Abstract
Adolescent and young adults (AYAs) face challenges in having their cancers recognized, diagnosed, treated, and monitored. Monitoring AYA cancer survival is of interest because of the lack of improvement in outcome previously documented for these patients as compared with younger and older patient outcomes. AYA patients 15–39 years old, diagnosed during 2000–2008 with malignant cancers were selected from the SEER 17 registries data. Selected cancers were analyzed for incidence and five-year relative survival by histology, stage, and receptor subtypes. Hazard ratios were estimated for cancer death risk among younger and older ages relative to the AYA group. AYA survival was worse for female breast cancer (regardless of estrogen receptor status), acute lymphoid leukemia (ALL), and acute myeloid leukemia (AML). AYA survival for AML was lowest for a subtype associated with a mutation of the nucleophosmin 1 gene (NPM1). AYA survival for breast cancer and leukemia remain poor as compared with younger and older survivors. Research is needed to address disparities and improve survival in this age group.
Cancer is one of the top five leading causes of death in the adolescent and young adult (AYA) population, those individuals diagnosed with cancer from 15 to 39 years of age (1). In 2010, 4.89% of cancers and 1.60% of cancer deaths occurred in this age group identified by the 18 registries from the National Cancer Institute’s (NCI’s) Surveillance, Epidemiology, and End Results (SEER) program or SEER-18 populations (2). Cancer is mainly a disease of mature adulthood, with more than half of new cases in 2009 occurring in those 65 and older. Recognition of the unique biology, cancer types, and lack of progress in treatment success in the AYA population has been evident over the last decade (3,4). To address the unique needs of AYAs with cancer, in 2006, NCI and LIVESTRONG convened a Progress Review Group (5) that created recommendations for improving outcomes in AYA cancer patients. The same year, NCI published a monograph describing cancer etiology, incidence and survival statistics particular to AYA patients, age 15–29. In 2009, the NCI held a workshop comparing the biology of acute lymphoblastic leukemia, breast cancer, and colon cancer in AYA patients to adult patients (6). In 2009, Seminars in Oncology published a two part series of articles on AYA cancers, age 20–39 (7).
These activities have made great strides toward improving awareness and understanding of AYA cancers. Cancer incidence patterns vary among childhood, AYA, and adult populations. Pediatric and AYA groups have higher proportions of leukemias and lymphomas than those 40 and older (see Table 1). While brain and central nervous system (CNS) cancers affect pediatric, AYA, and adult populations, proportions of the histological subtypes are often different between older and younger patient groups with pilocytic astrocytomas more common in pediatric populations, and glioblastomas more common with increasing age (1). There are many explanations for cancer survival differences in the AYA age group, including suggestions that the biology of breast and colon cancer may be different in AYAs (6), access to care (8,9), participation in clinical trials and whether the treatments are directed at children or adult populations (6,10), contrasting improvement in outcomes compared with younger and older patients (11), economic (12) and psychosocial issues (13).
Table 1.
Description of AYA cancer site ICD-O-3 histological codes*
| ALL | B cell, T cell, and unknown, from the SEER AYA site recode |
| AML | With genetic changes: ICD-O-3 9861, 9866, 9871, 9896, 9897; AML NOS: ICD-O-3 9840, 9867, 9872–9874, 9891; 9861; AML with nucleophosmin 1 (NPM 1) mutation: 9861 |
| Hodgkin lymphoma | SEER AYA site recode |
| Non-Hodgkin lymphoma | SEER AYA site recode |
| All CNS | SEER AYA site recode |
| Osseous sarcoma | SEER AYA site recode |
| Soft tissue sarcoma | SEER AYA site recode |
| Germ cell and trophoblastic neoplasms | Male germ cell site codes were confirmed as trophoblastic neoplasms and included testicular cancer, among other sites |
| Melanoma and skin carcinomas | SEER AYA site recode |
| Thyroid carcinoma | Include papillary (ICD-O-3 8050, 8260, 8340, 8341, 8343, 8344, 8350), follicular (ICD-O-3 8290: 8330–8332, 8335) and medullary ICD-O-3 8345, 8346, 8510) histological types (17) |
| Carcinoma of breast | Breast cancer was restricted to ductal and lobular neoplasms (ICD-O-3 8500-8549) |
| Carcinoma of colon and rectum | Include only adenomas and adenocarcinomas (ICD-O-3 8140-8389) |
| Carcinoma of cervix and uterus | Squamous cell (ICD-O-3 8050-8089) and adenomas/adenocarcinomas (ICD-O-3 8140-8389) were combined for cervix and uterine cancer because these histologies demonstrated similar trends |
* ALL = acute lymphoid leukemia; AML = acute myeloid leukemia; CNS = central nervous system; AYA = adolescents and young adults; ICD-O-3 =International Classification of Diseases for Oncology, Third Edition; NOS = not otherwise specified; SEER = Surveillance, Epidemiology, and End Results.
