Abstract
Introduction
Reducing cancer disparities is a major public health objective. Disparities often are discussed in terms of either race and ethnicity or socioeconomic status (SES), without examining interactions between these variables.
Methods
Surveillance, Epidemiology, and End Results (SEER)-18 data, excluding Alaska Native and Louisiana registries, from 2002 to 2008, were used to estimate five-year, cause-specific survival by race/ethnicity and census tract SES. Differences in survival between groups were used to assess absolute disparities. Hazard ratios were examined as a measure of relative disparity. Interactions between race/ethnicity and neighborhood SES were evaluated using proportional hazard models.
Results
Survival increased with higher SES for all racial/ethnic groups and generally was higher among non-Hispanic white and Asian/Pacific Islander (API) than non-Hispanic black and Hispanic cases. Absolute disparity in breast cancer survival among non-Hispanic black vs non-Hispanic white cases was slightly larger in low-SES areas than in high-SES areas (7.1% and 6.8%, respectively). In contrast, after adjusting for stage, age, and treatment, risk of mortality among non-Hispanic black cases compared with non-Hispanic white cases was 21% higher in low-SES areas and 64% higher in high-SES areas. Similarly, patterns of absolute and relative disparity compared with non-Hispanic whites differed by SES for Hispanic breast cancer, non-Hispanic black colorectal cancer, and prostate cancer cases. Statistically significant interactions existed between race/ethnicity and SES for colorectal and female breast cancers.
Discussion
In health disparities research, both relative and absolute measures provide context. A better understanding of the interactions between race/ethnicity and SES may be useful in directing screening and treatment resources toward at-risk populations.
The National Cancer Institute (NCI) defines cancer disparities as adverse differences in cancer outcomes between population groups (1). Each year, summary cancer survival statistics are updated for the major racial and ethnic groups (2,3). Research has focused largely on racial and ethnic inequalities. Less information is available on the role of socioeconomic status (SES) in cancer outcomes such as survival (4–6). Racial/ethnic disparities may be explained by SES (7,8), access to affordable health care coverage (9), or biological differences (10). If racial disparities are driven by inequalities (11) such as adverse social experiences and economic deprivation (12,13), it is possible that race/ethnicity and SES have an interactive effect on survival (8,14,15).
Minorities and the poor often experience worse health outcomes than non-Hispanic whites and more affluent individuals, respectively (16). Because cancer registries do not routinely collect data on SES, only a few studies of cancer survival in the United States have investigated interactions between income and education (17,18). To improve the value of its data, NCI’s Surveillance, Epidemiology, and End Results (SEER) Program recently developed a variable based on a census-tract level SES index (19).
Although most research on health disparities has focused on relative measures, such as hazard and rate ratios, absolute measures provide an intuitive metric, giving context to relative disparity measures (20). In this study, we used the census-tract level SES index to estimate absolute and relative survival disparities in lung, liver, kidney, colorectal, breast, and prostate cancer burdens. As an absolute measure of disparity, we examined the difference in five-year, cause-specific survival. As a relative measure of disparity, we present hazard ratio measures that are adjusted for race/ethnicity, SES, age, stage, and treatment.
Methods
Data Sources and Study Population
Disparities in five-year, cause-specific survival were estimated by race/ethnicity and SES. The study cohort included microscopically confirmed malignant cancers of the lung/bronchus (hereafter “lung”), liver/intrahepatic bile duct (hereafter “liver”), kidney/renal pelvis (hereafter “kidney”), colon and rectum (hereafter “colorectal”), prostate, and female breast (hereafter “breast”) diagnosed from January 1, 2002, through December 31, 2008, and followed through December 31, 2009.
Screening and treatment protocols and cancer survival rates differed for these cancer sites during the study period. For lung cancer, there were no population-based screening recommendations, the cancers typically were diagnosed in late stages, and survival rates were low (21). Similarly, although five-year relative survival rates for liver cancer tripled from less than 5% in the 1970s to 15% in 2003, this cancer often was diagnosed at regional or distant stages (2). In contrast, more than 60% of kidney cancer cases were diagnosed at a localized stage, and survival increased from 50% to 75% during 1970–2003 (2). For colorectal cancer, there were well-established screening protocols, decreasing trends in incidence, and increasing trends in survival. The five-year survival estimates for breast cancer were above 85%, and nearly 72.4% of women were screened yearly after age 40 per recommended guidelines (22). Finally, for prostate cancer, the five-year survival rate was above 90% and, despite changes in screening recommendations, approximately 45% of men over age 75 were screened in 2008 (23).
Sociodemographics
Race/ethnicity was classified as non-Hispanic white, non-Hispanic black, non-Hispanic Asian or Pacific Islander (API), American-Indian or Alaska Native (AI/AN), and Hispanic. Age at diagnosis was categorized as greater than 45 years of age, in 10-year intervals from ages 45–85, and age 85 years or older.
