Abstract
Study Design:
Prospective study.
Objective:
To investigate the efficacy of transcranial electrically stimulated muscle-evoked potentials (TcE-MsEPs) for predicting postoperative segmental upper extremity palsy following cervical laminoplasty.
Summary of Background Data:
Postoperative segmental upper extremity palsy, especially in the deltoid and biceps (so-called C5 palsy), is the most common complication following cervical laminoplasty. Some papers have reported that postoperative C5 palsy cannot be predicted by TcE-MsEPs, although others have reported that it can be predicted.
Methods:
This study included 160 consecutive cases that underwent open-door laminoplasty, and TcE-MsEP monitoring was performed in the biceps brachii, triceps brachii, abductor digiti minimi, tibialis anterior, and abductor hallucis. A >50% decrease in the wave amplitude was defined as an alarm point. According to the monitoring alarm, interventions were performed, which include steroid administration, foraminotomies, etc.
Results:
Postoperative deltoid and biceps palsy occurred in 5 cases. Among the 155 cases without segmental upper extremity palsy, there were no monitoring alarms. Among the 5 deltoid and biceps palsy cases, 3 had significant wave amplitude decreases in the biceps during surgery, and palsy occurred when the patients awoke from anesthesia (acute type). In the other 2 cases in which the palsy occurred 2 days after the operation (delayed type), there were no significant wave decreases. In all of the cases, the palsy was completely resolved within 6 months.
Discussion:
The majority of C5 palsies have been reported to occur several days after surgery, but some of them have been reported to occur immediately after surgery. Our results demonstrated that TcE-MsEPs can predict the acute type, whereas the delayed type cannot be predicted.
Conclusions:
A >50% wave amplitude decrease in the biceps is useful to predict acute-type segmental upper extremity palsy. Further examination about the interventions for monitoring alarm will be essential for preventing palsy.
Key Words: cervical laminoplasty, intraoperative neurophysiological monitoring, postoperative C5 palsy
Postoperative segmental upper extremity motor paresis in the deltoid muscle and biceps brachii muscle (so-called “C5 palsy”) is one of the most common postoperative complications after cervical laminoplasty.1,2 In the review article by Sakaura et al,3 the incidence of postoperative C5 palsy after cervical laminoplasty was reported to be 4.7% (range, 0%–30%).
Intraoperative detection of C5 palsy using intraoperative neurophysiological monitoring (IONM) has been attempted. Specifically, transcranial electrically stimulated muscle-evoked potentials (TcE-MsEPs) are expected to detect C5 palsy during surgery, but controversy remains whether TcE-MsEPs can detect C5 palsy during surgery or not. Whereas some papers have reported that postoperative C5 palsy can be detected,4–7 others reported that it cannot be detected by TcE-MsEPs.8,9
In this paper, we describe our prospective study for predicting postoperative C5 palsy using TcE-MsEPs and discuss the efficacy of the TcE-MsEPs.
METHODS
This prospective study included 160 consecutive cases that underwent open-door laminoplasty for cervical myelopathy with TcE-MsEP monitoring at our institute between October 2007 and May 2012. Informed consent was obtained from all of the patients. All surgeries were performed by a single surgeon (Y.F.). All patients underwent single-door laminoplasty including C3–6 or C4–6. A bone graft was applied to prevent lamina reclosure. The left-side open procedure was usually performed, but in cases with radiculo-myelopathy, the symptomatic side was opened and a foraminotomy was applied.
Anesthesia
Muscle relaxant was not used during surgery, including tracheal intubation. Propofol was administered by the target-controlled infusion technique, and the calculated effect site concentration of propofol was maintained between 1.5 and 3 µg/mL. Remifentanil was administered in the infusion rate of 0.2–0.5 µg/kg/min. The infusion rate of propofol and remifentanil was adjusted according to the Entropy monitor between 40 and 60.
TcE-MsEP Procedure
IONM was performed using a 16-channel Axon Epoch XP (Axon systems Inc.). The stimulation electrodes were placed 2 cm anterior and 4 cm lateral to the Cz over the cerebral cortex motor area. A transcranial 5-train pulse electrical stimulation was performed with an intensity of 400–600 V. The TcE-MsEPs were recorded from the muscles using a needle. To cover all neurological segments, the evoked muscles were selected as follows: biceps (C5, 6), triceps (C6–C8), abductor digiti minimi (C8, Th1), tibialis anterior (L4, 5), and abductor hallucis (L5, S1).
