Abstract
The constantly growing incidence of obesity represents a risk of health complications for individuals, and is a growing economic burden for health care systems and society. The aim of this study was to evaluate the efficacy of bariatric surgery, specifically laparoscopic greater curve plication, laparoscopic sleeve gastrectomy, and Roux-en-Y gastric bypass, in patients with type 2 diabetes mellitus. The effect of bariatric surgery on the changes in blood pressure before, and 12 months after, surgery and in pharmacotherapy in the 12 months after surgery was analyzed. For achieving this purpose, 74 patients from the Obesity and Surgery Department of Vitkovice Hospital in Ostrava in the Czech Republic, were monitored. They were operated in 2011 and 2012. The Bonferroni method was used to test hypotheses about the impact of surgery on blood pressure and pharmacotherapy. One year after the surgery, systolic and diastolic blood pressure values decreased, both with no statistically significant difference between surgery types. Improvement was observed in 68% of cases, with 25% of patients discontinuing pharmacotherapy entirely.
Keywords: type 2 diabetes mellitus, bariatric surgery, blood pressure, pharmacotherapy
Introduction
There are more than 2.1 billion obese people worldwide, amounting to approximately 30% of the world population. Further increases are expected in the future, with an estimated 50% of the population becoming obese by 2030. Body weight is generally assessed with the body mass index (BMI). Excess body weight is defined as a BMI between 25.0 and 29.9 kg/m2. A BMI of 30 or higher is considered obesity. Obesity affects 2.5 times as many people as malnutrition. Obesity is responsible for 5% of all deaths, and its economic impact corresponds to 2.8% of the gross domestic product, making its effects comparable to those of smoking, war, and terrorism.1 Obesity not only increases the risk of health complications for the individual but increasing obesity rates also represent a significant and steadily growing burden for health care systems and society as a whole.
Maglione et al2 investigated the outcomes of four surgical procedures in patients with a BMI over 30 kg/m2: laparoscopic adjustable gastric banding (LAGB), Roux-en-Y gastric bypass (RYGB), biliopancreatic diversion with duodenal switch, and laparoscopic sleeve gastrectomy (LSG). These studies confirmed the high efficacy of these methods compared to conventional methods and the greater efficacy of RYGB and LAGB in providing significant, relatively permanent weight loss. Fried et al3 also confirmed the effect of surgical procedures on obesity. Surgery resulted in long-term success (ie, lasting 5 years or longer) in over 80% of patients, whereas conservative treatment failed in approximately 80% of patients. It has also been shown that metabolic surgery significantly decreases mortality among obese patients and also decreases the risk of obesity-related comorbidities.4 The current standard therapy for type 2 diabetes mellitus (T2DM) is pharmacotherapy. Several randomized controlled trials (RCTs) performed in obese patients showed remission of T2DM after bariatric surgery. Recent RCTs have shown bariatric procedures to produce a similar effect in nonmorbidly and nonseverely obese insulin-dependent T2DM patients, suggesting the procedures currently used in bariatric surgery as new therapeutic approaches in patients with T2DM.5 Obesity is considered a risk factor for the development of T2DM.6 For patients with class 2 obesity (BMI >35 kg/m2) with comorbidities and patients with class 3 obesity (BMI >40 kg/m2), bariatric surgery is recommended when conservative attempts do not result in substantial weight loss. Existing evidence, such as that of the DiaSurg study suggest that bariatric surgery not only leads to substantial weight loss but also to T2DM remission in 42%–78% of patients who have undergone bariatric surgery.7,8
The aim of this study is to evaluate the therapeutic efficacy of three bariatric surgery procedures, the laparoscopic greater curve plication (LGCP), LSG, and RYGB methods, in obese patients with T2DM. The following variables were assessed 12 months after surgery and compared to those values preoperatively: changes in blood pressure; the effect of bariatric therapy on pharmaceutical therapy; and the effect of bariatric therapy on T2DM.
