daytime (DT, 4 pm to 7 pm) patterns of active and inactive periods obtained 1 day pre-injury or anesthesia (day −1) and on day 4 post-TBI in focal and diffuse brain-injured piglets were compared to those of time-matched sham piglets experiencing only the anesthesia portion of the protocol. After a full set of data were collected for a piglet, the number of 0.1-g crossings on day −1 and day 4 were determined for every minute epoch, and any epoch with ≤3 crossings was designated as “inactive,” and epochs with more crossings were designated as “active,” with those active minutes with ≥100 crossings additionally defined as “high activity.” Because of some battery failures, and a variety of pre-injury days of measurement, these days were selected for analysis to include the most animals in each study group.
The most commonly derived metrics from human actigraphy data11 were evaluated or adapted to capture daytime drowsiness and nighttime active disruptions: “settle” onset time (SOT) after lights-out in the animal facility (NT, 7 pm to 7 am), defined as the number of minutes until the first of three consecutive minute-long inactive epochs; the density or number of active periods per hour in the DT and in the NT after SOT; average duration of consecutive active periods in DT and NT after SOT; the inactive efficiency, defined as the percent of NT after SOT or DT spent in inactive; and the fraction of high-activity epochs in DT and NT normalized to total number of active epochs for that day and time. Downloading the data from USB flashdrive to computer, processing and application of the actigraphy algorithm, calculating clinically relevant activity parameters, generating plots displaying results, and exporting the calculated parameters to a user-friendly platform (Microsoft Excel, Redmond, WA) were operationalized and partially automated by using a PigFit, our lab-designed Matlab application.
Statistical analysis
The data from all 23 animals on day −1 was pooled to create a single robust pre-injury or pre-anesthesia experience baseline. All metrics are presented in box and whiskers format, with a horizontal line within the box at the median value, and the 75th and 25th percentile responses represented by the upper and lower dimensions of the box. The ends of the whiskers are the most extreme values, and filled symbols depict outlier values. Box plots were examined qualitatively for increase or decrease in metric variability (box size). Difference between NT and DT values (NT-DT) for each metric were evaluated, and the means and 95% confidence intervals (CIs) for those differences were determined. Positive metric difference (NT-DT) values represent instances where nighttime magnitude was greater than daytime, whereas negative differences represent larger daytime values. Significant NT-DT differences were defined when CIs did not include zero (and indicated with an asterisk in box plots and Table 1). To examine variation across groups, CIs of NT-DT differences were compared (and indicated with a dagger in Table 1). To examine whether NT and DT values differed significantly within a day, a quantitative Wilcoxon's rank-sum test was performed for four groups: the consolidated group on day −1 and for each of the three groups separately on day +4, with significance defined as p ≤ 0.05 (and indicated with a horizontal line in the box plots).
Table 1.
Night- and Daytime Differences in Actigraphy Metrics
| Metric | Day | Group | N | Mean value ± SD (95% CI) |
|---|---|---|---|---|
| Inactive efficiency (NT-DT), unitless fraction | −1 | All* | 23 | 0.126 ± 0.0317 (0.0605, 0.192) |
| +4 | Sham | 6 | −0.055 ± 0.0717 (−0.239, 0.1295) | |
| +4 | CCI*,† | 8 | −0.18 ± 0.0736 (−0.354, −0.0057) | |
| +4 | RNR | 9 | −0.00541 ± 0.0476 (−0.115, 0.104) | |
| Fraction high activity per active time (NT-DT) | −1 | All | 23 | −0.0481 ± 0.0383 (−0.127, 0.0313) |
| +4 | Sham | 6 | −0.0199 ± 0.0847 (−0.238, 0.198) | |
| +4 | CCI | 8 | −0.1 ± 0.1078 (−0.355, 0.155) | |
| +4 | RNR* | 9 | 0.1058 ± 0.0409 (0.0115, 0.2) | |
| Mean active interval length (NT-DT), min | −1 | All* | 23 | −3.472 ± 0.75 (−5.03, −1.92) |
| +4 | Sham | 6 | −0.905 ± 1.343 (−4.36, 2.55) | |
| +4 | CCI | 8 | 0.992 ± 1.269 (−2.01, 3.99) | |
| +4 | RNR | 9 | 0.267 ± 0.964 (−1.96, 2.49) | |
| Density of active intervals (NT-DT), no./h | −1 | All | 23 | 0.775 ± 0.484 (−0.228, 1.779) |
| +4 | Sham | 6 | 0.958 ± 1.223 (−2.186, 4.103) | |
| +4 | CCI | 8 | 0.698 ± 0.911 (−1.455, 2.851) | |
| +4 | RNR | 9 | −0.444 ± 0.794 (−2.275, 1.386) | |
| Settle onset time (NT only), min | −1 | All* | 23 | 12.74 ± 4.23 (3.97, 21.51) |
| +4 | Sham | 6 | 8.83 ± 6.06 (−6.74, 24.4) | |
| +4 | CCI | 8 | 22.38 ± 16.46 (−16.56, 61.31) | |
| +4 | RNR | 9 | 19.33 ± 10.44 (−4.73, 43.4) |
All values are mean ± SD with 95% CI in parentheses.
