Abstract
Background
It is often assumed that increases in cancer survival reflect true progress against cancer. This is true when these increases are accompanied by decreased burden of disease: Fewer people being diagnosed or dying from cancer (ie, decreased incidence and mortality). But increased survival can also occur even when incidence is increasing and mortality is unchanged.
Objective
To use trends in cancer burden—incidence and mortality—to illustrate when changes in survival reflect true progress.
Methods
Using data from 1975 to 2010 collected by the Surveillance, Epidemiology, and End Results Program (incidence, survival) and the National Center for Health Statistics (mortality), we analyzed US trends in five-year relative survival, age-adjusted incidence, and mortality for selected cancers to identify patterns that do and do not reflect progress.
Results
Among the nine common cancers examined, survival increased in seven, and changed little or not at all for two. In some cases, increased survival was accompanied by decreased burden of disease, reflecting true progress. For example, from 1975 to 2010, five-year survival for colon cancer patients improved (from 48% to 68%) while cancer burden fell: Fewer cases (incidence decreased from 60 to 41 per 100000) and fewer deaths (mortality decreased from 28 to 16 per 100000), a pattern explained by both increased early detection (with removal of cancer precursors) and more effective treatment. In other cases, however, increased survival did not reflect true progress. In melanoma, kidney, and thyroid cancer, five-year survival increased but incidence increased with no change in mortality. This pattern suggests overdiagnosis from increased early detection, an increase in cancer burden.
Conclusions
Changes in survival must be interpreted in the context of incidence and mortality. Increased survival only represents progress when accompanied by a reduction in incidence, mortality, or ideally both.
Changes in cancer survival are increasingly being used as a measure to track progress against cancer and appear frequently in medical journal articles, news media reports, and statements from policymakers and advocacy groups. For example, a recent medical journal article argued that higher cancer costs in the United States versus Europe are “worth it” based on increased survival rates (1). And the Komen Foundation’s breast cancer awareness campaign has repeatedly used higher breast cancer survival for screened versus unscreened women as a central argument for why women should undergo mammography (2).
Survival statistics are popular because they seem intuitively obvious: increasing survival sounds like good news, decreasing survival sounds like bad news. But the story is more complicated. Improved survival represents progress when it is accompanied by a decreased burden of disease: fewer people being diagnosed or dying from cancer. But improved survival can also occur even when disease burden is increased. Survival is the proportion of cancer patients who are alive at a given time after diagnosis, and in fact it is a complex measure which can be affected by several biases. These biases are introduced by early detection, typically from screening or incidental detection with advanced imaging. Early detection inflates survival by moving back the time of diagnosis (lead time bias), by identifying relatively slow growing, good prognosis cancers (length bias), or by finding cancers never destined to progress at all or which progress so slowly that the person dies of other causes (overdiagnosis). These biases explain how survival can increase even if no deaths are delayed or prevented. Unfortunately, it is impossible to disentangle the effects of early diagnosis solely based on improved survival in population-based cancer surveillance data because cancer registries do not routinely collect the mode of diagnosis (ie, symptomatic, screened, or incidental).
To understand whether improved survival represents true progress, it needs to be interpreted in the context of cancer burden: How many people are diagnosed with cancer (incidence) and how many die from it (mortality). Building on the work of Dickman and Adami (3), we analyzed trends in cancer survival for major cancer sites in the United States, together with incidence and mortality. We also illustrate survival and incidence trends by stage at diagnosis. For mortality we provide an example of how incidence-based mortality (IBM) (4) by stage at diagnosis, which is obtained from deaths that occurred from cases reported to the cancer registry, can add in the interpretation of the trends.
Materials and methods
We compared changes in five-year relative survival with changes in the burden of cancer—as age-adjusted incidence and mortality rates—from 1975 to 2010 for nine cancer sites: lung, prostate, female breast, colorectal, Hodgkin lymphoma, cervix, melanoma, kidney, and thyroid. We describe which patterns of changes in survival and cancer burden do and do not represent progress.
Measures and Data Sources
For incidence and survival, we used cases diagnosed in 1975 through 2010 from the Surveillance, Epidemiology, and End Results (SEER)-9 cancer registry areas (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah) which covers about 9.4% of the US population. In survival calculations, cases were followed up through December 31, 2010; however, cases diagnosed in 2010 were not included in the calculations because of greater uncertainty in the ascertainment of death. For mortality, we used US population data from National Center for Health Statistics (NCHS) (available in the SEER*Stat software).
