Abstract
The established urinary antibiotic nitroxoline has recently regained considerable attention, due to its potent activities in inhibiting angiogenesis, inducing apoptosis and blocking cancer cell invasion. These features make nitroxoline an excellent candidate for anticancer drug repurposing. To rapidly advance nitroxoline repurposing into clinical trials, the present study performed systemic preclinical pharmacodynamic evaluation of its anticancer activity, including a methyl thiazolyl tetrazolium assay in vitro and an orthotopic urological tumor assay in vivo. The current study determined that nitroxoline exhibits dose-dependent anti-cancer activity in vitro and in urological tumor orthotopic mouse models. In addition, it was demonstrated that the routine nitroxoline administration regimen used for urinary tract infections was effective and sufficient for urological cancer treatment, and 2 to 4-fold higher doses resulted in obvious enhancement of anticancer efficacy without corresponding increases in toxicity. Furthermore, nitroxoline sulfate, one of the most common metabolites of nitroxoline in the urine, effectively inhibited cancer cell proliferation. This finding increases the feasibility of nitroxoline repurposing for urological cancer treatment. Due to the excellent anticancer activity demonstrated in the present study, and its well-known safety profile and pharmacokinetic properties, nitroxoline has been approved to enter into a phase II clinical trial in China for non-muscle invasive bladder cancer treatment (registration no. CTR20131716).
Keywords: nitroxoline, 5-nitro-8-hydroxy-quinoline, bladder cancer, renal cancer, anticancer activity, drug repurposing
Introduction
Nitroxoline (5-nitro-8-hydroxy-quinoline) is an established antibiotic that has been widely used in European, Asian and African countries for >50 years (1). It is particularly effective for the treatment of urinary tract infections (UTI) due to its unique pharmacokinetic properties (1). When administered orally, nitroxoline is rapidly absorbed into the plasma and then excreted into urine (2). It also has a long retention time in urine (2), thus, making it ideal for UTI treatment. Recently, nitroxoline has gained considerable attention due to its potent anticancer properties. It was first identified as an effective inhibitor of angiogenesis by two parallel screens: A target-based screen for methionine aminopeptidase-2 (MetAP-2) inhibitors from a library of 175,000 chemical compounds and a cell-based screen using the Johns Hopkins Drug Library to identify currently used clinical drugs that can also inhibit human umbilical vein endothelial cell (HUVEC) proliferation (3). Thereafter, more studies confirmed the anticancer activity of nitroxoline and further demonstrated its anticancer mechanism (3–5). In particular, nitroxoline demonstrated potent anticancer activity against various types of cancer cell, including lymphoma, leukemia, glioma, bladder cancer, breast cancer, pancreatic cancer and ovarian cancer cells (3–5). As well as inhibiting angiogenesis, nitroxoline was also able to induce cancer cell apoptosis (4), and suppress cancer cell migration and invasion (6). Taken together, as an established drug for UTI treatment, nitroxoline has exhibited great promise as a novel candidate for anticancer treatment.
Drug repurposing, the process of identifying novel uses for existing drugs, has been gaining popularity in recent years. The major advantage of this approach is that the pharmacokinetic, pharmacodynamic and toxicity profiles of known drugs are generally well known due to of years of clinical history (1,7). Thus, exploring established non-cancer drugs for anticancer activity provides an opportunity to rapidly advance therapeutic strategies into clinical trials. To rapidly advance nitroxoline repurposing for anticancer treatment into clinical trials, the present study performed systemic preclinical pharmacodynamic evaluation of nitroxoline, with specific aims to confirm the anticancer activity of nitroxoline, select cancer types suitable for nitroxoline treatment and provide a reference dosage regimen for future clinical application.
Materials and methods
Drugs and materials
Nitroxoline (Lot: KLL-20110601 J) and its metabolite nitroxoline sulfate (Lot: 5209-005A1), which was isolated and purified from the urine of nitroxoline-treated mice, were provided by Jiangsu Asieris Pharmaceuticals Co., Ltd. (Taizhou, China). Cisplatin (Lot: F20100420) was purchased from Qilu Pharmaceutical Co., Ltd. (Jinan, China).
Cell lines and cell culture
The human cell lines used for the in vitro and in vivo anticancer activity assays are listed in Table I. Among them, HUVEC, HepG2, A549, LoVo, MCF7, T24, 5637 and J82 cell lines were obtained from American Type Tissue Culture Collection (ATCC; Manassas, VA, USA), and cultured in their respective ATCC-specified medium. KCC853, SGC-7901 and human embryonic lung fibroblast (HELF) cell lines were purchased from China Infrastructure of Cell Line Resources (Beijing, China), and cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). All cells were maintained in a humidified incubator with 5% CO2 at 37°C.
Table I.
Anti-cancer activity of nitroxoline in vitro.
| Cell line | Origin of cell line | Inhibition rate at 80 µM, % | IC50, µM | Inhibition rate at 10 µM, % |
|---|---|---|---|---|
| HUVEC | Human umbilical vein endothelial cells | 97.1±1.2 | 7.06±1.64 | 66.1±7.5 |
| T24 | Bladder transitional cell carcinoma | 99.4±0.5 | 1.68±0.09c | 94.0±2.4b |
| 5637 | Bladder grade II carcinoma | 98.0±1.9 | 2.45±0.23b | 87.9±1.9b |
| KCC853 | Clear cell renal cell carcinoma | 96.1±2.3 | 2.96±0.89a | 86.0±7.8a |
| HepG2 | Hepatocellular carcinoma | 95.3±2.2 | 5.75±1.04 | 57.4±0.6 |
| SGC-7901 | Gastric carcinoma | 96.1±2.9 | 7.18±0.19 | 43.6±2.5b |
| A549 | Lung adenocarcinoma | 75.1±1.6c | 11.43±2.07 | 53.6±6.7 |
| MCF7 | Breast adenocarcinoma | 74.1±0.9c | 20.58±4.83c | 51.1± 2.1a |
| J82 | Bladder transitional cell carcinoma | 57.8±1.2c | 20.83±5.47b | 46.8±7.4a |
| LoVo | Colorectal carcinoma | 45.0±1.1c | 5.99±0.56 | 33.6±1.4c |
| HELF | Human embryonic lung fibroblasts | 39.8±1.2c | 13.26±2.61 | 2.9±0.8c |
Data are presented as mean ± standard deviation.