Prospective analyses were performed for predicting C5 palsy using the alarm criteria of a >50% decrease in the wave amplitude from the baseline.5 When the monitoring wave attenuation occurred, interventions including resting surgery, steroid administration, washing with warm saline, and additional foraminotomies at C4–5 and C5–6 were applied. When none of those interventions were effective, the surgeon decided whether surgery would be continued or not.
Clinical Evaluation
Muscle power strength was measured by manual muscle testing (MMT) preoperatively, when patient awoke from anesthesia, and every day after the operation for 1 week. C5 palsy was defined as a >1 level postoperative MMT deterioration in the deltoid and biceps. The C5 palsy cases were followed up with MMT until full recovery, and the duration was evaluated until full recovery.
According to the onset of muscle weakness, C5 palsy cases were separated into 2 groups: the acute type in which C5 palsy occurred when patients awoke from anesthesia and the delayed type in which C5 palsy did not occur when patients awoke but started later.
The Japanese Orthopaedic Association Score (JOA score; range −2 to 17) was also evaluated preoperatively and at the final follow-up. The recovery rate was calculated by Hirabayashi’s method as: (postoperative JOA score−preoperative JOA score)/(17−preoperative JOA score)×100.10 Statistical analysis was performed by using Statcel 3 (OMS Ltd, Saitama, Japan). The Wilcoxon signed-rank test was used for comparison of preoperative and postoperative JOA scores. A P value of <0.05 was considered statistically significant.
Accuracy Test
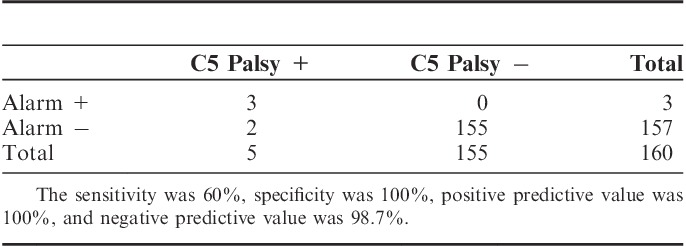
The accuracy of the monitoring alarm was evaluated according to the occurrence of C5 palsy. We classified the monitoring results into 4 groups. First, the true positive cases had C5 palsy with a monitoring alarm using the >50% decrease criterion. The false negative cases had C5 palsy without an alarm. The false positive cases did not have C5 palsy, although there was an alarm. The true negative cases included the cases without either C5 palsy or an alarm. The sensitivity, specificity, positive predictive value, and negative predictive value of the >50% decrease criterion were calculated.
RESULTS
Clinical Features
There were 5 postoperative C5 palsy cases among 160 cases that underwent cervical laminoplasty (3.1%). In 155 cases without C5 palsy, there was no wave amplitude decrease of >50% (true negative results).
Among the 5 C5 palsy cases, C5 palsy occurred within 24 hours in 3 cases (acute type) and later than 24 hours in 2 cases (delayed type). The details of these 5 cases are listed in the Table 1. All cases showed postoperative unilateral muscle weakness of the deltoid and biceps on the unilateral side but did not show muscle weakness of the contralateral deltoid or biceps and the other muscles including the triceps, wrist, and hand muscles. There was no deterioration of sensory function, walking ability, and bladder function.
TABLE 1.
Clinical Details in Patients With C5 Palsy
The mean preoperative JOA score was 11.5, which had significantly improved to 15.2 at the final follow-up. The recovery rate calculated by the Hirabayashi method was 64.3%. There were no differences in recovery rate between the acute type and the delayed type.
Acute-type C5 Palsy
All 3 acute-type C5 palsy cases showed a >50% decrease in the wave amplitude in the biceps (true positive results). The clinical course in each of these 3 cases was similar. The TcE-MsEP waves in the biceps decreased when the lamina was opened. Interventions including resting surgery, steroid administration, washing with warm saline and foraminotomies at C4–5 and C5–6 were performed, but the TcE-MsEP waves did not recover. Therefore, surgery was continued as originally planned. Immediately after the operation, unilateral muscle power of the deltoid and biceps were slightly weak (1-level deterioration in MMT), and the following day, the MMT decreased by at least 2.
Uniquely, in those 3 cases, the triceps wave amplitude on the same side also decreased at the same time with the biceps wave deterioration, but no motor weakness occurred in the triceps in any of these cases. There was no wave decrease in the other muscles including biceps and triceps on the contralateral side. The typical waveform changes of case 1 and case 2 were shown in Figures 1 and 2, respectively.
FIGURE 1.
(Continued)
FIGURE 2.
(Continued)
FIGURE 1.