The NCHS US cancer mortality data is derived from information recorded on death certificates, and it lacks information pertaining to the onset of disease, for example, stage at diagnosis. However, population-based registries, by linking individuals incidence data to their mortality outcomes, allows for creation of a file containing mortality information (deaths) among those person diagnosed with cancer and reported to the registry. IBM (4) rates are calculated as the deaths from the cases reported to the cancer registry divided by the registry population. In this paper, we calculated IBM rates for female breast cancer by stage at diagnosis in the SEER-9 area to illustrate recent declines in mortality rates by stage. Incidence, mortality and IBM are reported as age-adjusted annual rates per 100000, using the 2000 US standard population. For more information on the standard population see http://seer.cancer.gov/stdpopulations/.
For cancer stage, we used the SEER historical stage A variable which consists of three categories: localized, regional, and distant, and provides consistent staging information over the years. For breast, we also considered ductal carcinoma in situ. Due to coding changes in lung and prostate, consistent staging is only available after 1988 for lung cancer and after 1995 for prostate cancer (see http://seer.cancer.gov/analysis/stage.html for detailed information).
Trend Analyses
We analyzed trends in incidence, mortality, and survival by gender (for cancers that occur in both sexes) and report combined results except where there were differences (lung and thyroid cancer).
To better characterize the trends and changes over time, we fitted joinpoint models to the observed data. Trends in incidence and mortality were modeled using joinpoint regression models that use year of diagnosis (year of death for mortality) as a covariate to estimate times at which statistically significant changes in rates occurred (5,6). For IBM trends, we fit joinpoint models after 1995 (year of death), which is 20 years after the start of cancer registration in 1975, to include all breast cancer deaths reported to the registry (see Discussion for more details).
A different joinpoint model is used to analyze trends in survival. Joinpoint survival models, fit linear segments to the probabilities (hazard) of dying (7). In this work, we report estimated five-year relative survival, rather than observed survival, based on the models from 1975 to 2010; for patients diagnosed 2006 or later, the survival estimates reflect projected results (because five-year follow-up is not available).
Results
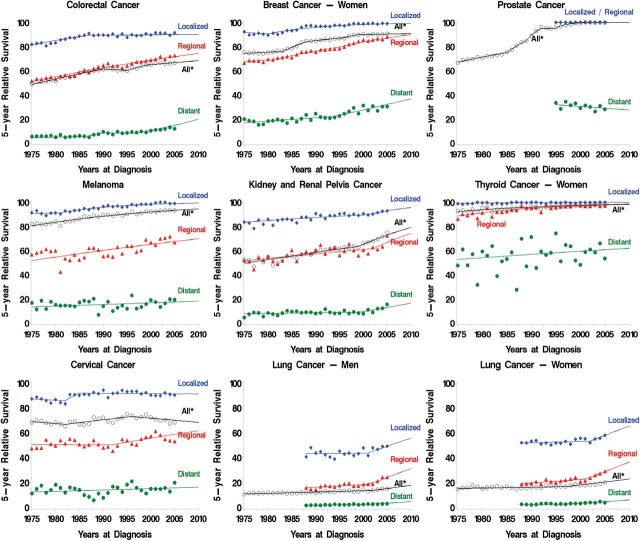
Between 1975 and 2005 increase in survival is observed for seven cancer sites (colorectal, breast, prostate, melanoma, kidney, thyroid cancer, and Hodgkin lymphoma) and projected to continue increasing from 2006 through 2010 (Figure 1). Although, survival changed little for cervical and lung cancer, there is some evidence of an increase in survival after 2001 for men and women diagnosed with localized and regional lung cancer.
Figure 1.
Trends in five-year relative survival by stage (local, regional, distant and all stages)—selected cancer sites (Hodgkin lymphoma not shown). Symbols represent observed values, lines represent estimated values from the joinpoint survival models. *All= All stages.
Considering changes in survival in the context of changes in cancer burden revealed different patterns representing progress, mixed progress, and no progress against cancer. These patterns are summarized in Table 1 and displayed in more detail in Figures 2–6. While multiple scenarios may occur simultaneously for a given cancer, the illustrative examples below focus on the predominant one.
Table 1.
Expected patterns in five-year survival, incidence, and mortality under various scenarios
| Pattern | Time trends | Progress? | Cancer example* | ||
|---|---|---|---|---|---|
| 5-year survival | Incidence | Mortality | |||
| Increase in survival, lower disease burden | |||||
| Treatment more effective | ↑ | ↔ | ↓ | Yes | Hodgkin lymphoma |
| Treatment more effective + increased early detection (screening or incidental detection) without overdiagnosis | ↑ | ↑ ↓ temporary increases and returns to baseline or below baseline for a time | ↓ | Yes | Colorectal cancer† |
| Little or no increase in survival, lower disease burden | |||||
| Detection and removal of cancer precursor | ↔ | ↓ all stages | ↓ | Yes | Cervical cancer‡ |
| Decrease in true occurrence of disease by decrease in risk factors (assuming unchanged cancer aggressiveness) | ↔ | ↓ all stages | ↓ | Yes | Lung cancer (men after 1990) |
| Increase in survival, mixed disease burden | |||||
| Treatment more effective + increased early detection (screening or incidental detection) with overdiagnosis | ↑ | ↑ increase sustained (mostly early stage) | ↓ | Mixed | Breast cancer§, prostate cancer |
| Increase in survival, higher disease burden | |||||
| Treatment not more effective + increased early detection (screening or incidental detection) with overdiagnosis | ↑ | ↑ increase sustained (mostly early stage) | ↔ | No | Melanoma, kidney cancer, thyroid cancer |
| Little or no increase in survival, higher disease burden | |||||
| Increase in true occurrence of disease by increase in risk factors (assuming unchanged cancer aggressiveness) | ↔ | ↑ all stages | ↑ | No | Lung cancer (women after 1980) |
* Examples were selected for illustrative purpose but multiple scenarios may occur simultaneously for a given cancer.