NT-DT difference is significantly different from 0. †Value on day +4 is significantly different from overall group on day −1. Negative values indicate larger daytime magnitudes than nighttime.
NT, nighttime; DT, daytime; RNR, rapid nonimpact head rotation; CCI, controlled cortical impact; SD, standard deviation; CI, confidence interval.
Results
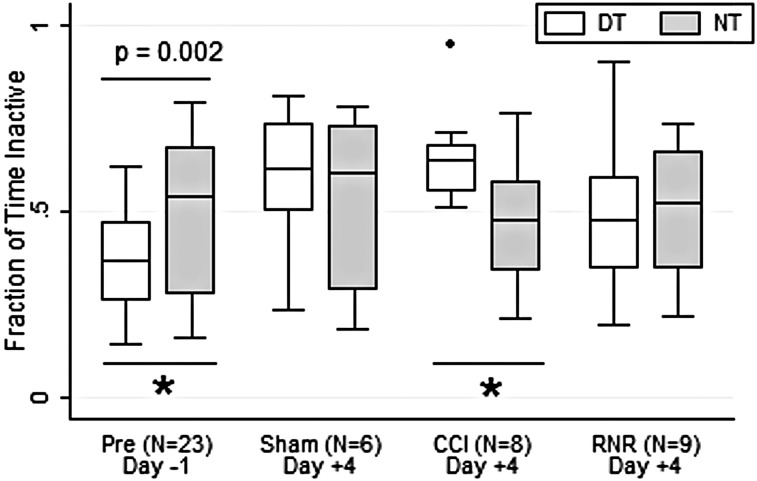
The inactive efficiency, or fraction of time spent inactive (Fig. 2), was significantly larger in NT than DT on Day −1, before injury or anesthesia, and after acclimation to the animal facility. Interestingly, the normal diurnal pattern was extinguished by day +4 in shams and the RNR group. In contrast, the CCI group had a significant pattern reversal on day +4 from the consolidated group on day −1. By day +4, the CCI group spent a significantly larger and more consistent fraction of time inactive in DT, which is a pattern consistent with daytime lethargy after focal TBI.
FIG. 2.
Fraction inactive time. Daytime (white) and nighttime (gray) variation differs significantly before anesthesia (day −1) and in the CCI group on day +4 (see text for details). DT, daytime; NT, nighttime; CCI, controlled cortical injury; RNR, rapid nonimpact head rotation.
