Abstract
Soft tissue defects are relatively common, yet currently used reconstructive treatments have varying success rates, and serious potential complications such as unpredictable volume loss and reabsorption. Human adipose-derived stem cells (ASCs), isolated from liposuction aspirate have great potential for use in soft tissue regeneration, especially when combined with a supportive scaffold. To design scaffolds that promote differentiation of these cells down an adipogenic lineage, we characterized changes in the surrounding extracellular environment during adipogenic differentiation. We found expression changes in both extracellular matrix proteins, including increases in expression of collagen-IV and vitronectin, as well as changes in the integrin expression profile, with an increase in expression of integrins such as αVβ5 and α1β1. These integrins are known to specifically interact with vitronectin and collagen-IV, respectively, through binding to an Arg-Gly-Asp (RGD) sequence. When three different short RGD-containing peptides were incorporated into three-dimensional (3D) hydrogel cultures, it was found that an RGD-containing peptide derived from vitronectin provided strong initial attachment, maintained the desired morphology, and created optimal conditions for in vitro 3D adipogenic differentiation of ASCs. These results describe a simple, nontoxic encapsulating scaffold, capable of supporting the survival and desired differentiation of ASCs for the treatment of soft tissue defects.
Introduction
Soft tissue defects are caused by a number of sources, including trauma, deep burns, tumor removal, and liposarcomas.1 Current standard reconstruction treatments of these defects include alloplastic implants and autologous fat transplants. While these methods have shown some success, they come with serious potential complications, including foreign body reaction, donor-site morbidity, and migration of implants.1 The likely occurrence of reabsorption in autologous fat transplants (currently the most commonly used treatment) makes this option less than optimal. It is believed that the benefits demonstrated by fat transplants are attributed primarily to a specific population of stem cells present in the tissue.2,3 Therefore, a need exists for improved, consistent reconstruction strategies capable of treating these soft tissue defects.
Adipose tissue, which is a rich source of easily isolated adipose-derived stem cells (ASCs), can be frequently harvested in large quantities utilizing standard liposuction procedures. These procedures have been shown to be effective and safe with low risk of donor-site morbidity.4,5 Additionally, ASCs are readily obtained in significant quantities from a patient, are robust, and capable of self-renewal. ASCs are multipotent mesenchymal stem cells that have the ability to differentiate down various lineages, including adipogenic, osteogenic, chondrogenic, muscular, cardiac, and endothelial, much like bone marrow-derived mesenchymal stem cells (BM-MSCs).1,6
While ASCs and BM-MSCs share many similarities beyond their potential lineages, including the majority of their confirmed in vitro immunophenotypes,7 it has been shown that ASCs have a higher proliferation potential with a more consistent growth rate in culture8 and are at least 10 times more abundant than BM-MSCs.9 Due to the accessibility of ASCs, the ease of cell culture and the short expansion times after isolation, it is feasible to use these cells to create autologous, patient-specific treatments, avoiding the potential complications of immune rejection. To reduce treatment outcome variability and promote targeted tissue regeneration, implanting only cells that provide the positive benefits (ASCs), in an engineered environment, is thought to be a viable approach.
By implanting the desired cell population, volume loss and reabsorption should be kept to a minimum, as all cells present retain the potential to proliferate as well as differentiate. The cells in the scaffold will be influenced by the native tissue and may encourage the cells of the host's tissue to regenerate.10,11 A synthetic scaffold that promotes preferential differentiation and supports survival of this population of cells would be critical to the effectiveness of such a treatment. Such a scaffold would need to provide the necessary mechanical support for the graft as well as the long-term survival of the grafted cells.
Poly(ethylene-glycol) (PEG) hydrogels have promising potential to serve as basic scaffolding materials in regenerative medicine.12–15 The inert backbone of PEG hydrogels, and the relative ease to functionalize sites mimicking extracellular matrix (ECM) proteins to help direct cell fate, make this type of hydrogel appealing for soft tissue reconstruction.16–18 ECM proteins contain sites that cells can bind to and use to interact with their surrounding environment, including neighboring cells. The sites a cell interacts with can affect it in a variety of ways, including inducing proliferation, signaling a specific pathway for differentiation, or initiating apoptosis.19–22
A binding site that numerous ECM proteins have been shown to contain is the Arg-Gly-Asp (RGD) sequence. Originally identified as a critical cell–ECM adhesion component in fibronectin (FN),23–25 RGD has since been identified in numerous other ECM proteins, such as vitronectin, collagen I, and collagen IV.26,27 It has been shown that adipocytes in vivo are surrounded by an ECM that includes many similar proteins such as multiple collagens (types I and IV included), multiple laminins, fibronectin, and others.28 The expression levels of some of these proteins have previously been shown to change, in vitro, during differentiation of ASCs down various lineages.1,29–31 During osteogenic differentiation increased deposition of collagens I and IV have been observed,1,29 whereas chondrogenic differentiation shows increases in collagen II.2,3,32 Changes in ECM expression of adipogenically differentiated mesenchymal cells isolated from bone marrow have been studied previously.4,5,27–29,33 However, little work has been done examining adipogenesis in the more readily abundant cell population from fat lipoaspirate.
Integrin receptors are one of the primary methods used by cells to recognize, and attach to, these RGD sequences and other peptide sequences in ECM proteins.1,6,25,34–36 These receptors are heterodimeric transmembrane proteins made up of an alpha and beta subunit. They interact with the surrounding ECM and relay messages from the extracellular environment to the cells, as well as from the cell to the ECM.7,34 Based on previous work showing that BM-MSCs change their integrin expression profile during other types of differentiation,8,29–31,37 we hypothesized that the integrin profile of ASCs being directed toward an adipogenic lineage will alter in response to a changing ECM environment. We further hypothesize that we can utilize various small integrin-interacting peptides, incorporated into a three-dimensional (3D) scaffold, to promote adipogenic differentiation of this stem cell population.
Materials and Methods
Cell lines and culture
Cells were isolated from two donors and a third line was purchased from Life Technologies (San Diego, CA). Donor cells were isolated from lipoaspirate by methods previously established.5,6,9,38–42 Briefly, 1–4 L of lipoaspirate was repeatedly washed with an equivalent volume of phosphate-buffered saline (PBS) containing 10 U/mL penicillin–streptomycin (Life Technologies) until the PBS layer was mostly clear. The washed lipoaspirate was then aliquoted into 225-cm2 flasks containing 0.15% collagenase type I solution (220.00 U/mg; Life Technologies) and 20 U/mL penicillin–streptomycin antibiotics. The flasks were incubated at 37°C on a slow shaker for 2 h, with additional vigorous shaking every 15 min. The collagenase was neutralized by the addition of fetal bovine serum (FBS; Atlas Biologicals, Fort Collins, CO) to a final concentration of 10%.