† Decreased colorectal cancer mortality and increased survival reflects both removal of cancer precursors (polyps) and more effective treatment of all stages.
‡ The trends also reflect decrease in risk factors by early detection and removal of cancer precursor.
§ Decreased breast cancer mortality reflects both more effective treatment of all stages and any benefit from screening.
Figure 2.
Progress: Increase in survival, lower disease burden. Symbols represent observed values, lines represent estimated values from the joinpoint regression models. Five-year relative survivals are estimated from the joinpoint survival models and projected values are italicized.
Figure 6.
No progress: Little or no increase in survival, higher disease burden. Symbols represent observed values, lines represent estimated values from the joinpoint regression models. Five-year relative survivals are estimated from the joinpoint survival models and projected values are italicized.
Progress Against Cancer
Increase in Survival, Lower Disease Burden.
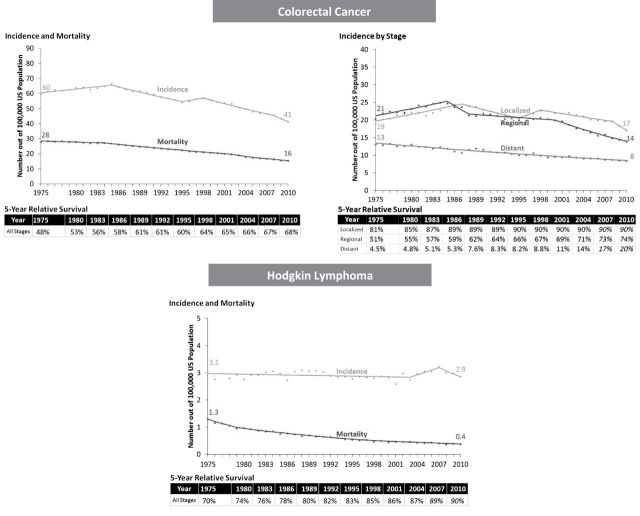
The combination of increased survival and lower disease burden represents unambiguous progress against cancer. Trends in colorectal cancer exemplify this pattern: five-year survival for colorectal cancer patients increased from 48% in 1975 to 68% in 2010 (survival table in Figure 2), while cancer burden in the population fell—incidence and mortality declined from 60 to 41 per 100000 and 28 to 16 per 100000, respectively (Figure 2). The decline in incidence—and increased survival—was most pronounced for regional and distant stages (Figure 1).
A similar pattern was observed for Hodgkin lymphoma. From 1975 through 2010, five-year survival increased from 70% to 90%, while burden fell: Incidence did not change (3 per 100000) but mortality decreased from 1.3 to 0.4 per 100000 (Figure 2).
Little or No Increase in Survival, Lower Disease Burden.
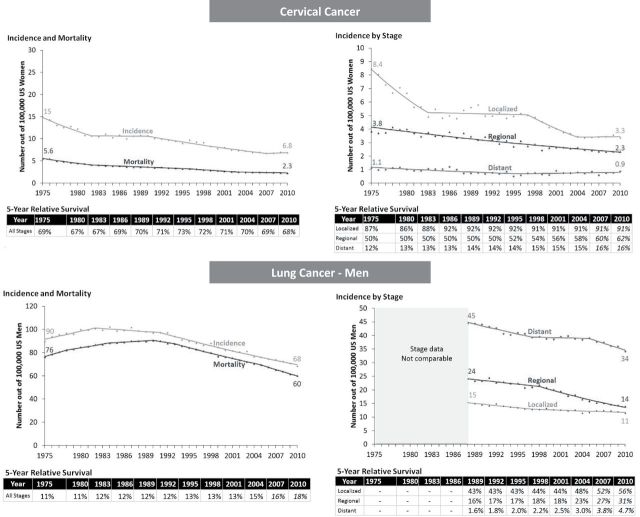
Progress was also observed when survival changed only a little. For cervical cancer, survival was unchanged (all stages combined), but the burden of disease fell substantially, reflecting early detection and removal of cancer precursors (Figure 3). Incidence declined for all stages, survival improved most for regional stage. However, Figure 1 panel for cervical cancer, shows the large variability in survival for regional stage cervical cancer.