Methods
This study evaluated outcomes in 74 patients with T2DM who underwent LGCP, LSG, or RYGB in 2011 and 2012 at the Obesitology Outpatient Unit and Department of Surgery at the Vítkovice Hospital in Ostrava, Czech Republic. Patient selection for the surgical treatment of obesity followed guidelines of the International Federation for the Surgery of Obesity, ie, BMI ≥40 kg/m2, BMI ≥35 kg/m2 with associated comorbidities, or BMI <35 kg/m2 and a medical history of weight loss after intensive obesity treatment, followed by weight regain.3 For each of the procedures, the following hypotheses were tested:
Hypothesis 1: Blood pressure will decrease 12 months after surgery, without a significant difference among individual procedure groups. The dose of antihypertensive medication will be reduced, commensurate with the decrease in blood pressure.
Hypothesis 2: T2DM will improve 12 months after surgery, with commensurate dose reduction of oral hypoglycemic agents and insulin.
The hypotheses were tested with the Bonferroni method.9 This method compares the means of all possible pairs, ie, it compares I(I−1)/2 pairs. Two median values µi and µj then differ at the significance level α, if it holds true that:
| (1) |
where tα/m(N−I) is the Student distribution quantil and m represents the number of all possible comparisons, m = I(I−1)/2.
Normality of data distribution was assessed on the basis of skewness and kurtosis. Data with normal distribution were tested with the paired t-test, while data without normal distribution were tested with the paired Wilcoxon test. The statistical tests were evaluated at a 5% level of significance.
The research set included 74 patients, 23 men and 51 women. Patient age ranged from 33 to 70 years, with a mean of 52.7±9.12 months. The mean age of the men was 54.2 years and that of the women was 52.0 years. The most populous group was the 51–60-year bracket, which reflects the fact that people of this age are often obese. Moreover, most patients who undergo bariatric procedures have obesity of a higher class. There were no patients under 30 years of age in the study, which corresponds with the fact that occurrence of T2DM in the population increases with age. Approximately 39% of the patients underwent LGCP, 38% underwent RYGB, and 23% underwent LSG. The study was approved by the ethical committee at the Faculty of Medicine, University of Ostrava, in accordance with the ethical standards of the Helsinki Declaration of 1975, as revised in 2000. All subjects were carefully instructed both verbally and in writing about the aims of the study and provided written, informed consent.
Results
The impact of bariatric surgery on T2DM, changes in hypertension, and changes in pharmaceutical therapy are described.
Changes in hypertension
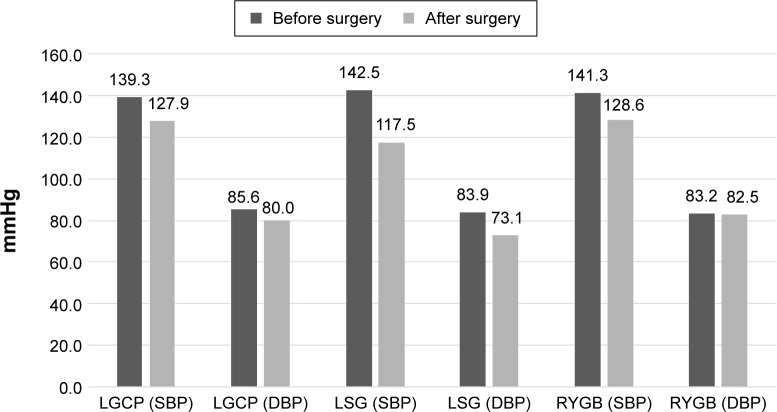
Blood pressure was recorded before surgery for all patients; however, values 12 months after surgery were recorded for less than half of them. Average systolic blood pressure before surgery for the entire set was 141 mmHg (σ=16.21). By 12 months after surgery, blood pressure had decreased by 16 mmHg, on average (σ=8.99). One year after LGCP and RYGB surgery, the average systolic blood pressure was 127.9 mmHg (σ=6.99–8.99). LGS patients had an average blood pressure of 117.5 mmHg 12 months after surgery (σ=8.86). According to the Czech Diabetes Society (CDS),10 a decrease in blood pressure to below 130/80 mmHg is considered a success in the treatment of hypertensive diabetic patients. Average systolic blood pressure values 12 months after surgery were below 130 mmHg for all procedure groups. Diastolic pressure was 80 mmHg on average (Figure 1).