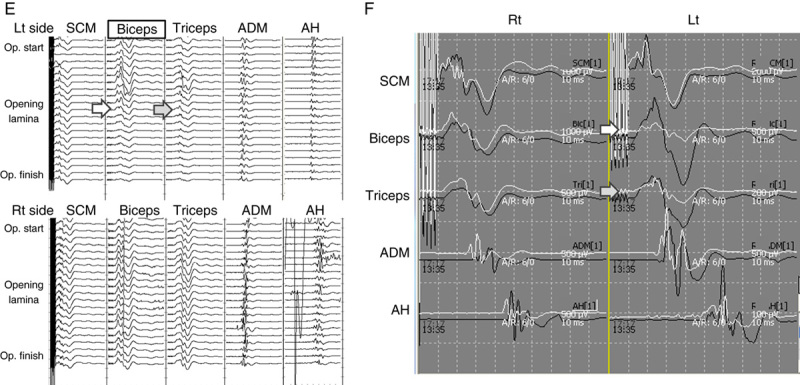
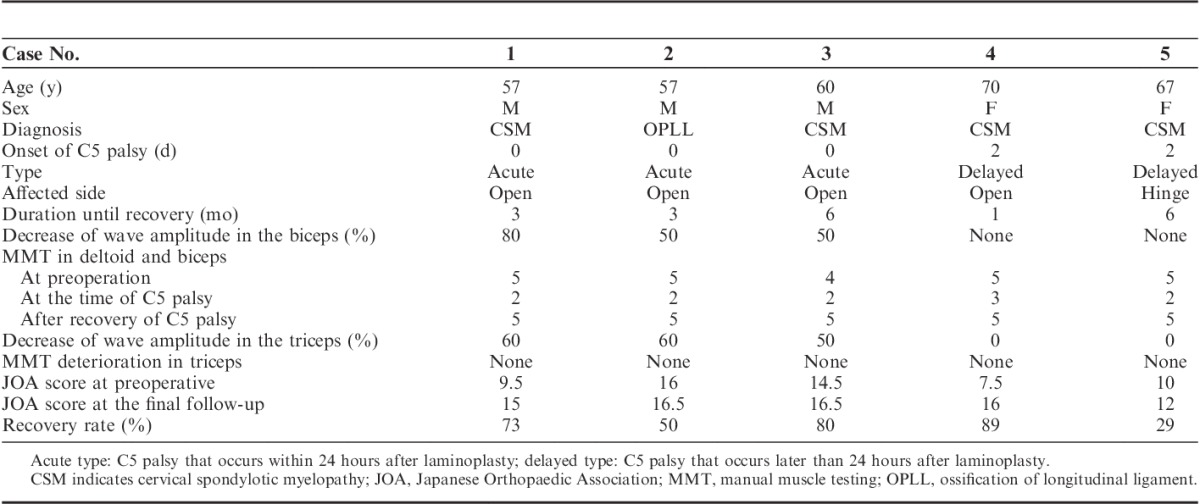
Case 1. A 57-year-old man with cervical spondylotic myelopathy. Left-sided open-door laminoplasty at C4–6 was performed. A, Sagittal image in preoperative MRI. B–D, Sagittal image and axial images at C4–5 and C5–6 in postoperative MRI. E, TcE-MsEP wave amplitudes in the biceps (white arrow) and triceps (gray arrow) decreased when the lamina was opened. Interventions including foraminotomies were not effective to recover the wave amplitude. F, Compared with the control waves, the final wave amplitude decreased 80% in the biceps and 60% in the triceps. (The black wave indicates the control wave and the white wave indicate the wave at the end of surgery.) C5 palsy occurred immediately after surgery. The muscle power recovered to MMT 5 by 3 months after surgery. MMT indicates manual muscle testing; MRI, magnetic resonance imaging; TcE-MsEP, transcranial electrically stimulated muscle-evoked potential.
FIGURE 2.
