Abstract
Background
To isolate progress against cancer from changes in competing causes of death, population cancer registries have traditionally reported cancer prognosis (net measures). But clinicians and cancer patients generally want to understand actual prognosis (crude measures): the chance of surviving, dying from the specific cancer and from competing causes of death in a given time period.
Objective
To compare cancer and actual prognosis in the United States for four leading cancers—lung, breast, prostate, and colon—by age, comorbidity, and cancer stage and to provide templates to help patients, clinicians, and researchers understand actual prognosis.
Method
Using population-based registry data from the Surveillance, Epidemiology, and End Results (SEER) Program, we calculated cancer prognosis (relative survival) and actual prognosis (five-year overall survival and the “crude” probability of dying from cancer and competing causes) for three important prognostic determinants (age, comorbidity [Charlson-score from 2012 SEER-Medicare linkage dataset] and cancer stage at diagnosis).
Result
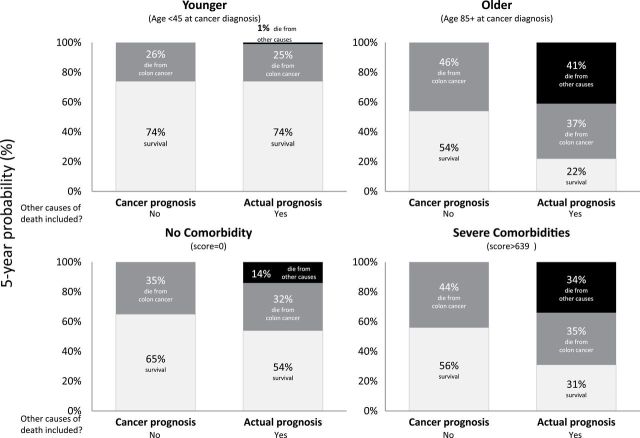
For younger, healthier, and earlier stage cancer patients, cancer and actual prognosis estimates were quite similar. For older and sicker patients, these prognosis estimates differed substantially. For example, the five-year overall survival for an 85-year-old patient with colorectal cancer is 54% (cancer prognosis) versus 22% (actual prognosis)—the difference reflecting the patient’s substantial chance of dying from competing causes. The corresponding five-year chances of dying from the patient’s cancer are 46% versus 37%. Although age and comorbidity lowered actual prognosis, stage at diagnosis was the most powerful factor: The five-year chance of colon cancer death was 10% for localized stage and 83% for distant stage.
Conclusion
Both cancer and actual prognosis measures are important. Cancer registries should routinely report both cancer and actual prognosis to help clinicians and researchers understand the difference between these measures and what question they can and cannot answer. We encourage them to use formats like the ones presented in this paper to communicate them clearly.
To isolate progress against cancer from changes in competing causes of death, population cancer registries have traditionally reported cancer prognosis. In the statistical literature, cancer prognosis measures are called “net” because they cancel out competing causes of death. Relative survival, for example, is a net measure—it is calculated as the ratio of overall survival for cancer patients to the expected survival of a comparable group of cancer-free individuals (1,2). Since a cohort of cancer-free individuals is difficult to obtain, general population life tables are used instead (3).
Cancer prognosis measures are especially useful for researchers and policy makers because they clarify whether increased survival among cancer patients reflects improvements in the prevention or treatment of cancer versus the prevention or treatment of competing causes of death such as cardiovascular disease. But clinicians and cancer patients generally have a different interest. They want to know actual prognosis: what is likely to happen to a person diagnosed with cancer. Specifically, what is their chance of dying from cancer, dying from other causes, or surviving?
Actual prognosis—also called “crude” prognosis in the statistical literature—accounts for the fact that, for people diagnosed with cancer, two competing causes of death determine survival: death from cancer and death from other causes. Survival estimates that account for competing risk of death are especially important for individual decision making where clinicians and patients need to balance the benefits and toxicities of cancer therapy. For patients with early stage low-risk cancers found by screening, for example, the chance of cancer death may be so low that patients might decide potential treatment harms exceed possible benefit. Similarly, very old patients with severe comorbidities may not be able to experience benefit from cancer treatment because the chance of death from competing causes increases substantially with advancing age (4–6).