Figure 3.
Progress: Little or no increase in survival, lower disease burden. Symbols represent observed values, lines represent estimated values from the joinpoint regression models. Five-year relative survivals are estimated from the joinpoint survival models and projected values are italicized.
Lung cancer in men also demonstrated this pattern: five-year survival changed little, while both incidence and mortality decreased (90 to 68 cases per 100000 and 76 to 60 deaths per 100000) reflecting successful prevention efforts in smoking reduction (8,9) (Figure 3). Incidence declined for all stages but was most pronounced for regional and distant. Overall survival was almost unchanged in the first two decades and started to improve slightly after 2001. From 1989 to 2010, survival by stage improved for localized (from 43% to 56%); regional (from 16% to 31%); and distant (from 1.6% to 4.7%).
Mixed Progress Against Cancer
Increase in Survival, Increased Incidence, Decreased Mortality.
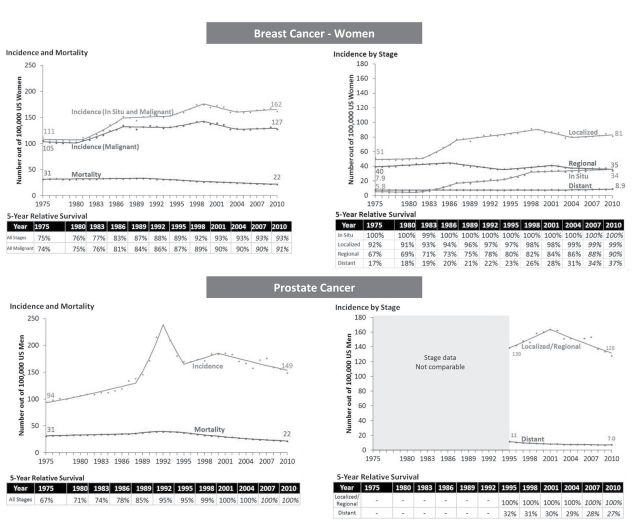
Improved survival can also occur with mixed changes in burden. Some of the largest absolute increases in survival occurred in breast (74% to 91%) and prostate cancer (67% to 100%). For both cancers, mortality has decreased from 1975 to 2010—both breast and prostate cancer mortality fell from 31 to 22 per 100000—representing a decreased burden of disease (Figure 4). But at the same time, incidence increased substantially—breast cancer (in situ and malignant) increased from 111 to 162 per 100000; prostate cancer increased from 94 to 149 per 100000—representing an increased burden of disease.
Figure 4.
Mixed progress: Increase in survival, increased incidence, decreased mortality. Symbols represent observed values, lines represent estimated values from the joinpoint regression models. Five-year relative survivals are estimated from the joinpoint survival models and projected values are italicized.
The increased incidence for breast cancer occurred almost entirely in early stage disease (ductal carcinoma in situ and localized stage), reflecting the introduction and adoption of screening mammography in the mid 1980s and overdiagnosis. The five-year survival increased most for regional and distant stages, likely reflecting more effective treatment during this time period.
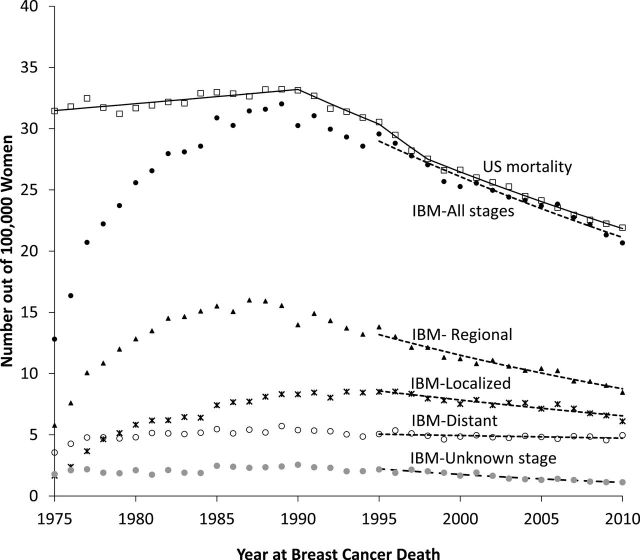
Figure 7 shows female breast cancer IBM by stage at diagnosis. The pattern clearly shows mortality reduction in all stages, including unknown stage. In particular, a significant mortality reduction in the regional stage is observed. This figure also displays US mortality from the NCHS. Trends from US and SEER IBM breast cancer mortality are parallel after 1995, clearly demonstrating the need of 20 years after start of cancer registration to analyze breast cancer IBM trends.