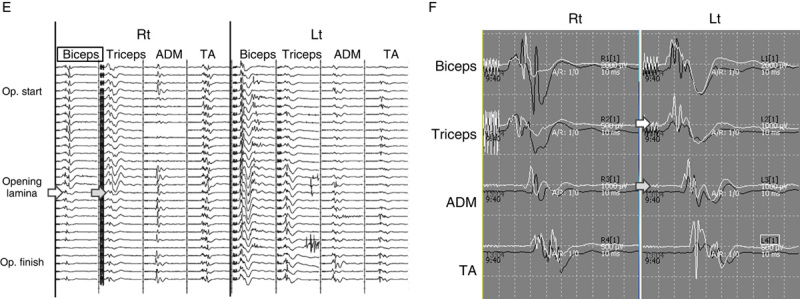
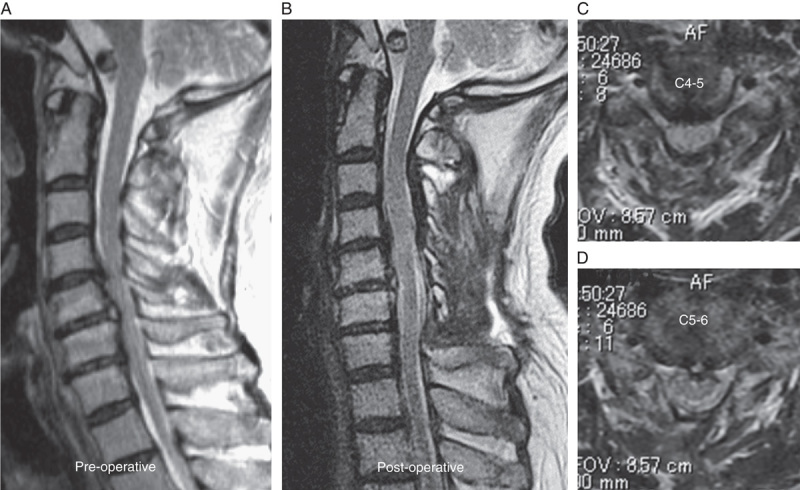
Case 2. A 57-year-old man with cervical ossification of the posterior longitudinal ligament. Right-sided open-door cervical laminoplasty at C4–6 was performed. A, Sagittal image in preoperative MRI. B–D, Sagittal image and axial images at C4–5 and C5–6 in postoperative MRI. E, The wave amplitude decreased in the left biceps (white arrow) and triceps (gray arrow), observed after opening the lamina. Interventions including foraminotomies were not effective to recover the wave amplitude. F, Compared with the control waves, the final wave amplitude decreased 50% in the biceps and 60% in the triceps. (The black wave indicates the control wave and the white wave indicate the wave at the end of surgery.) C5 palsy occurred immediately after surgery. The muscle power recovered to MMT 5 by 3 months after surgery. MMT indicates manual muscle testing; MRI, magnetic resonance imaging.
In the postoperative computed tomography scan, there was no dislocation of the opened lamina and grafting bone. The postoperative magnetic resonance imaging scan showed that the spinal cord was well decompressed. In all the cases, steroid administration and rehabilitation were performed, and the muscle strength of deltoid and biceps recovered completely within 6 months.
Delayed-type C5 Palsy
None of the 2 delayed C5 palsy cases showed significant wave amplitude decreases during surgery. The typical TcE-MsEP waveform of case 5 is shown in Figure 3. Immediately after surgery, there was no muscle power weakness of the deltoid and biceps. However, weakness of the deltoids and biceps occurred 2 days after surgery without sensory deficit. In the postoperative computed tomography and magnetic resonance imaging, the spinal canal and spinal cord were well decompressed. Therefore, steroid administration and rehabilitation were performed, and muscle weakness recovered completely within 6 months.
FIGURE 3.
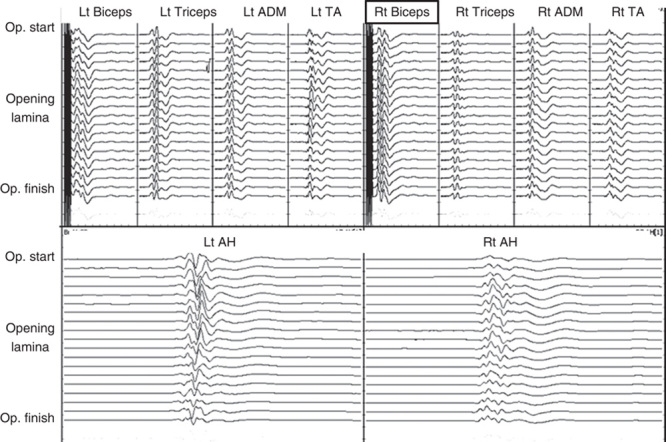
Case 3. A 67-year-old woman with cervical myelopathy. Left-sided open-door cervical laminoplasty at C4–6 was performed. Although there was no decrease in the wave amplitude during surgery and no muscle weakness immediately after surgery, C5 palsy on the right side (hinge side) started 2 days after surgery. The muscle power subsequently recovered to MMT 5 at 6 months after surgery. MMT indicates manual muscle testing.