The cell solution was then centrifuged at 1000 g for 10 min to pellet the stem cell dense fraction. The supernatant was removed and the cell pellet was resuspended in 160 mM ammonium chloride solution to lyse any red blood cells present. Cells were then centrifuged at 1000 g for 10 min, and the resulting pellets were resuspended in media containing 60% Dulbecco's modified Eagle's medium (DMEM; Life Technologies) with 10% FBS and 40% MesenPRO medium (Life Technologies). Cells were passed through 50-mL sterile vacuum 60 μm Nylon cell strainers (Millipore, Billerica, MA), counted through hemocytometer, and then cryopreserved in a 3:2 mixture of DMEM +10% FBS:MesenPRO with 10% dimethyl sulfoxide. For culturing and expansion, cells were thawed into 3:2 DMEM +10% FBS:MesenPro media and switched to 100% MesenPRO 24–48 h later. The cells were characterized using flow cytometry for immunophenotypes in accordance with guidelines set forth by the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT).43 The cultures were determined to be CD44, CD90, and CD105 positive as well as CD45 and CD31 negative. All cells were passaged at least one time before use in experiments to ensure a population of only plastic-adherent ASCs. Cells were transduced with a constitutive mCherry–luciferase reporter plasmid (generously provided by Dr. Byron Hann, UCSF) and selected for using neomycin to obtain a population with only fluorescent-expressing cells, to facilitate visualization of cells in 3D culture. All subsequent experiments were preformed on cells between passage 2 and passage 5.
Adipogenic differentiation
ASCs were dissociated using TrypLE Select (Life Technologies) at 37°C for 5–10 min and inactivated by dilution with MesenPRO, as per the TrypLE protocol. The cells were spun down at 1200 rpm and resuspended in MesenPRO then seeded into appropriate vessels at varying densities for two-dimensional (2D) or 3D analysis. Cells in 2D cultures were grown in MesenPRO on uncoated tissue culture plastic until confluent and then switched to adipogenic differentiation medium. Cells for 3D cultures were treated in the same manner and then encapsulated in hydrogels at a high confluence, 2 × 106–2 × 107 cells/mL of hydrogel as explained below, and were placed directly into adipogenic differentiation media. Adipogenic differentiation medium consisted of alpha-modified minimum essential medium (Sigma-Aldrich, St. Louis, MO), supplemented with 10% FBS (Atlas Biologicals), 2 mM L-Glutamine (Life Technologies), 100 μM indomethacin (Sigma-Aldrich), 10 μg/mL insulin (Sigma-Aldrich), 1 μM dexamethasone (Sigma), 500 μM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), and 10 U/mL penicillin–streptomycin (Life Technologies).10,11,39,40,44
Two-dimensional and 3D cultures were allowed to differentiate for 21 days before analysis. Two-dimensional cultures were stained (“Immunocytochemistry,” below) and imaged on an Olympus IX70 (Olympus, Shinjuku, Tokyo) at 20× magnification. Three-dimensional cultures were stained with Hoechst 33342 (2 μg/mL; Invitrogen), LipidTOX (1:150; Life Technologies) for detection of lipid vacuoles, and CellMask (1:150; Life Technologies) to enhance mCherry reporter and visualize cell bodies. Three-dimensional gels were then imaged on an Olympus FluoView 1000 Spectral Confocal microscope. Images were analyzed using Imaris software (Bitplane, South Windsor, CT). The “surface object” module was utilized to create a 3D representation of the stack for each channel imaged and generate objects for the nuclei, lipid vacuoles, cellular membranes, and the tagged peptides independently. Threshold settings were optimized for each channel to allow for unbiased counting of analyzed surface types. The distance tool in the software was also used to determine which cells contained lipids. The size of the lipid vacuoles was determined using size functions in the Imaris software.
Immunocytochemistry
ASCs at passage 2 were seeded at 2.3 × 104 cells/cm2 on uncoated tissue culture plastic 12-well plates and grown to 100% confluence in MesenPRO. Cells were then either fixed or switched to adipogenic differentiation medium and differentiated for 21 days. Cells were fixed through incubation with 4% paraformaldehyde in 0.2 M sodium cacodylate buffer for 10 min and stored in 0.4% paraformaldehyde at 4°C for no more than 6 months. Cells were blocked using 5% bovine serum albumin (BSA) and permeabilized using 0.2% Triton X-100 in 1× PBS for 1 h at 4°C. Cells were stained overnight at 4°C with primary antibodies diluted in blocking buffer (See Table 1 for a list of primary antibodies and concentrations used). Cells were subsequently washed with PBS and stained with AlexaFluor secondary antibodies (20 μg/mL) and Hoechst 33342 (2 μg/mL; Life Technologies) for 1 h at room temperature before being imaged.
Table 1.
A List of Primary Antibodies Used in This Study
| Antibody | Species | Concentration (μg/mL) | Manufacturer | Catalog number |
|---|---|---|---|---|
| Integrin α1 | Mouse monoclonal | 2 | EDM Millipore, Temecula, CA | MAB1973Z-20 |
| Integrin α3 | Mouse monoclonal | 2 | EDM Millipore | MAB1952Z-20 |
| Integrin α5 | Mouse monoclonal | 2 | EDM Millipore | MAB 1956Z-20 |
| Integrin α6 | Rat monoclonal | 2 | EDM Millipore | MAB1378-20 |
| Integrin αV | Mouse monoclonal | 2 | EDM Millipore | MAB1953Z-20 |
| Integrin β1 | Mouse monoclonal | 2 | EDM Millipore | MAB1951Z-20 |
| Integrin β4 | Mouse monoclonal | 2 | EDM Millipore | MAB2060-20 |
| Integrin β5 | Rabbit polyclonal | 2 | EDM Millipore | AB1926-20 |
| Fibronectin | Rabbit polyclonal | 10 | One World Lab, San Diego, CA | C0195 |
| Collagen type IV | Rabbit polyclonal | 4 | Abcam, Cambridge, MA | ab19808 |
| Laminin-α4 | Rabbit polyclonal | 10 | One World Lab, San Diego, CA | bs-11055R |
| Collagen Type 1 | Rabbit polyclonal | 10 | Rockland, Inc., Pottstown, PA | 600-401-103-0.1 |
| Vitronectin | Rabbit polyclonal | 5 | Abcam, Cambridge, MA | ab113700 |
3D hydrogels
A solution of 4-arm PEG-Thiol (Creative PEGWorks, Chapel Hill, NC) at 10 wt% was prepared in 37°C cell culture media. A solution of divinyl sulfone (DVS) crosslinker (Sigma-Aldrich) was prepared in 4°C cell culture media. Custom ECM-based peptides were synthesized by BioMatik (linear RGD and vitronectin-derived peptides) and received as a kind gift from the laboratory of Erkki Ruoslathi, UC Santa Barbara (cycloRGD) with FITC (linear RGD and vitronectin-derived) and FAM (cycloRGD) fluorescent markers attached. Gels were functionalized with peptides at 80 μM concentrations before DVS crosslinker addition. Cells were expanded on uncoated tissue culture plastic after transduction of the mCherry plasmid and selection with G418.
After dissociation with TrypLE Select and pelleting, cells were resuspended in PEG/peptide solution and the DVS crosslinker was added at a 10:1 ratio (DVS:PEG) immediately before plating. Fifty microliters of gel solution was then plated into each well of a Teflon mold. The polymerizing PEG solution was incubated 30 min at 37°C then each gel was placed directly into appropriate culture media (adipogenic differentiation or MesenPRO) in separate wells of a 24-well plate.
QGel without RGD (ref. 1004; QGel, Lausanne, Switzerland) was used following their recommended protocol. Briefly, ASCs were harvested and pelleted in the same manner as in the DVS-PEG gels and resuspended in 125 μL of MesenPRO with peptide added at 80 μM. One vial of QGel powder was dissolved in 375 μL of Buffer A and quickly vortexed. The hydrogel mixture was then added to the cell suspension and quickly plated into molds in 50 μL aliquots and allowed to polymerize at 37°C for 30 min then immediately transferred into culture media.