To improve the quality of area-based SES estimates, our analysis is based on a new census-tract level linkage created by SEER and an index variable of SES status in 16 registries, after excluding the Alaska Native and Louisiana registries, where census tract SES data were not available. Cases were mapped to their year 2000 census tract of residence based on their address at time of diagnosis. Validated indices of socioeconomic position were developed using factor analysis from census tract-level estimates from the 2000 census, and from five-year estimates from the 2005–2009 American Community Survey (ACS) (24). Both indices were extracted from the same seven SES measures identified by Yost et al. (25). to represent three components of SES (occupation, education, and income). The variables included: proportion with a blue-collar job, proportion older than age 16 in the workforce without a job, education index, median household income, proportion below 200% of the poverty level, median rent, and median house value. We assigned an index value to each census tract and categorized them into quintiles. The first quintile (Q1, lowest neighborhood SES) corresponded to the 20th percentile or less of SES, and the fifth quintile (Q5, highest neighborhood SES) corresponded to the 80th percentile or higher. For cases diagnosed in 2002–2003, the 2000 census index was used; for cases diagnosed in 2004–2008, the 2005–2009 ACS index was used. (This research resource is accessible by submitting a suitable concept to the NCI SEER Program, care if the corresponding author or M. Yu.) Cases with unknown race/ethnicity or addresses that could not be mapped to a census tract were excluded from the analysis.
Cancer Staging and Treatment
Cancer survival is strongly associated with stage at diagnosis and treatment. Staging was based on SEER Summary Stage 2000 (1998+). Summary staging, although not of clinical utility, was used as a measure of population-based cancer control efforts. Stage was categorized as localized, regional, distant, or unknown/unstaged. For prostate cancer, localized and regional stages were combined according to American Joint Committee on Cancer staging procedures (26). First-course cancer-directed surgery and radiation therapy were determined from the SEER variables “cancer-directed surgery” and “radiation sequence with surgery.”
Statistical Analysis
Cancer survival was studied using both absolute and relative disparity measures (27). To assess disparities on the absolute scale, a survival rate difference was calculated based on Kaplan–Meier estimates of five-year, cause-specific survival by race/ethnicity and SES using SEER*Stat software (28). Because suitable life tables were not available for the SES subgroups, cause-specific death classification was used (29), with other causes of death censored. Pairwise comparisons were considered statistically significant when the Z-test statistic had a P value of less than .05. Sensitivity analyses were used to compare age-standardized and unadjusted survival.
Relative disparities were assessed by calculating hazard ratios for various race/ethnicity and SES comparisons, as well as their interactions, for each cancer site. Stratified Cox proportional hazard models were developed with SAS v.9.3 software (30). Hazard ratios reflected relative risk of death in the 5 years after diagnosis adjusted for confounding factors. Each site-specific model was stratified by age at diagnosis and tumor stage after inspection of the [log, −log] plot of the survival function revealed signs of non-proportionality, as recommended by Breslow (31). Models also were adjusted for surgery and radiation treatment. Cases with missing or unknown treatment status were coded as having no reported treatment. Wald χ2 tests were used to assess the statistical significance of the interaction between SES and race/ethnicity. Comparisons of survival are presented by SES and racial/ethnic group. For pairwise comparisons, confidence intervals (CIs) that did not include 1.0 were considered statistically significant.
Results
