Abstract
Isolated methylmalonic acidemia (MMA), a group of autosomal recessive inborn errors of metabolism, is most commonly caused by complete (mut0) or partial (mut−) deficiency of the enzyme methylmalonyl-CoA mutase (MUT). The severe metabolic instability and increased mortality experienced by many affected individuals, especially those with mut0 MMA, has led centers to use elective liver transplantation as a treatment for these patients. We have previously demonstrated the efficacy of systemic adeno-associated viral (AAV) gene delivery as a treatment for MMA in a murine model and therefore sought to survey AAV antibody titers against serotypes 2, 8, and 9 in a group of well-characterized MMA patients, accrued via a dedicated natural history study (clinicaltrials.gov ID: NCT00078078). Plasma samples provided by 42 patients (8 mut− and 34 mut0; 10 had received organ transplantation), who ranged in age between 2 and 31 years, were analyzed to examine AAV2 (n = 35), AAV8 (n = 41), and AAV9 (n = 42) antibody titers. In total, the seroprevalence of antibodies against AAV2, AAV8, or AAV9 was 20%, 22%, and 24%, respectively. We observed a lower-than-expected seropositivity rate (titers ≥1:20) in the pediatric MMA patients (2–18 years) for both AAV2 (p < 0.05) and AAV8 (p < 0.01) neutralizing antibodies (NAbs) compared with historical controls. Those with positive NAb titers were typically older than 18 years (p < 0.05 all serotypes) or had received solid organ transplantation (p < 0.01 AAV8, AAV9). The mut0 patients who had not been transplanted (n = 24)—that is, the subset with the greatest need for improved treatments—represented the seronegative majority, with 21 out of 24 patients lacking Abs against all AAV capsids tested. The unexpected lack of NAbs against AAV in this patient population has encouraging implications for systemic gene delivery as a treatment for mut MMA.
Introduction
Methylmalonic acidemia (MMA) is a severe autosomal recessive inborn error of metabolism characterized by lethal metabolic instability and multiorgan pathology. The disorder exhibits genetic heterogeneity and is most commonly caused by complete (mut0) or partial (mut−) deficiency of the enzyme methylmalonyl-CoA mutase (MUT), which isomerizes methylmalonyl-CoA into succinyl-CoA for entry into the Krebs cycle1 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/hum). Affected patients are medically fragile and suffer from numerous complications such as recurrent metabolic crises, “metabolic stroke” or infarction of the basal ganglia, pancreatitis, chronic renal failure, impaired growth, osteoporosis, optic nerve atrophy, and intellectual impairment.2
Patients with MMA experience significant morbidity and mortality, and the prognosis for long-term survival is poor. This has been established since the first studies were published in the early 1980s, and three decades later the outcomes are still dismal. The mortality of mut MMA was 60–88% in the first reports in the 1980s and 1990s3,4 and has improved somewhat to ∼40% by the first decade in the 2000s.5,6 This has led centers to pursue elective liver and combined liver–kidney transplantation as a treatment for the metabolic instability that eventually causes death.7–11 When successful, solid organ transplantation can eliminate the frequent episodes of metabolic acidosis, but has numerous practical limitations that include procedural availability, surgical mortality and morbidity, expense, a finite donor pool, and the need for life-long immune suppression. Alternative approaches to restore enzyme activity to the liver and other tissues in patients with MMA are clearly needed.
We have therefore studied viral gene therapy as treatment for MMA, using preclinical cellular12,13 and animal models,14–19 to gather efficacy and safety data. After proof-of-concept experiments using lentiviral13 and adenoviral delivery,12,19 we have developed and tested adeno-associated viral (AAV) vectors as gene therapy agents for MMA.14,16–18 AAV has emerged as an efficacious gene therapy vector for the delivery of small transgenes to somatic tissues in vivo and further displays substantial tissue tropism(s) conferred by the capsid.20 We have used AAVs of serotypes 2, 8, and 9 that express the mouse Mut or human MUT gene under the control of the enhanced chicken beta-actin promoter (CBA)16–18,21 or the liver-specific thyroid-binding globulin promoter (TBG)18 and administered them to Mut−/− mice in the neonatal period. The results of these studies are striking: whereas the untreated Mut−/− mice uniformly perish in early life, the treated Mut−/− mice have near-normal long-term survival and growth parameters, display an ameliorated metabolic phenotype, and demonstrate enzymatic activity longer than one year after a single treatment with an AAV8 or AAV9 vector. Surprisingly, the systemic delivery of an AAV9 vector also resulted in modest transduction of the kidney and long-term preservation of renal function in the treated mutants.18 Although genotoxicity has been observed in the mouse studies with some vectors, we recently demonstrated that manipulating regulatory elements and AAV dosing could allow for the potential clinical application of systemic AAV gene delivery as a new treatment for mut MMA.21
Given the well-recognized barrier to in vivo gene transfer imposed by preexisting cellular and humoral immunity to AAV capsids,22–24 we have surveyed a large cohort of well-characterized MMA patients for AAV neutralizing antibody (NAb) titers against serotypes 2, 8, and 9. We anticipate that these data will inform the selection of an optimal serotype to use in a future gene therapy clinical trial. Our results suggest that patients with the most severe forms of isolated MMA display a low prevalence of seropositivity against AAV2, AAV8, and AAV9 capsids during the first two decades of life, and would be suitable candidates for a future AAV gene therapy clinical trial. Whether the low seroprevalence reflects a decreased incidence of exposure to AAV or a generalized impairment in humoral immunity as a consequence of the underlying metabolic block is unknown.