Adhesion assay
The ECM-based peptides were covalently conjugated to amine-modified Primaria 96-well plates (353872; Corning Inc., Corning, NY) through an N-hydroxysuccinimide ester (NHS) NHS-PEG12-Maleimide bifunctional linker (PI22112; Thermo Scientific Pierce, Carlsbad, CA). NHS groups on the linker were reacted with amines on the plates to form amine bonds, and consequently attach the linker to the plates through incubation with 100 μM NHS-PEG12-Maleimide in PBS for 30 min at room temperature. Peptides were diluted to 20 μg/mL in PBS and conjugated overnight at 4°C to react the free cysteine on the peptides with the maleimide groups on the linker and create thioester bonds.12–15,45 Plates were then blocked for 2 h at room temperature with 1% BSA and washed with PBS to remove excess BSA. ASCs were detached from tissue culture plastic after expansion using TrypLE Select (Life Technologies), diluted for inactivation in serum-free cell culture media, pelleted, resuspended in serum-free media and then stained in suspension with Hoechst 33342 (2 μg/mL; Invitrogen) for 10 min at room temperature for subsequent nuclear visualization.
Cells were counted on a hemocytometer, then seeded in triplicate for each peptide at 2.9 × 104 cells/cm2 and allowed to adhere to the modified plates for 3 h at 37°C.16–18,23 It was determined that a shorter amount of time did not allow for strong-enough adhesion to withstand even gentle washing. Nonadherent cells were removed by very gently washing plates three times with room temperature PBS. A negative control of no linker/no peptide and linker/no peptide were included. Each well was imaged immediately after the 3-h attachment period on an Olympus IX70 fluorescent microscope (Olympus) at 10× magnification at the center of each well. Fluorescent nuclei were counted and cell area was calculated using ImageJ software (National Institute of Health). Statistical analysis was performed using a two-tailed Student's t-test for this and all subsequent assays.
Culture of ASCs on full-length ECM proteins
All proteins were used at a final concentration of 10 μg/mL. Protein dilutions were made fresh and 24-well culture plates were coated overnight at 4°C. Laminin (L4544; Sigma-Aldrich), fibronectin (4305-FN-200; R&D systems, Minneapolis, MN), vitronectin (2308-VN-050; R&D systems), collagen type I (6220-CL; R&D Systems), and collagen type IV (CC076; Millipore) full-length proteins were used. Passage 4 ASCs were thawed from cryopreservation and seeded at a density of 3 × 104 cells/cm2. Cells were harvested at days 1, 3, 5, and 7 using TrypLE Select and counted using the Scepter Handheld Automated Cell Counter (Millipore). Media were harvested from the cultures at each time point before cell harvest and snap frozen in liquid nitrogen, for ELISA testing. The Adiponectin, Leptin, Basic Fibroblast Growth Factor (bFGF), Vascular Endothelial Growth Factor (VEGF), Hepatocyte Growth Factor (HGF) (Life Technologies), and Stromal Cell-Derived Factor-1 (R&D Systems) ELISA Kits were used according to the manufacturer's protocol to assay the amount of each factor present in the spent media at various time points.
Proliferation assay
QGel was prepared as described in the 3D hydrogel section above. ASCs were seeded at a density of 10 × 106 cells/mL and cultured after polymerization in 24-well plates in MesenPRO media for 7, 14, and 21 days. At each time point, as well as on day 0, QGels were placed in individual 1.5-mL Eppendorf tubes and frozen at −80°C. Once all samples had been collected, QGels were digested using Proteinase K (0.5 mg/mL) overnight at 60°C and DNA content was determined following the CyQUANT NF Cell Proliferation Assay protocol for nonadherent cells (Life Technologies).
Peptide-functionalized PEG-DVS hydrogels were made as described above in the 3D hydrogels section. Cells were seeded at a density of 10 × 106 cells/mL and cultured in MesenPRO media for 0, 7, 14, or 21 days. Because the PEG-DVS gels were unable to be digested to quantify DNA content the hydrogels were instead stained with Hoechst, to visualize nuclei, and imaged on an Olympus FluoView Spectral Confocal microscope. The average number of nuclei for each peptide condition, in three fields, for each of three individual gels was determined at desired time points using Imaris software (Bitplane, South Windsor, CT). The spots module was used to create a 3D image of the 150 μm Z-stacks and a spot was created based on intensity value of nuclei visualized in the DAPI channel, allowing determination of individual nuclei. Threshold settings were optimized and used for impartial counting of nuclei, independent of peptide condition. Nuclei counts were averaged for each gel and then for each peptide condition. A Student's t-test was used to determine statistical significance.
Results
As previously described, ASCs isolated from human lipoaspirate can be differentiated down multiple lineages, including adipogenic, chondrogenic, osteogenic, and endothelial.1,6,19–22,46 The differentiation process of many cell types has been shown to be affected by the extracellular environment.19–21,23–25,47,48 During differentiation, the surrounding extracellular environment must change to properly support the new, maturing cell population. In an effort to understand this process during adipogenic differentiation, we used immunocytochemistry to examine the expression of ECM proteins and integrin subunits in undifferentiated ASCs and in ASCs that had undergone adipogenic differentiation for 21 days.
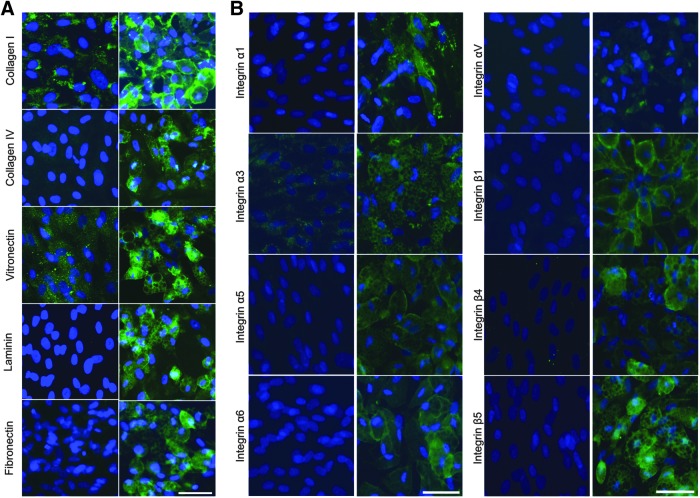
As shown in Figure 1A certain ECM proteins, such as collagen I and vitronectin, were expressed at relatively low levels by undifferentiated ASCs. After 21 days of adipogenic differentiation there was a change in the expression pattern of these proteins as well as an increase in overall expression. Other proteins, such as collagen IV, laminin-α4, and fibronectin were not found to be present in undifferentiated ASC cultures, but could be readily detected in the ASC-derived adipocyte population. Still other ECM proteins such as elastin (not shown) were detected, but showed no change in immunoreactivity after adipogenic differentiation.
FIG. 1.
Analysis of ECM protein and integrin expression in ASCs before and after adipogenic differentiation. (A) ECM proteins indicated (green) were detected by immunocytochemistry in undifferentiated ASCs (left) and ASCs subjected to adipogenic differentiation for 21 days (right). Nuclei (blue) were detected by Hoechst staining. Scale bar = 50 μm. (B) Integrin subunits indicated (green) were detected by immunocytochemistry in undifferentiated ASCs (left) and ASCs subjected to adipogenic differentiation for 21 days (right). Nuclei (blue) were detected by Hoechst staining. Scale bars = 50 μm. ASCs, adipose-derived stem cells; ECM, extracellular matrix.