In this paper, we devote our attention to survival statistics relevant to the AYA population ages 15–39. Using recent SEER data, first we examine the distribution of incident cancers in AYAs compared with younger and older populations. Then we selected 10 of the more common cancers among AYAs to investigate differences in relative survival rates for AYAs compared with younger and older populations. In these analyses, we aim to identify areas where cancer survival differs for AYAs to offer pathways for research aimed at reducing disparities in the future.
Data and Variables
We selected patients diagnosed with primary malignant cancers between 2000 and 2008 from the November 2011 submission of the SEER-17 registries, including San Francisco-Oakland, Connecticut, Detroit, Hawaii, Iowa, New Mexico, Seattle, Utah, Atlanta, San Jose-Monterey, Los Angeles, Rural Georgia, Greater California, Kentucky, Louisiana, New Jersey, and Greater Georgia. Alaskan Native Registry cases were excluded to avoid over representation of this population. Patients were followed for 5 years from diagnosis or until December 2009. Patients diagnosed on death certificate or autopsy and with unknown age only were excluded.
In order to assess incidence rates and compare AYA survival with the older adults and younger children, we focused on cancer sites that occurred in AYAs and other age groups. For all age groups, the AYA site recode (14,15) from the SEER data was used to identify the first primary cancer site. The AYA site recode is a classification scheme that was devised specifically for AYA patients after the realization that codes in the International Classification of Childhood Cancer (ICCC) scheme have a different distribution in the AYA age group (14). The AYA site recode variable is intended to rely more on morphology rather than topography, and minimize the number of malignancies grouped as “other.” The AYA cancer sites were categorized into (Tables 1 and 2): carcinomas of the colon and rectum, cervix and uterus, breast, thyroid, melanoma and skin, germ cell and trophoblastic neoplasms, sarcomas of the CNS, osseous, and soft tissue sites, ALL, acute myeloid leukemia (AML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), all other carcinomas, miscellaneous and unspecified neoplasms. Cervix and uterus were left combined as they are in the current iteration of this classification (14) to permit comparisons. To reduce bias in differential diagnosis between age groups we considered common histologies from the International Classification of Diseases for Oncology, Third Edition [ICD-O-3 (15)] which are described in Table 1. Male germ cell site codes were confirmed as trophoblastic neoplasms and included testicular cancer, among other sites. Kaposi sarcoma (KS) cases were excluded because KS is a consequence of infection with HIV. There were slight differences in the cancer sites considered in the incidence and survival analyses. HL and NHL were included in the incidence analyses, however NHL was excluded from the survival analyses because more than 50% of NHL deaths are AIDS-associated among those 30–40 years old (16). Both the osseous and soft tissue sites were not considered for survival analysis because incidence rates were too low in all age groups (Table 2).
Table 2.
Incidence rates per 100 000 and percent of malignant cancers diagnosed 2000–2009, SEER17 by age at diagnosis*
| 0–14 | % | 15–39 | % | 40–64 | % | 65+ | % | |
|---|---|---|---|---|---|---|---|---|
| Males | ||||||||
| Leukemias | 5.34 | 33.18 | 3.48 | 6.99 | 13.61 | 2.38 | 78.71 | 2.71 |
| Lymphomas (HL and NHL) | 1.89 | 11.52 | 8.56 | 17.13 | 29.35 | 5.10 | 113.95 | 3.97 |
| All CNS | 3.22 | 19.91 | 3.13 | 6.27 | 9.23 | 1.60 | 24.31 | 0.86 |
| Osseous sarcoma | 0.84 | 5.10 | 1.10 | 2.22 | 1.07 | 0.18 | 2.33 | 0.08 |
| Soft tissue sarcoma | 1.04 | 6.42 | 2.30 | 4.61 | 5.85 | 1.01 | 22.35 | 0.77 |
| Germ cell and trophoblastic neoplasms | 0.51 | 3.19 | 10.31 | 20.96 | 5.46 | 0.92 | 0.93 | 0.03 |
| Melanoma and skin carcinomas | 0.20 | 1.22 | 5.64 | 11.10 | 34.56 | 6.02 | 124.74 | 4.35 |
| Thyroid carcinoma | 0.10 | 0.63 | 2.68 | 5.28 | 8.41 | 1.46 | 12.35 | 0.45 |
| Carcinoma of breast | 0.00 | 0.00 | 0.06 | 0.11 | 1.23 | 0.22 | 6.09 | 0.21 |