Materials and Methods
Patients and samples
The patients were evaluated at the NIH Clinical Center under the protocol “Clinical and Basic Investigations of Methylmalonic Acidemia and Related Disorders” (clinicaltrials.gov identifier: NCT00078078). The study was approved by the National Human Genome Research Institute (NHGRI) Institutional Review Board, and the research adhered to the tenets of the Declaration of Helsinki. Informed consent from patients and/or guardians was obtained. This protocol evaluates clinical and genetic features of patients with MMA and allows for research specimen collection. All patients were clinically characterized, and the subtype of MUT deficiency (mut0, mut−) was determined by a combination of propionate incorporation, complementation studies (Dr. David Rosenblatt, McGill University), and molecular genetic analysis of MUT (GeneDx, Gaithersburg, MD). Blood samples were drawn in Na heparin tubes and centrifuged at 1000–2000 × g at 4°C for 10 min. Plasma was removed and aliquoted in 1 ml volumes, and samples were stored at −80°C (mean 4.3 years stored; range 0.5–9.7) until use for this study. Plasma samples were thawed on wet ice. From the thawed samples, one aliquot was removed for AAV8 and 9 NAb analysis. In the subset of patients with remaining serum in the aliquot who were tested for AAV2 NAbs, another freeze–thaw cycle was needed to obtain a third sample for AAV2 NAb testing. NAbs were measured in 41 patients for both AAV8 and AAV9 NAbs, with an additional sample tested only for AAV9 NAbs. Thirty-five samples were also assayed for the presence of AAV2 NAbs, including most of those seropositive against AAV8 and AAV9, as well as an additional subset of clinically severe or older mut0 patients, aged 2–28. We were unable to measure AAV2 titers in all patients because of limited amounts of plasma.
Cell culture, AAV vectors, and neutralizing antibody assay
The methods have been described previously.25 In brief, the human hepatoma cell line Huh7 was maintained in Dulbecco's modified Eagle's medium (DMEM; Cellgro) supplemented with 10% fetal bovine serum (FBS; Hyclone). Cells were cultured at 37°C in an atmosphere of 5% CO2 in air. AAV vectors in the single-strand configuration contained the gene encoding β-galactosidase (LacZ), which was driven by a cytomegalovirus (CMV) promoter (AAV.CMV.LacZ). All AAV.CMV.LacZ vectors used in this study were prepared by the Vector Core of the University of Pennsylvania (Philadelphia, PA) as previously described.26 Recombinant AAV genomes equipped with AAV2 inverted terminal repeats (ITRs) were packaged by triple transfection of 293 cells with cis-plasmid, adenovirus helper plasmid, and a chimeric packaging construct in which the AAV2 rep gene was fused with cap genes of different AAV2, AAV8, or AAV9. All recombinant vectors were purified by CsCl sedimentation. Genome copy titers of AAV vectors were determined by TaqMan analysis (Applied Biosystems), using probes and primers targeting a bovine growth hormone polyadenylation signal. Plasma samples were heat inactivated at 56°C for 35 min.
Recombinant AAV.CMV.LacZ (109 genomic copies/well) was diluted in serum-free DMEM and incubated with 2-fold serial dilutions (initial dilution, 1:5) of heat-inactivated serum samples on DMEM for 1 hr at 37°C. Subsequently, the serum–vector mixture was added to 96-well plates seeded with 1 × 105 Huh7 cells/well that had been infected 2 hr earlier with wild-type HAdV5 (50 viral particles/cell). After 1 hr, each well was supplemented with an equal volume of 20% FBS DMEM and incubated for 18–22 hr at 37°C and 5% CO2. Then, cells were washed twice in PBS and lysed, and the lysate was developed with the mammalian β-galactosidase assay kit for bioluminescence, in accordance with manufacturer's protocol (Applied Biosystems), and measured in a microplate luminometer (Clarity; BioTek). The NAb titer was reported as the highest serum dilution that inhibited AAV.CMV.LacZ transduction (β-gal expression) by >50%, compared with the mouse serum control (Sigma S3509). Samples were determined to be positive for antibodies against AAV capsids 2, 8, and 9 when a titer of 1:5 or higher dilution of serum inhibited AAV transduction over 50%.25,27 Seropositivity for NAbs was defined as titers of ≥1:20 dilution.
Statistics
The sample storage age dependence of the seropositivity was assessed using an unpaired Student's t-test. Chi-square statistics were used to compare observed vs. expected seropositivity rates for NAbs against serotype 2 and 8 capsids in the pediatric MMA patients. Fisher's exact test was used to compare number of null alleles, enzymatic subtype, and transplant status.
Results
The salient demographic features of the patient cohort, which lists the age, enzymatic subtype, genotype, and AAV antibodies assayed, are presented in Tables 1 and 2, and Supplementary Table S1. Samples from 42 patients with mut MMA between age 2 and 31 years were studied to examine AAV2 (n = 35), AAV8 (n = 41), and/or AAV9 (n = 42) AAV antibody titers. The cohort included 34 mut0 and 8 mut− patients; 10 mut0 patients had previously received a kidney, liver, or combined liver–kidney transplant.
Table 1.
MMA patient demographics
| AAV2 (n = 35) | AAV8 (n = 41) | AAV9 (n = 42) | |
|---|---|---|---|
| Mean age, years (min, max) | 11.91 (2.4, 28.5) | 12.27 (2.3, 31.6) | 12.09 (2.3, 31.6) |
| Sex, n (%) | |||
| Male | 21 (60) | 23 (56) | 23 (56) |
| Female | 14 (40) | 18 (44) | 19 (44) |
| Race, n (%) | |||
| White | 18 (51) | 22 (54) | 22 (52) |
| Other | 17 (49) | 19 (46) | 20 (48) |
| Type, n (%) | |||
| mut0 | 28 (80) | 33 (80) | 34 (81) |
| mut− | 7 (20) | 8 (20) | 8 (19) |
AAV, adeno-associated viral; MMA, methylmalonic acidemia.
Table 2.