The increase in expression of select ECM components, specifically collagen IV, vitronectin, and fibronectin, which were not detected in undifferentiated ASCs, supports the idea that the differentiated cells demonstrated increased utilization of integrin receptors that recognize Arg-Gly-Asp (RGD) sequences present in all of these proteins.26,27,49–53 Laminins containing the α4 subunit are recognized by different integrins not involved in RGD signaling. There are at least eight known integrins that can bind to laminin28,54 and adipocytes use a small number of these.33
While integrin expression of BM-MSC-derived adipocytes has been examined previously,29 the expression of integrins in adipocytes derived from lipoaspirate, compared to their undifferentiated counterparts, has yet to be examined fully. Using immunocytochemistry, we characterized the expression of integrin subunits in undifferentiated ASCs and ASC-derived adipocytes that had been differentiated for 21 days (Fig. 1B). We detected several integrin subunits in ASC-derived adipocytes, but not in undifferentiated ASCs (specifically α1, α5, α6, αV, β4, and β5), as well as some integrin subunits (α3 and β1) that were expressed in both the differentiated and undifferentiated cells, although at higher levels in the ASC-derived adipocytes than the undifferentiated ASCs. In addition, there were subunits that were found to have no discernible change in expression after 21 days of adipogenic differentiation, such as α2, α4, and β3 (not shown). These results indicate that both ECM and ECM receptors are dynamically regulated during adipogenic differentiation.
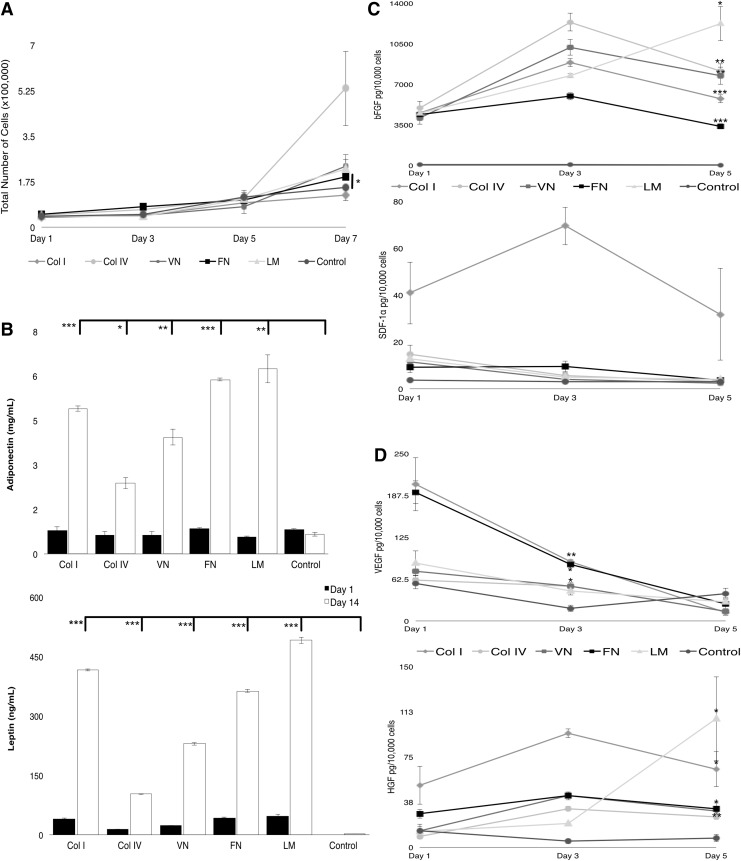
To examine how the ECM environment might affect the adipogenic differentiation of ASCs, we cultured cells grown on full-length proteins in 2D and assessed their proliferation and expression of adipogenic and endothelial markers over time (Fig. 2). These ECM proteins were selected based on changes in expression of ECM proteins and integrins observed during adipogenic differentiation of ASC cultures, with the goal of mimicking the normal adipose ECM environment and promoting this pathway. Proliferation of undifferentiated ASCs cultured in maintenance conditions on selected ECM proteins was assessed over one week. In general, cell proliferation was relatively slow in the low-serum media, both on ECM proteins as well as tissue culture plastic (control). However, ASCs cultured on collagen IV showed a significant increase in cell number compared to the control (Fig. 2A) (p < 0.05). The only protein that showed a significant, although slight, increase in proliferation at day 7 compared to uncoated tissue culture plastic was fibronectin (p < 0.05). All other ECM proteins tested supported proliferation rates similar to that of ASCs cultured on untreated tissue culture plastic. Next, we examined secreted factors important in adipogenic differentiation (specifically leptin and adiponectin) using an ELISA.
FIG. 2.
Effects of full-length ECM proteins on ASCs. (A) Proliferation on different substrates was quantified (*p < 0.05) (B) Secretion of adipogenic factors (adiponectin and leptin) by ASCs cultured on different ECM proteins was quantified by ELISA at day 1 (dark bars) and day 14 (light bars). Secretion of bFGF and SDF1- α (C) and VEGF and HGF (D) by ASCs cultured on different ECM proteins was quantified by ELISA over time (***p < 0.001; **p < 0.01; *p < 0.05). bFGF, basic fibroblast growth factor; Col I, collagen I; Col IV, collagen IV; FN, fibronectin; HGF, hepatocyte growth factor; LM, Laminin; SDF-1α, stromal cell-derived factor 1α; VEGF, vascular endothelial growth factor; VN, vitronectin.
We found that after 14 days of culture in maintenance conditions on selected ECM proteins ASCs grown on all proteins secreted significantly higher levels of both leptin and adiponectin relative to ASCs cultured on untreated tissue culture plastic (Fig. 2B) (p < 0.001). ASCs cultured on fibronectin or laminin secreted the highest levels of adiponectin and cells on collagen IV secreted the lowest levels. Secretion of four angiogenic factors was also investigated: bFGF, stromal cell-derived factor 1α (SDF-1α) (Fig. 2C), VEGF, and HGF (Fig. 2D). Because these factors mediate the formation of new blood vessels, recruit endothelial progenitors, and stimulate vasculogenesis and mitogenesis, their expression during adipogenic differentiation would be useful for eventual clinical applications using ASC-derived adipocytes in tissue grafts and transplants. Results showed that secretion of bFGF and HGF was higher at day 5 on all protein substrates as compared to uncoated control. SDF-1α levels were higher on protein substrates at day one (especially collagen I), but did not significantly differ from uncoated controls at day 5. VEGF levels decrease over time, but were higher, compared to uncoated controls, after 3 days on all substrates. Taken together, these results show that ECM protein could be utilized to improve adipogenic differentiation in a 3D culture.