Table 1 presents the demographic and clinical distribution of cases by cancer site. Total numbers of cases ranged from a low of 32 571 for liver cancer to a high of 357 078 for prostate cancer. Excluding prostate cancer, the site with the highest percentage in men was liver cancer (71.7%), and the site with the lowest percentage in men was colorectal cancer (50.9%). Excluding breast cancer, the site with the highest percentage in women was colorectal cancer (49.1%) and the lowest percentage in women was liver cancer (28.3%). Most lung (64%), colorectal (58%), and prostate cancer (64%) cases were diagnosed among individuals aged 65 years and older; and most liver (55.6%), kidney (55.5%), and breast (62.2%) cancer cases occurred in individuals aged less than 65 years. Stage at diagnosis varied by site: the majority of breast and kidney cancers were localized (60.3% and 62.3%, respectively), whereas 56.4% of lung cancer cases were diagnosed at distant stages.
Table 1.
Frequency of cases by cancer site and proportion by demographic characteristics*
| Cancer site | ||||||
|---|---|---|---|---|---|---|
| Lung | Liver | Kidney | CRC | Prostate | Breast† | |
| N | 266 439 | 32 571 | 62 371 | 213 274 | 357 078 | 313 482 |
| Gender, % | ||||||
| Men | 53.8 | 71.7 | 61.4 | 50.9 | 100 | — |
| Women | 46.2 | 28.3 | 38.7 | 49.1 | — | 100 |
| Age at diagnosis, % | ||||||
| 0–44 | 2.3 | 5.9 | 10.8 | 5.8 | 0.7 | 13.6 |
| 45–54 | 10.3 | 21.5 | 18.4 | 14.3 | 9.6 | 24.0 |
| 55–64 | 23.2 | 28.2 | 26.3 | 21.2 | 31.2 | 24.6 |
| 65–74 | 31.4 | 22.4 | 23.2 | 24.3 | 35.7 | 19.0 |
| 75–84 | 26.0 | 17.0 | 16.6 | 24.0 | 19.1 | 14.1 |
| 85+ | 6.9 | 5.0 | 4.7 | 10.4 | 3.8 | 4.7 |
| Stage, % | ||||||
| Localized | 15.1 | 40.3 | 62.3 | 39.4 | 92.8‡ | 60.3 |
| Regional | 22.0 | 26.3 | 16.8 | 36.2 | —‡ | 32.7 |
| Distant | 56.4 | 18.5 | 17.1 | 19.7 | 4.1 | 5.1 |
| Unstaged | 6.5 | 14.8 | 3.7 | 4.7 | 3.1 | 1.9 |
| Cancer-directed surgery, % | ||||||
| Yes | 20.9 | 24.0 | 82.0 | 85.8 | 38.7 | 92.8 |
| Radiation therapy, % | ||||||
| Yes | 7.6 | 1.3 | 3.3 | 10.4 | 2.9 | 47.7 |
| Race/ethnicity,% | ||||||
| NHW | 78.2 | 51.8 | 71.5 | 71.6 | 70.5 | 72.7 |
| NHB | 10.9 | 11.8 | 10.5 | 11.3 | 13.6 | 10.1 |
| API | 5.1 | 17.6 | 4.5 | 7.0 | 4.4 | 6.8 |
| AI/AN | 0.4 | 1.1 | 0.8 | 0.5 | 0.3 | 0.5 |
| HISP | 5.2 | 17.4 | 12.1 | 8.9 | 8.3 | 9.4 |
| Unknown | 0.2 | 0.3 | 0.6 | 0.7 | 2.8 | 0.6 |
| SES, % | ||||||
| Q1 (low) | 17.4 | 19.2 | 14.7 | 14.9 | 12.5 | 12.2 |
| Q2 | 17.6 | 18.3 | 16.6 | 16.8 | 15.0 | 15.1 |
| Q3 | 17.4 | 18.1 | 17.6 | 17.6 | 17.0 | 17.3 |
| Q4 | 16.5 | 17.8 | 17.9 | 18.1 | 18.7 | 19.5 |
| Q5 (high) | 14.3 | 15.0 | 18.0 | 17.9 | 22.6 | 22.7 |
| Missing | 16.7 | 11.7 | 15.3 | 14.6 | 14.2 | 13.3 |
* AI/AN = American Indian/Alaska Native; API = Asian/Pacific Islander; CRC = colorectal cancer; HISP = Hispanic; NHB = non-Hispanic blacks; NHW = non-Hispanic whites; Q = quintile; SES = socioeconomic status.
† Breast cancer restricted to women.
‡ Localized, regional combined.
Overall, most cancer cases occurred in non-Hispanic whites, followed by non-Hispanic blacks. With the exception of prostate cancer cases (at 2.8%), less than 1% of all cancer cases were excluded due to unknown race. A disproportionately high percentage of liver cancer cases occurred among API and Hispanic individuals (17.6% and 17.4%, respectively).
The frequency of lung and liver cancer cases decreased from Q1 (low SES) census tracts to Q5 (high SES) census tracts. This pattern was reversed for kidney, colorectal, prostate, and breast cancers. SES data were missing for between 11.7% (liver) and 16.7% (lung) of cases for each site. These cases were excluded.
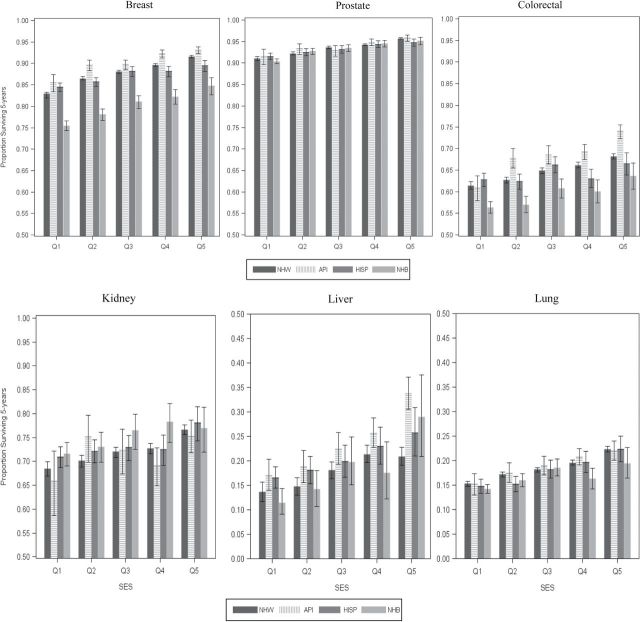
Five-year, cause-specific survival is presented for all six cancer sites by race/ethnicity and SES in Table 2 and Figure 2. Overall survival was lowest for lung and liver cancers (18.3% and 19.3%, respectively), intermediate for colorectal and kidney cancers (64.7% and 72.6%, respectively), and highest for breast and prostate cancers (87.9% and 93.7%, respectively). Survival increased from low- to high-SES groups, with the smallest difference between Q1 and Q5 occurring for prostate cancer (−4.8%) and the largest difference between Q1 and Q5 occurring for breast (−9.8%) and liver cancers (−10.4%). Among racial/ethnic groups, APIs experienced the best five-year, cause-specific survival for all SES strata for each cancer site except kidney, for which non-Hispanic blacks had the best survival. Non-Hispanic blacks had the lowest survival rates for liver (15.1%), colorectal (58.2%), and breast (78.7%) cancers. Figure 1 depicts race/ethnicity- and SES-stratified survival with 95% CIs. Sensitivity analyses, with age-standardized survival, showed that variation from these results was small, at between 1% and 3% (data not shown) .
Table 2.