P<0.05
P<0.01
P<0.001 vs. HUVEC cells (one-way analysis of variance with Tukey's post-hoc test). IC50, half maximal inhibitory concentration.
Cytotoxicity assay
Cells were seeded in 96-well plates at a density of 3,000 cells per well. After 24 h culture in normal growth medium, the cells were exposed to graded concentrations (84, 42, 21, 10.5, 5.25, 2.63, 1.32 and 0.66 µM) of nitroxoline or nitroxoline sulfate for 96 h. Following treatment, the viability of the cells was determined by performing a methyl thiazolyl tetrazolium (MTT) assay. Briefly, MTT (0.5 mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added and cells were incubated for 4 h at 37°C. After removing the supernatant, the formazan crystals produced were dissolved in 200 µl dimethyl sulfoxide (Sigma-Aldrich) and the absorbance at 570 nm was determined using a microplate reader (Multiskan Ascent; Thermo Labsystems; Thermo Fisher Scientific, Inc.). Data are presented as the mean ± standard deviation (SD) derived from quadruplicate samples of at least two independent experiments. The half maximal inhibitory concentration (IC50) was determined by logistic non-linear regression analysis of the dose-response curves (Origin software, version 7.0; OriginLab Corporation, Northampton, MA, USA).
In vivo anti-cancer effect assay
All animal care and experimental procedures conformed to the Guide for the Care and Use of Laboratory Animals (8) as adopted and promulgated by Beijing Medical Experimental Animal Care Commission. The present study was approved by The Laboratory Animal Ethics Committee of Beijing Institute of Radiation Medicine (Beijing, China; certificate no., BIRMSPF-120125A). A total of 220 male athymic nude mice (Balb/c nu/nu; 8 weeks old) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China), housed in controlled specific pathogen-free conditions (25±1°C constant temperature, 40–60% relative humidity, 12-h light/dark cycle) and allowed free access to food and water during the study period.
A total of 140 nude mice were used to generate mouse models of orthotopic bladder cancer, as previously described (3,9,10). Briefly, mice were anesthetized by intraperitoneal injection of 100 mg/kg ketamine/10 mg/kg xylazine (Sigma-Aldrich). A superficial 6–0 polypropylene purse-string suture [Medico (Huaian) Co., Ltd., Huaian, China] was placed around the urethral meatus prior to passing a lubricated 24-gauge intravenous catheter [Medico (Huaian) Co., Ltd.] through the urethra into the bladder. Subsequent to aspirating urine and irrigating the bladder with phosphate-buffered saline (PBS; Invitrogen; Thermo Fisher Scientific, Inc.), a stylet needle was used to slightly impair the bladder urothelium by gently scraping; this action also facilitated tumor cell seeding. Thereafter, T24 or 5637 bladder carcinoma cells (2×106) were instilled into the bladders of nude mice (70 mice per tumor model) as single-cell suspensions in 200 µl PBS and the purse-string suture was tied down for a 2.5-h period, during which the mice were kept anesthetized.
A total of 80 nude mice were used to establish a mouse model of orthotopic renal cancer by subrenal capsule implantation. First, 5×106 KCC853 clear cell renal carcinoma cells in 200 µl PBS were subcutaneously injected into the back of nude mice. When the subcutaneous tumor volume reached 400–600 mm3, tumor tissues were removed from the hosts and cut into small sections measuring ~2×2×2 mm3 in size under sterile conditions. The tumor sections were then implanted into the subcapsular area of the right kidney of the nude mice, as previously described (11,12).
Each of the three mouse model groups were sorted into 5 or 6 groups (n=7–14 per group) with almost equal mean body weight 2 weeks after establishment of the orthotopic tumor models. The groups included the control group, the cisplatin group and the nitroxoline groups. Cisplatin was used as the positive control drug by intravenous injection at a dose of 5 mg/kg once a week. Mice were orally administered with nitroxoline as the treatment group, according to the dosage regimens described in Tables II–IV. The control group was orally administered with normal saline (10 ml/kg) twice a day. Animals were sacrificed by CO2 asphyxiation 3–4 weeks after treatment to allow necropsy, and the whole bladders or kidneys were harvested and assessed for tumor weight. The whole weight of the bladder and orthotopic tumor was calculated as bladder tumor weight, as the majority of the orthotopic tumors infiltrated into the muscles of the bladders and separate of the two tissues was difficult. Renal tumor weight was calculated by subtracting the weight of the left normal kidney from that of the right tumor-bearing kidney. The tumor growth inhibitory rate was calculated as follows: Inhibitory rate (%) = [1 - (mean tumor weight of treated group / mean tumor weight of control group)] × 100.
Table II.