Figure 7.
Age-adjusted breast cancer mortality rates for women diagnosed with breast cancer in the Surveillance, Epidemiology, and End Results (SEER)-9 areas. Includes all breast cancer deaths from women diagnosed with breast cancer between 1975 and 2010. US mortality is obtained from data at the National Center for Health Statistics (NCHS) and incidence-based mortality (IBM) is based on incidence diagnosed from 1975 from SEER-9 area. Symbols represent observed values, lines represent estimated values from the joinpoint regression models. For IBM, joinpoint regression models were fitted to the deaths observed after 1995 to account for follow-up years need to mirror the US mortality rates from NCHS.
Prostate cancer incidence peaked after widespread introduction of prostate-specific antigen screening test in early 1990s and rapidly declined as screening use stabilized. Incidence, however, is still higher in 2010 than in the prescreening era (149 vs 94 per 100000), consistent with overdiagnosis. Due to the lack of consistent staging information, we do not know how stage-specific incidence and survival changed with the introduction of prostate-specific antigen screening.
No Progress Against Cancer
Increase in Survival, Higher Disease Burden.
Between 1975 and 2010, survival increased for melanoma (80% to 94%), kidney cancer (49% to 79%), and thyroid cancer in women (92% to 98%) (Figure 5). At the same time, however, there was an increase in the burden of disease. Although mortality did not change, incidence increased dramatically. Melanoma incidence tripled from 7.9 to 24 per 100000, kidney cancer increased from 7.1 to 15 per 100000, and thyroid cancer in women increased from 6.5 to 21 per 100000—sky rocketing recently (rising on average 6.6% each year after 1996). Most of the increased incidence was from an increase in localized disease (except for thyroid cancer where incidence of regional stage also increased). This pattern suggests overdiagnosis from increased early detection either from screening or incidental detection.
Figure 5.
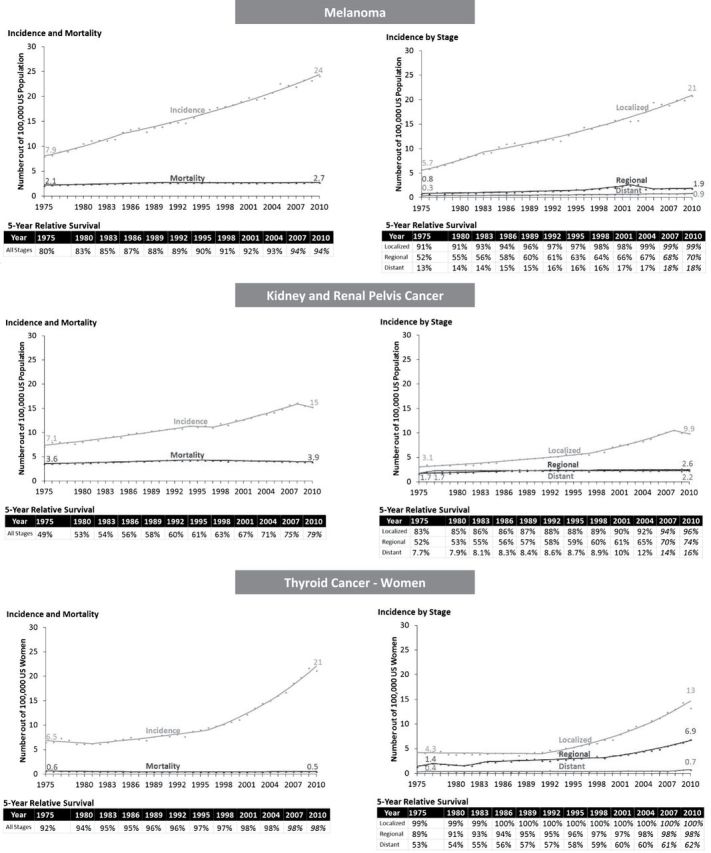
No progress: Increase in survival, higher disease burden. Symbols represent observed values, lines represent estimated values from the joinpoint regression models. Five-year relative survivals are estimated from the joinpoint survival models and projected values are italicized.
Little or No Increase in Survival, Higher Disease Burden.