Five-year, cause-specific survival by race/ethnicity and census tract socioeconomic status (SES), absolute (rate difference) and relative (hazard ratio) disparity, low (Q1) vs high (Q5) SES strata*
| Race/ethnicity | Five-year, cause-specific survival, % | RD† HR Q1 vs Q5 | ||||||
|---|---|---|---|---|---|---|---|---|
| All SES strata | Q1 low | Q2 | Q3 | Q4 | Q5 high | AD rate difference Q1–Q5 | ||
| Lung | ||||||||
| Overall | 18.3 | 15.0 | 17.0 | 11.3 | 19.5 | 22.3 | −7.3 | |
| NHW | 18.6 | 15.3 | 17.2 | 18.2 | 19.6 | 22.4 | −7.1‡ | 1.24‡ |
| NHB | 15.7 | 14.2 | 16.0 | 18.6 | 16.3 | 19.5 | −5.3‡ | 1.15‡ |
| API | 19.4 | 15.2 | 17.5 | 19.0 | 20.8 | 22.0 | −6.8‡ | 1.20‡ |
| AI/AN | 11.8 | 11.3 | 11.0 | 11.3 | 9.0 | 26.7 | −15.4‡ | 1.19 |
| HISP | 17.2 | 14.8 | 15.2 | 18.2 | 19.7 | 22.3 | −7.5‡ | 1.22‡ |
| Liver | ||||||||
| Overall | 19.3 | 14.6 | 16.2 | 19.3 | 22.5 | 25.0 | −10.4 | |
| NHW | 18.2 | 13.7 | 14.8 | 18.1 | 21.4 | 20.9 | −7.2‡ | 1.32‡ |
| NHB | 15.1 | 11.5 | 14.2 | 19.8 | 17.6 | 29.0 | −17.5‡ | 1.29‡ |
| API | 24.4 | 17.0 | 18.8 | 22.5 | 25.7 | 33.8 | −16.8‡ | 1.32‡ |
| AI/AN | 16.1 | 14.3 | 14.1 | 16.9 | 19.4 | 28.6 | −14.3 | 1.28 |
| HISP | 19.3 | 16.6 | 18.1 | 19.9 | 23.0 | 25.8 | −9.2‡ | 1.36‡ |
| Kidney | ||||||||
| Overall | 72.6 | 69.6 | 71.0 | 72.3 | 72.7 | 76.6 | −7.0 | |
| NHW | 72.5 | 68.5 | 70.1 | 72.0 | 72.7 | 76.6 | −8.1‡ | 1.23‡ |
| NHB | 74.1 | 71.6 | 73.1 | 76.5 | 78.3 | 77.0 | −5.4‡ | 1.00 |
| API | 72.2 | 65.9 | 75.2 | 72.4 | 69.1 | 75.3 | −9.4‡ | 1.26 |
| AI/AN | 68.5 | 69.3 | 75.5 | 62.0 | 66.1 | 51.9 | 17.4 | 0.88 |
| HISP | 72.6 | 70.9 | 72.2 | 72.9 | 72.5 | 78.1 | −7.2‡ | 1.13 |
| CRC | ||||||||
| Overall | 64.7 | 60.5 | 62.4 | 65.0 | 66.0 | 68.5 | −8.0 | |
| NHW | 65.1 | 61.4 | 62.7 | 64.9 | 66.1 | 68.2 | −6.8‡ | 1.28‡ |
| NHB | 58.2 | 56.4 | 57.0 | 60.8 | 60.1 | 63.7 | −7.3‡ | 1.17‡ |
| API | 69.3 | 60.9 | 67.8 | 68.6 | 69.3 | 74.0 | −13.1‡ | 1.36‡ |
| AI/AN | 58.9 | 50.7 | 59.5 | 60.7 | 74.2 | 57.9 | −7.2 | 1.26 |
| HISP | 63.9 | 62.8 | 62.4 | 66.2 | 63.1 | 66.5 | −3.7‡ | 1.18‡ |
| Breast | ||||||||
| Overall | 87.9 | 81.6 | 85.9 | 87.9 | 89.4 | 91.4 | −9.8 | |
| NHW | 88.8 | 82.6 | 86.5 | 88.1 | 89.6 | 91.6 | −9.0‡ | 1.75‡ |
| NHB | 78.7 | 75.5 | 78.1 | 81.1 | 82.2 | 84.8 | −9.3‡ | 1.40‡ |
| API | 91.0 | 85.5 | 89.6 | 89.8 | 92.3 | 93.1 | −7.6‡ | 1.34‡ |
| AI/AN | 83.6 | 80.9 | 78.5 | 85.6 | 92.6 | 87.0 | −6.1 | 1.62 |
| HISP | 86.5 | 84.5 | 85.7 | 88.2 | 88.2 | 89.5 | −5.0‡ | 1.44‡ |
| Prostate | ||||||||
| Overall | 93.7 | 90.9 | 92.4 | 93.5 | 94.3 | 95.7 | −4.8 | |
| NHW | 93.9 | 91.1 | 92.2 | 93.6 | 94.2 | 95.7 | −4.6‡ | 1.56‡ |
| NHB | 92.5 | 90.4 | 92.8 | 93.5 | 94.6 | 95.2 | −4.8‡ | 1.31‡ |
| API | 94.3 | 91.6 | 93.4 | 92.9 | 94.9 | 95.9 | −4.3‡ | 1.28 |
| AI/AN | 89.6 | 81.1 | 93.9 | 90.7 | 92.0 | 97.4 | −16.3‡ | 1.80 |
| HISP | 93.0 | 91.6 | 92.5 | 93.2 | 94.4 | 94.8 | −3.2‡ | 1.13 |
* AD = absolute disparity; AI/AN = American Indian/Alaska Native; API = Asian/Pacific Islander; CRC = colorectal cancer; HISP = Hispanic; HR = hazard ratio; NHB = non-Hispanic blacks; NHW = non-Hispanic whites; Q = quintile; RD = relative disparity; SES = socioeconomic status.