Accuracy Testing
When using the >50% decrease criterion, the sensitivity was 60%, specificity was 100%, positive predictive value was 100%, and negative predictive value was 98.7% (Table 2).
TABLE 2.
The Accuracy of the >50% Decrease Criterion for Predicting C5 Palsy
DISCUSSION
The incidence of C5 palsy was 3.1% in the current study and was similar to incidence percentages in previous reports. All of the C5 palsies in our study recovered within 6 months, and myelopathy symptom improved, as has been reported in previous papers.11,12 However, C5 palsy is still a big problem because patients have to spend several months in rehabilitation and there also have been some unsatisfactory recovery cases reported in the previous papers. Furthermore, C5 palsy not only occurs after a cervical laminoplasty but also after a laminectomy, anterior cervical fusion, etc.3 Therefore, predicting C5 palsy is very important in cervical spine surgery.
Predicting C5 Palsy Using TcE-MsEP Monitoring
Controversy remains whether TcE-MsEPs can detect C5 palsy during surgery or not. Previous reports of C5 palsy after cervical spine surgery are as follows (Table 3): (1) Fan et al6 reported that 2 C5 palsy cases could be detected by an >75% unilateral amplitude decrease of deltoid/biceps muscles after cervical laminectomy; (2) Bose et al5 reported that 10 of 12 C5 palsy cases could be detected by a >50% decrease of TcE-MsEP amplitude in the deltoid and biceps muscle during anterior cervical surgery; (3) Yanase et al13 reported that 1 C5 palsy case showed a >50% decrease of the biceps wave amplitude, but 3 cases did not show the significant wave decrease; (4) Tanaka et al8 and (5) Nakamae et al9 reported that there were no abnormal TcE-MsEP findings in the deltoid and biceps in C5 palsy cases after cervical laminoplasty; (6) Sakaki et al7 reported that 2 C5 palsy cases showed a >70% decrease of the deltoid and biceps wave amplitude after 1 anterior spinal surgery and 1 posterior laminoplasty; (7) Bhalodia et al4 reported that TcE-MsEPs showed a >65% wave decrease in 5 acute-onset–type C5 palsies, but did not show significant wave changes in 7 delayed-onset C5 palsies.
TABLE 3.
Previous Reports of TcE-MsEP Monitoring for C5 Palsy
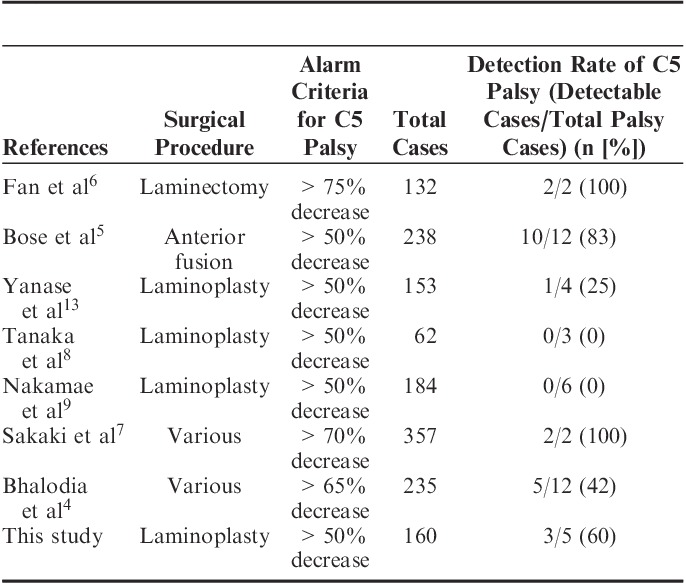
In the current study, C5 palsy could be detected in 3 cases using the alarm criterion with a >50% decrease of wave amplitude in the biceps, but in 2 cases, it could not be detected. All detectable C5 palsies occurred immediately after surgery (acute type), and all undetectable cases occurred 2 days after the surgery (delayed type). It is consistent with the Bhalodia et al4 result.
The majority of C5 palsies is known to occur several days after surgery,3 but some C5 palsies occurred immediately after surgery. Chiba and colleagues reported 15 postoperative C5 palsy cases with 2 of them occurring on the surgical day and 2 others occurring 1 day after surgery. The undetectable C5 palsy cases, reported by Tanaka et al,8 Nakamae et al,9 and Yanase et al,13 were the delayed type. Therefore, this difference in onset is speculated to be one of the biggest reasons for the controversy in previous studies. It will be practical to consider that TcE-MsEPs can detect acute-type C5 palsy but cannot detect delayed-type C5 palsy because neural damage may not occur during surgery in the delayed type.