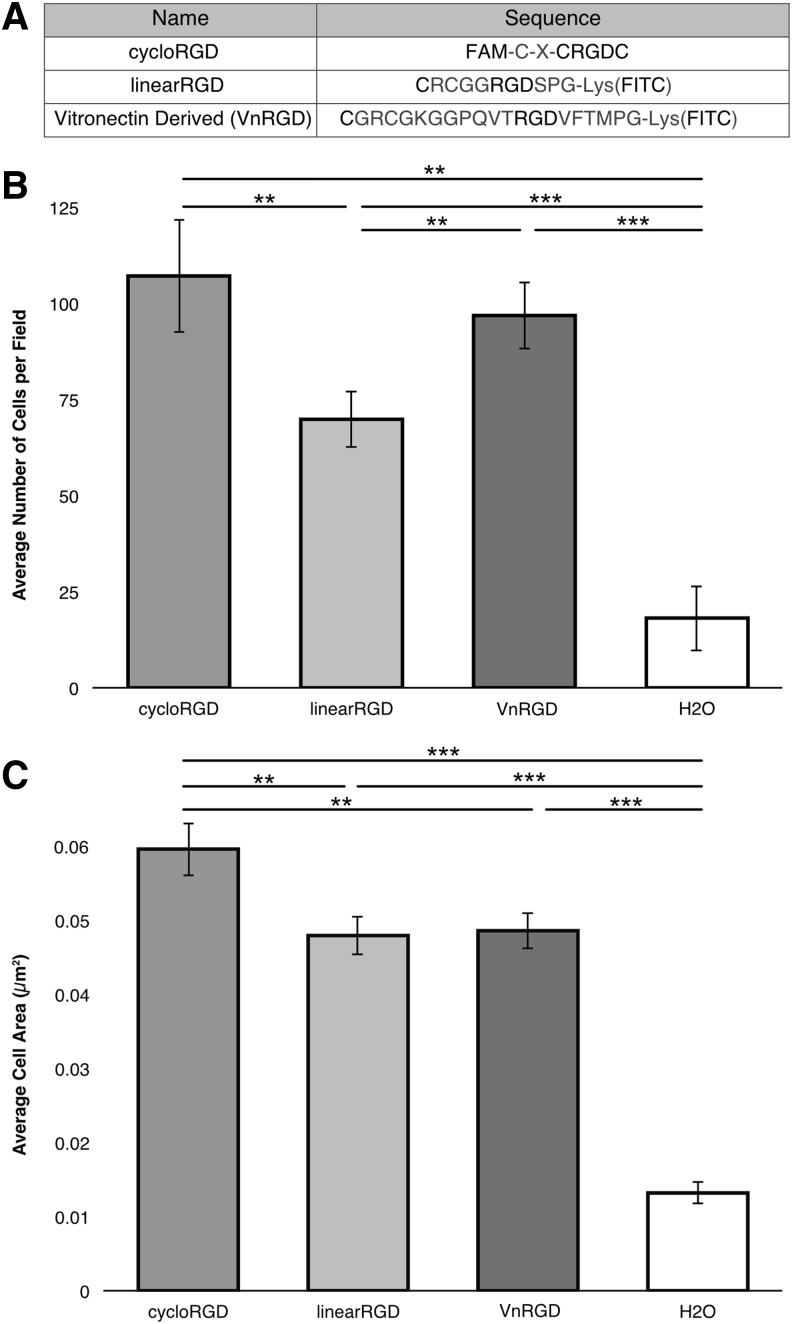
Since four out of the five ECM proteins examined above contain functional RGD peptide sequences, we chose to investigate the ability of different RGD-containing peptides to promote adhesion of ASCs. We investigated peptides with varying adhesivity: a cyclized RGD peptide (cycloRGD), a linear RGD peptide (linearRGD), and a vitronectin-derived RGD peptide (VnRGD) (Fig. 3A). It has been previously shown that cells adhere to cycloRGD significantly more strongly (10 times) than to the linearized form.55 The VnRGD peptide was derived from the full-length vitronectin protein sequence, containing the RGD sequence56 based on work done previously.57 To investigate the adhesive properties of each RGD peptide on ASCs, we performed 3 h-long adhesion assays.
FIG. 3.
ASC Adhesion and spreading on RGD peptides. (A) Sequence of peptides. (B) Average number of cells that attach to peptides in two-dimensional culture, after 3 h, showing varying adhesion, compared to no-peptide control. (C) Average area of cells after attachment on various peptides showing extent of spreading after 3 h of attachment compared to no-peptide control (**p < 0.01, ***p < 0.001).
We found that significantly greater number of ASCs adhered to cycloRGD compared to linearRGD (p = 0.04), but similar number of ASCs adhered to VnRGD and cycloRGD (p = 0.55) (Fig. 3B). ASCs adhered to each RGD peptide in significantly greater number compared to the no-peptide control (p < 0.001). The extent of the ASC spreading on the RGD peptides, which is indicative of the strength of adhesion of the ASCs on the surface, was also quantified (Fig. 3C). We found that individual ASCs spread over significantly greater distances when plated on cycloRGD compared to linearRGD, VnRGD, and no-peptide controls (p = 0.008, p = 0.009, and p < 0.001, respectively). However, no difference in the amount of spreading was observed when comparing ASCs assayed on linearRGD and VnRGD (p = 0.85). These results indicate that cycloRGD allows for both strong initial attachment and spreading of ASCs and that the VnRGD peptide provides good initial attachment, but less cell spreading of ASCs. Overall, these three RGD peptides were found to support varying degrees of ASC adhesion and cell spreading, giving us the ability to investigate the effects of varied adhesion on adipogenic differentiation of ASCs in 3D.
We next incorporated the RGD peptides into 3D hydrogels to examine possible effects on proliferation and adipogenic differentiation of the ASCs. We initially used QGel, which is a commercially available gel that has previously been used successfully in in vivo studies58 to examine whether peptides could be incorporated, retained, and whether ASCs could survive and be differentiated down an adipogenic lineage in 3D. Peptides with a free cysteine were added into QGel solution just before cells were encapsulated at a concentration of 80 μM. Using a CyQUANT assay to determine the DNA content of the QGels after 21 days in maintenance media, it was shown that the undifferentiated ASCs could in fact survive in 3D cultures and that the cycloRGD peptide provided the best condition for the ASCs to attach and proliferate in the Qgels (Fig. 4A) with significantly more growth at day 21 than VnRGD and no-peptide conditions (p = 0.0002 and p = 0.0018).
FIG. 4.
QGel 3D Hydrogel proliferation and adipogenic differentiation. (A) Proliferation of the ASCs in maintenance conditions in 3D culture as determined by a CyQUANT assay. (B) 3D reconstructions of ASCs adipogenically differentiated for 21 days in Qgel containing various attachment peptides. (C) The average number of nuclei visualized per field showing the overall number of cells present after 21 days of adipogenic culture. (D) The percent of total cells present in each field that are associated with a lipid vacuole. (E) The average size of all the lipid vacuoles present for each condition (*p < 0.05, **p < 0.01, ***p < 0.001). 3D, three-dimensional.
All three peptides supported proliferation of ASCs in 3D cultures more than gels without attachment sites. After culture for 21 days in QGel with adipogenic differentiation media, all three peptides were still present, as shown by the continued presence of fluorescent signal (Fig. 4B; green). Cells were detected under all conditions, including those cultured in QGel without peptides, as shown by staining for nuclei. Likewise, all conditions allowed for adipogenesis, as shown by a LipidTOX stain for neutral lipids in lipid vacuoles (Fig. 4B; orange). Cell membranes were visualized using the fluorescent reporter transfected into the cells before encapsulation and enhanced for clarity using CellMask (Fig. 4B; red). Quantification of cell numbers showed that all gels contained about the same number of cells, regardless of the peptide (Fig. 4C).