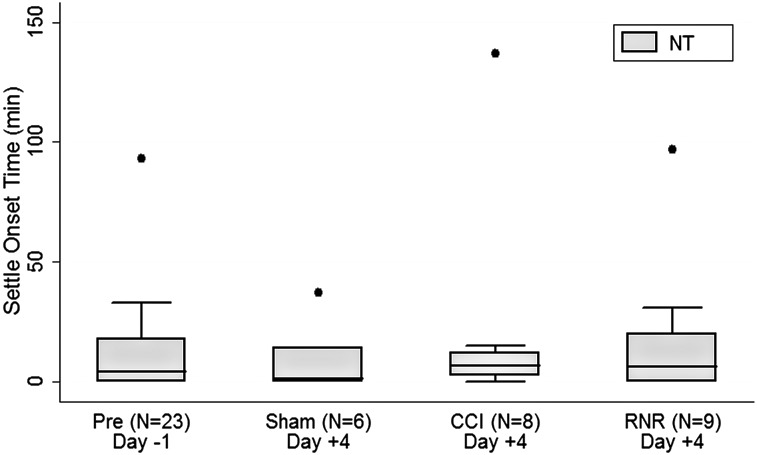
On day −1, piglets needed a significant period of time to settle down and have three inactive epochs in a row after the lights were turned off in the facility. Perhaps evidence of accomodation to their environment, by day +4, the SOTs (Fig. 3) for all groups were no longer significantly different from zero. Sham piglets settled on day +4 most consistently (smallest whisker expanse), even more consistent than the consolidated group on day −1, perhaps reflecting an increasing comfort with their surroundings.
FIG. 3.
Nighttime settle onset time. On day −1, settle onset time was positive. In all groups on day +4, settle onset time did not differ from zero. NT, nighttime; CCI, controlled cortical injury; RNR, rapid nonimpact head rotation.
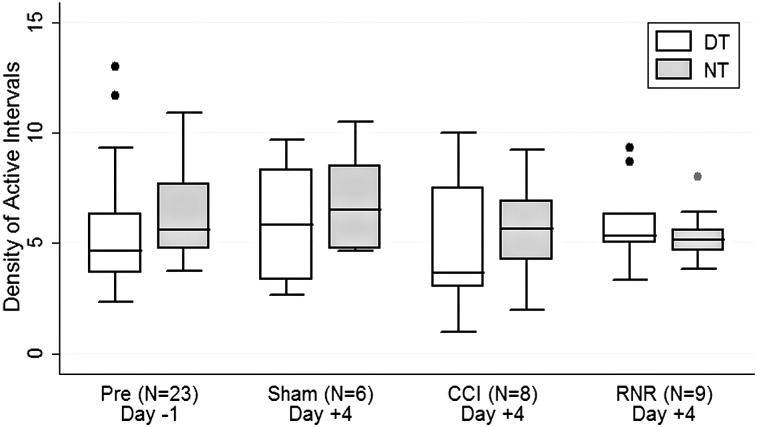
The density of active intervals (Fig. 4) did not differ between day or night on any day for any group studied. The NT-DT difference CI was smallest post-RNR, with consistently smaller variability (smaller whisker span), indicating the development of a pattern that blurred distinctions between day and night. In general, the denisty of active periods became less variable in all groups during the day on day +4 and more variable at nighttime post-CCI.
FIG. 4.
Density of active intervals. Density of active intervals did not differ between daytime or nighttime or across groups or days of study. DT, daytime; NT, nighttime; CCI, controlled cortical injury; RNR, rapid nonimpact head rotation.
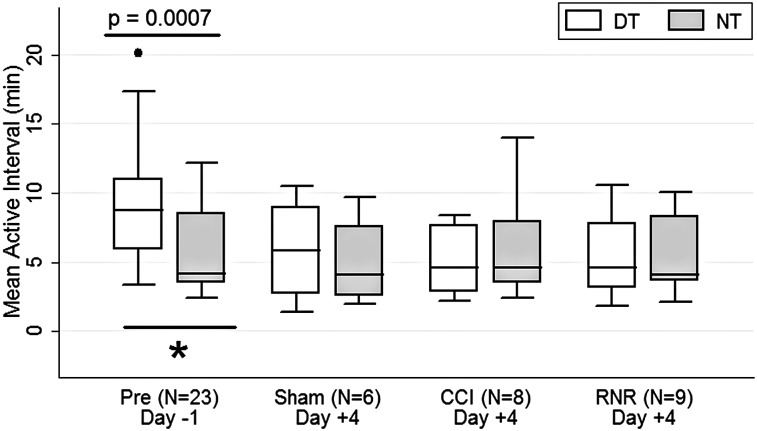
As a consequence of the diurnal pattern established on day −1 when there was a larger fraction of inactivity during the night than day (Fig. 2), but with a similar density of active periods during night and day (Fig. 4), the mean length of consecutive active periods (Fig. 5) on day −1 was significantly longer during the daytime than nighttime. On day +4, this distinction between day- and nighttime was eliminated, and this difference from pre-anesthesia pattern nearly reached signficance for the RNR group, for which the NT-DT CI barely overlaps with that of the consolidated group on day −1.