Figure 1.
Comparison of average blood pressure values before, and 12 months after surgery.
Abbreviations: LGCP, laparoscopic greater curve plication; LSG, laparoscopic sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Blood pressure in 11 of 17 patients (65%) was below 130/80, and was below 140/90 in an additional five patients (29%). Bonferroni comparisons revealed no statistically significant differences among individual procedure types in blood pressure reduction after surgery.
Changes in pharmaceutical therapy
The last area we investigated was the effect of bariatric surgery on pharmaceutical therapy of patients. The research set included 74 patients, both men and women. Fifty patients had been taking medication for high blood pressure, and data about the course of their treatment was recorded 12 months after surgery for 44 patients. Within the entire set, medication was reduced in 68% of cases, and 25% of patients discontinued medication altogether. Grouped by surgery type, LGCP resulted in medication reduction in 53% of patients, of whom 20% discontinued medication altogether. After LSG, medication was reduced in 70% of patients, of whom 30% discontinued medication altogether. As for RYGB, 79% of patients had reduced medication 12 months after surgery, and 26% of these had discontinued medication altogether (Table 1).
Table 1.
Overview of changes in antihypertensive pharmacotherapy 12 months after the surgery
| Type of surgery | Changes in antihypertensive pharmacotherapy
|
||||
|---|---|---|---|---|---|
| 0% | ≤49% | 50%–99% | 100% | Total | |
| LGCP | |||||
| n | 7 | 2 | 3 | 3 | 15 |
| % | 46.7 | 13.3 | 20.0 | 20.0 | 100.0 |
| LSG | |||||
| n | 3 | 1 | 3 | 3 | 10 |
| % | 30.0 | 10.0 | 30.0 | 30.0 | 100.0 |
| RYGB | |||||
| n | 4 | 1 | 9 | 5 | 19 |
| % | 21.1 | 5.3 | 47.4 | 26.3 | 100.0 |
| Total | |||||
| n | 14 | 4 | 15 | 11 | 44 |
| % | 31.8 | 9.1 | 34.1 | 25.0 | 100.0 |
| Symmetry test, P<0.001 | |||||
Notes: Overall, there was a statistically significant difference in the doses of drugs before and after treatment (P<0.001). When evaluating various types of operations, the difference in doses of drugs before and after treatment was not significant in LSG (P=0.072), a statistically significant difference was found in other types of operations (LGCP, P=0.046; RYGB, P=0.002). In case of types of surgery detected a statistically significant difference (LGCP, P=0.046; RYGB, P=0.002).
Abbreviations: LGCP, laparoscopic greater curve plication; LSG, laparoscopic sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass.
Of the 74 total patients, 64 used oral hypoglycemic agents and 23 used insulin. Data on the use of oral hypoglycemic agents 12 months after surgery were recorded for 56 patients. Within this set, 82% had reduced medication, and 39% had discontinued medication altogether. The CDS (2012)10 considers a 50% reduction in the use of oral hypoglycemic agents a therapeutic success. This reduction was achieved by 90%, 81%, and 82% of LGCP, LSG, and RYGB patients, respectively (Table 2).
Table 2.
Overview of changes taking oral antidiabetic drugs 12 months after surgery
| Type of surgery | Change in oral antidiabetic 12 months after surgery
|
||||
|---|---|---|---|---|---|
| 0% | ≤49% | 50%–99% | 100% | Total | |
| LGCP | |||||
| n | 2 | 0 | 9 | 10 | 21 |
| % | 9.5 | 0.0 | 42.9 | 47.6 | 100.0 |
| LSG | |||||
| n | 2 | 0 | 6 | 3 | 11 |
| % | 18.2 | 0.0 | 54.5 | 27.3 | 100.0 |
| RYGB | |||||
| n | 6 | 0 | 9 | 9 | 24 |
| % | 25.0 | 0.0 | 37.5 | 37.5 | 100.0 |
| Total | |||||
| n | 10 | 0 | 24 | 22 | 56 |
| % | 17.9 | 0.0 | 42.9 | 39.3 | 100.0 |
| Symmetry test, P<0.001 | |||||
Notes: The results of statistical calculations show statistically significant differences in all surgery types of P<0.001, P<0.011, and P<0.001 for LGCP, LSG, and RYGB, respectively.