While all conditions allowed for adipogenic differentiation, it was found that the cycloRGD peptide yielded a significantly lower percentage of cells containing lipid vacuoles (p = 0.04) when compared to the VnRGD condition (Fig. 4D). The percentage of cells containing lipid vacuoles was similar for ASCs differentiated with the linearRGD, VnRGD, and QGel without peptides. Using Imaris software we were also able to measure the 3D area of the lipid vacuoles and found that the lipid vacuoles formed by ASCs differentiated with cycloRGD were significantly smaller than those formed in QGel without peptides (p = 0.04) (Fig. 4E). The size of the lipid vacuoles formed by ASCs differentiated with linearRGD, VnRGD, and QGel without peptides were not significantly different in size.
We next incorporated RGD peptides in a simple, encapsulating, PEG-based hydrogel with a DVS crosslinker (PEG-DVS),59 and tested proliferation and adipogenic differentiation of ASCs. ASCs were seeded into these PEG-DVS gels at 10 × 106 cells/mL, with consistent peptide concentrations as in the QGels. Undifferentiated ASCs were grown in the PEG-DVS gels, in MesenPRO, for up to 21 days and imaged using a confocal microscope to determine the rate of proliferation in each of the peptide conditions. At day 21 it was found that the linearRGD peptide actually supported proliferation significantly better than all other peptides, and no-peptide conditions (cycloRGD, p = 0.009; VnRGD, p = 0.004; H2O, p = 0.0001) (Fig. 5A). In this hydrogel system the cycloRGD and vitronectin-derived peptides supported the same (limited) proliferation and both were more supportive than no peptide.
FIG. 5.
PEG-DVS 3D hydrogel proliferation and adipogenic differentiation. (A) Proliferation of the ASCs in maintenance conditions in 3D culture over 21 days. (B) 3D reconstructions of ASCs adipogenically differentiated for 21 days in PEG-DVS gels containing various attachment peptides. (C) The average number of nuclei visualized per field showing the overall number of cells present after 21 days of adipogenic culture. (D) The percent of total cells present, in each field that are associated with a lipid vacuole. (E) The average size of all the lipid vacuoles present for each condition (*p < 0.05, **p < 0.01, ***p < 0.001). DVS, divinyl sulfone; PEG, poly(ethylene-glycol).
When ASCs were seeded in PEG-DVS gels and grown in adipogenic media for 21 days, all peptide conditions, including the gels without peptide, supported adipogenesis (Fig. 5B), as indicated by staining of neutral lipids with LipidTOX (Fig. 5B; orange). Additionally, high levels of peptide incorporation were still apparent after 21 days in culture, as assessed by the visualization of the FITC/FAM tag on the peptides (Fig. 5B; green). Unlike with the QGel system, we observed a difference in the total number of cells present in the PEG-DVS systems after 21 days of adipogenic differentiation, with a significantly greater number of cells present in the VnRGD system compared to the linearRGD system (p = 0.004) (Fig. 5C). Also differing from the results using QGel, we found that there was no significant difference in the percentage of cells containing lipid vacuoles (Fig. 5D). However, the size of the lipid vacuoles in the VnRGD and linearRGD systems were both significantly larger compared to the size of the lipid vacuoles in the cycloRGD system (VnRGD, p = 0.001; linearRGD, p = 0.04) (Fig. 5E). Overall, our results with the PEG-DVS system agree with the Qgel data in that the VnRGD peptide was the most supportive of adipogenic differentiation.
Discussion
In this study, we have shown that the ECM profile of ASCs changes over 21 days of adipogenic differentiation. Increases in collagens type I and type IV, as well as in vitronectin and fibronectin indicate a likely increase in RGD sites throughout the culture. This change in proteins present outside of the cells correlates to the changing integrin profile of the cells. Integrin α1β1 has been shown to be involved in the attachment to collagen IV and laminins containing the α4 subunit,30,60 while α3β1, α6β1, and α6β4 have all been shown to interact specifically with laminins.60–62 αVβ5, αVβ1, and α5β1 along with several other integrin heterodimers have been shown to interact with RGD sequences from varying ECM proteins, including collagen I, fibronectin, and vitronectin.30,35,63 The increase of each of these subunits in adipogenically differentiated cultures, along with increases in ECM proteins that they are able to interact with, supports the hypothesis that these integrins may be important to the differentiation process.
It has been found that BM-MSCs adipogenically differentiated for 21 days only showed a significant increase in α6 integrin subunit and significant decreases most notably in α3 and β4.29 Although there are a few common changes of integrin subunit expression between BM-MSCs and ASCs (like the increase in α6), there are more notably some potentially important differences. One such difference is the lack of increase in α5 and αV in the BM-MSCs. β1 can dimerize with a large number of α subunits, including α5 and αV, which are both used for binding of RGD sequences in fibronectin. The increase in expression we observed in β1, coupled with the expression of α5 and αV in the derived adipocytes further supports the importance of interaction with RGD-containing ECM proteins in adipogenic differentiation of ASCs. Such changes can be potentially utilized in the design of a synthetic 3D environment to enhance adipogenic differentiation.
We found that ECM protein substrates in a 2D environment increase the secretion of factors favorable for adipogenic differentiation (relative to uncoated controls) such as adiponectin and leptin.64–68 Other secreted factors important in angiogenesis and vasculogenesis, specifically bFGF, sDF1α, VEGF, and HGF69–72 were also increased. These data are particularly important, as the survival of a 3D tissue graft in vivo will most likely require the formation of some vasculature to bring blood and essential nutrients to the cells present in the tissue graft. While the expression of these factors is clearly essential for developing tissue grafts, it is also important to keep in mind that ASCs themselves are capable of differentiating into endothelial cells,46,73 which would allow these cells to potentially survive in vivo by differentiating into both adipocytes and endothelial cells capable of creating their own vasculature.
By examining the important links between how the changing extracellular environment of differentiating ASCs affects the expression of integrins, we were able to determine candidate peptides for incorporation into our 3D structures. Based on the expression of multiple ECM proteins that contain RGD sequences in the adipogenically differentiated cultures, we decided to examine how different RGD peptides might affect adipogenic differentiation in a 3D system. Knowing that mature adipocytes are natively found in a relatively soft environment,74 we chose three RGD-based peptides to provide different strengths of adhesion to examine the effect of this initial adhesion strength on the undifferentiated ASCs for their eventual adipogenic differentiation. The initial interaction of these cells with their environment may provide important cues for their eventual differentiation.19,21
We found that the greatest number of undifferentiated ASCs adhered to cycloRGD, although this was not significantly greater than the number of cells adhered to VnRGD. The difference between the cycloRGD and VnRGD peptides was seen in the extent to which the ASCs spread out during the adhesion period. The size of the undifferentiated ASCs grown on the VnRGD over 3 h was less than that on the cycloRGD and similar to cell spreading on linearRGD. Overall, while both cycloRGD and VnRGD provide good sites for initial attachment of undifferentiated ASCs, the VnRGD peptide keeps the cells in a more rounded formation, which may be favorable for adipogenic differentiation.75,76
The strong initial adhesion and reduction in spread morphology of the undifferentiated ASCs on the VnRGD peptide conditions were found to be beneficial to adipogenic differentiation in a 3D environment as well, in both the commercially available QGel as well as a novel formulation of a PEG-DVS hydrogel. We saw that in the QGel the number of cells with lipid vacuoles was significantly lower in the cycloRGD gel, which promoted spreading. While the percentage of lipid vacuole-containing cells did not differ in the PEG-DVS gels between the different peptides used, the size of the lipid vacuoles did vary with cells cultured in the VnRGD-containing gels typically forming significantly larger lipid vacuoles. This production of larger lipid vacuoles is potentially due to the more rounded morphology of the cells differentiated in the VnRGD-containing hydrogels.