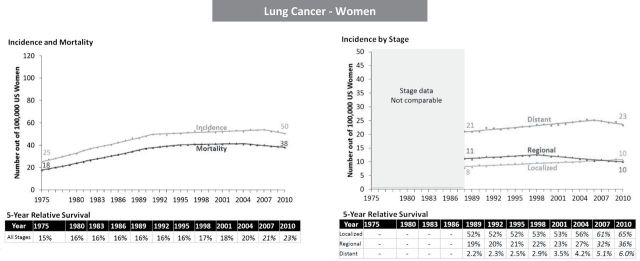
Lung cancer in women illustrates another pattern: From 1975 to 2010 there was a little change in overall survival (15% to 23%) but an unambiguous increase in disease burden (incidence increased from 25 to 50 per 100000 and mortality increased from 18 to 38 deaths per 100000), reflecting the later uptake of cigarette smoking among women compared with men in United States (10,11) (Figure 6). However, trends in recent years show some increase in survival and downturn in disease burden. Overall survival shows almost no change until 1998, followed by a steady modest increase. From 1989 to 2010, survival by stage also increased for localized (from 52% to 65%); regional (from 19% to 36%); and distant (from 2.2% to 6%) stage cancers. (Figure 6). Increase in incidence and mortality were pronounced in 1980s and leveled off in 1990s, and slightly decreasing in later 2000s (after 2007 for incidence and 2004 for mortality).
Discussion
This paper shows that survival increased for seven cancer sites and changed little for two cancer sites. For some cancers, survival increased dramatically, even though more people were diagnosed with the cancer (breast, prostate, thyroid, kidney cancer, and melanoma). For other cancers, survival only increased a little (lung cancer in men) or was unchanged (cervical cancer), despite a substantial decrease in disease burden. Looking at changes in disease burden, however, improves interpretation of increases in survival.
Survival is a complex measure that can be affected by important biases due to early detection (3,12). These biases explain how survival can increase even if no deaths are delayed or prevented. Figure 8 illustrates changes in survival time and lead time bias. In Figure 8A, the first arrow illustrates the natural history of a cancer diagnosis because of symptoms. The survival time is the time from diagnosis to death from the cancer. When treatment is more effective, survival time is extended and death is postponed. If the treatment is not curative, the person still dies of the cancer, while with curative treatment, the person dies from other causes of death. In Figure 8B, the second arrow shows how early detection finds the cancer before symptoms develop and creates extra time—the extra time cause by early detection is called “lead time.” In this scenario, earlier treatment is no more effective than later treatment so the time of death is the same. But the survival time is longer solely due to the lead time. Sometimes earlier treatment is more effective, as in the third arrow. Here survival time is longer because of both lead time and the life-prolonging effect of treatment. Because treatment is not curative, the person still dies of the cancer. The fourth arrow shows the case of curative treatment. Screening also introduces length bias. Cancers have heterogeneous growth rates. The slowest growing cancers have the longest asymptomatic phase, making them most likely to be picked up by screening. By definition, these cancers have the most favorable prognosis, a phenomenon called length bias. In contrast, rapid growing, aggressive cancers have the shortest asymptomatic phase and are least likely to be picked up by screening. As a result of length bias, screen-detected cancers will tend to have the longest survival times. The most extreme case of length bias is overdiagnosis. Here screening detects cancers never destined to cause symptoms or death because death from other causes occurs before the cancer progresses.
Figure 8.
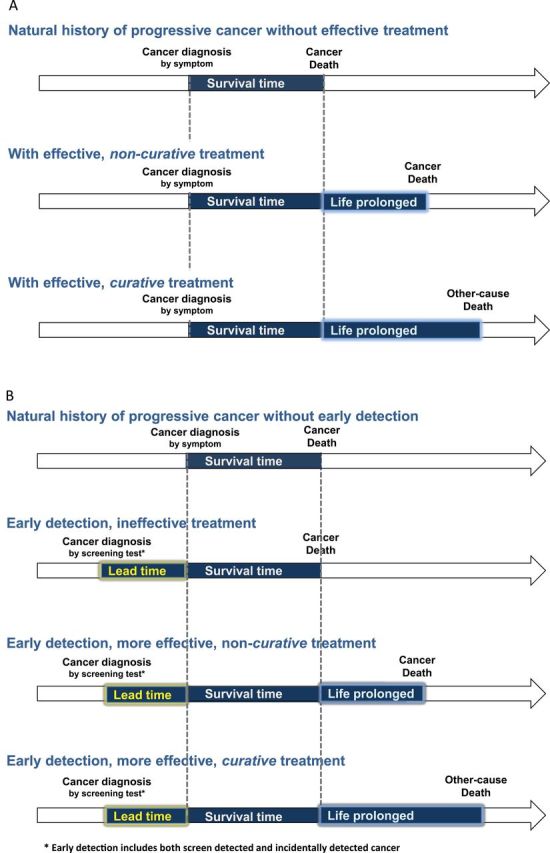
Changes in survival time. Survival time extended are highlighted in light blue (life prolonged) and yellow (lead time). The scenarios assume that cancer diagnosis shorten life expectancy. A) Survival time extended with effective treatment assuming no early detection. B) Survival time extended with lead time bias under early detection.