Abbreviations: LGCP, laparoscopic greater curve plication; LSG, laparoscopic sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass.
Twenty-three patients in the research set used insulin. All of these patients experienced improvement 12 months after surgery, and more than half of them had discontinued insulin altogether. A 25% reduction in the use of insulin, considered a therapeutic success by the CDS (2012), was achieved in all patients, regardless of procedure. The RYGB group contained the largest proportion of patients using both insulin and oral hypoglycemic agents (Table 3).
Table 3.
Overview of changes in the insulin therapy 12 months after the surgery
| Type of surgery | Change in insulin 12 months after surgery
|
||||
|---|---|---|---|---|---|
| 0% | ≤49% | 50%–99% | 100% | Total | |
| LGCP | |||||
| n | 0 | 1 | 1 | 2 | 4 |
| % | 0.0 | 25.0 | 25.0 | 50.0 | 100.0 |
| LSG | |||||
| n | 0 | 0 | 2 | 2 | 4 |
| % | 0.0 | 0.0 | 50.0 | 50.0 | 100.0 |
| RYGB | |||||
| n | 0 | 0 | 7 | 8 | 15 |
| % | 0.0 | 0.0 | 46.7 | 53.3 | 100.0 |
| Total | |||||
| n | 0 | 1 | 10 | 12 | 23 |
| % | 0.0 | 4.3 | 43.5 | 52.2 | 100.0 |
| Symmetry test, P<0.001 | |||||
Abbreviations: LGCP, laparoscopic greater curve plication; LSG, laparoscopic sleeve gastrectomy; RYGB, Roux-en-Y gastric bypass.
RYGB yielded a less prominent reduction in oral hypoglycemic agents compared to the other procedures. It should be noted, however, that discontinuation of insulin alone should be considered a success.
Impact of bariatric surgery on T2DM
This section summarizes the results of previous measurements independent of type of surgery. Patients selected for the research were diabetics treated with diet, oral antidiabetics (OADs), or insulin. The outcome of the treatment was seen in only 57 patients 12 months after surgery. Twenty-three patients were treated with a combination of insulin and OADs, and 34 received only OADs. A complete withdrawal of drugs was registered in 19 patients (33.3%), four patients completely stopped taking insulin and OADs. Fifteen patients taking OADs before surgery stopped taking these drugs 12 months from the surgery. Only one diabetic who was taking OADs had no changes in health. About 82% of the entire sample showed changes in OADs to ≤50% of the original dose before the surgery. About 60% of patients showed changes in insulin dose to ≤25% of the OADs and insulin.
Serum glucose concentration was evaluated in 60 patients before and 12 months after surgery. Physiological glucose values (<6 mmol/L) were reached by 40 patients, ie, 67% of this group, 12 months after surgery. The value of HbA1c 12 months after the surgery was analyzed in 46 patients, values <4.8% were achieved by 38 patients (83%). Approximately 77% (24 patients out of 31) of the patients achieved blood glucose values ≤6 mmol/L and HbA1c <4.8%. The HbA1c values, 12 months after the surgery were <4.8%, thus leading to withdrawal of the OADs and insulin in 80% of patients (8 out of 10). Nine patients from the complete set of ten (90%) achieved remission of diabetes.
Summary
In evaluating the study hypotheses, we can say that Hypothesis 1 and 2 showed differences among individual bariatric procedures.
Hypothesis 1
Blood pressure will decrease 12 months after surgery, without a significant difference among individual procedure groups. The dose of antihypertensive drugs will be reduced commensurate with the decrease in blood pressure.