Effects of nitroxoline on 5637 cell bladder cancer orthotopic xenografts in nude mice.
| Drug administration | Anti-cancer activity | Toxicity | |||||
|---|---|---|---|---|---|---|---|
| Drug | Dose, mg/kg | Schedule | Route | Tumor weight, mga | Inhibition rate, % | Body weight loss, %a | Mortalities/total mice, n |
| Control | Vehicle | BID for 21 days | p.o. | 164±61 | 14.8±4.9 | 4/14 | |
| Cisplatin | 5 | QW for 3 weeks | i.v. | 79±41c | 52.0 | 19.8±9.1 | 2/14 |
| Nitroxoline | 30 | BID for 21 days | p.o. | 88±32b | 46.4 | 14.9±3.7 | 1/7 |
| Nitroxoline | 60 | BID for 21 days | p.o. | 63±24c | 61.4 | 12.0±3.8 | 2/14 |
| Nitroxoline | 120 | BID for 21 days | p.o. | 50±21c | 69.6 | 7.4±3.2 | 5/14 |
| Nitroxoline | 240 | BID for 21 days | p.o. | 43±19c | 73.6 | 12.2±4.7 | 2/7 |
Data are presented as mean ± standard deviation.
P<0.01
P<0.001 vs. vehicle control (one-way analysis of variance with Tukey's post-hoc test). BID, twice a day; QW, once a week; p.o., oral; i.v. intravenous.
Table IV.
Effects of nitroxoline on KCC853 cell renal tumor orthotopic xenografts in nude mice.
| Drug administration | Anti-cancer activity | Toxicity | |||||
|---|---|---|---|---|---|---|---|
| Drug | Dose, mg/kg | Schedule | Route | Tumor weight, mga | Inhibition rate, % | Body weight loss, %a | Mortalities/total mice, n |
| Control | Vehicle | BID for 21 days | p.o. | 183±73 | 11.8±4.3 | 2/16 | |
| Cisplatin | 5 | QW for 3 weeks | i.v. | 99±35b | 46.0 | 15.0±3.3 | 1/16 |
| Nitroxoline | 60 | BID for 21 days | p.o. | 96±41b | 47.5 | 13.2±5.7 | 2/16 |
| Nitroxoline | 120 | BID for 21 days | p.o. | 89±23b | 51.4 | 9.2±3.7 | 4/16 |
| Nitroxoline | 240 | BID for 21 days | p.o. | 67±28b | 63.7 | 7.7±5.4 | 2/16 |
Data are presented as mean ± standard deviation.
P<0.001 vs. vehicle control (one-way analysis of variance with Tukey's post-hoc test). BID, twice a day; QW, once a week; p.o., oral; i.v. intravenous.
Immunohistochemical staining
The tumor tissues were fixed in formalin (Wuhan Boster Biological Technology, Ltd., Wuhan, China), embedded in paraffin (Wuhan Boster Biological Technology, Ltd.) and sectioned into 4-mm sections. The tissue sections were deparaffinized and rehydrated as follows: Sections were incubated in three washes of xylene (Sigma-Aldrich) for 5 min each; sections were sequentially incubated in 100, 95, 90, 80 and 70% ethanol (Sinopharm Chemical Reagent Co., Ltd., Beijing, China) for 10 min each; and sections were washed twice in distilled water for 5 min each. The tissues were then blocked with 3% H2O2 to quench the endogenous peroxidase activity. Antigen retrieval was performed by boiling the slides in sodium citrate buffer (10 mM, pH 6.0; Wuhan Boster Biological Technology, Ltd.) for 20 min. Slides were then incubated with non-specific binding blocking buffer (PBS + 5% bovine serum albumin + 0.1% Tween-20; Sigma-Aldrich) at room temperature for 1 h, followed by rabbit anti-human polyclonal Ki67 (1:50 dilution; cat. no. BA2888; Wuhan Boster Biological Technology, Ltd.), rabbit anti-human polyclonal survivin (1:50 dilution; cat. no. BA14055; Wuhan Boster Biological Technology, Ltd.) and rabbit anti-human polyclonal cluster of differentiation (CD)31 (1:50 dilution; cat. no. BA1346; Wuhan Boster Biological Technology, Ltd.) antibodies overnight at 4°C. After washing with PBS, slides were then incubated with goat anti-rabbit polyclonal horseradish peroxidase-conjugated secondary antibody (1:200 dilution; cat. no. SV0002; Wuhan Boster Biological Technology, Ltd.) at room temperature for 1 h. The immunohistochemistry reaction was developed with a DAB substrate kit (Wuhan Boster Biological Technology, Ltd.) prior to counterstaining the slides with hematoxylin. Hematoxylin and eosin (H&E) staining was performed using a H&E staining kit in accordance with the manufacturer's protocol (Wuhan Boster Biological Technology, Ltd.). Briefly, deparaffinized and rehydrated tissue slides were incubated in hematoxylin solution for 2 min. Subsequent to washing twice with distilled water for 5 min each, the slides were sequentially immersed in acid alcohol, distilled water and ammonia solution for 10 sec each. Slides were then washed twice with distilled water for 5 min each, immersed in 80% ethanol for 10 min, incubated in eosin solution for 15 sec, and then sequentially immersed in 90, 95 and 100% ethanol for 10 min each. Finally, the slides were incubated twice in xylene for 5 min each and mounted with DPX Mountant for histology (Sigma-Aldrich). Negative controls were treated the same way except the primary antibody was replaced by the isotype-matched anti-human IgG. The immunohistochemical staining was examined using an EVOS X1 microscope (Advanced Microscopy Group; Thermo Fisher Scientific, Inc.).