| Carcinoma of colon and rectum | 0.02 | 0.10 | 3.60 | 6.94 | 59.06 | 10.36 | 305.82 | 10.66 |
| All other carcinomas and misc. and unspecified neoplasms | 2.96 | 18.72 | 9.54 | 18.38 | 401.25 | 70.64 | 2146.13 | 75.90 |
| Total | 16.12 | 100.00 | 50.40 | 100.00 | 569.08 | 100.00 | 2837.70 | 100.00 |
| Females | ||||||||
| Leukemias | 4.49 | 31.58 | 2.58 | 3.19 | 8.66 | 1.56 | 42.63 | 2.60 |
| Lymphomas (HL and NHL) | 1.06 | 7.34 | 6.90 | 8.58 | 20.06 | 3.61 | 79.43 | 4.76 |
| All CNS | 2.97 | 20.72 | 2.52 | 3.13 | 6.17 | 1.10 | 16.09 | 0.95 |
| Osseous sarcoma | 0.75 | 5.20 | 0.80 | 1.00 | 0.87 | 0.15 | 1.46 | 0.09 |
| Soft tissue sarcoma | 0.87 | 6.06 | 2.31 | 2.85 | 6.74 | 1.20 | 13.56 | 0.81 |
| Germ cell and trophoblastic neoplasms | 0.59 | 4.15 | 1.05 | 1.32 | 0.24 | 0.04 | 0.09 | 0.01 |
| Melanoma and skin carcinomas | 0.23 | 1.60 | 9.40 | 11.59 | 26.15 | 4.62 | 50.13 | 2.99 |
| Thyroid carcinoma | 0.36 | 2.50 | 13.40 | 16.52 | 24.95 | 4.36 | 19.35 | 1.11 |
| Carcinoma of breast | 0.00 | 0.02 | 20.45 | 24.37 | 215.91 | 38.58 | 409.63 | 23.97 |
| Carcinoma of cervix and uterus | 0.01 | 0.06 | 9.11 | 11.05 | 50.63 | 9.10 | 85.03 | 4.94 |
| Carcinoma of colon and rectum | 0.02 | 0.13 | 3.24 | 3.91 | 45.15 | 8.18 | 224.40 | 13.61 |
| All other carcinomas and misc. and unspecified neoplasms | 2.89 | 20.65 | 10.33 | 12.50 | 151.27 | 27.51 | 739.01 | 44.17 |
| Total | 14.24 | 100.00 | 82.10 | 100.00 | 556.79 | 100.00 | 1680.81 | 100.00 |
* CNS = central nervous system; HL = Hodgkin lymphoma; NHL = non-Hodgkin lymphoma.
Results were stratified by SEER historic stage [localized, regional, or distant (17)], a strong predictor of cancer survival. For female breast cancer, we also examined estrogen receptor (ER) status. For HL, where historic stage was not available, we restricted the analysis to more recent data (2004–2009) and stratified by American Joint Committee on Cancer (AJCC) 6th Edition [2004 (18)], forming two groups: early stage (I–II, above the peritoneum) and late stage (III–IV, below the peritoneum).
AML and acute lymphoid leukemia (ALL) were classified according to the World Health Organization (WHO) Classification of Tumors (19) and further collapsed into fewer groups. While AML type 9861/3 has a described genetic abnormality involving mutations of the nucleophosmin 1 (NPM1) gene (19), the five-year relative survival varied enough to warrant a separate analysis group. ALL was stratified by cell of origin, B cell or T cell, according to the 2012 Hematopoietic and Lymphoma Neoplasms manual (20). CNS cancer sites were included as a group, and sarcoma was defined as either osseous or soft tissue (14).
Incidence and Survival Methods
Incidence and survival results were obtained using SEER*stat software version 7.1.0. Age-adjusted incidence rates were reported as counts per 100 000 person years. Relative survival, an estimate of cancer survival, is the ratio of all-causes survival and the expected survival from comparable US life tables of the same age, gender, year, and race as the respective cancer patients group. We used the 1970–2007 life tables which are available from SEER*Stat software (21), estimated from the National Center for Health Statistics decennial and annual life tables. Cansurv software was used to estimate hazard ratios (HRs) (22), from relative survival data. The relative risks of cancer death (HR estimates) are presented for patients diagnosed at age 0–14, 40–64, and 65–79 years old compared with the AYA group, ages 15–39. If the HR is less than 1.00, the patients of that age group have a lower risk of cancer death compared with AYAs; if the HR is greater than 1.00, patients of that age group have a higher risk of cancer death compared with AYAs. HRs were considered significantly different when the 95% confidence intervals did not overlap (P < .05).
Results
Incidence rates per 100 000 and frequencies of cancer sites are compared between AYA’s and other age groups in Table 2. The relative risk of cancer death 5 years after diagnosis for patients diagnosed at ages 0–14, 40–64, and 65–79 compared with the AYA group, age 15–39 are presented in Table 3. Five-year relative survival estimates by five-year groups are presented in Figures 1 and 2 for selected cancer sites.
Table 3.