Patient cohort, enzymology, molecular genetics, and anti-AAV antibody status
| Patient | Age (years) | Sex | Subtype | Date of sample | Allele 1 | Allele 2 | Transplant status | AAV2 Ab titer | AAV8 Ab titer | AAV9 Ab titer |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.3 | M | mut− | 12/13/11 | c.1846C>T p.R616C | c.1846C>T p.R616C | <1:5 | <1:5 | <1:5 | |
| 2 | 2.4 | F | mut− | 2/28/06 | c.91C>T p.R31X | c.323G>A p.R108H | <1:5 | <1:5 | <1:5 | |
| 3 | 2.5 | M | mut− | 12/5/11 | c.277C>T p.R93C | c.1106G>A p.R369H | <1:5 | <1:5 | <1:5 | |
| 4 | 3.2 | M | mut− | 12/10/09 | c.1207C>T p.R403X | c.2150G>T p.G717V | <1:5 | <1:5 | <1:5 | |
| 5 | 3.3 | M | mut0 | 5/12/10 | c.1399C>T p.R467X | c.693C>G p.Y231X | ND | <1:5 | <1:5 | |
| 6 | 3.5 | F | mut− | 8/19/05 | c.281G>T p.G94V | c.2150G>T p.G717V | <1:5 | <1:5 | <1:5 | |
| 7 | 4.2 | F | mut0 | 8/10/10 | c.682C>T p.R228X | c.1106G>A p.R369H | LT | <1:5 | 1:40 | 1:40 |
| 8 | 4.4 | M | mut0 | 5/20/08 | c.91C>T p.R31× | c.1181dupT p.L394FfsX30 | <1:5 | <1:5 | <1:5 | |
| 9 | 4.4, 2.3 | F | mut0 | 5/9/07, 4/15/05 | c.607G>A p.G203R | c.682C>T p.R228X | <1:5 | <1:5 | <1:5 | |
| 10 | 4.5 | F | mut0 | 5/9/07 | c.1207C>T p.R403X | c.2008G>C p.G670R | ND | <1:5 | <1:5 | |
| 11 | 4.6 | F | mut0 | 12/8/10 | c.2080C>T p.R694W | c.643G>T p.G215C | <1:5 | ND | 1:20 | |
| 12 | 5.6 | M | mut0 | 6/14/11 | c.322C>T p.R108C | c.682C>T p.R228X | <1:5 | <1:5 | <1:5 | |
| 13 | 6.0 | F | mut0 | 6/4/07 | c.323G>A p.R108H | c.1867G>C p.G623R | ND | <1:5 | <1:5 | |
| 14 | 6.1 | M | mut0 | 2/23/06 | c.1741C>T p.R581× | c.753 + 2T>A p.? | <1:5 | <1:5 | <1:5 | |
| 15 | 6.5 | F | mut0 | 5/3/11 | c.878A>C p.Q293P | c.878A>C p.Q293P | <1:5 | <1:5 | <1:5 | |
| 16 | 6.6 | M | mut0 | 8/4/09 | c.2179C>T p.R727X | c.2179C>T p.R727X | <1:5 | <1:5 | <1:5 | |
| 17 | 7.2 | M | mut0 | 6/17/09 | c.281G>T p.G94V | c.1867G>C p.G623R | <1:5 | <1:5 | <1:5 | |
| 18 | 7.8 | M | mut0 | 7/3/06 | c.1106G>A p.R369H | c.1778_1782delAAAGT p.S594RfsX11 | LT | <1:5 | <1:5 | <1:5 |
| 19 | 8.6 | M | mut0 | 4/20/10 | c.1022dupA p.N341KfsX17 | c.671_678dup p.V227NfsX16 | <1:5 | <1:5 | <1:5 | |
| 20 | 9.4 | M | mut0 | 8/26/08 | c.1105C>T p.R369C | c.1207C>T p.R403X | <1:5 | <1:5 | <1:5 | |
| 21 | 9.7, 8.2 | M | mut0 | 4/17/12, 9/22/10 | c.1038_1040delTCT p.347delL |
c.349G>T p.E117X | LKT at 8.7 | <1:5 | <1:5 | <1:5 |
| 22 | 10.4 | F | mut0 | 11/13/14 | c.2179C>T p.R727X | c.2179C>T p.R727X | <1:5 | <1:5 | <1:5 | |
| 23 | 10.6 | M | mut0 | 4/17/07 | c.670G>T p.E224X | c.682C>T p.R228X | LKT | <1:5 | <1:5 | <1:5 |
| 24 | 10.9 | M | mut0 | 9/8/05 | c.322C>T p.R108C | c.322C>T p.R108C | 1:5 | <1:5 | <1:5 | |
| 25 | 11.3 | M | mut0 | 6/1/08 | c.682C>T p.R228X | c.682C>T p.R228X | LKT | <1:5 | 1:10 | 1:10 |
| 26 | 11.8, 4.6 | F | mut0 | 7/27/10, 6/8/04 | c.1942G>C p.G642R | c.1942G>C p.G642R | <1:5 | <1:5 | <1:5 | |
| 27 | 11.9, 8.0 | F | mut0 | 6/18/12, 8/25/08 | c.927G>A p.W309X | c.983T>C p.L328P | <1:5 | <1:5 | <1:5 | |
| 28 | 13.9, 11.9 | M | mut0 | 6/11/09, 6/27/07 | c.1106G>A p.R369H | c.1106G>A p.R369H | LT | <1:5 | <1:5 | <1:5 |
| 29 | 13.2, 8 | F | mut0 | 8/10/11, 7/11/06 | c.2053dupCTC p.685insL | c.91C>T p.R31X | ND | <1:5 | <1:5 | |
| 30 | 17, 10.5, 12.6 | F | mut0 | 10/19/11, 7/17/07, 6/20/05 | c.1658delT p.V553GfsX17 | c.29dupT p.L11TfsX38 | ND | <1:5 | <1:5 | |