Pathogenesis of C5 Palsy
Sakaura et al3 speculated, in their review article, that the 5 pathologic mechanisms of C5 palsy are as follows: (1) inadvertent injury to the nerve root during surgery; (2) nerve root traction caused by consecutive shifting of the cord after decompression surgery1; (3) spinal cord ischemia due to decreased blood supply from radicular arteries; (4) segmental spinal cord disorder; and (5) reperfusion injury of the spinal cord.14,15
In the current study, all of the wave decreases occurred immediately after the lamina opening procedure and the muscle strength recovered fully within 6 months. In the acute-type C5 palsy, direct nerve root injury (mechanism 1) was strongly suspected. Although there was no obvious neural damage according to the intraoperative surgical findings, other kinds of injury, such as heat injury or traction injury (mechanism 2), cannot be detected during surgery. Therefore, it is impossible to clarify the mechanism in this study and further investigation will be necessary.
Choice of Monitoring Muscle
In this study, the biceps wave was used for detecting C5 palsy and the deltoid wave was not. Deltoid muscles are known to be innervated more selectively by the C5 nerve root than in the biceps, but deltoid and biceps palsy is reported to occur not only due to C5 disorder but also to C6 disorder or a combination of both C5 and C6 disorders.16 Although it would be ideal to monitor both muscles, previous papers by Fan et al,6 Bose et al,5 Sakaki et al7 and Bhalodia et al4 demonstrated that the wave amplitude in the biceps decreased as much as in the deltoid in C5 palsy cases. Moreover, Yanase et al13 predicted C5 palsy using only the biceps wave without deltoid monitoring. Our study demonstrates that the TcE-MsEP wave in the biceps can detect C5 palsy.
TcE-MsEP Alarm Criteria for C5 Palsy
The alarm criterion for TcE-MsEP monitoring is still controversial. There are various TcE-MsEP alarm criteria used for predicting postoperative motor deterioration, such as threshold elevation,17 a 50%–80% decrease of the wave amplitudes,18–20 wave disappearance,21 a 10% delay of the wave latency,18 or waveform changes.22,23
In the current study, C5 palsy occurred after a 50% decrease of the wave amplitude in 2 cases and an 80% decrease in 1 case. Tamaki and Kubota24 and Iwasaki et al25 have reported the difficulty of predicting segmental upper extremity palsies by TcE-MsEP monitoring, compared with spinal tract injury. Tsutsui et al26 reported in an animal study that only a 30% decrease of the wave amplitude occurring after cutting 1 nerve root, and a 70% decrease of the wave amplitude occurring even after cutting 2 nerve roots, although cutting spinal tract fibers caused a 90% decrease of the wave amplitude. The pathologic mechanism underlying this phenomenon is speculated as follows: in the normal spinal cord, all muscles are innervated by >2 segments (Fig. 4A). In cases with typical spinal cord injuries, all pathways can be easily injured at the same time (Fig. 4B). However, in cases with segmental injuries, such as a gray matter injury or nerve root injury (Fig. 4C), there is a possibility that some pathways can be preserved, and the TcE-MsEP waves will not disappear in these cases.
FIGURE 4.
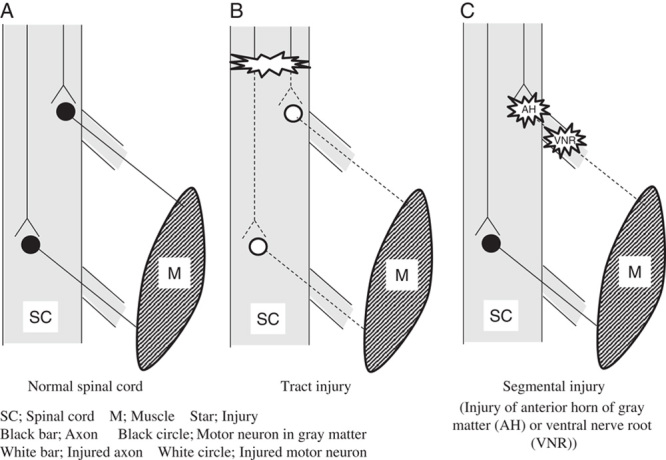
The proposed mechanisms of tract injury and segmental injury. A, In the normal spinal cord, all muscles are controlled by multiple segments. B, In cases with tract injury, all pathways can be easily injured at the same time. C, In cases with segmental injuries including a gray matter injury or nerve root injury, there is a possibility that some pathways can be preserved, and the TcE-MsEP waves will not disappear in these cases. TcE-MsEP, transcranial electrically stimulated muscle-evoked potential.