Statistical analysis
Data are presented as the mean ± SD. Equal variance was assessed by Bartlett's test, and the statistical significance of differences between groups was compared by one-way analysis of variance test followed by Tukey's post-hoc test (GraphPad Prism 5.0; GraphPad Software Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
In vitro sensitivities of various cancer cell lines to the anticancer activity of nitroxoline
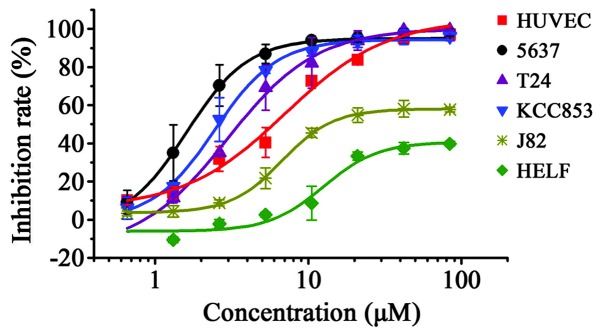
Nitroxoline inhibited the growth of all cell lines in a dose-dependent manner (Table I). However, there were marked differences among its effects on individual cell lines. At the maximum concentration (80 µM), nitroxoline caused complete (>95%) growth inhibition of the HUVEC, T24, 5637, KCC853, HepG2 and SGC7901 cell lines (Table I). Among them, the T24, 5637 and KCC853 urological cancer cell lines demonstrated an obvious left shift in their dose-response curves (Fig. 1) and significant 2–4-fold decreases in IC50 values compared with the well-known target cell line HUVEC (Table I) (3). These results indicate nitroxoline is more effective at directly inhibiting the proliferation of certain urological cancer cells than others. Considering that the antimicrobial activity of nitroxoline has been demonstrated at concentration of >10 µM (13), and that daily nitroxoline dosage of 400–750 mg (for adults) resulted in ≤10 µM nitroxoline retention in human plasma and urine after 24 h (2), the present study next analyzed nitroxoline from a translational perspective by comparing the anticancer effects of 10 µM nitroxoline on the aforementioned cell lines. T24, 5637 and KCC853 exhibited 86–94% growth inhibition upon 10 µM nitroxoline treatment. Nitroxoline was significantly more effective and sensitive than in HUVEC cells, which exhibited only ~66% growth inhibition. However, not all bladder cancer cell lines were equally sensitive to nitroxoline. J82 cells exhibited ~47% growth inhibition following 10 µM nitroxoline treatment, and its IC50 value was 7–10-fold higher than those of T24, 5637 and KCC853 (Table I and Fig. 1). In addition, HELF, a non-cancerous cell line, was insensitive to the cytotoxic effect of nitroxoline, exhibiting just ~2.9% growth inhibition at 10 µM nitroxoline; this result confirmed the excellent safety profile of nitroxoline, as established by its 50-year clinical history. Taken together, the results indicate that certain urological cancer cell lines, such as T24, 5637 and KCC853, are sensitive to nitroxoline treatment and are suitable for establishing urological tumor orthotopic mouse models that use the unique pharmacokinetic property of nitroxoline (high accumulation in urinary tract) to their advantage. Therefore, T24, 5637 and KCC853 urological cancer cell lines were selected for further in vivo pharmacodynamic analysis.
Figure 1.
Dose-dependent proliferation inhibition rates of nitroxoline on various cell types, as determined by methyl thiazolyl tetrazolium assay. Different cell lines exhibit differential sensitivities to nitroxoline treatment in vitro. Dose-response curves were obtained using a logistic nonlinear regression analysis model. Data are presented as mean ± standard deviation from quadruplicate samples of at least two independent experiments. HUVEC, human umbilical vein endothelial cells; HELF, human embryonic lung fibroblast.
Effects of nitroxoline on the growth of urological tumor orthotopic xenografts
Nitroxoline dose-dependently inhibited the growth of 5637 cell bladder tumor orthotopic xenografts (Fig. 2A; Table II). The lowest dose (30 mg/kg, twice a day) of nitroxoline used for cancer treatment in nude mice was equivalent to the common dose (750 mg/day) used for human UTI treatment, according to equivalent dose calculation based on the body surface area of different species (14). This low dose of nitroxoline (30 mg/kg) resulted in ~46% tumor growth inhibition compared with the vehicle control. As the dose of nitroxoline increased, tumor growth inhibition rates demonstrated corresponding increases and amounted to ~74% at the highest dose of 240 mg/kg. The T24 cell bladder tumor orthotopic xenograft it exhibited a more sensitive response to nitroxoline treatment (Fig. 2B; Table III), with as high as 83.9% of growth inhibition at the dose of 120 mg/kg. Even at the lowest dose (30 mg/kg), nitroxoline significantly inhibited the growth of T24 bladder tumors by 58.5%. The effect on tumor growth was not specific to bladder tumors; nitroxoline was also effective at inhibiting the growth of KCC853 cell renal tumor orthotopic xenografts, resulting in 47.5, 51.4 and 63.7% of growth inhibition at doses of 60, 120 and 240 mg/kg, respectively (Fig. 2C; Table IV). Cisplatin, the positive control drug, inhibited the growth of 5637, T24 and KCC853 orthotopic tumors by 46.4, 86.6 and 46.0%, respectively, suggesting that the anticancer effects of 5 mg/kg cisplatin in nude mice were equivalent to that of 60–120 mg/kg nitroxoline. Taken together, nitroxoline appears to effectively inhibit the growth of orthotopic urological tumors in a dose-dependent manner.
Figure 2.