Five-year hazard ratios of cancer death with adolescent and young adult (AYA) patients as the comparison group*
| Hazard ratios | |||||||
|---|---|---|---|---|---|---|---|
| Cancer | Histology | Subgroup | N (%AYA) | 0–14 | 15–39 | 40–64 | 65–79 |
| Female breast cancer | Ductal and lobular | Localized | 199 231 (5%) | — | 1.00 | ‡ | ‡ |
| (Relative survival) | neoplasms | Regional | 113 050 (9%) | — | 1.00 | 0.69† | 0.79† |
| Distant | 18 165 (9%) | — | 1.00 | 1.04 | 1.43† | ||
| ER+ | 230 195 (5%) | — | 1.00 | 0.54† | 0.46† | ||
| ER− | 67 763 (11%) | — | 1.00 | 0.87† | 0.96 | ||
| ER unknown | 34 630 (6%) | — | 1.00 | 0.69† | 0.75 | ||
| Female breast cancer | Ductal and lobular | Localized | 198 665 (5%) | — | 1.00 | 0.57† | 0.74† |
| (Cause-specific survival) | neoplasms | Regional | 112 506 (9%) | — | 1.00 | 0.73† | 0.95 |
| Distant | 17 957 (9%) | — | 1.00 | 1.06 | 1.44† | ||
| ER+ | 229 406 (5%) | — | 1.00 | 0.64† | 0.88† | ||
| ER− | 67 411 (11%) | — | 1.00 | 0.90† | 1.09† | ||
| ER unknown | 34 421 (6%) | — | 1.00 | 0.71† | 0.83† | ||
| Colorectal | Adenomas and adenocarcinomas | Localized | 81 701 (3%) | — | 1.00 | 1.12 | 2.09† |
| Regional | 66 447 (4%) | — | 1.00 | 0.96 | 1.35† | ||
| Distant | 35 640 (4%) | — | 1.00 | 1.10† | 1.53† | ||
| Cervix and uterine cancer | Adenomas, adenocarcinomas, and squamous cell | Localized | 55 477 (12%) | — | 1.00 | 0.80† | 0.92 |
| Regional | 18 315 (12%) | — | 1.00 | 0.79† | 1.12† | ||
| Distant | 5609 (9%) | — | 1.00 | 0.93 | 1.16† | ||
| Melanoma | Localized | 86 403 (18%) | — | 1.00 | — | — | |
| Regional | 10 627 (15%) | — | 1.00 | 1.60† | 2.05† | ||
| Distant | 3740 (9%) | — | 1.00 | 1.12 | 1.15 | ||
| Male germ cell carcinoma | Localized | 12 997 (69%) | 6.93† | 1.00 | 0.71 | — | |
| Regional | 3386 (70%) | 1.4† | 1.00 | 1.25† | — | ||
| Distant | 2194 (73%) | 0.57 | 1.00 | 1.26† | — | ||
| Hodgkin lymphoma | AJCC stages I and II | 5691 (61%) | — | 1.00 | 2.46† | 10.89† | |
| AJCC stages III and IV | 3588 (49%) | — | 1.00 | 2.91† | 8.71† | ||
| Acute lymphoid leukemia | B cell | 6000 (28%) | 0.13† | 1.00 | — | — | |
| T cell | 961 (44%) | 0.36† | 1.00 | — | — | ||
| Unknown | 1420 (23%) | 0.15† | 1.00 | — | — | ||
| Acute myeloid leukemia | With recent genetic abnormalities | 10 525 (18%) | 0.67† | 1.00 | 1.90† | 4.29† | |
| 9861/3 | 8143 (13%) | 0.6† | 1.00 | 1.71† | 3.48† | ||
| NOS | 4756 (16%) | 0.64† | 1.00 | 1.74† | 3.52† | ||
| CNS | 20 735 (19%) | 0.98 | 1.00 | 3.87† | 9.01† | ||
| Sarcoma | Osseous | Localized | 1262 (37%) | 0.88 | 1.00 | 1.01 | 2.39† |
| Regional | 1283 (39%) | 0.62† | 1.00 | 1.04 | 1.79† | ||
| Distant | 624 (41%) | 0.58† | 1.00 | 1.34† | 2.01† | ||
| Sarcoma | Soft tissue | Localized | 7,639 (22%) | 0.82 | 1.00 | 1.50† | 2.11† |
| Regional | 3,590 (21%) | 0.63† | 1.00 | 1.21† | 1.92† | ||
| Distant | 2,252 (19%) | 0.57† | 1.00 | 1.51† | 1.93† | ||
* AJCC = American Joint Committee on Cancer; CNS = central nervous system, ER = estrogen receptor; NA = not available; NOS = not otherwise specified.
† P < .05
‡ Did not converge.
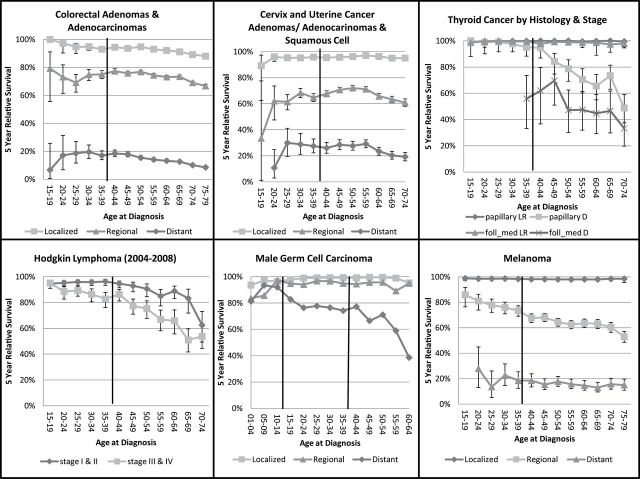
Figure 1.