| 31 | 18.5, 16.4 | M | mut0 | 4/3/07, 3/21/05 | c.572C>A p.A191E | c.655A>T p.N219Y | <1:5 | <1:5 | <1:5 | |
| 32 | 22.5 | M | mut0 | 8/4/05 | c.322C>T p.R108C | c.1106G>A p.R369H | KT | ND | 1:40 | 1:80 |
| 33 | 22.7, 24 | M | mut0 | 9/12/07, 6/28/06 | c.572C>A p.A191E | c.682C>T p.R228X | 1:5 | 1:5 | 1:5 | |
| 34 | 24.7 | M | mut0 | 1/27/09 | c.655A>T p.N219Y | c.1048C>T p.H350Y | <1:5 | <1:5 | <1:5 | |
| 35 | 26.8, 19.9 | F | mut0 | 8/22/11, 8/25/04 | c.1106G>A p.R369H | c.1106G>A p.R369H | LKT | 1:160 | 1:40 | 1:40 |
| 36 | 25.4, 25.4 | F | mut0 | 5/9/06, 5/8/06 | c.935G>T p.G312V | c.1909G>A p.G637R | <1:5 | <1:5 | <1:5 | |
| 37 | 26.1, 24.2 | M | mut0 | 10/1/11, 10/7/09 | c.1741C>T p.R581X | c.1741C>T p.R581X | KT | 1:160 | 1:80 | 1:320 |
| 38 | 26.5, 25.9 | M | mut− | 9/13/10, 12/3/09 | c.1760A>C p.Y587S | c.2150G>T p.G717V | 1:80 | 1:10 | 1:10 | |
| 39 | 27.5, 20.6 | F | mut0 | 7/27/11, 8/17/04 | c.2053dupCTC p.685insL | c.2053dupCTC p.685insL | <1:5 | <1:5 | <1:5 | |
| 40 | 28.5 | F | mut− | 8/22/11 | c.2150G>T p.G717V | c.2150G>T p.G717V | 1:80 | 1:5 | 1:20 | |
| 41 | 28.7 | F | mut− | 5/23/07 | c.2150G>T p.G717V | unknown | ND | <1:5 | <1:5 | |
| 42 | 31.6, 27.5 | F | mut0 | 10,29/08, 9/17/04 | c.826G>T p.E276× | c.1106G>A p.R369H | LKT | 1:40 | 1:10 | 1:80 |
Shaded boxes indicate AAV Ab-positive samples. In seropositive patients (33, 35, 37, 38, and 42), the first age listed represents the date of assay for AAV8 and 9 Abs, whereas the later date indicates the age tested for AAV2 Abs.
Ab, antibody; KT, kidney transplant; LKT, combined liver–kidney transplant; LT, liver transplant; ND, not determined because of lack of material.
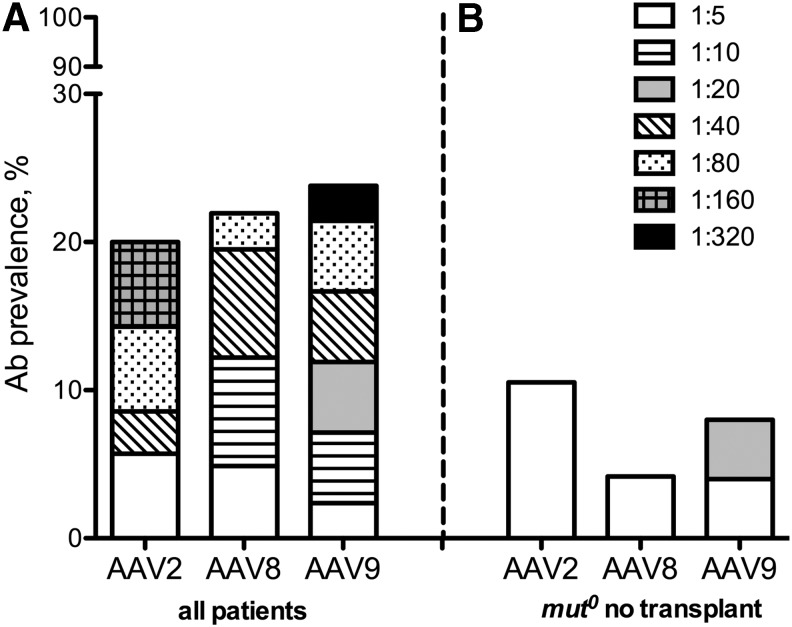
Figure 1A shows the AAV antibody titer distributions among the patients. The seroprevalence in the MMA cohort screened against AAV2, AAV8, or AAV9 was 7/35 (20%), 9/41 (22%), and 10/42 (24%), respectively (Figure 1A and Table 2). Overall, 31/42 (74%) of the collective cohort were seronegative for NAbs against AAV2, AAV8, and AAV9. Furthermore, in those patients who had AAV antibodies, the majority (6/11) were only weakly seropositive (<1:20) for at least one serotype28 (Fig. 1A). When only the nontransplanted group of mut0 MMA patients (n = 24) was considered, the resulting seroprevalence rates were lower and the humoral responses weaker (Fig. 1B).
Figure 1.
(A) AAV antibody seroprevalance within the entire MMA cohort (n = 42) that includes both nontransplanted and transplanted (liver, kidney, and/or combined liver–kidney; n = 10) patients. The overall seroprevalance, defined as ≥1:5, is depicted (20–24%) along with the titer distribution of antibodies against AAV2 (n = 35), AAV8 (n = 41), and AAV9 (n = 42) capsids. The seroprevalence is subdivided into titer dilution levels from weak (1:5) NAb titers to strong (1:320). (B) AAV antibody seropositivity in the subgroup of patients with mut0 MMA who were not transplanted (n = 24), which shows low NAb prevalence (4–10%) for the cohort of severe, nontransplanted patients who would be ideal candidates for gene therapy. AAV, adeno-associated viral; Ab, antibody; MMA, methylmalonic acidemia; NAb, neutralizing antibody.