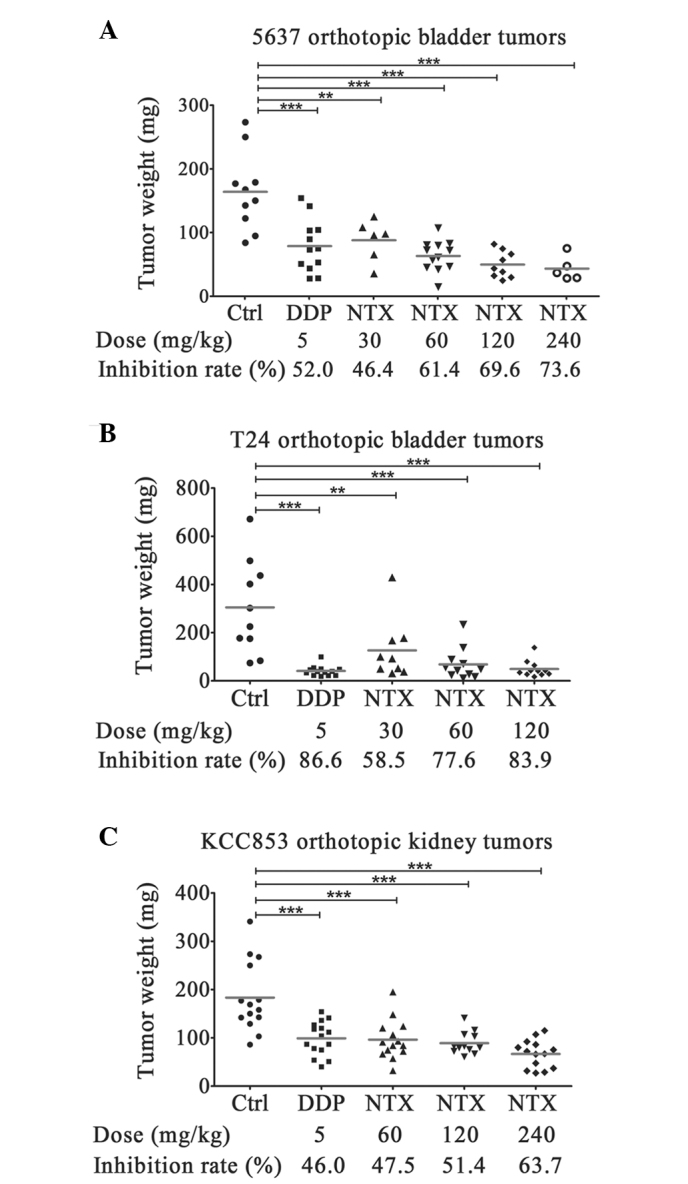
Dose-dependent anti-cancer effects of NTX on urological tumor orthotopic xenografts, as determined by tumor weight. (A) 5637 orthotopic bladder tumors; (B) T24 orthotopic bladder tumors; (C) KCC853 orthotopic kidney tumors. **P<0.01, ***P<0.001 vs. vehicle controls (one-way analysis of variance with Tukey's post-hoc test). Ctrl, control; DDP, cisplatin; NTX, nitroxoline.
Table III.
Effects of nitroxoline on T24 cell bladder cancer orthotopic xenografts in nude mice.
| Drug administration | Anti-cancer activity | Toxicity | |||||
|---|---|---|---|---|---|---|---|
| Drug | Dose, mg/kg | Schedule | Route | Tumor weight, mga | Inhibition rate, % | Body weight loss, %a | Mortalities/total mice, n |
| Control | Vehicle | BID for 28 days | p.o. | 304±195 | 18.0±2.6 | 4/14 | |
| Cisplatin | 5 | QW for 4 weeks | i.v. | 41±21 | 86.6 | 23.1±7.3 | 1/14 |
| Nitroxoline | 30 | BID for 28 days | p.o. | 126±125b | 58.5 | 22.1±5.4 | 5/14 |
| Nitroxoline | 60 | BID for 28 days | p.o. | 69±65c | 77.6 | 16.7±2.6 | 3/14 |
| Nitroxoline | 120 | BID for 28 days | p.o. | 49±35c | 83.9 | 14.3±2.3 | 3/14 |
Data are presented as mean ± standard deviation.
P<0.01
P<0.001 vs. vehicle control (one-way analysis of variance with Tukey's post-hoc test). BID, twice a day; QW, once a week; p.o., oral; i.v. intravenous.
Toxic effects were concurrently evaluated by mouse mortality and body weight loss rates. These factors were not exhibited in a corresponding dose-dependent manner (Tables II–IV). In fact, no significant differences in mouse mortality and body weight loss rates were observed between each group, suggesting that these outcomes may not occur as side-effects of nitroxoline but due to unavoidable factors, such as postoperative complications or exacerbation of the orthotopic tumors.
Overall, low doses of nitroxoline (30 mg/kg, twice a day) were sufficient to significantly inhibit urological tumor growth in orthotopic mouse models. Higher doses of nitroxoline (60–240 mg/kg, twice a day) demonstrated obviously enhanced anticancer efficacy without corresponding increases in toxicity.