In addition to the above-mentioned biases, which affect survival for all stages combined, stage migration can spuriously increase stage-specific survival (13). Stage migration is the upstaging of cancers because of more sensitive imaging tests. Stage migration is possible because there is spectrum of aggressiveness among cancers within each stage. Higher resolution imaging reclassifies the most aggressive localized stage cancers—the ones that have just barely crossed into the next stage—into regional stage cancers. As a result, the average aggressiveness of the remaining localized cancers is reduced, and the average aggressiveness of the regional cancers is also reduced because the newly reclassified (formerly localized) cancers are less aggressive than the “traditional” regional cancers. So stage migration increases survival for both localized and regional cancers [the “Will Rogers effect” (13)].
Ideally one would like to estimate cancer survival corrected for biases due to early detection. While this is possible using data from randomized screening trials (14–16), it is not possible from cancer registries that do not routinely collect the mode of diagnosis. Research initiatives to improve the collection of clinical data at the time of diagnosis by increasing use of electronic medical records in cancer registry data collection, for example, may allow improved estimation of cancer survival statistics correcting for some of the early detection biases. Simulation modeling of population trends, such as done in the Cancer Intervention and Surveillance Modeling Network (CISNET), is another useful way to help interpret trends since lead time, length bias, and overdiagnosis can be modeled explicitly and fit to population trends. For example, the CISNET prostate cancer modeling group previously estimated overall estimates of overdiagnosis and lead time (17), and these work could be potentially extended and be used for interpreting survival trends. This remains as a future research.
The best way to interpret survival in terms of a cancer progress measure is to look at survival, mortality, and incidence together. This approach makes it possible to identify the variety of “signatures” such as overdiagnosis (improved survival, increased incidence—especially for early stages and unchanged mortality), improved treatment (improved survival, unchanged incidence, decreased mortality), or removal of cancer precursors (improved survival, decreased incidence, decreased mortality).
Incidence and survival by stage can provide further insight as for example, an increase in early stage incidence is a potential indicator of overdiagnosis. In this study, we have reported US mortality but did not show US mortality by stage due to the lack of information regarding stage at diagnosis from the NCHS mortality data. Instead IBM rates (4) constructed from the deaths of the reported cases in a cancer registry can provide such an information, and we have shown an example using female breast cancer mortality (Figure 7). We show that breast cancer IBM rates are declining for all stages, including unknown stage. This provides more conclusive evidence of progress, than if mortality for one stage is declining but for others increasing with overall mortality declining. However, there is a limitation of IBM trends which requires a caution when using them. IBM trends are reliable for smaller number of years than US mortality rates. Since IBM only includes deaths that occurred from cases reported to the cancer registry and some cancer death may occur many years after cancer diagnosis, it is underestimated in the early years of registry operations. As shown an example in Figure 7, 20 or more years of after the start of registration was required for breast cancer IBM to mirror the US breast cancer mortality rates. The years of follow-up required for the IBM to mirror the US mortality rates depends on the cancer site considered (4). IBM by other tumor characteristic could be used for any incidence characteristics; as for example, by estrogen receptor (ER) expression could be used to show different patterns in mortality for ER-positive and ER-negative breast cancers (18). However analyzing IBM by ER expression would be complicated and require some effort since ER status is only available after 1990 with unknown ER status need to be imputed for the analysis.
We fitted joinpoint models to describe trends in incidence, mortality, and survival. The model incorporates statistically significant changes in trends by identifying change points (called joinpoints). However, we have used the modeling more as a tool to better characterize trends rather than characterizing and interpreting estimated change points. Interpreting specific change points need to be considered together with dissemination of intervention or cancer control policies, and it is not within the scope of this paper. The joinpoint survival model assumes that the hazards of death at different years after diagnosis are proportional. However, it is possible that survival in the 1 year after diagnosis has changed more substantially than in later years after diagnosis and may also have different change points in trends. More flexible survival models will be investigated in future research.
The results of this paper not only apply to temporal comparisons of cancer survival but also to comparisons of cancer survival among different populations. The importance of integrating survival, incidence and mortality, or other indicators is especially important given the recent increase in collaborative studies that compare relative cancer survival across countries and registries (19–23). Survival differences across countries and registries need to be interpreted cautiously because of different screening practices in different populations and the resulting biases due to early detection and other unmeasured confounders (20,22). Focusing on stage-specific comparisons (particularly of more advanced stages) or survival comparison of cancer sites without screening or early detections should be less affected by these biases. Inclusion of stage-specific incidence rates, mortality rates, and proportion of cases diagnosed at early stage versus late stage can improve interpretation of the results. Similar design displaying survival trends together with incidence and mortality trends are reported for bladder, pancreas, and corpus uteri cancers and leukemia in the overview chapter of this monograph (24).
In conclusion, survival is an important cancer prognosis measure for clinicians and their patients. To be correctly used as a cancer policy measure and to correctly understand temporal changes in survival, changes in incidence and mortality, or other indicators need to be considered simultaneously. Improved survival does represent progress when accompanied by a reduction in mortality, incidence, or ideally both.