FIG. 5.
Active interval length. On day −1, active interval length was significantly longer in the daytime than nighttime (see text for details). DT, daytime; NT, nighttime; CCI, controlled cortical injury; RNR, rapid nonimpact head rotation.
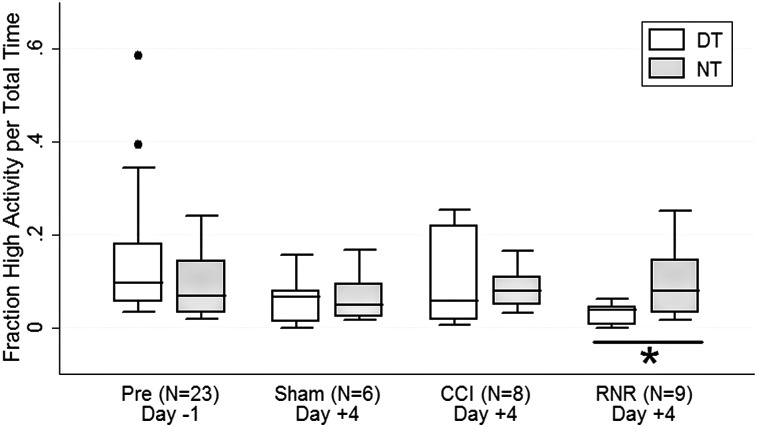
Interestingly, the fraction of active epochs spent engaged in high-level activity (e.g., walking continuously or running) was similar between night and day on day −1 (Fig. 6). On day +4, CCI piglets demonstrated a dramatic increase in daytime high-activity levels, and the NT-DT difference CI was large. The fraction of high activity in the daytime was consistently lowest in the RNR group, resulting in a significantly larger fraction of high activity occurring during nighttime hours rather than daytime, consistent with a nighttime restlessness after diffuse TBI.
FIG. 6.
Fraction of time spent at high-activity levels. The fraction of time spent engaged in high level activities (running, walking continuously) did not differ between day and night on day −1. On day +4, only the RNR group had significant differences, with a larger fraction of active intervals in the night in high-level activities than in the daytime. DT, daytime; NT, nighttime; CCI, controlled cortical injury; RNR, rapid nonimpact head rotation.
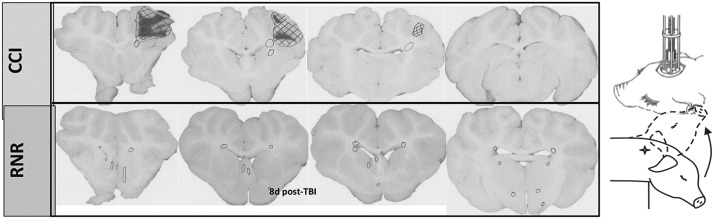
Average lesion volume (Vinj) was 0.36% ± 0.08% in the RNR group and 3.5% ± 1.4% in the CCI group, normalized to total cerebrum volume, and no animals had adverse clinical outcomes. Selected representative images reveal persistent widespread white matter injury at 8 days post-RNR (circles, Fig. 7), with a similar anatomic pattern, albeit with smaller lesion volume than we have published previously in this age group for the same head rotational velocity and direction within hours and 1 day post-TBI.17,24 The focal brain injury included large regions of infarct, characterized by ischemic changes on H&E (hatched regions in Fig. 7), with persistent hemorrhagic regions (dark areas) and axonal injury documented on β-APP-positive regions in penumbral areas (circles, Fig. 7). Lesion volume at 8 days post-CCI was similar in size to our previously published values at 1 day post-CCI.17
FIG. 7.
Schematic of two head injury models. Focal injuries produced by controlled cortical impact (top) and diffuse brain injuries produced by a sagittal rapid nonimpact rotation (RNR) of the head (bottom). Representative coronal sections shown with regions of axonal injury indicated with circles, and infarct/ischemic/hemorrhagic lesions with hatched marks. CCI, controlled cortical impact; d, day; TBI, traumatic brain injury.