Five-year relative survival by age group and cancer site. The adolescent and young adult (AYA) group is marked by the solid vertical lines.
Figure 2.
Five-year relative survival by age group and cancer site. The adolescent and young adult (AYA) group is marked by the solid vertical lines.
Solid Malignancies
Carcinomas are more common in patients over age 40 (Table 2). The top incident sites among AYA males include germ cell neoplasms, lymphomas (including both HL and NHL), and melanoma and skin carcinomas. Among AYA females, breast, thyroid, cervix and uterine cancers are the most common carcinomas. Breast cancer continues to dominate incidence rates among females older than 40, while carcinomas of other sites along with miscellaneous and unspecified neoplasms dominate incidence rates among males older than 40.
Patients above 40 years of age with localized and regional breast cancer had a significantly lower risk of cancer death than AYAs 15–39 (Table 3), while distant stage patients older than 40 had a higher risk of death that is significant among those 65–79 years of age. For most age subgroups, relative survival rates by stage (Figure 1) tended to be slightly worse for AYAs 20–29 years of age than those 30 and older. For localized stage, 20–24-year old survival rates are 90% and gradually approach 100% by age 65–69 years (Figure 1, top left). Regional stage shows a similar trend for younger (75% survival) to mid-life (90% survival) age groups, then starts to decline after 64 years (85% survival) but not fully to the rate of AYAs. For distant stage, survival is below 30% for AYAs aged 20–29, peaks to 40% at age 40–49 then gradually drops below 30% again after age 69. Estimates of the HRs (Table 3) show significantly lower risk of death for patients age 40–64 compared with AYA across ER status (ER+, ER−, ER unknown: HRs range 0.54–0.87 for relative survival). Risk of death was also lower by ER status for patients aged 65–79, especially for those with ER+ tumors (HR = 0.46, significant). Five-year relative survival among AYA subgroups by ER status (Figure 1) is indistinguishable for those 20–29 years of age, but improves for those with ER+ tumors beginning with those diagnosed at 30–34 years of age through age 70–74. Five-year relative survival for ER− and ER unknown tumors was similar and hovered around 80%.
Because relative survival for breast cancer tends to be overestimated for local stage (16) as older populations are screened more often than AYA populations, we evaluated cause-specific survival for breast cancer. The results are very similar to the results based on relative survival (Table 3).
For most of the other carcinomas (colorectal cancer, cervix and uterine cancer, melanoma, and male germ cell carcinoma), AYAs have better survival outcomes compared with older and younger patients (Table 3). Five year-relative survival for colorectal cancer by stage gradually declines with increasing age. For localized colorectal cancer, HRs indicate double the risk of cancer related death in the oldest ages compared with AYAs (localized HR = 2.09, P < .05 for those 65–79 years, Table 3). Cervix and uterine cancer HRs show a lower risk of death for those aged 40–64 with localized stage disease (HR = 0.80, P < .05) compared with AYAs but the HRs reverse for 65–79 year olds with more advanced stages of regional and distant stage disease (HR = 1.12, 1.16, respectively, P < .05). Melanoma relative survival rates are consistently high (>95%) across age groups for localized stage. For regional stage disease, five-year relative survival has a steady decline with increasing age (HR = 1.60 for age 40–64 and 2.05 for age 65–79, compared with AYAs P < .05). Distant stage melanoma is rare among AYAs (9% of cases) and survival rates are consistently low across age groups. These data indicate no difference in the risk of cancer death by age group for late stage melanoma (Table 3). Male germ cell survival rates are above 90% for localized and regional stage disease across age groups (see Figure 2). For distant stage disease, the risk of cancer death is lower for children under 15 (0.57) and higher for older adults 40–64 years (HR = 1.26, P < .05) compared with AYAs (Table 3).
Thyroid cancers were divided into papillary and follicular/medullary subgroups and further stratified by early and late stage disease. Early stage cancers are more common in AYAs than distant stage disease and those cancers have high survival rates (above 90%) regardless of histology and age, see Figure 2. Survival for distant papillary thyroid cancer starts to decline in the 40’s reaching 50% in the 70–74-year-old age group. Distant stage follicular/medullary cancer is rare among those younger than 35 years of age. Relative survival rates for ages 35–39 are just under 60%. HRs could not be estimated due to instability of the estimates from the data available, not shown in Table 3.
Hematopoietic Malignancies
Hematopoietic conditions (including HL and leukemias) represent approximately 40% of all incident cancers for patients under age 15 (23) (Table 2), but this proportion decreases throughout young adulthood and the older age groups. For both early (I–II) and late (III–IV) stage HLs among the AYAs have better five-year relative survival than patients over 40, (HR > 2, P < .05, Table 3). Late stage five-year relative survival is 95% at age 15–19 years and drops to 85% by age 35–39 years. After age 40, relative survival for late stage cancer drops more rapidly, finally reaching 50% by age 65–69 (Figure 2).