Nitroxoline-treated orthotopic urological tumors exhibit low proliferative characteristics
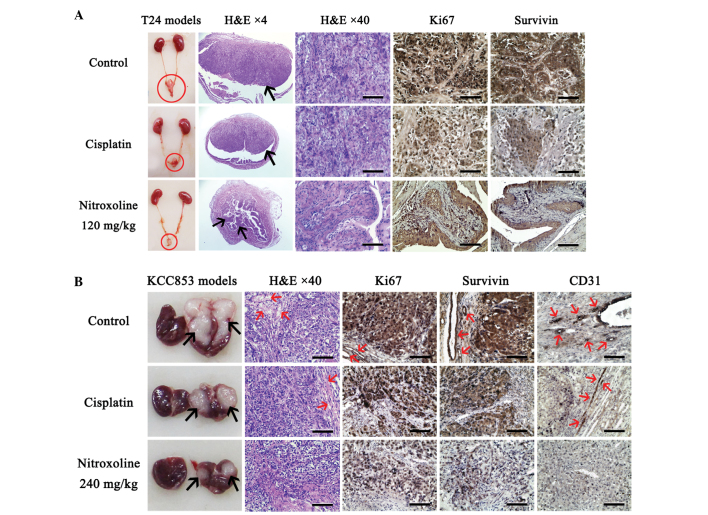
Due to the high sensitivity of T24 cells to nitroxoline treatment in vitro and in vivo (Table I; Fig. 3), further histological analysis and cytotoxic assays were performed using T24 cells or T24 xenograft tumors. Histological examination by H&E staining revealed that T24 orthotopic bladder tumors from the vehicle control and cisplatin groups had features common in non-papillary urothelial carcinoma (15), characterized by the presence of neoplastic urothelial nests (Fig. 3A). However, the majority of bladder tumors removed from nitroxoline-treated mice had features of papillary urothelial carcinoma (15), showing fused papillae and disordered architecture (Fig. 3A). Therefore, nitroxoline appears to significantly delay malignant progression of T24 tumors. Immunohistochemistry further demonstrated that the nested tumor cells in control T24 tumors had strong Ki67 (16) and survivin (17) staining, while markedly weaker staining of both proliferative markers were observed in tumors treated with cisplatin or nitroxoline (Fig. 3A). Taken together, the results indicate that nitroxoline may delay the malignant progression of T24 orthotopic bladder tumors by inhibiting their proliferative activity.
Figure 3.
Nitroxoline-treated urological tumor orthotopic xenografts demonstrate low proliferative characteristics. Representative anatomy, H&E staining and immunohistochemical (DAB and hematoxylin) staining images of (A) T24 bladder tumor and (B) KCC853 renal tumor orthotopic xenografts with different treatments. (A) Red circles in anatomy images indicate bladders. Arrows in H&E staining images (magnification, ×4) indicate tumor areas in the bladders that stained more intensively compared with normal bladder cells. Dark brown immunohistochemical staining for Ki67 and survivin indicate the strong proliferative characteristics of cancer cells. (B) Arrows in anatomy images indicate tumor areas in the right kidneys. Left kidneys in the same anatomy images were from the same mice and were used as self-control normal kidneys. Red arrows in Ki67 and survivin staining images indicate endothelial cells with strong proliferative activity. Red arrows in CD31 staining images indicate microvessels. Scale bar, 100 µm. H&E, hematoxylin and eosin; CD, cluster of differentiation.
All the H&E-stained KCC853 orthotopic renal tumors showed features of clear cell renal cell carcinoma (Fig. 3B) (18). The difference between each group was the density of microvessels in the tumors. KCC853 orthotopic tumors of the vehicle control and cisplatin groups had numerous erythrocyte-filled blood vessels, while microvessels were rarely observed in the nitroxoline-treated tumors, particularly tumors in the highest dose (240 mg/kg) group (Fig. 3B). Immunohistochemistry further confirmed the inhibitory effect of nitroxoline on angiogenesis. Ki67 and survivin exhibit strong staining in proliferative tumor and proliferative endothelial cells; this strong staining was obviously observed in both cells types of control KCC853 tumor samples (Fig. 3B). By contrast, cisplatin- and nitroxoline-treated tumors only demonstrated weak Ki67 and survivin staining in tumor cells, and no visible staining in endothelial cells (Fig. 3B). Furthermore, immunohistochemical staining of CD31, a direct marker of blood vessels (19), confirmed that the density of microvessels was very low in nitroxoline-treated tumors compared with that in the vehicle control tumors (Fig. 3B). Taken together, the results indicate that nitroxoline may effectively inhibit angiogenesis and proliferation of KCC853 orthotopic renal tumors.
Nitroxoline sulfate, a metabolite of nitroxoline, also exhibits anticancer activity
Following oral administration, ~99% of the excreted nitroxoline is eliminated as metabolites in the urine and only 1.0% is eliminated as the parent component (2,20). Thus, the present study aimed to determine whether the metabolites of nitroxoline also exhibit anticancer activity. According to the relative peak area signals, the two major metabolites of nitroxoline, nitroxoline sulfate and glucuronide, contribute most to the urinary excretion of nitroxoline (20). Due to the poor stability of nitroxoline glucuronide in vitro, it is rarely used the in vitro evaluation of anticancer activity. However, nitroxoline sulfate can be successfully collected from the urine of nitroxoline-treated nude mice with high purity (99%) and good stability (stable at 37°C for at least 96 h). Thus, the present study performed an MTT assay using nitroxoline and nitroxoline sulfate, and demonstrated that nitroxoline sulfate is able to inhibit the growth of HUVEC and T24 cells, but with ~30% decreases in the maximum inhibition rates and 2–3-fold increases in IC50 values compared with its parent drug, nitroxoline. However, due to the high concentration and excretion levels of nitroxoline sulfate in the urine (30–60-fold higher than that of nitroxoline) (20), urinary nitroxoline sulfate level may be sufficient to inhibit tumor growth (Fig 4).
Figure 4.