Therefore, Sakaki et al7 recommended using the 2 kinds of alarm criteria, used for cervical spine surgery: the wave disappearance criterion for tract injury and the >70% decrease criterion for segmental injury. In this study, a >50% decrease of the wave amplitude in the biceps was an adequate criterion to predict C5 palsy.
However, caution is necessary because a >50% decrease of the triceps wave amplitude is not adequate to predict triceps palsy. The reason for this is unclear, but we speculate that the triceps waves might be affected by a C6 segment disorder because the triceps brachii muscle is innervated by C6, C7, and C8 nerve roots. Previous papers did not mention the triceps wave in C5 palsy cases, and Tsutsui et al26 did not check for any palsy after cutting nerve roots in their animal study. Therefore, further investigations, including animal studies, will be necessary to confirm the pathomechanism of this phenomenon.
Interventions for Monitoring Alarms
In this series, we could predict the occurrence of C5 palsy during surgery in 3 cases, but we could not prevent the occurrence of palsy despite the interventions stated above. This is one of the serious limitations of IONM, but we believe that there will be a better chance of preventing C5 palsy if we can predict C5 palsy during surgery. For example, Nakamae et al9 reported 1 case in which a foraminotomy, according to monitoring alarm, recovered the TcE-MsEP wave and prevented C5 palsy; however, foraminotomies27,28 were not useful in our series. Further examinations about the intervention for monitoring alarm will be essential to prevent C5 palsy.
CONCLUSIONS
Among 160 cases with cervical laminoplasty, postoperative C5 palsy occurred in 5 cases. TcE-MsEP monitoring using the >50% decrease criterion in the biceps could detect 3 cases of acute-type C5 palsy, but could not detect 2 cases of delayed C5 palsy.
ACKNOWLEDGMENTS
The authors appreciate Mr Vincent John Hykel for his devoted support.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Tsuzuki N, Abe R, Saiki K, et al. Paralysis of the arm after posterior decompression of the cervical spinal cord. II. Analyses of clinical findings. Eur Spine J. 1993;2:197–202 [DOI] [PubMed] [Google Scholar]
- 2.Yonenobu K, Hosono N, Iwasaki M, et al. Neurologic complications of surgery for cervical compression myelopathy. Spine (Phila Pa 1976). 1991;16:1277–1282. [DOI] [PubMed] [Google Scholar]
- 3.Sakaura H, Hosono N, Mukai Y, et al. C5 palsy after decompression surgery for cervical myelopathy: review of the literature. Spine (Phila Pa 1976). 2003;28:2447–2451. [DOI] [PubMed] [Google Scholar]
- 4.Bhalodia VM, Schwartz DM, Sestokas AK, et al. Efficacy of intraoperative monitoring of transcranial electrical stimulation-induced motor evoked potentials and spontaneous electromyography activity to identify acute-versus delayed-onset C-5 nerve root palsy during cervical spine surgery: clinical arti. J Neurosurg Spine. 2013;19:395–402. [DOI] [PubMed] [Google Scholar]
- 5.Bose B, Sestokas AK, Schwartz DM. Neurophysiological detection of iatrogenic C-5 nerve deficit during anterior cervical spinal surgery. J Neurosurg Spine. 2007;6:381–385. [DOI] [PubMed] [Google Scholar]
- 6.Fan D, Schwartz DM, Vaccaro AR, et al. Intraoperative neurophysiologic detection of iatrogenic C5 nerve root injury during laminectomy for cervical compression myelopathy. Spine (Phila Pa 1976). 2002;27:2499–2502. [DOI] [PubMed] [Google Scholar]
- 7.Sakaki K, Kawabata S, Ukegawa D, et al. Warning thresholds on the basis of origin of amplitude changes in transcranial electrical motor-evoked potential monitoring for cervical compression myelopathy. Spine (Phila Pa 1976). 2012;37:E913–E921. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka N, Nakanishi K, Fujiwara Y, et al. Postoperative segmental C5 palsy after cervical laminoplasty may occur without intraoperative nerve injury: a prospective study with transcranial electric motor-evoked potentials. Spine (Phila Pa 1976). 2006;31:3013–3017. [DOI] [PubMed] [Google Scholar]