To assess whether the length of storage might influence the lower-than-expected seroprevalance, we analyzed the age of each sample in the antibody-positive versus antibody-negative groups (Supplementary Fig. S2) for each capsid. There was no difference in the sample age between the groups for any serotype (p = 0.77 AAV2 NAbs and p = 0.83 AAV8/9 NAbs). This is consistent with the observations from other studies that have documented the stability of antibodies to varied pathogens, such as malaria and adenovirus,28 as well as a wider range of anti-immunotherapeutic antibodies.29
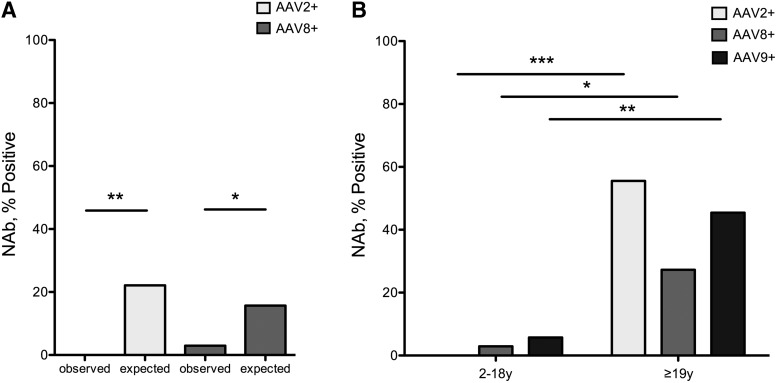
We next compared the seropositivity rate, defined as a titer of ≥1:20, of NAbs AAV2 and 8 in a subset of our MMA cohort, aged 18 and under, to historical data from a reference pediatric population.27 A lower-than-expected seropositivity rate was observed in the pediatric MMA patients for both AAV2 (p = 0.0066) and AAV8 (p = 0.0408) NAbs (Fig. 2A and Supplementary Table S2). Within the MMA cohort, a significant difference in the seroprevalance of all three NAbs was documented between children and adults (AAV2 p = 0.0004; AAV8 p = 0.0399; AAV9 p = 0.0054) (Fig. 2B). In the group of those aged 18 years and less, only 2 patients were seropositive for any AAV serotype, and every patient was seronegative for at least one of the capsids tested (Table 2).
Figure 2.
(A) Seropositivity, as defined as ≥1:20, in the pediatric MMA cohort compared with historical controls. There was a lower-than-expected AAV2 (p = 0.0066) and AAV8 (p = 0.0408) NAb seroprevalance in MMA individuals ≤18 years. (B) Neutralizing antibody prevalence to AAV serotypes 2, 8, and 9 by age. The seroprevalance of AAV2, AAV8, and AAV9 NAbs between children and adults was significantly different for all serotypes with a higher incidence of seropositivity in adults (7/11). *p < 0.05, **p < 0.01, ***p < 0.001.
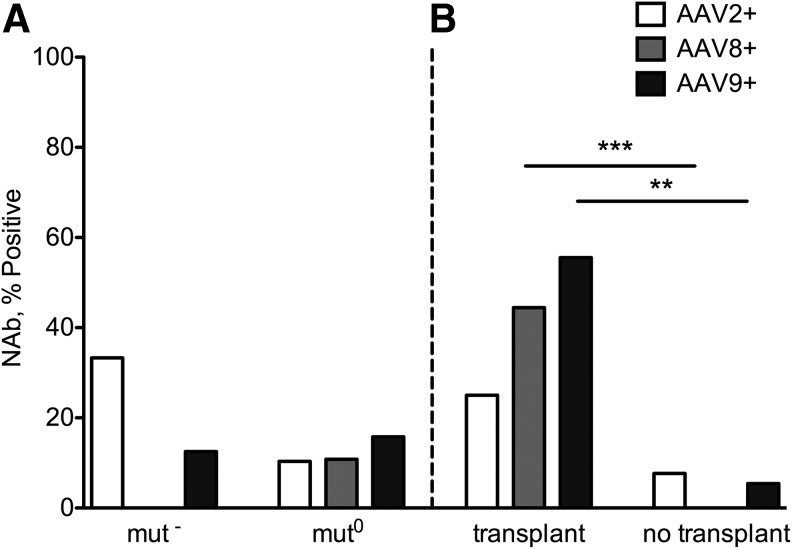
To investigate the clinical characteristics shared by the seropositive group, the cohort was subdivided on the basis of molecular genetics, cellular enzymology, and transplant status (Fig. 3). First, we compared the prevalence of null alleles (nonsense, frameshift, splice mutations) between the AAV NAb-positive and NAb-negative groups and found no statistically significant difference between them (6/16 alleles [38%] vs. 29/68 alleles [42%]; p = 0.7837). A similar incidence of seropositive titers was noted between enzymatic subtypes, mut0 and mut− (AAV2 p = 0.1952; AAV8 p = 1.0; AAV9 p = 1.0) (Fig. 3A). However, when the cohort was stratified based on transplant status, there was a statistically significant difference between the transplanted and nontransplanted groups for NAbs against AAV8 (p = 0.0008) and AAV9 (p = 0.0016) but not AAV2 (p = 0.3007) (Fig. 3B). The 24 nontransplanted mut0 patients were almost entirely seronegative, with only 1 patient (patient 11) seropositive at a 1:20 dilution against AAV9 (Table 2).
Figure 3.
Subgrouping of the MMA cohort by enzymatic subtype (A) and transplant status (B). (A) The seroprevalance was similar in mut− and mut0 groups (AAV2 p = 0.1952; AAV8 p = 1.0; AAV9 p = 1.0). (B) The seroprevalence was significantly different in the transplanted and nontransplanted groups for AAV8 (p = 0.0008) and AAV9 (p = 0.0016) but not AAV2 (p = 0.3007) with 6/10 of the transplanted patients seropositive against AAV2, 8, and/or 9. **p < 0.01, ***p < 0.001.