Discussion
In naïve 4-week-old piglets acclimated to the animal facility, piglets take time to settle after the lights are turned out, and they spend a larger fraction of the nighttime in quiet rest than in the daytime, with shorter active intervals at night that occur at the same frequency as the daytime and contain a similar proportion of high-level activities. Taken together, these data suggest that naïve piglets fluctuate between longer intervals of inactivity and shorter intervals of activity frequently during the night, and they fluctuate between longer active bouts and short inactive periods during the day.
By day +4, sham animals consistently settled down quickly, and any distinction between day- and nighttime activity and rest levels disappeared. In domestic pigs of a similar age housed in environments with enrichment materials, piglets became less active and spent the majority of the observed time period inactive.25,26 Whereas many of these same actigraphy metric transitions were observed in the two injury groups as in the sham animals, TBI was associated with several significant disruptive behaviors in activity. Specifically, the injured groups spent a larger proportion of their time inactive during the day than the night (CCI) and a larger portion of their activity was high-level movement during the night than the day, consistent with a portrait in the brain-injured piglet of prolonged inactivity during the day and disrupted nighttime rest.
Our findings are similar to patient studies documenting sleep and activity disturbance accelerometer data in assessments post-TBI,27–30 where differences between sleep patterns in control and patient groups have been found in both the acute and long-term stages of recovery from mild-to-moderate TBI. Common trends across most studies have shown that injured patients generally sleep 2 h more over the 24-h period (hypersomnia), but their sleep is less efficient and has more awakenings ≥5 min, and they report more daytime sleepiness.27 Although typically used to monitor active and inactive periods, actigraphy has been found to be a sensitive and accurate monitor of sleep, however with poor specificity compared to traditional PSG methods31; therefore, caution should be used in inferring sleep patterns from actigraphic data. Further, typical sensor location is on the wrist, using watch-like devices on the nondominant limb; if motor complications are present post-TBI, alternative sensor placement should be considered and new acceleration thresholds would need to be developed to distinguish active and inactive periods.
Although actigraphy has the advantage of being an integrative outcome metric already in use in humans of all ages that requires no animal acclimation or task training and presents minimal disruption to the piglet daily routine, our study has several limitations deserving mention. First, during the development of our activity acceleration thresholds, inactive periods were visually defined as periods when piglets were lying down, typically supine, and making little movement other than position adjustment; all movements beyond that were identified as active periods. These low acceleration levels designated as inactive are not further discriminated into quiet alert periods or various levels of sleep. Future work may include PSG measurements. Second, our actigraphy measurements were captured for only 4 days after injury, and for relatively mild TBIs. Future studies should investigate longer intervals post-TBI and more severe deficits. Third, our small group sizes were not powered to perform statistical analyses of subtle changes across groups on day +4. Finally, our activity crossing threshold developed for 4-week-old pigs and values of crossing associated with high levels of activity are specific to the location of the sensor (on the back, secured with a harness) and should be validated before use in pigs of other ages or in other sensor locations. Regardless, this small study revealed significant changes in daytime activity and nighttime inactive patterns 4 days after mild focal and diffuse TBI.
In summary, we have developed and implemented a noninvasive, objective neurofunctional assessment for evaluating the sustained effects of TBI in piglets with both diffuse and focal injury types. Derived from commercial actigraphy methods in humans, this assessment continuously monitors the day/night activity of piglets using close-fitting jackets equipped with tri-axial accelerometers to monitor movements of the thorax. In naïve piglets, acceleration metrics were correlated with video images to define values associated with a range of activities, from recumbence (inactive) to running. Both focal and diffuse brain injury produced alterations in activity that were significant 4 days post-TBI. Compared with shams (who acclimated to the animal facility 4 days after an anesthesia experience by blurring the distinction between day and night activity), time-matched animals with TBI had larger fractions of inactive periods during the day than nighttime, and had larger fractions of active time spent in high activity (e.g., constant walking, intermittent running) during the night than during the day. These persistent disturbances in rest and activity are similar to those observed in human adults and children post-TBI, establishing actigraphy as a translational metric, used in both humans and large animals, for the assessment of injury severity, progressions, and intervention.