Results for acute lymphoid leukemia (ALL) show worse relative survival for AYAs compared with younger children by cell type (HR = 0.13 for B cell, 0.36 for T cell, P < .05, Table 3). There appears to be a slight interaction effect of age and cell type on five-year relative survival. Among children ages 1–9 years old, T-cell ALL has worse survival than B-cell ALL, however, AYAs with T-cell ALL tend to have similar survival to B-cell ALL AYAs (Figure 1). For B-cell ALL, five-year relative survival drops from a high of more than 90% among 1–9 year olds, to a low of 30% for those 35–39 years old. Five-year relative survival for T-cell ALL has a smaller range, from a high of nearly 80% among those 5–9 years old to nearly 35% among 35–39 year olds (Figure 1).
HRs were more favorable with a lower risk of cancer death for all AML subtypes in the childhood group, ages 0–14, compared with the older age groups, P value less than .05, regardless of type (Table 3). Survival favors the AML subtype with genetic abnormalities across age groups, though this is not significant (Figure 1). For all subtypes, five-year relative survival drops almost continuously from the youngest age group (60–70% relative survival, age 0–4) to the oldest age group (10% relative survival, age 65–69).
HRs for central nervous system (CNS) tumors indicated that those in childhood and the AYA age group had a lower risk of death than those in the 40–64 years age group or the 65–79 years age group. Risk of dying among 40–64 years olds was nearly four times that of AYAs (HR = 3.87, P < .05), and nine times as high in the 65–79 year age group (HR = 9.01, P < .05). More than a third of the osseous sarcoma cases occurred in the AYA age group, however risk of dying increased with age, especially among the 65–79 years old group (Table 3). The proportion of soft tissue sarcomas occurring in the AYA population was less, at approximately 20%, however a steady increase in the risk of dying was evident by age and stage, with the risk of dying for those 65–79 nearly double that of the AYA group (HRs = 2.11, 1.92, 1.93 for localized, regional, and distant stages, respectively, P < .05).
Discussion
Cancer occurs mostly among those 65 and older; however, cancer incidence and survival evaluation in younger ages is important to determine cancer frequency and survival in groups that are sometimes overlooked. In this article, we assessed incident cancer rates for several carcinomas, melanoma, hematopoietic, and other sites among AYAs, and evaluated five-year relative survival by specific cancer characteristics and age using data from 2000 to 2009. Results from these analyses revealed that only breast cancer and leukemias had worse survival for AYAs, than older and younger age groups, respectively. Relative survival relies on the assumption that the life table estimates represent survival from other-cause death for the cohort of cancer patients. Studies have shown that screened populations may have a better overall survival than life tables (24). For breast cancer patients, we also calculated HRs using cause-specific survival from the SEER cause of death classification variable. The overestimation of relative survival for localized stage breast cancer has a differential effect across age (16), where survival among older age groups is more likely to be overestimated than among AYAs. This is because older populations are more heavily screened than AYA’s who are less likely to be screened (16). We compared cause-specific and relative survival for breast cancer, and found that relative survival was slightly overestimated in the older age groups for localized (after age 45) and regional stage (after age 55) (data not shown). This indicates that there is some differential bias across age groups due to life tables not representing the background mortality in those cohorts because of a “healthy screener effect.”
Despite adjustment for stage and ER status we found that young women with early stage breast cancer had 10–20% worse five-year relative survival than older patients. Reasons for these differences may be influenced by confounders such as race and ethnicity, and biologic characteristics that need further exploration in AYAs (6). Shavers et al. (25) found that African-American women under 35 have a higher incidence rate, worse prognosis and survival outcomes even after controlling for demographic and treatment differences. Cancer detection method may also influence these differences, as younger patients are less likely to have cancers detected by mammography (26), so survival for older ages may be artificially higher due to overdiagnosis, length and lead time biases. Johnson et al. analyzed breast cancer trends among women aged 25 and older (27). An increasing trend in incidence of distant stage breast cancer was noted among younger women between the ages of 25 and 39 between 1976 and 2009. Explanations include stage migration, i.e. more recently diagnosed cases assigned to later stage groups and an increase in ER positive subtypes.
For ALL, there was more than a 30% gap in five-year relative survival between younger children and AYAs. AML showed a similar trend with relative survival rates declining with increasing age. Since ICD-O-3 9861/3 AML is often assigned as a working or preliminary diagnosis until a more definitive diagnosis can be made, this may delay disease management and ultimately result in less favorable outcomes (19,28). Some of the discrepancy in survival rates for patients with leukemia may also be explained by more aggressive treatment for younger people, particularly children diagnosed with ALL. Successful pediatric protocols in children with ALL have been implemented for young adult patients within the last 10 years, and there have been improvements in outcomes. Older AYA patients may have more comorbidity that could affect disease management (29).