This hypothesis cannot be rejected. One year after surgery, both systolic and diastolic blood pressure values had decreased, without a statistically significant difference among individual procedure types. For all procedures, blood pressure values remained below 130/80 mmHg 12 months after surgery. Within the entire set, 68% of cases had reduced their medication, and 25% had discontinued medication altogether.
Hypothesis 2
T2DM will improve 12 months after surgery, with a commensurate dose reduction of oral hypoglycemic agents and insulin.
One year after surgery, 33% of patients had discontinued oral hypoglycemic agents and insulin altogether. Approximately 79% of patients experienced improvement. Eighty-three percent of patients had attained the target value of HbA1c (<4.8%). If discontinuation of insulin and oral hypoglycemic agents concurrent with HbA1c levels below 4.8% are the criteria for diabetes remission, then T2DM remission was achieved in eight of ten patients for whom data were available. Average HbA1c values decreased below 4.5% (σ=0.76). T2DM improved. Thus, the hypothesis was not rejected.
Discussion
In addition to T2DM and dyslipidemia, many of the patients in this study had hypertension. The average systolic blood pressure values 12 months after surgery did not represent a critical cardiovascular system load, ie, they had decreased below the 130 mmHg criterion proposed by the CDS (2012). There was no statistically significant difference in mean systolic blood pressure change among the individual procedure types. One year after surgery, 65% of the set had attained optimal values (<130/80 mmHg), and an additional 29% of the set had attained improvement of hypertension (<140/90 mmHg). These values correlate with the findings of a meta-analysis by Buchwald et al,11 who authors reported that optimum blood pressure values were attained by 62% of patients after bariatric surgery and that an additional 17% improved. Sugerman et al12 researched the effect of gastric bypass on hypertension. The authors reported that blood pressure had returned to normal in 69% of patients 12 months after surgery. DePaula et al13 researched the relationship between sleeve gastrectomy and hypertension. Their study found that 88% of patients attained normal blood pressure. Based on the abovementioned studies, there exists a correlation, albeit slight, between blood pressure values after surgery and the type of procedure performed. The fact that no significant differences were found between individual procedure groups in this study may result from the small number of patients whose blood pressure was recorded after bariatric surgery.
One year after surgery, 33% of patients had discontinued oral hypoglycemic agents and insulin altogether. Improvement, ie, decrease of oral hypoglycemic agents by at least 50% and decrease of insulin dose by at least 25% (according to the recommendations given by the CDS14), was reported in 79% of patients. Normal HbA1c levels (<4.8%) were attained by 83% of patients. These results are similar to those of the meta-analysis by Buchwald et al.11 If discontinuation of insulin and oral hypoglycemic agents concurrent with reducing HbA1c below 4.8% are considered as the criteria for diabetes remission, then T2DM remission was achieved in eight out of ten patients for whom data were available. However, if remission is defined as HbA1c decrease below 4.8% without discontinuation of medication, as proposed by Svačina,15 then T2DM remission was achieved by 83% of patients in this study. Svačina15 reported that 50% of gastric bypass and sleeve gastrectomy patients achieved T2DM remission. The other half of the patients experienced improvement in diabetes symptoms. All patients discontinued insulin. The majority, however, including patients with remission, continued metformin therapy. The decision not to discontinue oral hypoglycemic agents may be made because patients for whom surgical therapy is indicated often have diabetes with poorer compensation and of longer duration.15
The lack of data on the exact duration of diabetes is a disadvantage in this study, because the longer diabetes lasts, the lower the probability of remission.15 Adjustments in pharmaceutical therapy after surgery also depend on the willingness of the patient to address the issue with the attending physician. The difference between our results and those of Svačina15 could have been caused by this factor. A further challenge lies in the lack of consensus in the literature concerning the definition of T2DM.16
This study did not find statistically significant differences among individual procedure types regarding the effect on T2DM. However, previous studies have found differences among the procedures. Schauer et al17 described one such difference between LSG and RYGB. The target HbA1c value (≤6%) was attained by 37% patients after LSG and by 42% after RYGB. Although the authors stated that there was no significant difference between the patient groups for the two bariatric procedures, an important fact is that the RYGB patients attained this value without any medication, whereas 28% of the LSG patients used at least one oral hypoglycemic agent. And it was precisely the need for oral hypoglycemic agents that decreased significantly after both surgery types. There was a 4% decrease in the need for insulin in the RYGB group and an 8% decrease in the LSG group. From this viewpoint, gastric bypass was more effective for diabetes remission.