- 9.Nakamae T, Tanaka N, Nakanishi K, et al. Investigation of segmental motor paralysis after cervical laminoplasty using intraoperative spinal cord monitoring with transcranial electric motor-evoked potentials. J Spinal Disord Tech. 2012;25:92–98. [DOI] [PubMed] [Google Scholar]
- 10.Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976). 1981;6:354–364. [DOI] [PubMed] [Google Scholar]
- 11.Currier BL. Neurological complications of cervical spine surgery: C5 palsy and intraoperative monitoring. Spine (Phila Pa 1976). 2012;37:E328–E334. [DOI] [PubMed] [Google Scholar]
- 12.Imagama S, Matsuyama Y, Yukawa Y, et al. C5 palsy after cervical laminoplasty: a multicentre study. J Bone Joint Surg Br. 2010;92:393–400. [DOI] [PubMed] [Google Scholar]
- 13.Yanase M, Matsuyama Y, Mori K, et al. Intraoperative spinal cord monitoring of C5 palsy after cervical laminoplasty. J Spinal Disord Tech. 2010;23:170–175. [DOI] [PubMed] [Google Scholar]
- 14.Chiba K, Toyama Y, Matsumoto M, et al. Segmental motor paralysis after expansive open-door laminoplasty. Spine (Phila Pa 1976). 2002;27:2108–2115. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa K, Homma T, Chiba Y. Upper extremity palsy following cervical decompression surgery results from a transient spinal cord lesion. Spine (Phila Pa 1976). 2007;32:E197–E202. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara Y, Tanaka N, Fujimoto Y, et al. Surgical outcome of posterior decompression for cervical spondylosis with unilateral upper extremity amyotrophy. Spine (Phila Pa 1976). 2006;3120:E728–E732. [DOI] [PubMed] [Google Scholar]
- 17.Raynor BL, Bright JD, Lenke LG, et al. Significant change or loss of intraoperative monitoring data: a 25-year experience in 12,375 spinal surgeries. Spine (Phila Pa 1976). 2013;38:E101–E108. [DOI] [PubMed] [Google Scholar]
- 18.Sutter M, Eggspuehler A, Grob D, et al. The validity of multimodal intraoperative monitoring (MIOM) in surgery of 109 spine and spinal cord tumors. Eur spine J. 2007;16(suppl 2):S197–S208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi S, Matsuyama Y, Shinomiya K, et al. A new alarm point of transcranial electrical stimulation motor evoked potentials for intraoperative spinal cord monitoring: a prospective multicenter study from the Spinal Cord Monitoring Working Group of the Japanese Society for Spine Surgery and Related. J Neurosurg Spine. 2014;20:102–107. [DOI] [PubMed] [Google Scholar]
- 20.Langeloo DD, Lelivelt A, Louis Journée H, et al. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: a study of 145 patients. Spine (Phila Pa 1976). 2003;28:1043–1050. [DOI] [PubMed] [Google Scholar]
- 21.Sala F, Palandri G, Basso E, et al. Motor evoked potential monitoring improves outcome after surgery for intramedullary spinal cord tumors: a historical control study. Neurosurgery. 2006;58:1129–1143. [DOI] [PubMed] [Google Scholar]
- 22.Ito Z, Imagama S, Sakai Y, et al. A new criterion for the alarm point for compound muscle action potentials. J Neurosurg Spine. 2012;17:348–356. [DOI] [PubMed] [Google Scholar]
- 23.Quiñones-Hinojosa A, Lyon R, Zada G, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005;56:982–993. [PubMed] [Google Scholar]
- 24.Tamaki T, Kubota S. History of the development of intraoperative spinal cord monitoring. Eur Spine J. 2007;16(suppl 2):S140–S146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasaki H, Tamaki T, Yoshida M, et al. Efficacy and limitations of current methods of intraoperative spinal cord monitoring. J Orthop Sci. 2003;8:635–642. [DOI] [PubMed] [Google Scholar]
- 26.Tsutsui S, Tamaki T, Yamada H, et al. Relationships between the changes in compound muscle action potentials and selective injuries to the spinal cord and spinal nerve roots. Clin Neurophysiol. 2003;114:1431–1436. [DOI] [PubMed] [Google Scholar]
- 27.Komagata M, Nishiyama M, Endo K, et al. Prophylaxis of C5 palsy after cervical expansive laminoplasty by bilateral partial foraminotomy. Spine J. 2004;4:650–655. [DOI] [PubMed] [Google Scholar]
- 28.Sasai K, Saito T, Akagi S, et al. Preventing C5 palsy after laminoplasty. Spine (Phila Pa 1976). 2003;28:1972–1977. [DOI] [PubMed] [Google Scholar]