It is not surprising to find better prognosis in AYAs with thyroid cancer, HL, and melanoma than among the older groups, since these are cancers that are known to have high success rates among all ages with little relapse and low cancer mortality. Thyroid cancer has been noted for dramatic increases in incidence and steady mortality rates, and younger patients have more cervical lymph node metastasis and a decreased likelihood of dying (30). Of interest however, is the development of nomograms to predict relapse and death from thyroid cancer in populations of all ages (31). Our findings indicate that five-year relative survival for HL is above 80% for early and late stage disease for the AYA group. The natural history and outcomes from treatment are similar in young and older age groups for HL, with dual goals to maximize cure rates and limit toxicity due to radiation and chemotherapy exposures (32). Even though melanoma incidence is increasing in AYA patients, mortality is decreasing (33), and a Swedish study found that the most prominent five-year relative survival differences between AYAs and older patients was for Stage II (34), just as our results indicate better survival for regional disease through age 39 as compared with the older group. It may be that AYAs rebound slightly better after cancer treatment because of better overall health and less other-cause mortality. Despite lower case counts, colorectal cancer (except for distant stage disease) had better prognosis for AYAs. Male germ cell cancer also had better prognosis for AYAs across age groups, perhaps due to higher numbers of cases with high rates of treatment success and urgency to start treatment (35).
Among limitations, our relative survival rates may slightly over-estimate cancer survival in AYA’s. Younger patients tend to relocate, but reasonable response rates for survival studies are possible with extensive searches (36). Since loss to follow-up affects known vital status, minimizing the amount of loss to follow-up becomes important to assess adherence to treatment. The implications and assumptions associated with differential loss to follow-up are fully described in another article in this monograph (37). We did not consider prognostic measures like conditional survival which is also an important statistic for long term survivors, or genetic profiles which may explain some of the variability of the results.
Overall, our data suggest that AYAs, in general, are doing very well as compared with their younger and older counterparts. However, our findings among AYAs with breast cancer and leukemia emphasize the importance of investigating the biology of both of these diseases in AYA patients as compared with older adults or children. The finding that distant stage breast cancer incidence is increasing in women 25–39 years of age highlights the need to investigate the genetic etiology, as well as timing of diagnosis, treatment efficacy, and clinical trial involvement. Identifying and educating those AYA women who are at higher risk could improve outcomes for these women. Similarly, for leukemia, additional data regarding genetic differences in the types of leukemia occurring in AYA patients as compared with older and younger age ranges will provide insight and may suggest potential treatment targets (38). Continued development of clinical trials building on the recent successes in the treatment of AYA ALLs is crucial to improving outcomes for these patients. Our data suggest that outcomes are not the same across cancer types and that future of AYA oncology research needs to track individual cancer types along with treatment and outcome success by age group.
References
- 1. SEER*Stat Database: SEER-18 November 2012 Katrina Rita Adjustment. Bethesda, MD: National Cancer Institute; 2012. http://seer.cancer.gov. Accessed September 9, 2014. [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. [DOI] [PubMed] [Google Scholar]
- 3. Bleyer A, Barr R, Hayes-Lattin B, et al. The distinctive biology of cancer in adolescents and young adults. Nature. 2008;8:288–298. [DOI] [PubMed] [Google Scholar]
- 4. Bleyer A, Budd T, Montello M. Adolescents and young adults with cancer. Cancer. 2006;107(7 suppl):1645–1655. [DOI] [PubMed] [Google Scholar]
- 5.LIVESTRONG Young Adult Alliance Steering Committee. Closing the gap: a strategic plan: addressing the recommendations of the Adolescent and Young Adult Oncology Progress Review Group. http://www.livestrong.org/pdfs/LAF-YAA-Report-pdf. Published 2006. Accessed September 10, 2014.
- 6. Tricoli JV, Seibel NL, Blair DG, Albritton K, Hayes-Lattin B. Unique characteristics of adolescent and young adult acute lymphoblastic leukemia, breast cancer, and colon cancer. J Natl Cancer Inst. 2011;103(8):628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barr R, Bleyer A, Eds. Impact of Malignant Disease on Young Adults. Seminars in oncology. 2009;36(3 (Part I) and 5 (Part II)):A1-A6,191–288; A1-A10, 373–490. [Google Scholar]
- 8. Keegan TH, Tao L, DeRouen MC, et al. ; AYA HOPE Study Collaborative Group. Medical care in adolescents and young adult cancer survivors: what are the biggest access-related barriers? J Cancer Surviv. 2014;8(2):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aizer AA, Falit B, Mendu ML, et al. Cancer-specific outcomes among young adults without health insurance. J Clin Oncol. 2014; 32:2025–2030. 10.1200/JCO.2013.54.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harrison CJ, Martineau M, Secker-Walker LM. The Leukaemia research Fund/United Kingdom Cancer Cytogenetics Group Karyotype Database in acute lymphoblastic leukaemia: a valuable resource for patient management. Br J Haematol. 2001;113(1):3–10. [DOI] [PubMed] [Google Scholar]