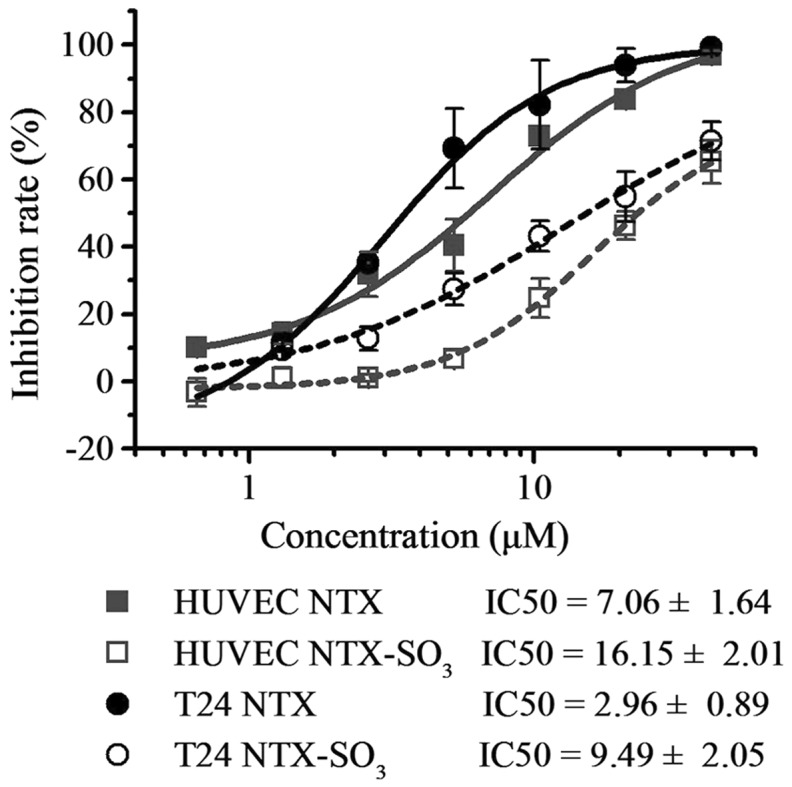
NTX-SO3, a metabolite of NTX, exhibits anti-cancer activity in vitro. Dose-response curves were obtained using a logistic nonlinear regression analysis model. Compared with the parent drug (NTX), NTX-SO3 shows increased IC50 values in HUVEC cells (P<0.001) and T24 cells (P<0.01). Data are presented as mean ± standard deviation from quadruplicate samples of at least two independent experiments. HUVEC, human umbilical vein endothelial cells; NTX, nitroxoline; NTX-SO3, nitroxoline sulfate.
Discussion
Drug repurposing, alternatively termed ‘new uses for old drugs’ or ‘drug repositioning’, has gained considerable attention over the past decade, and become a powerful alternative strategy for identifying and developing novel anticancer drugs (1,7,21). The majority of non-cancer drugs approved for anticancer treatment have common features, including well-defined pharmacokinetic and pharmacodynamics properties, and well-characterized cancer targets (1,7,21). Nitroxoline has an excellent safety profile and well-defined pharmacokinetic properties that have been established through its 50-year clinical history (1,2,6). Furthermore, its anticancer activity has been demonstrated to be associated with angiogenesis inhibition by targeting MetAP2 and sirtuin 1/2 (3), blocking cancer cell migration and invasion by targeting cathepsin B (6), and directly inducing apoptosis (4). Thus, the most important and imperative study required to advance nitroxoline repurposing into clinical trials for anticancer treatment was systemic preclinical pharmacodynamic evaluation of its anticancer activity. The current in vitro study compared the sensitivities of various cell lines to nitroxoline treatment, from which three sensitive cell lines with urological origins (T24, 5637 and KCC853) were selected for further in vivo analysis. As expected, nitroxoline effectively and dose-dependently inhibited the growth of these urological tumors in orthotopic mouse models. The low dose (30 mg/kg, twice a day) of nitroxoline used in nude mice, equivalent to the common dose (750 mg/day) used for human UTI treatment, inhibited the growth of urological xenografts by 40–60%. Higher dose of nitroxoline (120–240 mg/kg, twice a day) resulted in 50–85% growth inhibition without corresponding increase in toxicity. Therefore, the routine nitroxoline administration regimen used for human UTI treatment was sufficient for urological cancer treatment, and higher dose of nitroxoline may aid cancer patients with good general conditions.
In addition, the present study demonstrated that nitroxoline sulfate, one of the most common metabolites of nitroxoline in the urine, may effectively inhibit the proliferation of T24 and HUVEC cells. Sulfate conjugation increases the aqueous solubility of the parent drug nitroxoline to allow for renal excretion; however, it decreases the lipid solubility of nitroxoline, making it difficult for nitroxoline sulfate to pass across the cell membrane and exhibit its anticancer activity. Although nitroxoline sulfate demonstrated relatively low anticancer activity compared with parent drug nitroxoline in the present study, its high concentration and excretion levels in the urine (30–60-fold higher than that of nitroxoline) (20) mean that it may be sufficient to inhibit tumor growth. The results of the present study largely increased the feasibility of nitroxoline repurposing for clinical anticancer application, particularly for bladder cancer treatment.
In summary, the current study demonstrated that nitroxoline and its metabolite nitroxoline sulfate in the urine exhibit powerful anti-cancer potential against urological tumors, and the routine nitroxoline administration regimen for human UTI treatment appeared to be effective and sufficient for urological cancer treatment. These results, in addition to the well-known safety profiles of nitroxoline and well-defined pharmacokinetic properties, successfully advanced nitroxoline repurposing into a phase II clinical trial in China for non-muscle invasive bladder cancer treatment (registration no. CTR20131716).
Acknowledgements
The present study was partially supported by the National Key Technologies R&D Program for New Drugs (grant no. 2012ZX09301003-001).