Acknowledgments
The authors are grateful to Melissa Byro and Kortne Hudick for their technical assistance and animal handling, and to Carole Marcus and Lisa Meltzer for discussions about metrics and methods for assessing sleep irregularities in children. The study was supported by the NIH grants U01NS069545 and R01NS39679, the CHOP Critical Care Endowment Fund, and the Stephenson Chair Fund.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Institute of Medicine (IOM) and National Research Council (NRC). (2014). Sports-Related Concussions in Youth: Improving the Science, Changing the Culture. National Academies Press: Washington, DC: [PubMed] [Google Scholar]
- 2.Eonta S.E., Carr W., McArdle J.J., Kain J.M., Tate C., Wesensten N.J., Norris J.N., Balkin T.J., and Kamimori G.H. (2011). Automated neuropsychological assessment metrics: repeated assessment with two military samples. Aviat. Space Environ. Med. 82, 34–39 [DOI] [PubMed] [Google Scholar]
- 3.Gioia G.A., Schneider J.C., Vaughan C.G., and Isquith P.K. (2009). Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? Br. J. Sports Med. 43, Suppl. 1, i13–i22 [DOI] [PubMed] [Google Scholar]
- 4.Lovell M.R., and Collins M.W. (1998). Neuropsychological assessment of the college football player. J. Head Trauma Rehabil. 13, 9–26 [DOI] [PubMed] [Google Scholar]
- 5.Rosen C.L., Auckley D., Benca R., Foldvary-Schaefer N., Iber C., Kapur V., Rueschman R., Zee P., and Redline S. (2012). A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep 35, 757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hekler E.B., Buman M.P., Grieco L., Rosenberger M., Winter S.J., Haskell W., and King A.C. (2015). Validation of physical activity tracking via android smartphones compared to ActiGraph accelerometer: laboratory-based and free-living validation studies. JMIR mHealth uHealth 3, e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ancoli-Israel S., Cole R., Alessi C., Chambers M., Moorcroft W., and Pollak C.P. (2003). The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26, 342–392 [DOI] [PubMed] [Google Scholar]
- 8.Meltzer L.J., Walsh C.M., Traylor J., and Westin A.M. (2012). Direct comparison of two new actigraphs and polysomnography in children and adolescents. Sleep 35, 159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zollman F.S., Cyborski C., and Duraski S.A. (2010). Actigraphy for assessment of sleep in traumatic brain injury: case series, review of the literature and proposed criteria for use. Brain Inj. 24, 748–754 [DOI] [PubMed] [Google Scholar]
- 10.Kempf J., Werth E., Kaiser P.R., Bassetti C.L., and Baumann C.R. (2010). Sleep-wake disturbances 3 years after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 81, 1402–1405 [DOI] [PubMed] [Google Scholar]
- 11.Kaufman Y., Tzischinsky O., Epstein R., Etzioni A., Lavie P., and Pillar G. (2001). Long-term sleep disturbances in adolescents after minor head injury. Pediatr. Neurol. 24, 129–134 [DOI] [PubMed] [Google Scholar]
- 12.Chiu H.Y., Chen P.Y., Chen N.H., Chuang L.P., and Tsai P.S. (2013). Trajectories of sleep changes during the acute phase of traumatic brain injury: a 7-day actigraphy study. J. Formos. Med. Assoc. 112, 545–553 [DOI] [PubMed] [Google Scholar]
- 13.Chen P.Y., Tsai P.S., Chen N.H., Chaung L.P., Lee C.C., Chen C.C., Chiu H.T., Lu Y.J., Wei K.C., and Chiu H.Y. (2015). Trajectories of sleep and its predictors in the first year following traumatic brain injury. J. Head Trauma Rehabil. 30, E50–E55 [DOI] [PubMed] [Google Scholar]
- 14.Baumann C.R., Werth E., Stocker R., Ludwig S., and Bassetti C.L. (2007). Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain 130, 1873–1883 [DOI] [PubMed] [Google Scholar]