When comparing the commercially available QGel and the PEG-DVS gels, we found that while both systems incorporate and maintain the presence of the RGD peptides well, the PEG-DVS gels seem to be more supportive of adipogenic growth and differentiation of ASCs compared to QGel. It may be that while the basic thiolene chemistry employed in both gels is acceptable for differentiation of ASCs into adipocytes, the somewhat different PEG-DVS gel system provides a slightly more conducive environment for the growth and differentiation of ASCs to adipocytes, possibly due to fewer crosslinking events.
We were able to determine that when ASCs isolated from lipoaspirate were encapsulated into a PEG-based hydrogel, they were able to not only survive for at least 3 weeks, but also able to proliferate in an undifferentiated state as well as differentiate into adipocytes. The incorporation of RGD-based peptides into these gels affected the efficiency of adipogenic differentiation. Specifically, we found that a strongly adhesive peptide, cycloRGD, may not provide ideal adipogenic-inducing interactions for ASCs in vitro. VnRGD, however, may create the most inductive environment for adipogenic differentiation; while it supported significant initial adhesion of undifferentiated ASCs, it still allowed the cells to remain more rounded. It also proved to offer the most beneficial environment for adipogenic differentiation in 3D hydrogels, with cells differentiated in its presences showing large lipid vacuoles, indicating more mature adipocytes. These data show that the ECM-based attachment sites provided within a 3D environment have a direct effect on the efficiency of adipogenic differentiation of ASCs in vitro. Although these conditions may not precisely reflect the in vivo environment, they represent an important foundation to developing potential treatments. The incorporation of RGD-containing peptides into tissue grafts might later be utilized for improved adipogenic differentiation of ASCs, with the ultimate goal of soft tissue reconstruction in vivo.
Acknowledgments
This work was supported by grant bio09R-156745 from the University of California Discovery grant and the Industry–University Cooperative Research Program. Additional support came from the California Institute for Regenerative Medicine (CIRM; grants DR1-01444, CL1-00521, TB1-01177, TG2-01151 [D.O.C.]), CIRM Major Facilities Grant (FA1-00616), and the University of California Santa Barbara Institute for Collaborative Biotechnologies from the U.S. Army Research Office (Grant W911NF-09–0001). The content within does not necessarily reflect the position or policy of the government, and endorsement should not be inferred. The authors acknowledge the use of the NRI-MCDB Microscopy Facility and the Spectral Laser Scanning Confocal supported by the Office of The Director, National Institutes of Health of the NIH under Award # S10OD010610. We thank Dr. Byron Hann for providing the mCherry-luciferase vector. T.N.C. is a CIRM Scholar.
Authors' Contribution
T.N.C.: Conception and design, collection and/or assembly of data, data analysis and interpretation, and article writing. C.R.H. and R.K.A.R.: Conception and design, collection and/or assembly of data, data analysis and interpretation. K.S.: Collection and/or assembly of data, data analysis and interpretation. D.J.B.: Conception and design. C.J.H.: Conception and design, financial support. D.M. and D.V.E.: Conception and design, collection and/or assembly of data, data analysis and interpretation, article writing, and financial support. D.O.C.: Conception and design, collection and/or assembly of data, data analysis and interpretation, article writing, final approval of article, and financial support.
Disclosure Statement
No conflicting financial interests exist.
References
- 1.Gimble J.M., Katz A.J., and Bunnell B.A. Adipose-derived stem cells for regenerative medicine. Circ Res 100, 1249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimura K., Sato K., Aoi N., Kurita M., Hirohi T., and Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 32, 48, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimura K., Asano Y., Aoi N., Kurita M., Oshima Y., Sato K., et al. . Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J 16, 169, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., et al. . Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13, 4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oedayrajsingh-Varma M.J., van Ham S.M., Knippenberg M., Helder M.N., Klein-Nulend J., Schouten T.E., et al. . Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8, 166, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Astori G., Vignati F., Bardelli S., Tubio M., Gola M., Albertini V., et al. . “In vitro” and multicolor phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med 5, 55, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baer P.C. Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. WJSC 6, 256, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng L., Jia Z., Yin X., Zhang X., Liu Y., Chen P., et al. . Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev 17, 761, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Zhu X., Shi W., Tai W., and Liu F. The comparition of biological characteristics and multilineage differentiation of bone marrow and adipose derived Mesenchymal stem cells. Cell Tissue Res 350, 277, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Wu I., Nahas Z., Kimmerling K.A., Rosson G.D., and Elisseeff J.H. An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg 129, 1247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domenis R., Lazzaro L., Calabrese S., Mangoni D., Gallelli A., Bourkoula E., et al. . Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res Ther 6, 2, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alhadlaq A., Tang M., and Mao J.J. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng 11, 556, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Burdick J.A., and Anseth K.S. Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials 23, 4315, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Hwang N.S., Kim M.S., Sampattavanich S., Baek J.H., Zhang Z., and Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells 24, 284, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Williams C.G., Kim T.K., Taboas A., Malik A., Manson P., and Elisseeff J. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 9, 679, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Cukierman E., Pankov R., Stevens D.R., and Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science 294, 1708, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Peyton S.R., Raub C.B., Keschrumrus V.P., and Putnam A.J. The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials 27, 4881, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Salinas C.N., Cole B.B., Kasko A.M., and Anseth K.S. Chondrogenic differentiation potential of human mesenchymal stem cells photoencapsulated within poly(ethylene glycol)–arginine-glycine-aspartic acid-serine thiol-methacrylate mixed-mode networks. Tissue Eng 13, 1025, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Daley W.P., Peters S.B., and Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci 121, 255, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Engler A.J., Sen S., Sweeney H.L., and Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Guilak F., Cohen D.M., Estes B.T., Gimble J.M., Liedtke W., and Chen C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5, 17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metallo C.M., Mohr J.C., Detzel C.J., dePablo J.J., VanWie B.J., and Palecek S.P. Engineering the stem cell microenvironment. Biotechnol. Prog 23, 18, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Pierschbacher M., Hayman E.G., and Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A 80, 1224, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierschbacher M.D., and Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Ruoslahti E., and Pierschbacher M.D. Arg-Gly-Asp: a versatile cell recognition signal. Cell 44, 517, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Taubenberger A.V., Woodruff M.A., Bai H., Muller D.J., and Hutmacher D.W. The effect of unlocking RGD-motifs in collagen I on pre-osteoblast adhesion and differentiation. Biomaterials 31, 2827, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Ullah M., Sittinger M., and Ringe J. Extracellular matrix of adipogenically differentiated mesenchymal stem cells reveals a network of collagen filaments, mostly interwoven by hexagonal structural units. Matrix Biol 32, 452, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Mariman E.C.M., and Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci 67, 1277, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frith J.E., Mills R.J., Hudson J.E., and Cooper-White J.J. Tailored integrin–extracellular matrix interactions to direct human mesenchymal stem cell differentiation. Stem Cells Dev 21, 2442, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goessler U.R., Bugert P., Bieback K., Stern-Straeter J., Bran G., Hörmann K., et al. . Integrin expression in stem cells from bone marrow and adipose tissue during chondrogenic differentiation. Int J Mol Med 21, 271, 2008 [PubMed] [Google Scholar]