Conflict of interest
The authors declare the following competing financial interests: Dr Kevin Pan is one of the founders of Jiangsu Asieris Pharmaceuticals Co., Ltd., which has a financial interest in nitroxoline repurposing for anticancer application.
References
- 1.Shim JS, Liu JO. Recent advances in drug repositioning for the discovery of new anticancer drugs. Int J Biol Sci. 2014;10:654–663. doi: 10.7150/ijbs.9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mrhar A, Kopitar Z, Kozjek F, Presl V, Karba R. Clinical pharmacokinetics of nitroxoline. Int J Clin Pharmacol Biopharm. 1979;17:476–481. [PubMed] [Google Scholar]
- 3.Shim JS, Matsui Y, Bhat S, Nacev BA, Xu J, Bhang HE, Dhara S, Han KC, Chong CR, Pomper MG, et al. Effect of nitroxoline on angiogenesis and growth of human bladder cancer. J Natl Cancer Inst. 2010;102:1855–1873. doi: 10.1093/jnci/djq457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazovic J, Guo L, Nakashima J, Mirsadraei L, Yong W, Kim HJ, Ellingson B, Wu H, Pope WB. Nitroxoline induces apoptosis and slows glioma growth in vivo. Neuro Oncol. 2015;17:53–62. doi: 10.1093/neuonc/nou139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang H, Taggart JE, Zhang X, Benbrook DM, Lind SE, Ding WQ. Nitroxoline (8-hydroxy-5-nitroquinoline) is more a potent anti-cancer agent than clioquinol (5-chloro-7-iodo-8-quinoline) Cancer Lett. 2011;312:11–17. doi: 10.1016/j.canlet.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirković B, Renko M, Turk S, Sosič I, Jevnikar Z, Obermajer N, Turk D, Gobec S, Kos J. Novel mechanism of cathepsin B inhibition by antibiotic nitroxoline and related compounds. Chem Med Chem. 2011;6:1351–1356. doi: 10.1002/cmdc.201100098. [DOI] [PubMed] [Google Scholar]
- 7.Gupta SC, Sung B, Prasad S, Webb LJ, Aggarwal BB. Cancer drug discovery by repurposing: Teaching new tricks to old dogs. Trends Pharmacol Sci. 2013;34:508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 8.National Research Council: Guide for the Care and Use of Laboratory Animals. 8th. The National Academies Press; Washington DC: 2011. [Google Scholar]
- 9.Hadaschik BA, Black PC, Sea JC, Metwalli AR, Fazli L, Dinney CP, Gleave ME, So AI. A validated mouse model for orthotopic bladder cancer using transurethral tumour inoculation and bioluminescence imaging. BJU Int. 2007;100:1377–1384. doi: 10.1111/j.1464-410X.2007.07165.x. [DOI] [PubMed] [Google Scholar]
- 10.Kang MR, Yang G, Charisse K, Epstein-Barash H, Manoharan M, Li LC. An orthotopic bladder tumor model and the evaluation of intravesical saRNA treatment. J Vis Exp pii. 4207:2012. doi: 10.3791/4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao H, Nolley R, Chen Z, Peehl DM. Tissue slice grafts: An in vivo model of human prostate androgen signaling. Am J Pathol. 2010;177:229–239. doi: 10.2353/ajpath.2010.090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thong AE, Zhao H, Ingels A, Valta MP, Nolley R, Santos J, Young SR, Peehl DM. Tissue slice grafts of human renal cell carcinoma: An authentic preclinical model with high engraftment rate and metastatic potential. Urol Oncol. 2014;32:43.e23–e30. doi: 10.1016/j.urolonc.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murugasu-Oei B, Dick T. In vitro activity of the chelating agents nitroxoline and oxine against Mycobacterium bovis BCG. Int J Antimicrob Agents. 2001;18:579–582. doi: 10.1016/S0924-8579(01)00437-X. [DOI] [PubMed] [Google Scholar]
- 14.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 15.Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: A summary and commentary. Int J Surg Pathol. 2005;13:143–153. doi: 10.1177/106689690501300203. [DOI] [PubMed] [Google Scholar]
- 16.Santos L, Amaro T, Costa C, Pereira S, Bento MJ, Lopes P, Oliveira J, Criado B, Lopes C. Ki-67 index enhances the prognostic accuracy of the urothelial superficial bladder carcinoma risk group classification. Int J Cancer. 2003;105:267–272. doi: 10.1002/ijc.11049. [DOI] [PubMed] [Google Scholar]
- 17.Shariat SF, Ashfaq R, Karakiewicz PI, Saeedi O, Sagalowsky AI, Lotan Y. Survivin expression is associated with bladder cancer presence, stage, progression, and mortality. Cancer. 2007;109:1106–1113. doi: 10.1002/cncr.22521. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Beltran A, Scarpelli M, Montironi R, Kirkali Z. 2004 WHO classification of the renal tumors of the adults. Eur Urol. 2006;49:798–805. doi: 10.1016/j.eururo.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): A novel vascular cell-cell adhesion molecule. J Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagenlehner FM, Münch F, Pilatz A, Bärmann B, Weidner W, Wagenlehner CM, Straubinger M, Blenk H, Pfister W, Kresken M, Naber KG. Urinary concentrations and antibacterial activities of nitroxoline at 250 milligrams versus trimethoprim at 200 milligrams against uropathogens in healthy volunteers. Antimicrob Agents Chemother. 2014;58:713–721. doi: 10.1128/AAC.02147-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boguski MS, Mandl KD, Sukhatme VP. Drug discovery. Repurposing with a difference. Science. 2009;324:1394–1395. doi: 10.1126/science.1169920. [DOI] [PubMed] [Google Scholar]