- 15.Gonçalves B.S., Cavalcanti P.R., Tavares G.R., Campos T.F., and Araujo J.F. (2014). Nonparametric methods in actigraphy: an update. Sleep Sci. 7, 158–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lind N.M., Moustgaard A., Jelsing J., Vajta G., Cumming P., and Hansen A.K. (2007). The use of pigs in neuroscience: modeling brain disorders. Neurosci. Biobehav. Rev. 31, 728–751 [DOI] [PubMed] [Google Scholar]
- 17.Margulies S.S., Kilbaugh T., Sullivan S., Smith C., Propert K., Byro M., Saliga K., Costine B.A., and Duhaime A.C. (2015). Establishing a clinically relevant large animal model platform for TBI therapy development: using cyclosporin A as a case study. Brain Pathol. 25, 289–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornum B.R., and Knudsen G.M. (2011). Cognitive testing of pigs (Sus scrofa) in translational biobehavioral research. Neurosci. Biobehav. Rev. 35, 437–451 [DOI] [PubMed] [Google Scholar]
- 19.Jean-Louis G., von Gizycki H., Zizi F., Fookson J., Spielman A., Nunes J., Fullilove R., and Taub H. (1996). Determination of sleep and wakefulness with the Actigraph Data Analysis Software (ADAS). Sleep 19, 739–743 [PubMed] [Google Scholar]
- 20.Kuipers M., and Whatson T.S. (1979). Sleep in piglets: an observational study. Appl. Anim. Ethol. 5, 145–151 [Google Scholar]
- 21.Robert S., and Dallaire A. (1986). Polygraphic analysis of the sleep-wake states and the REM sleep periodicity in domesticated pigs (Sus scrofa). Physiol. Behav. 37, 289–293 [DOI] [PubMed] [Google Scholar]
- 22.Tobler I. (1995). Is sleep fundamentally different between mammalian species? Behav. Brain Res. 69, 35–41 [DOI] [PubMed] [Google Scholar]
- 23.Dickerson J., and Dobbing J. (1967). Prenatal and postnatal growth and development of the central nervous system of the pig. Proc. Roy. Soc. Lond. B Biol. Sci. 166, 384–395 [DOI] [PubMed] [Google Scholar]
- 24.Weeks D., Sullivan S., Kilbaugh T., Smith C., and Margulies S.S. (2014). Influences of developmental age on the resolution of diffuse traumatic intracranial hemorrhage and axonal injury. J. Neurotrauma 31, 206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillmann E., von Hollen F., Bunger B., Todt D., and Schrader L. (2003). Farrowing conditions affect the reactions of piglets towards novel environment and social confrontation at weaning. Appl. Anim. Behav. Sci. 81, 99–109 [Google Scholar]
- 26.Van de Weerd H.A., Docking C.M., Day J.E.L., Avery P.J., and Edwards S.A. (2003). A systematic approach towards developing environmental enrichment for pigs. Appl. Anim. Behav. Sci. 84, 101–118 [Google Scholar]
- 27.Baumann C.R., Werth E., Stocker R., Ludwig S., and Bassetti C.L. (2007). Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study. Brain 130, 1873–1883 [DOI] [PubMed] [Google Scholar]
- 28.Chiu H.Y., Chen P.Y., Chen N.H., Chuang L.P., and Tsai P.S. (2013). Trajectories of sleep changes during the acute phase of traumatic brain injury: a 7-day actigraphy study. J. Formos. Med. Assoc. 112, 545–553 [DOI] [PubMed] [Google Scholar]
- 29.Kaufman Y., Tzischinsky O., Epstein R., Etzioni A., Lavie P., and Pillar G. (2001). Long-term sleep disturbances in adolescents after minor head injury. Pediatr. Neurol. 24, 129–134 [DOI] [PubMed] [Google Scholar]
- 30.Zollman F.S., Cyborski C., and Duraski S.A. (2010). Actigraphy for assessment of sleep in traumatic brain injury: case series, review of the literature and proposed criteria for use. Brain Inj. 24, 748–754 [DOI] [PubMed] [Google Scholar]
- 31.Meltzer L.J., Hiruma L.S., Avis K., Montgomery-Downs H., and Valentin J. (2015). Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep 38, 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]