† Stratified by stage and age with adjustment for sex, cancer-directed surgery, radiation therapy.
‡ P < 0.05.
Figure 2.
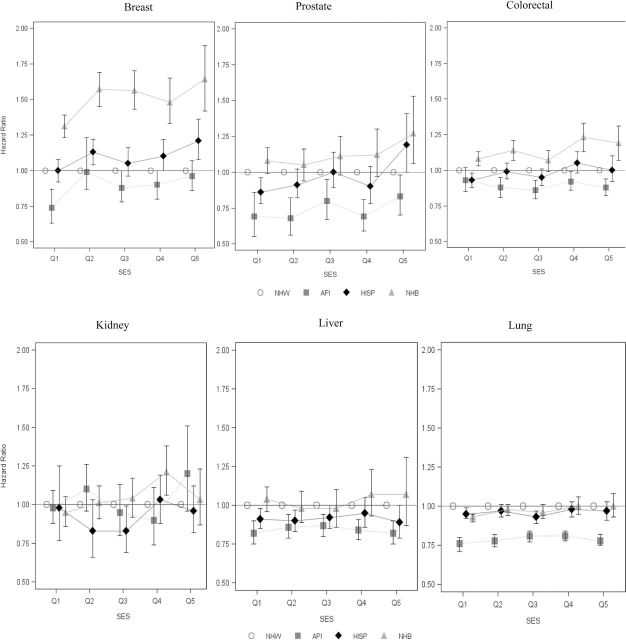
Relative disparity (hazard ratio) between race/ethnicity compared with non-Hispanic white (NHW) within socioeconomic status (SES) groups. Proportional hazards model stratified by stage and age at diagnosis with adjustment for sex, cancer-directed surgery, and radiation therapy and interaction between race/ethnicity and SES. Referent: NHW cases in same SES strata. Overall model interaction term P value for lung = .11, liver = .97, kidney = .09, colorectal = .05, prostate = .13; breast = 0. Results for American Indians and Alaska Natives not presented due to small numbers. API = Asian/Pacific Islander; HISP = Hispanic; NHB = non-Hispanic blacks; Q = quintile.
Figure 1.
Five-year, cause-specific survival estimates (and 95% confidence intervals) by site, race/ethnicity, and census tract-level socioeconomic status (SES). API = Asian/Pacific Islander; HISP = Hispanic; NHB = non-Hispanic blacks; NHW = non-Hispanic whites; Q = quintile.
Absolute and relative cancer survival disparities by SES within racial/ethnic groups are presented in the last two columns of Table 2. For lung cancer, the absolute difference in survival between Q1 and Q5 within racial/ethnic groups ranged from −15.4 (American Indian/Alaska Native [AI/AN]) to −5.3 (non-Hispanic blacks). The relative disparity comparison, based on stratified Cox proportional hazards models, shows a 24% increase in mortality risk for Q1 compared with Q5 for non-Hispanic whites and a 15% increase for non-Hispanic blacks. Except for lung and prostate cancer among AI/AN cases and kidney cancer among all minority groups, significant SES absolute disparities within racial/ethnic groups aligned with significant relative disparities.
Absolute and relative disparities between racial/ethnic groups by SES are presented in Table 3. Compared with non-Hispanic whites, the absolute disparity in breast cancer survival among non-Hispanic blacks was larger in Q1 than Q5, with −7.1% and −6.8% lower five-year survival, respectively. In contrast, the relative disparity in breast cancer survival between non-Hispanic black cases and non-Hispanic white cases, based on adjusted hazard ratios, was larger in Q5 than in Q1 (64% higher risk compared with 31% higher risk, respectively). Similar patterns were seen among non-Hispanic black and non-Hispanic white colorectal and prostate cancer cases.
Table 3.