Funding support
Supported by the National Cancer Institute.
We thank Martin Krapcho and Deborah G. Hacker of Information Management Services, Inc, for computational support and assistance in compiling the data used in this article.
References
- 1. Philipson T, Eber M, Lakdawalla DN, Corral M, Conti R, Goldman DP. An analysis of whether higher health care spending in the United States versus Europe is ‘worth it’ in the case of cancer. Health Aff (Millwood). 2012;31(4):667–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woloshin S, Schwartz LM. How a charity oversells mammography. BMJ. 2012;345:e5132. [DOI] [PubMed] [Google Scholar]
- 3. Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260(2):103–117. [DOI] [PubMed] [Google Scholar]
- 4. Chu KC, Miller BA, Feuer EJ, Hankey BF. A method for partitioning cancer mortality trends by factors associated with diagnosis: an application to female breast cancer. J Clin Epidemiol. 1994;47(12):1451–1461. [DOI] [PubMed] [Google Scholar]
- 5. Joinpoint: Joinpoint Regression Program [computer program]. Version 4.0.4. http://surveillance.cancer.gov/joinpoint/ Bethesda, MD: Surveillance Research Program, National Cancer Institute; 2013. [Google Scholar]
- 6. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 7. Yu B, Huang L, Tiwari RC, Feuer EJ, Johnson KA. Modelling population-based cancer survival trends using join point models for grouped survival data. J R Stat Soc Ser A Stat Soc. 2009;172(2):405–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moolgavkar SH, Holford TR, Levy DT, et al. Impact of reduced tobacco smoking on lung cancer mortality in the United States during 1975-2000. J Natl Cancer Inst. 2012;104(7):541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thun MJ, Jemal A. How much of the decrease in cancer death rates in the United States is attributable to reductions in tobacco smoking? Tob Control. 2006;15(5):345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris JE. Cigarette smoking among successive birth cohorts of men and women in the United States during 1900-80. J Natl Cancer Inst. 1983;71(3):473–479. [PubMed] [Google Scholar]
- 11. Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100(23):1672–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA. 2000;283(22):2975–2978. [DOI] [PubMed] [Google Scholar]
- 13. Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N Engl J Med. 1985;312(25):1604–1608. [DOI] [PubMed] [Google Scholar]
- 14. Duffy SW, Nagtegaal ID, Wallis M, et al. Correcting for lead time and length bias in estimating the effect of screen detection on cancer survival. Am J Epidemiol. 2008;168(1):98–104. [DOI] [PubMed] [Google Scholar]
- 15. Lawrence G, Wallis M, Allgood P, et al. Population estimates of survival in women with screen-detected and symptomatic breast cancer taking account of lead time and length bias. Breast Cancer Res Treat. 2009;116(1):179–185. [DOI] [PubMed] [Google Scholar]
- 16. Shen Y, Yang Y, Inoue LY, Munsell MF, Miller AB, Berry DA. Role of detection method in predicting breast cancer survival: analysis of randomized screening trials. J Natl Cancer Inst. 2005;97(16):1195–1203. [DOI] [PubMed] [Google Scholar]
- 17. Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jatoi I, Chen BE, Anderson WF, Rosenberg PS. Breast cancer mortality trends in the United States according to estrogen receptor status and age at diagnosis. J Clin Oncol. 2007;25(13):1683–1690. [DOI] [PubMed] [Google Scholar]
- 19. Berrino F, Gatta G, Chessa E, Valente F, Capocaccia R. Introduction: the EUROCARE II Study. Eur J Cancer. 1998;34(14 Spec No):2139–2153. [DOI] [PubMed] [Google Scholar]
- 20. Coleman MP, Gatta G, Verdecchia A, et al. ; EUROCARE Working Group. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol. 2003;14(suppl 5):v128–v149. [DOI] [PubMed] [Google Scholar]
- 21. Coleman MP, Quaresma M, Berrino F, et al. ; CONCORD Working Group. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9(8):730–756. [DOI] [PubMed] [Google Scholar]
- 22. Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R; EUROCARE Working Group. EUROCARE-4. Survival of cancer patients diagnosed in 1995-1999. Results and commentary. Eur J Cancer. 2009;45(6):931–991. [DOI] [PubMed] [Google Scholar]
- 23. Thomson CS, Forman D. Cancer survival in England and the influence of early diagnosis: what can we learn from recent EUROCARE results? Br J Cancer. 2009;101(suppl 2):S102–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mariotto AB, Noone AM, Howlader N, et al. Cancer survival: an overview of measures, uses, and interpretation. J Natl Cancer Inst Monogr. 2014;49:145–186. [DOI] [PMC free article] [PubMed] [Google Scholar]