We also examined co-seroprevalance of AAV antibodies (titer ≥1:5), because AAV antibody cross-reactivity can cause neutralization across a range of AAV serotypes.30 As expected, co-prevalence of antibodies to AAV capsids within individuals was also noted in our cohort. Out of the 11 patients who had AAV antibodies, 9 were tested for all three serotypes and 6 had AAV antibodies against all three capsids (Table 3 and Supplementary Fig. S3). As shown, 100% of individuals who had antibodies against AAV8 capsid (n = 9) also had antibodies against AAV9 (n = 9). Three individuals who had antibodies against AAV8 or 9 capsids lacked antibodies against AAV2, and only one individual showed low titer (1:5) for AAV2 and was negative for AAV8 and AAV9 antibodies (Table 2).
Table 3.
Coprevalence of antibodies against AAV types 2, 8, and 9 capsids
| AAV2+ | AAV8+ | AAV9+ | |
|---|---|---|---|
| AAV2+ | 88% AAV8+ (n = 8/9) | 90% AAV9+ (n = 9/10) | |
| AAV8+ | 66% AAV2+ (n = 6/9) | 100% AAV9+ (n = 9/9) | |
| AAV9+ | 70% AAV2+ (n = 7/10) | 100% AAV8+ (n = 9/9) |
Discussion
Because we envision systemic AAV gene therapy as a potential treatment for MMA patients, we surveyed the presence of AAV antibodies in a large and diverse patient cohort, assembled over a 10-year period at our center through a dedicated natural history study. The current investigation was focused on assessing the humoral immune response to various AAV capsids by retrospectively surveying antibody titers against serotypes that we have previously studied in a preclinical MMA model.14,16–18 All participants were characterized by molecular genetic analysis and/or enzymology and presented a spectrum of mutations in MUT: there were 42 distinct alleles that included 22 missense, 11 nonsense, 6 frameshift, 1 splice, and 2 in-frame insertion/deletion variants. Although cross-reacting material (CRM) status has not been assessed in cell lines from these patients, it is likely to be present in a significant proportion given that 67% of the patients harbored at least one missense or in-frame insertion/deletion mutation (n = 28). Five different alleles (c.572C>A p.A191E, c.655A>T p.N219Y, c.1106G>A p.R369H, c.2080C>T p.R694W, and c.2150G>T p.G717V) were present in 16 different individuals and all are predicted to produce residual enzyme based on a recent study.31 The large number of missense mutations is promising for future gene therapy efforts since these individuals would have a lower chance of developing an immune response to MUT encoded by an AAV vector. Although the participants were largely from the United States, and may therefore harbor an environmental and exposure profile distinct from patients in other countries, this cohort reflects a level of molecular and enzymatic heterogeneity similar to what has been previously described.32–35
Preexisting immunity against the AAV capsids has emerged as a significant barrier to effective systemic in vivo transduction in animal models and human clinical trials.36–38 More specifically, the presence of low-titer NAbs has been proven to interfere with hepatic transduction in macaques,37,39 in which significant differences in factor IX (FIX) expression level after AAV8-mediated systemic hepatic gene delivery were noted between control animals with NAb titers <1:5 and animals with preinjection titers of 1:5, 1:10, and >1:10.39 In an extension of these observations to humans, Nathwani et al. described a blunted response to an AAV8 vector administered to a hemophilia patient who had low-level NAb titers.40 In this patient, FIX activity was not restored after systemic AAV8 gene delivery, presumably because hepatic transduction was impeded by AAV8 NAbs.
Given the importance of preexisting NAbs as a factor in determining the in vivo efficacy of systemic gene therapy, several investigators have surveyed human populations to define the epidemiology of AAV capsid exposure and antibody responses. The seroprevalence data have been largely derived from adult blood donors in whom seropositivity has been defined as NAb titers ≥1:20 dilution. Higher seroprevalence rates were noted against AAV2, 1, and 6 (59%, 50.5%, and 37%) capsids, whereas serotypes AAV5, 8, and 9 were lower (3.2%, 19%, and 33.5%).31 A study conducted in newborns, children, and adolescents showed that NAb prevalence to AAV2 and AAV8 was moderate at birth, decreased from 7 to 11 months to almost undetectable levels, and then progressively increased through childhood and adolescence.27 Only a small number of studies have examined NAb prevalence in patient cohorts. Longitudinal studies in the hemophilia population have recognized that after an initial decline from maternal NAbs in the first year of life,41 the seropositivity rate of NAbs against AAV2 increases over time; ∼16% of the tested group presented as seropositive when entering the study, which increased to ∼44% by age ≥5 years.42 Additionally, it has been recognized that reactivity of NAbs to other capsids, such as 5 and 8, paralleled the trend for AAV2 seroconversion. In fact, ∼50% of the AAV2 seropositive patients also had NAbs against AAV5 and 8 in a related study.43 Similarly, surveys of the cystic fibrosis (CF) population have indicated that about 30% of adults were seropositive against AAV2, whereas children with CF had markedly lower rates (∼4–15%),41 and in the case of MPS VI, 42% of children and young adults were seropositive against AAV8.44
In the patients studied here, we initially observed an overall seroprevalance of 22–24% against AAV8 or 9 capsids, with 100% co-seropositivity between those who were tested for both AAV8 and AAV9 antibodies. A single patient was seropositive for only AAV9 antibodies, and specimen volume did not allow us to test for AAV8. Although constrained by sample availability, we extended our observations by examining AAV NAbs against serotype 2 in a smaller subset of patients who had an overall seroprevalance of 20%. In total, there were 11 individuals who had detectable AAV antibodies. In these 11 patients, 9 were tested for both AAV2 and AAV8, 10 were tested for AAV2 and AAV9, and 9 were tested for both AAV8 and AAV9. Six of 9 tested for all three were tri-seropositive (Table 3). Although our numbers are small, the co-seropositivity rates we observe approximate estimates seen in other studies.30,41,42 It should be noted that most of our patients displayed variable titers when positive, even against AAV2 (Fig. 1).