- 11. Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of allogeneic heamtopoietic cell transplantation for adolescent and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2012;18:861–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guy GP, Yabroff R, Ekwueme DU, et al. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Affairs. 2014;33(6):1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kwak M, Zebrack B, Meeske KA, et al. Prevalence and predictors of post-traumatic stress symptoms in adolescent and young adult cancer survivors: a 1-year follow-up study. Psycho-Oncology. 2013;22:1798–1806. [DOI] [PubMed] [Google Scholar]
- 14. Barr RD, Holowaty EJ, Birch JM. Classification schemes for tumors diagnosed in adolescents and young adults. Cancer. 2006;106(7):1425–1430. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization. International Classification of Diseases for Oncology, 3rd ed. Geneva, Switzerland; 2000. [Google Scholar]
- 16. Howlader N, Ries LA, Mariotto AB, et al. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shambaugh EM WM, Axtell LM. (eds). Summary Staging Guide. US Department of Health, Education, and Welfare, Pubic Health Service, National Institutes of Health; 1977. [Google Scholar]
- 18. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, Eds. American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 6th edition. New York: Springer-Verlag, 2002. [Google Scholar]
- 19. Swerdlow SH, Campo E, Harris NL, et al., eds. Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: International Agency for Research on Cancer, 2008. [Google Scholar]
- 20. Ruhl J, Adamo M, Dickie L, Sun L, eds. Hematopoietic and Lymphoid Neoplasms Case Reportability and Coding Manual. Bethesda, MD: National Cancer Institute; 2012. http://seer.cancer.gov/tools/heme/Hematopoietic_Instructions_and_Rules.pdf Accessed July 31, 2013. [Google Scholar]
- 21. Documentation for the expected survival life tables. Surveillance Epidemiology, and End Results Program Web site. http://seer.cancer.gov/expsurvival/documentation.html Accessed September 8, 2014. [Google Scholar]
- 22. Yu B, Tiwari RC, Cronin KA, McDonald C, Feuer EJ. CANSURV: a Windows program for population-based cancer survival analysis. Comput Methods Programs Biomed. 2005;80(3):195–203. [DOI] [PubMed] [Google Scholar]
- 23. Howlader N, Noone AM, Krapcho M, et al., eds. Cancer Statistics Review, 1975–2011. Bethesda, MD: National Cancer Institute; 2014. [Google Scholar]
- 24. Pinsky PF, Miller A, Kramer BS, et al. Evidence of a healthy volunteer effect in the Prostate, Lung, Colorectal, and Ovarian Screening Trial. Am J Epidemiol. 2007;165(8):874–881. [DOI] [PubMed] [Google Scholar]
- 25. Shavers VL, Harlan LC, Stevens JL. Racial/ethnic variation in clinical presentation, treatment, and survival among breast cancer patients under age 35. Cancer. 2003;97(1):134–147. [DOI] [PubMed] [Google Scholar]
- 26. Yabroff KR, Harlan LC, Clegg LX, et al. Is mode of breast cancer detection associated with cancer treatment in the United States? Cancer. 2008;112(5):1011–1019. [DOI] [PubMed] [Google Scholar]
- 27. Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309(8):800–805. [DOI] [PubMed] [Google Scholar]
- 28. Schlenk RF, Döhner K, Krauter J, et al. ; German-Austrian Acute Myeloid Leukemia Study Group. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358(18):1909–1918. [DOI] [PubMed] [Google Scholar]
- 29. Advani AS, Hunger SP, Burnett AK. Acute leukemia in adolescents and young adults. Semin Oncol. 2009;36(3):213–226. [DOI] [PubMed] [Google Scholar]
- 30. Ross DS. Predicting outcome in patients with thyroid cancer. J Clin Endocrinol Metab. 2013;98(12):4673–4675. [DOI] [PubMed] [Google Scholar]
- 31. Pathak K, Mazurat A, Lambert P, Klonisch T, Nason RW. Prognostic nomograms to predict oncological outcome of thyroid cancers. J Clin Endcrinol Metab. 2013;98:4768–4775. [DOI] [PubMed] [Google Scholar]
- 32. Jaglowski SM, Linden E, Termuhlen AM, Flynn JM. Lymphoma in adolescents and young adults. Semin Oncol. 2009;36:381–418. [DOI] [PubMed] [Google Scholar]
- 33. Howlader N, Noone AM, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 34. Plym A, Ullenhag G, Breivald M, Lambe M, Berglund A. Clinical characteristics, management and survival in young adults diagnosed with malignant melanoma: A population-based cohort study. Acta Oncologica. 2014;53:688–696. [DOI] [PubMed] [Google Scholar]
- 35. Cost NG. Testicular germ cell tumors. Current concepts and management strategies. Minerva Urol Nefrol. 2013;65(2):133–155. [PubMed] [Google Scholar]
- 36. Harlan LC, Lynch CF, Keegan TH, et al. ; AYA HOPE Study Collaborative Group. Recruitment and follow-up of adolescent and young adult cancer survivors: the AYA HOPE Study. J Cancer Surviv. 2011;5(3):305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinheiro PS, Liu L, Bungum TJ, Altekruse SF. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monogr. 2014;49:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Roberts KG, Payne-Turner D, Pei D, et al. Integrated genomic and mutational profiling of adolescent and young adult ALL identifies a high frequency of BCR-ABL1-like ALL with very poor outcome. Paper presented at: 55th Annual American Society of Hematology Meeting; December 7–10, 2013; New Orleans, LA.