Boza et al18 studied the effect of gastric bypass on T2DM. They reported that 53% of patients achieved full remission, an additional 10% achieved partial remission, and 26% had an improvement in T2DM. Glycemia changes after LSG were described also by Vidal et al.19 More than 75% of patients in that study experienced improvement of T2DM within 3 years of the procedure.
Surgical treatment of T2DM is a subject that has been discussed in depth in recent literature.20 Changes in the parameters of glucose metabolism in this study were not so surprising. Recently, many studies have shown a positive effect of LSG on the metabolism of glucose. Peterli et al21 have proved that, 1 week following LSG, significant hormonal changes occur, which are accompanied by increased insulin, GLP-1, and PYY levels. Those changes, as well as decreased Ghrelin levels can be a guide to explain the changes after LSG.22 However, the precise mechanisms responsible for these changes have not yet been completely understood. One of the explanations for the LSG effect on glucose mechanism is its influence on the production of insulinotropic GLP-1 hormone, secreted mainly by L and K intestinal cells in response to the presence of nutrients in the intestine lumina. Metabolic interventions (mainly malabsorption) dramatically increase the secretion of certain hormones, usually in the proximal parts of intestines, also intervening with the enteroinsular axis.23 However, a mere impact of incretins is perhaps not the only mechanism of metabolic intervention effect. Vagal nerve stimulation is also highly accountable, as well as alterations of vagal signals, followed by the effects of gastric evacuation capability.
The effect of LGCP on T2DM in this study was described; 18% of these patients achieved remission and 55% experienced improvement (weight loss summary results). In view of the presented findings, and considering the various factors that influence the results, it is very difficult to make a definitive statement about the efficacy of individual bariatric procedure types in the treatment of T2DM.
Acknowledgments
This study was supported by a grant from the Ministry of Education of the Czech Republic, allocated via the University of Ostrava under registration number SGS20/LF/2014 and projects FNHK and FIM.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dobbs R, Sawers C, Thompson F, et al. How the world could better fight obesity [Internet] [Accessed June 6, 2015]. (Report of the McKinsey Global Institute). Available from: http://www.mckinsey.com/insights/economic_studies/how_the_world_could_better_fight_obesity.
- 2.Maglione MA, Gibbons MM, Livhits M, et al. Bariatric Surgery And Nonsurgical Therapy in Adults with Metabolic Conditions And A Body Mass Index of 30.0 to 34.9 Kg/m2. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [Accessed June 20, 2015]. (Comparative Effectiveness Review 82 [AHRQ Publication No. 12(13)-EHC139-EF]). Available from: http://www.effectivehealthcare.ahrq.gov/ehc/products/227/1482/weight-loss-surgery-report-130529.pdf. [PubMed] [Google Scholar]
- 3.Fried M, Hainer V, Basdevant A, et al. Interdisciplinary European guidelines on surgery of severe obesity. Obes Facts. 2008;1:52–59. doi: 10.1159/000113937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenngott HG, Clemens G, Gondan M, et al. DiaSurg 2 trial – surgical vs. medical treatment of insulin-dependent type 2 diabetes mellitus in patients with a body mass index between 26 and 35 kg/m2: study protocol of a randomized controlled multicenter trial – DRKS00004550. Trials. 2013;14:183. doi: 10.1186/1745-6215-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meisinger C, Döring A, Thorand B, Heier M, Löwel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr. 2006;84(3):483–489. doi: 10.1093/ajcn/84.3.483. [DOI] [PubMed] [Google Scholar]