However, when the patients were stratified based on clinical features (age, molecular genetics, enzymatic subtype [mut0 vs. mut−], and transplant status), a striking trend was recognized. The large majority of the seropositive patients were older than 18 years (Fig. 2B) or had received organ transplants (Fig. 3B). Whether this pattern reflects the epidemiology of AAV infection or an improvement in the functional immune status of patients after transplant remains unknown. The latter seems more likely because after transplantation, MMA patients have improved metabolism, lower circulating metabolites, and increased protein tolerance. In contrast, the nontransplanted mut0 group, which represents the patients most likely to benefit from gene therapy, had only 1 patient out of the total group of 24 tested with a positive AAV NAb titer (≥1:20), against only one capsid (Table 2, patient 11).
When compared with pediatric historical controls,27 seropositivity rates in our cohort were lower than expected (Fig. 2A and Supplementary Table S2). Whether this is a reflection of an underlying immune disturbance is unknown, but unlikely to be caused by a decreased incidence of exposure to AAV, given the frequent hospitalizations experienced by this group. MMA patients are prone to bone marrow pan-suppression, including neutropenia and thrombocytopenia during periods of stress and infection.2 In earlier studies, over 50% of mut0 MMA patients had hematologic abnormalities (leukopenia, thrombocytopenia, and anemia) despite normal serum cobalamin values.3 Furthermore, there are several reports of MMA patients who experienced atypical infections, including gram-negative or fungal pathogens, and immunological studies have documented complex T- and B-cell defects.45–47 The immune system dysfunction in MMA warrants further investigation but it is possible that the underlying disorder may impact the ability to mount a robust humoral immune response after exposure to AAV capsids and potentially other antigens.
This report is the first to survey AAV antibody titers of any serotype in patients with MMA. The patterns observed show that patients who have not undergone liver or combined liver–kidney transplantation are very unlikely to harbor NAbs against the AAV capsids that have already been proven to be effective in liver-directed gene therapy in patients with hemophilia24,40 as well as mice with MMA.14,15,16–18,21 Furthermore, the very low frequency and titers of NAbs in the younger, severely affected MMA patients, of whom 21 out of 24 tested were seronegative against all 3 capsids, strongly support the application of systemic AAV gene delivery in this population, especially because the complications related to preexisting humoral immunity, as well as the interference with transduction it engenders, should be mitigated.
Supplementary Material
Acknowledgments
We thank all patients and their families for their participation, Isa Bernardini and Roxanne Fischer for processing patient samples, and Jean Gagne for assistance with data collection. This work was supported by the Intramural Research Program of the National Human Genome Research Institute.
Author Disclosure
J.M. Wilson is an advisor to REGENXBIO, Dimension Therapeutics, and Solid Gene Therapy, and is a founder of, holds equity in, and has a sponsored research agreement with REGENXBIO and Dimension Therapeutics; in addition, he is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies. No competing financial interests exist for the other authors.
References
- 1.Fenton WA, Gravel RA, Rosenblatt DS. Disorders of propionate and methylmalonate metabolism in the metabolic and molecular bases for inherited disease. In: The Metabolic and Molecular Bases of Inherited Disease (8th edn.). Scriver CR, Beaudet AL, Sly WS, Valle D, eds. McGraw-Hill, New York, NY: 2001; pp. 2165–2192 [Google Scholar]
- 2.Manoli I, Sloan JL, Venditti CP. Isolated Methylmalonic Acidemia. In: GeneReviews® [Internet]. Pagon RA, Adam MP, Ardinger HH, et al., eds. University of Washington, Seattle, WA: 2005. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1231/ [Google Scholar]
- 3.Matsui SM, Mahoney MJ, Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med 1983;308:857–861 [DOI] [PubMed] [Google Scholar]
- 4.van der Meer SB, Poggi F, Spada M, et al. . Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia. J Pediatr 1994;125:903–908 [DOI] [PubMed] [Google Scholar]
- 5.Horster F, Baumgartner MR, Viardot C, et al. . Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB). Pediatr Res 2007;62:225–230 [DOI] [PubMed] [Google Scholar]
- 6.Cosson MA, Benoist JF, Touati G, et al. . Long-term outcome in methylmalonic aciduria: A series of 30 French patients. Mol Genet Metab 2009;97:172–178 [DOI] [PubMed] [Google Scholar]
- 7.van't Hoff WG, Dixon M, Taylor J, et al. . Combined liver-kidney transplantation in methylmalonic acidemia. J Pediatr 1998;132:1043–1044 [DOI] [PubMed] [Google Scholar]
- 8.Kayler LK, Merion RM, Lee S, et al. . Long-term survival after liver transplantation in children with metabolic disorders. Pediatr Transplant 2002;6:295–300 [DOI] [PubMed] [Google Scholar]
- 9.Nagarajan S, Enns GM, Millan MT, et al. . Management of methylmalonic acidaemia by combined liver-kidney transplantation. J Inherit Metab Disease 2005;28:517–524 [DOI] [PubMed] [Google Scholar]
- 10.Morioka D, Kasahara M, Takada Y, et al. . Living donor liver transplantation for pediatric patients with inheritable metabolic disorders. Am J Transplant 2005;5:2754–2763 [DOI] [PubMed] [Google Scholar]
- 11.Morioka D, Kasahara M, Horikawa R, et al. . Efficacy of living donor liver transplantation for patients with methylmalonic acidemia. Am J Transplant 2007;7:2782–2787 [DOI] [PubMed] [Google Scholar]