Absolute disparity (rate difference) and relative disparity (hazard ratio) between racial/ethnic groups and non-Hispanic whites within the lowest (Q1) and highest (Q5) census tract-level socioeconomic status (SES)*
| Cancer site | Race/ethnicity | AD (rate difference) compared with NHWs in same SES group | RD (hazard ratio) compared with NHWs in same SES group | ||
|---|---|---|---|---|---|
| Q1 | Q5 | Q1 | Q5 | ||
| Lung | NHB | −1.1† | −2.9 | 0.93 (0.90–0.95) | 1.00 (0.93–1.08) |
| API | −0.1 | −0.4 | 0.76 (0.71–0.80) | 0.78 (0.75–0.82) | |
| HISP | −0.5 | −0.1 | 0.95 (0.92–0.99) | 0.97 (0.91–1.03) | |
| Liver | NHB | −2.2 | 8.1 | 1.04 (0.96–1.12) | 1.07 (0.87–1.31) |
| API | 3.3 | 12.9† | 0.82 (0.75–0.90) | 0.82 (0.75–0.90) | |
| HISP | 2.9 | 4.9 | 0.91 (0.85–0.98) | 0.89 (0.78–1.00) | |
| Kidney | NHB | 3.1† | 0.4 | 0.98 (0.88–1.09) | 1.20 (0.96–1.51) |
| API | −2.6 | −1.3 | 0.98 (0.77–1.25) | 0.96 (0.82–1.21) | |
| HISP | 2.4 | 1.5 | 0.95 (0.86–1.05) | 1.03 (0.87–1.23) | |
| CRC | NHB | −5.0† | −4.5† | 1.08 (1.03–1.13) | 1.19 (1.07–1.31) |
| API | −0.5 | 5.8† | 0.93 (0.85–1.02) | 0.88 (0.82–0.94) | |
| HISP | 1.4 | −1.7 | 0.93 (0.88–0.98) | 1.00 (0.92–1.10) | |
| Breast | NHB | −7.1† | −6.8† | 1.31 (1.23–1.39) | 1.64 (1.42–1.88) |
| API | 2.9† | 1.5† | 0.74 (0.63–0.87) | 0.96 (0.86–1.08) | |
| HISP | 1.9† | −2.1† | 1.00 (0.92–1.08) | 1.21 (1.08–1.36) | |
| Prostate | NHB | −0.7 | −0.5 | 1.08 (0.99–1.17) | 1.28 (1.07–1.53) |
| API | 0.5 | 0.2 | 0.69 (0.55–0.86) | 0.84 (0.71–0.99) | |
| HISP | 0.5 | −0.9 | 0.87 (0.79–0.96) | 1.20 (1.01–1.42) | |
* Stratified by stage and age at diagnosis with adjustment for sex, cancer-directed surgery, and radiation therapy and interaction between race/ethnicity and SES. Referent: Non-Hispanic white (NHW) cases in same SES strata. Overall model interaction term P value for lung = .11, liver = .97, kidney = .09, colorectal = .05, prostate = .13, breast = 0. Absolute difference (AD) in survival compared with NHWs. A negative value indicates that survival was higher than in the NHW group. API = Asian/Pacific Islander; CRC = colorectal cancer; HISP = Hispanic; NHB = non-Hispanic black; Q = quintile; RD = relative disparity.
† P < .05.
For lung, liver, and kidney cancers, only three of 18 absolute disparity comparisons (17%) in Table 3 were statistically significant. In contrast, 50% of absolute disparity comparisons for breast, colorectal, and prostate cancers were statistically significant. When Hispanic and non-Hispanic white cases were compared, breast, colorectal, and prostate cancer absolute disparities were greater than 1 for Hispanics in Q1 (better survival than non-Hispanic whites) and less than 1 in Q5 (worse survival than non-Hispanic whites). Hispanic cases experienced an increased risk of death compared with non-Hispanic whites in the highest SES category for breast cancer (hazard ratio of 1.21, 95% CI: 1.08 to 1.36) and prostate cancer (hazard ratio of 1.20, 95% CI: 1.01 to 1.42). Compared with non-Hispanic white cases, the risk of death for APIs was lower in both Q1 and Q5 for breast and prostate cancers and for colorectal cancer in Q5.
For colorectal and breast cancers, two of the three leading cancers with robust screening and treatment programs, there was a significant interaction between race/ethnicity and SES in the model (see Table 3). Supplementary Table 1 provides standard errors for Q1 and Q5 survival estimates and CIs for the hazard ratios in Table 3.
Discussion
In this cancer survival study, five-year, cause-specific survival increased with higher SES; and non-Hispanic white and API cases often experienced better cancer survival than non-Hispanic black, Hispanic, and AI/AN cases. These absolute disparities are consistent with other data (2) and support the minority poverty hypothesis, with disparities concentrated in low-SES minority groups. For breast, colorectal, and prostate cancers, the risk of death for non-Hispanic blacks increased with higher SES compared with non-Hispanic whites in the same SES strata. These relative disparities support the diminishing returns hypothesis, in which minorities do not achieve the same gains in health at high-SES levels as do their nonminority counterparts at equivalent SES levels (5,8,32). These findings demonstrate the value of evaluating both absolute and relative disparity measures with respect to cancer survival.
In disparities research, the measure, comparison group, and reference point influence both the direction and magnitude of survival disparities (27,32,33). For example, Harper et al. found that the absolute disparity in five-year, cause-specific mortality for breast cancer declined from 1987 to 2005, while the relative disparity increased (33), with mortality decreasing at a faster rate in more advantaged groups. Although our study evaluated a different outcome (cancer survival), we also found several inverse patterns between the absolute disparity and relative disparity, not only for breast cancer but also for prostate and colorectal cancers. The findings illustrate the need to carefully select disparity measures and comparison groups to develop the best inferences about disparities.
In this study, comparing Q1 and Q5 breast cancer survival differences between non-Hispanic black and non-Hispanic white cases leads to the conclusion that the absolute disparities are similar (−7.1% and −6.8%, respectively). However, hazard ratios indicated that there was a two-fold increase in risk of death for non-Hispanic black vs non-Hispanic white cases in Q5 compared with Q1. Similar differences in survival affect hazard ratios differently depending on whether survival is low or high, which can lead to misinterpretation of this relative disparity measure. As a third point of context, the overall 10.1% absolute disparity in breast cancer survival between non-Hispanic black and non-Hispanic white cases provides strong evidence of disparity between these cases. Disparities in colorectal cancer survival between non-Hispanic blacks compared with non-Hispanic whites followed a similar pattern. Further, the protective effect of API race was attenuated in high-SES strata for both prostate and breast cancers.