- 6.Fried M, Yumuk V, Oppert JM, et al. Interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. 2014;24(1):42–55. doi: 10.1007/s11695-013-1079-8. [DOI] [PubMed] [Google Scholar]
- 7.Fischer L, Wekerle AL, Bruckner T, et al. BariSurg trial: sleeve gastrectomy versus Roux-en-Y gastric bypass in obese patients with BMI 35–60 kg/m2 – A multi-centre randomized patient and observer blind non-inferiority trial. BMC Surg. 2015;15:87. doi: 10.1186/s12893-015-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer L, Hildebrandt C, Bruckner T, et al. Excessive weight loss after sleeve gastrectomy: a systematic review. Obes Surg. 2012;22(5):721–731. doi: 10.1007/s11695-012-0616-1. [DOI] [PubMed] [Google Scholar]
- 9.Anděl J. Fundamentals of Mathematical Statistics. Prague, Czech Republic: Matfy-Zpress; 2005. [Google Scholar]
- 10.The Czech Diabetes Society (CDS) Homepage of The Czech Diabetes Society. [Accessed June 6, 2015]. Available from: http://www.diab.cz/
- 11.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 12.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg. 1987;205(6):613–624. doi: 10.1097/00000658-198706000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DePaula AL, Stival AR, Halpern A, Vencio S. Surgical treatment of morbid obesity: mid-term outcomes of the laparoscopic ileal interposition associated to a sleeve gastrectomy in 120 patients. Obes Surg. 2011;21(5):668–675. doi: 10.1007/s11695-010-0232-x. [DOI] [PubMed] [Google Scholar]
- 14.Czech Diabetes Society Doporučeny postup peče o diabetes mellitus 2. Typu. [Recommended process of care for type 2 diabetes mellitus] [Accessed June 20, 2015]. Available from: http://www.diab.cz/dokumenty/dm2_12.pdf. Czech.
- 15.Svačina Š. Diabetes, bariatric surgery: a practical guide to the pros and cons. Postgrad Med. 2012;14:39–41. [Google Scholar]
- 16.Matoulek M, Svačina Š, Lajka J. Výskyt obezity a jejích komplikací v České republice [The incidence of obesity and its complications in the Czech Republic] Vnitřní lékařství. 2010;56(10):1019–1027. Czech. [PubMed] [Google Scholar]
- 17.Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boza C, Valderas P, Daroch DA, et al. Metabolic surgery: Roux-en-Y gastric bypass and variables associated with diabetes remission in patients with BMI <35. Obes Surg. 2014;24(8):1391–1397. doi: 10.1007/s11695-014-1218-x. [DOI] [PubMed] [Google Scholar]
- 19.Vidal JA, Ibarzabal F, Romero S, et al. Type 2 diabetes mellitus and the metabolic syndrome following sleeve gastrectomy in severely obese subjects. Obes Surg. 2008;18(9):1077–1082. doi: 10.1007/s11695-008-9547-2. [DOI] [PubMed] [Google Scholar]
- 20.Zimmet P, Alberti KG. Surgery or medical therapy for obese patients with type 2 diabetes? N Engl J Med. 2012;366(17):1635–1636. doi: 10.1056/NEJMe1202443. [DOI] [PubMed] [Google Scholar]
- 21.Peterli R, Wölnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy a prospective randomized trial. Ann Surg. 2009;250(2):234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- 22.Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab. 2009;35(6 Pt 2):518–523. doi: 10.1016/S1262-3636(09)73459-7. [DOI] [PubMed] [Google Scholar]
- 23.Fried M, Ribaric G, Buchwald JN, Svacina S, Dolezalova K, Scopinaro N. Metabolic surgery for the treatment of type 2 diabetes in patients with BMI 35 kg/m2: an integrative review of early studies. Obes Surg. 2010;20(6):776–790. doi: 10.1007/s11695-010-0113-3. [DOI] [PubMed] [Google Scholar]