- 31.Lam J., and Segura T. The modulation of MSC integrin expression by RGD presentation. Biomaterials 34, 3938, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., and Pittenger M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4, 415, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Noro A., Sillat T., Virtanen I., Ingerpuu S., Back N., Konttinen Y.T., et al. . Laminin production and basement membrane deposition by mesenchymal stem cells upon adipogenic differentiation. J Histochem Cytochem 61, 719, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giancotti F.G. Integrin signaling. Science 285, 1028, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12, 697, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Freitas V.M., Vilas-Boas V.F., Pimenta D.C., Loureiro V., Juliano M.A., Carvalho M.R., et al. . SIKVAV, a laminin alpha1-derived peptide, interacts with integrins and increases protease activity of a human salivary gland adenoid cystic carcinoma cell line through the ERK 1/2 signaling pathway. Am J Pathol 171, 124, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volloch V., and Olsen B.R. Why cellular stress suppresses adipogenesis in skeletal tissue, but is ineffective in adipose tissue: control of mesenchymal cell differentiation via integrin binding sites in extracellular matrices. Matrix Biol 32, 365, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adipose stem cell ioslation protocol. 2006. Available from: www.collaslab.com Accessed December20, 2012
- 39.Kern S., Eichler H., Stoeve J., Klüter H., and Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24, 1294, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Mitchell J.B., McIntosh K., Zvonic S., Garrett S., Floyd Z.E., Kloster A., et al. . Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24, 376, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Peroni D., Scambi I., Pasini A., Lisi V., Bifari F., Krampera M., et al. . Stem molecular signature of adipose-derived stromal cells. Exp Cell Res 314, 603, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Jürgens H.S., Neschen S., Ortmann S., Scherneck S., Schmolz K., Schüler G., et al. . Development of diabetes in obese, insulin-resistant mice: essential role of dietary carbohydrate in beta cell destruction. Diabetologia 50, 1481, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., et al. . Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 15, 641, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernacki S.H., Wall M.E., and Loboa E.G. Isolation of human mesenchymal stem cells from bone and adipose tissue. Methods Cell Biol 86, 257, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Meng Y., Eshghi S., Li Y.J., Schmidt R., Schaffer D.V., and Healy K.E. Characterization of integrin engagement during defined human embryonic stem cell culture. FASEB J 24, 1056, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao Y., Sun Z., Liao L., Meng Y., Han Q., and Zhao R.C. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun 332, 370, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Bodine S.C. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Kilian K.A., and Mrksich M. Directing stem cell fate by controlling the affinity and density of ligand-receptor interactions at the biomaterials interface. Angew Chem Int Ed 51, 4891, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dedhar S., Ruoslahti E., and Pierschbacher M.D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol 104, 585, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayman E.G., Pierschbacher M.D., and Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol 100, 1948, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruoslahti E., and Pierschbacher M.D. New perspectives in cell adhesion: RGD and integrins. Science 238, 491, 1987 [DOI] [PubMed] [Google Scholar]
- 52.Jin H., and Varner J. Integrins: roles in cancer development and as treatment targets. Br J Cancer 90, 561, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chillakuri C.R., Jones C., and Mardon H.J. Heparin binding domain in vitronectin is required for oligomerization and thus enhances integrin mediated cell adhesion and spreading. FEBS Lett 584, 3287, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Durbeej M. Laminins. Cell Tissue Res 339, 259, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Cheng S., Craig W.S., Mullen D., Tschopp J.F., Dixon D., and Pierschbacher M.D. Design and synthesis of novel cyclic RGD-containing peptides as highly potent and selective integrin alpha IIb beta 3 antagonists. J Med Chem 37, 1, 1994 [DOI] [PubMed] [Google Scholar]
- 56.Suzuki S., Oldberg A., Hayman E.G., Pierschbacher M.D., and Ruoslahti E. Complete amino acid sequence of human vitronectin deduced from cDNA. Similarity of cell attachment sites in vitronectin and fibronectin. EMBO J 4, 2519, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melkoumian Z., Weber J.L., Weber D.M., Fadeev A.G., Zhou Y., Dolley-Sonneville P., et al. . Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol 28, 606, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Fuerst A., Derungs S., Rechenberg von B., Auer J.A., Schense J., and Watson J. Use of a parathyroid hormone peptide (PTH(1–34))-enriched fibrin hydrogel for the treatment of a subchondral cystic lesion in the proximal interphalangeal joint of a warmblood filly. J Vet Med A Physiol Pathol Clin Med 54, 107, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Raeber G.P., Lutolf M.P., and Hubbell J.A. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J 89, 1374, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hall D.E., Reichardt L.F., Crowley E., Holley B., Moezzi H., Sonnenberg A., et al. . The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J Cell Biol 110, 2175, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soejima Y., Inoue M., Takahashi Y., Uozaki H., Sawabe M., and Fukusato T. Integrins αvβ6, α6β4 and α3β1 are down-regulated in cholangiolocellular carcinoma but not cholangiocarcinoma. Hepatol Res 44, E320, 2014 [DOI] [PubMed] [Google Scholar]
- 62.Ishikawa T., Wondimu Z., Oikawa Y., Gentilcore G., Kiessling R., Brage S.E., et al. . Laminins 411 and 421 differentially promote tumor cell migration via α6β1 integrin and MCAM (CD146). Matrix Biol 38, 69, 2014 [DOI] [PubMed] [Google Scholar]
- 63.Hersel U., Dahmen C., and Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385, 2003 [DOI] [PubMed] [Google Scholar]
- 64.Cristancho A.G., and Lazar M.A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 12, 722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharma A.M., and Staels B. Peroxisome proliferator-activated receptor and adipose tissue—understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab 92, 386, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Seo J.B., Moon H.M., Kim W.S., Lee Y.S., Jeong H.W., Yoo E.J., et al. . Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression. Mol Cell Biol 24, 3430, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosen E.D. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids 73, 31, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Lowe C.E., O'Rahilly S., and Rochford J.J. Adipogenesis at a glance. J Cell Sci 124, 2681, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Comoglio P.M. Structure, biosynthesis and biochemical properties of the HGF receptor in normal and malignant cells. EXS 65, 131, 1993 [PubMed] [Google Scholar]
- 70.Kazemi S., Wenzel D., Kolossov E., Lenka N., Raible A., Sasse P., et al. . Differential role of bFGF and VEGF for vasculogenesis. Cell Physiol Biochem 12, 55, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Mirshahi F., Pourtau J., Li H., Muraine M., Trochon V., Legrand E., et al. . SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res 99, 587, 2000 [DOI] [PubMed] [Google Scholar]
- 72.Villaschi S., and Nicosia R.F. Angiogenic role of endogenous basic fibroblast growth factor released by rat aorta after injury. Am J Pathol 143, 181, 1993 [PMC free article] [PubMed] [Google Scholar]
- 73.Szöke K., Beckstrøm K.J., and Brinchmann J.E. Human adipose tissue as a source of cells with angiogenic potential. Cell Transplant 21, 235, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Kochhar A., Wu I., Mohan R., Condé-Green A., Hillel A.T., Byrne P.J., et al. . A comparison of the rheologic properties of an adipose-derived extracellular matrix biomaterial, lipoaspirate, calcium hydroxylapatite, and cross-linked hyaluronic acid. JAMA Facial Plast Surg 16, 405, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., and Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell 6, 483, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Mathieu P.S., and Loboa E.G. Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Eng Part B Rev 18, 436, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]