- 12.Chandler RJ, Tsai MS, Dorko K, et al. . Adenoviral-mediated correction of methylmalonyl-CoA mutase deficiency in murine fibroblasts and human hepatocytes. BMC Med Genet 2007;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandler RJ, Sloan J, Fu H, et al. . Metabolic phenotype of methylmalonic acidemia in mice and humans: The role of skeletal muscle. BMC Med Genet 2007;8:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carrillo-Carrasco N, Chandler RJ, Chandrasekaran S, et al. . Liver-directed recombinant adeno-associated viral gene delivery rescues a lethal mouse model of methylmalonic acidemia and provides long-term phenotypic correction. Hum Gene Ther 2010;21:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler RJ, Chandrasekaran S, Carrillo-Carrasco N, et al. . Adeno-associated virus serotype 8 gene transfer rescues a neonatal lethal murine model of propionic acidemia. Hum Gene Ther 2011;22:477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandler RJ, Venditti CP. Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno-associated viral gene therapy. Mol Ther 2010;18:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandler RJ, Venditti CP. Pre-clinical efficacy and dosing of an AAV8 vector expressing human methylmalonyl-CoA mutase in a murine model of methylmalonic acidemia (MMA). Mol Genet Metab 2012;107:617–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senac JS, Chandler RJ, Sysol JR, et al. . Gene therapy in a murine model of methylmalonic acidemia using rAAV9-mediated gene delivery. Gene Ther 2012;19:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandler RJ, Venditti CP. Adenovirus-mediated gene delivery rescues a neonatal lethal murine model of mut(0) methylmalonic acidemia. Hum Gene Ther 2008;19:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zincarelli C, Soltys S, Rengo G, et al. . Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 2008;16:1073–1080 [DOI] [PubMed] [Google Scholar]
- 21.Chandler RJ, LaFave MC, Varshney GK, et al. . Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest 2015;125:870–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janelidze S, Nordstrom U, Kugler S, et al. . Pre-existing immunity to adeno-associated virus (AAV)2 limits transgene expression following intracerebral AAV2-based gene delivery in a 6-hydroxydopamine model of Parkinson's disease. J Gene Med 2014;16:300–308 [DOI] [PubMed] [Google Scholar]
- 23.Calcedo R, Wilson JM. Humoral immune response to AAV. Front Immunol 2013;4:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manno CS, Pierce GF, Arruda VR, et al. . Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 2006;12:342–347 [DOI] [PubMed] [Google Scholar]
- 25.Calcedo R, Vandenberghe LH, Gao G, et al. . Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Diseases 2009;199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao GP, Alvira MR, Wang L, et al. . Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A 2002;99:11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calcedo R, Morizono H, Wang L, et al. . Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol 2011;18:1586–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendriks J, Stals C, Versteilen A, et al. . Stability studies of binding and functional anti-vaccine antibodies. Bioanalysis 2014;6:1385–1393 [DOI] [PubMed] [Google Scholar]
- 29.Michaut L, Laurent N, Kentsch K, et al. . Stability of anti-immunotherapeutic antibodies in frozen human serum samples. Bioanalysis 2014;6:1395–1407 [DOI] [PubMed] [Google Scholar]
- 30.Boutin S, Monteilhet V, Veron P, et al. . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: Implications for gene therapy using AAV vectors. Hum Gene Ther 2010;21:704–712 [DOI] [PubMed] [Google Scholar]
- 31.Forny P, Froese DS, Suormala T, et al. . Functional characterization and categorization of missense mutations that cause methylmalonyl-CoA mutase (MUT) deficiency. Hum Mutat 2014;35:1449–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worgan LC, Niles K, Tirone JC, et al. . Spectrum of mutations in mut methylmalonic acidemia and identification of a common Hispanic mutation and haplotype. Hum Mutat 2006;27:31–43 [DOI] [PubMed] [Google Scholar]
- 33.Lempp TJ, Suormala T, Siegenthaler R, et al. . Mutation and biochemical analysis of 19 probands with mut0 and 13 with mut- methylmalonic aciduria: Identification of seven novel mutations. Mol Genet Metab 2007;90:284–290 [DOI] [PubMed] [Google Scholar]
- 34.Merinero B, Perez B, Perez-Cerda C, et al. . Methylmalonic acidaemia: Examination of genotype and biochemical data in 32 patients belonging to mut, cblA or cblB complementation group. J Inherit Metab Disease 2008;31:55–66 [DOI] [PubMed] [Google Scholar]
- 35.Acquaviva C, Benoist JF, Pereira S, et al. . Molecular basis of methylmalonyl-CoA mutase apoenzyme defect in 40 European patients affected by mut(o) and mut- forms of methylmalonic acidemia: Identification of 29 novel mutations in the MUT gene. Hum Mutat 2005;25:167–176 [DOI] [PubMed] [Google Scholar]
- 36.Nathwani AC, Rosales C, McIntosh J, et al. . Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther 2011;19:876–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Calcedo R, Wang H, et al. . The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther 2010;18:126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Wang H, Bell P, et al. . Systematic evaluation of AAV vectors for liver directed gene transfer in murine models. Mol Ther 2010;18:118–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Calcedo R, Bell P, et al. . Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther 2011;22:1389–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nathwani AC, Tuddenham EG, Rangarajan S, et al. . Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 2011;365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halbert CL, Miller AD, McNamara S, et al. . Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther 2006;17:440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C, Narkbunnam N, Samulski RJ, et al. . Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther 2012;19:288–294 [DOI] [PubMed] [Google Scholar]
- 43.Mimuro J, Mizukami H, Shima M, et al. . The prevalence of neutralizing antibodies against adeno-associated virus capsids is reduced in young Japanese individuals. J Med Virol 2014;86:1990–1997 [DOI] [PubMed] [Google Scholar]
- 44.Ferla R, Claudiani P, Savarese M, et al. . Prevalence of anti-adeno-associated virus serotype 8 neutralizing antibodies and arylsulfatase B cross-reactive immunologic material in mucopolysaccharidosis VI patient candidates for a gene therapy trial. Hum Gene Ther 2015;26:145–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Church JA, Koch R, Shaw KN, et al. . Immune functions in methylmalonic aciduria. J Inherit Metab Disease 1984;7:12–14 [DOI] [PubMed] [Google Scholar]
- 46.Oberholzer VG, Levin B, Burgess EA, et al. . Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch Dis Child 1967;42:492–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong SN, Low LC, Lau YL, et al. . Immunodeficiency in methylmalonic acidaemia. J Paediatr Child Health 1992;28:180–183 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.