Analysis of survival disparities should be guided by an understanding of the factors that affect survival, and caution against overinterpretation is advised. Overdiagnosis and lead-time bias can contribute to SES- and race-related disparities by inflating survival in some groups without any decrease in mortality, particularly for cancers diagnosed via screening. In this study, women in high-SES strata accounted for nearly twice the proportion of breast cancer cases (Q5 = 22.7%) as in low-SES strata (Q1 = 12.2%). Among patients in high-SES quintiles, breast, prostate, and colorectal cancer screening is more frequent among non-Hispanic whites than non-Hispanic blacks (34–36). If non-Hispanic white or API cases in high-SES strata have earlier diagnoses (lead-time bias) of nonfatal tumors (overdiagnosis) more frequently than non-Hispanic black, AI/AN, or Hispanic cases, an elevated hazard ratio might not accurately reflect actual mortality risk (37). Although such biases may be partially addressed by controlling for stage, treatment, and age, the potential exists for residual confounding. Elsewhere in this monograph, Cho and colleagues discuss how changes in screening and treatment affect survival and the interpretation of survival in the context of incidence, stage, and mortality (38).
In this study, for the five cancer sites other than kidney, API cases had the highest overall survival, and Hispanic cases shared the second or third highest overall survival with non-Hispanic white cases. The study by Pinheiro et al. (39) in this monograph provides evidence of a differential loss of follow-up among Hispanic and API cases. Lost follow-up after diagnosis may have been disproportionately distributed among minority cases in low-SES strata, contributing to statistically significant protective effects with respect to these five cancer sites.
Regardless of these potential biases, the rate differences between low- and high-SES groups and the elevated hazard ratio of high-SES minorities compared with their non-Hispanic white counterparts suggest the need for research into mechanisms of disparity among these subgroups. Although low-SES minority patients may lack fundamental access to care (such as for screening to detect earlier stage disease), minorities in higher SES neighborhoods should have greater access to care. If they fail to use this access because of social, cultural, linguistic, environmental, or even financial barriers (40,41), a diminishing return on health care access is possible among high-SES cases with screenable cancers (42,43). Access to culturally sensitive patient navigation and care coordination could be of benefit to affluent minority cancer patients and promote substantial disparity reductions in large populations (44). Priority should be given to interventions designed to reduce cancer survival disparities; focusing on low-SES individuals or neighborhoods also should continue to be a priority because the absolute difference in cancer survival is greatest in these groups.
This study has several strengths, including its population-based design that covers approximately one quarter of the US population and linkage to census tract SES measures. As in other studies (14,45,46), low-SES cases had higher rates of late-stage disease, and stage at diagnosis did not fully explain relative survival disparities. Study limitations include missing census tract and treatment data, uneven loss of follow-up across SES quintiles and racial/ethnic categories, and potential misclassification using area-based measures. Despite these limitations, the study provides insights into relationships between absolute and relative disparities in cancer survival and potential pitfalls of using a single disparity measure.
In summary, on the absolute scale, survival increased with higher SES across racial and ethnic groups. However, relative to non-Hispanic whites of similar SES, the risk of death among APIs, non-Hispanic blacks, and Hispanics was greatest in the high-SES strata for breast, colorectal, and prostate cancers. Efforts to reduce lagging survival among racial and ethnic minorities are needed in both low- and high-SES areas.
Funding
Provided by National Cancer Institute Division of Cancer Control and Population Sciences and Cancer Prevention Fellowship Program.
Supplementary Material
References
- 1. Cancer health disparities factsheet. National Cancer Institute Web site. http://www.cancer.gov/cancertopics/factsheet/disparities/cancer-health-disparities Published 2008. Accessed July 30, 2014. [Google Scholar]
- 2. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. [DOI] [PubMed] [Google Scholar]
- 3. Howlader N, Noone A, Krapcho M, et al. , eds. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute; 2012. http://seer.cancer.gov/csr/1975_2010 Published 2012. Accessed April 2013. [Google Scholar]
- 4. Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294(22):2879–2888. [DOI] [PubMed] [Google Scholar]
- 5. Braveman PA, Cubbin C, Egerter S, Williams DR, Pamuk E. Socioeconomic disparities in health in the United States: what the patterns tell us. Am J Public Health. 2010;100 (Suppl 1):S186–S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krieger N, Fee E. Measuring social inequalities in health in the United States: a historical review, 1900-1950. Int J Health Serv. 1996;26(3) :391–418. [DOI] [PubMed] [Google Scholar]
- 7. Kawachi I, Daniels N, Robinson DE. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24(2):343–352. [DOI] [PubMed] [Google Scholar]
- 8. Farmer MM, Ferraro KF. Are racial disparities in health conditional on socioeconomic status? Soc Sci Med. 2005;60(1):191–204. [DOI] [PubMed] [Google Scholar]
- 9. Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5–19. [DOI] [PubMed] [Google Scholar]
- 10. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109(9):1721–1728. [DOI] [PubMed] [Google Scholar]
- 11. Krieger N. A glossary for social epidemiology. J Epidemiol Community Health. 2001;55(10):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krieger N. Defining and investigating social disparities in cancer: critical issues. Cancer Causes Control. 2005;16(1):5–14. [DOI] [PubMed] [Google Scholar]
- 13. McLaren L, Hawe P. Ecological perspectives in health research. J Epidemiol Community Health. 2005;59(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brawley OW. Disaggregating the effects of race and poverty on breast cancer outcomes. J Natl Cancer Inst. 2002;94(7):471–473. [DOI] [PubMed] [Google Scholar]
- 15. LaVeist TA. Disentangling race and socioeconomic status: a key to understanding health inequalities. J Urban Health. 2005;82(2 Suppl 3):iii26–iii34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson W . Chicago, IL: University of Chicago Press; 1987. [Google Scholar]
- 17. Clegg LX, Li FP, Hankey BF, Chu K, Edwards BK. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162(17):1985–1993. [DOI] [PubMed] [Google Scholar]
- 18. Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979-2003. Cancer. 2011;117(14):3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471–482. [DOI] [PubMed] [Google Scholar]
- 20. Harper S, Lynch JW. Selected Comparisons of Measures of Health Disparities: A Review Using Databases Relevant to Healthy People 2010 Cancer-Related Objectives. NCI Cancer Surveillance Monograph Series, Number 7. Bethesda, MD: National Institutes of Health; 2007. NIH publication 07-6281. [Google Scholar]
- 21.US Preventative Services Task Force. Lung cancer screening: recommendation statement. http://www.uspreventiveservicestaskforce.org/3rduspstf/lungcancer/lungcanrs.htm Published May 2004. Accessed July 30, 2014. [Google Scholar]
- 22. Centers for Disease Control and Prevention. Cancer screening-United States, 2010. MMWR. 2012:61(3):41–45.22278157 [Google Scholar]
- 23. Prasad SM, Drazer MW, Huo D, Hu JC, Eggener SE. 2008 US Preventive Services Task Force recommendations and prostate cancer screening rates. JAMA. 2012;307(16):1692–1694. [DOI] [PubMed] [Google Scholar]
- 24. Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81–92. [DOI] [PubMed] [Google Scholar]
- 25. Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–711. [DOI] [PubMed] [Google Scholar]
- 26. American Journal of Clinical Oncology Cancer. . Philadelphia, PA: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 27. Harper S, Lynch J. Methods for Measuring Cancer Disparities: Using Data Relevant to Healthy People 2010 Cancer-Related Objectives. NCI Cancer Surveillance Monograph Series, Number 6. Bethesda, MD: National Cancer Institute, 2006. NIH publication 05-5777. [Google Scholar]
- 28. SEER*Stat [computer program]. Version 8.1. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 29. Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. SAS/STAT (for Windows) [computer program]. Version 9.3. Cary, NC: SAS Institute Inc; 2012. [Google Scholar]
- 31. Breslow NE. Analysis of survival data under the proportional hazards model. Int Stat Rev. 1975:43(1):45–57. [Google Scholar]
- 32. Schoendorf KC, Hogue CJ, Kleinman JC, Rowley D. Mortality among infants of black as compared with white college-educated parents. N Engl J Med. 1992;326(23):1522–1526. [DOI] [PubMed] [Google Scholar]
- 33. Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman MC. Trends in area-socioeconomic and race-ethnic disparities in breast cancer incidence, stage at diagnosis, screening, mortality, and survival among women ages 50 years and over (1987-2005). Cancer Epidemiol Biomarkers Prev. 2009;18(1):121–131. [DOI] [PubMed] [Google Scholar]
- 34. Carpenter WR, Howard DL, Taylor YJ, Ross LE, Wobker SE, Godley PA. Racial differences in PSA screening interval and stage at diagnosis. Cancer Causes Control. 2010;21(7):1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(2):389–394. [DOI] [PubMed] [Google Scholar]
- 36. Summers C, Saltzstein SL, Blair SL, Tsukamoto TT, Sadler GR. Racial/ethnic differences in early detection of breast cancer: a study of 250,985 cases from the California Cancer Registry. J Womens Health (Larchmt). 2010;19(2):203–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jørgensen KJ, Gøtzsche PC. Overdiagnosis in publicly organised mammography screening programmes: systematic review of incidence trends. BMJ. 2009;339:b2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cho H, Mariotto AB, Schwartz LM, Luo J, Woloshin S. When do changes in cancer survival mean progress? The insight from population incidence and mortality. J Natl Cancer Inst Monogr. 2014;49:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pinheiro AS, Morris CR, Liu L, Bungum TJ, Altekruse SF. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monogr. 2014;49:210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mayberry RM, Mili F, Ofili E. Racial and ethnic differences in access to medical care. Med Care Res Rev. 2000;57 (Suppl 1):108–145. [DOI] [PubMed] [Google Scholar]
- 41. Williams DR, Jackson PB. Social sources of racial disparities in health. Health Aff (Millwood). 2005;24(2):325–334. [DOI] [PubMed] [Google Scholar]
- 42. Masi CM, Blackman DJ, Peek ME. Interventions to enhance breast cancer screening, diagnosis, and treatment among racial and ethnic minority women. Med Care Res Rev. 2007;64(5 Suppl):195S–242S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61(4):237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30(3):427–32; discussion 433. [DOI] [PubMed] [Google Scholar]
- 45. Auvinen A, Karjalainen S. Possible explanations for social class differences in cancer patient survival. IARC Sci Publ. 1997;(138):377–397. [PubMed] [Google Scholar]
- 46. Singh GK, Miller BA, Hankey BF, et al. Area Socioeconomic Variations in U.S. Cancer Incidence, Mortality, Stage, Treatment, and Survival, 1975–1999. NCI Cancer Surveillance Monograph Series, Number 4 Bethesda, MD: National Cancer Institute, 2003. NIH publication 03-5417. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.