In this paper, we compare cancer and actual prognosis in the United States for four leading cancers: lung, breast, prostate, and colon—by age, comorbidity, and cancer stage to highlight the similarities and differences between these measures. To help nontechnical audiences (eg, policy makers and patients) understand the measures, we will use the terms cancer prognosis and actual prognosis instead of the more technical statistical terms net survival and crude survival, respectively. And we will provide guidance for how to select and interpret the most appropriate measure depending on the question of interest. This guidance is timely as cancer registries are beginning to present actual prognosis in addition to traditional cancer prognosis measures (7).
Methods
We use population-based Surveillance, Epidemiology, and End Results (SEER) data from 18 cancer registries, which represents roughly 28% of the total US population. For cancer and actual prognosis calculations, we analyzed the cohort of patients diagnosed with lung, breast, prostate, and colon cancer during 2000–2009 followed through December 31, 2010. Only malignant cases were included for analyses.
Calculating Cancer Prognosis
The most commonly used cancer prognosis measure is five-year relative survival: a ratio of overall survival for a cohort of cancer patients to expected survival in the general population (2). Because most cancers are relatively uncommon, the expected survival in the general population approximates a “cancer-free” population. The numerator—overall survival (also called observed survival)—is calculated using the actuarial method where all deaths are counted as events. The denominator—expected survival—is calculated from US life tables matched on age, sex, race, and year with the cancer cohort. For example, if the five-year overall survival was 80% for women diagnosed with malignant breast cancer, and the expected survival was 90% for the age, sex, race, and year matched population, then the five-year relative survival for women with malignant breast cancer would be 80%/90% = 89%.
Calculating Actual Prognosis
Although previous studies (8,9) used life tables to estimate actual prognosis, we could not do so since available US life tables do not account for differences in life expectancy from comorbidities. Instead, we used cause of death information to estimate the actual prognosis. In the absence of comorbidity-adjusted life tables, this approach may provide more accurate estimates (10–13).
We used the SEER cause-specific death classification variable (10,13) to estimate the probabilities of dying of cancer, dying of competing causes, and survival. Cancer registries use cause of death from death certificates, which are prone to misclassification errors. Also, determining whether a death is due to specific cancer can be difficult (eg, is a death from Kaposi sarcoma in an AIDS patient due to the cancer or to AIDS?). The “SEER cause-specific death classification” provides guidance about which deaths should be “attributable” to a specific cancer diagnosis. Because assigning cause of death is more difficult for patients with more than one cancer, the variable has different rules for assigning deaths for patients with only one cancer and those with more than one cancer diagnosis. For patients diagnosed with only one cancer, the variable classifies a death as due to a specific cancer if the death attributed to the same cancer site, to the same general organ system as specified by International Classification of Diseases for Oncology, Third Edition (eg, oral cavity and pharynx compared with lip), to all other malignant cancers (assuming that metastatic disease has been misclassified), or to AIDS with cancer (for selected cancer sites such as Kaposi’s sarcoma or non-Hodgkin lymphomas). For patients diagnosed with more than one cancer, the variable classifies a death as due to a specific cancer if the death was attributed to the same cancer site as the first diagnosis or to the general organ system of the site. Deaths from all other malignant cancers are censored because they are presumed to be due to the other cancer diagnosis (detailed information about this variable can be found Howlader et al. (10) and SEER Cause-specific Death Classification Variable Web site (13).
Statistical Analysis
At any point in time, a cohort of cancer patients can be classified into three mutually exclusive groups: 1) patients who died of cancer; 2) patients who died of causes other than cancer; and 3) patients who are alive. In mathematical terms, let be the number deaths in interval i for cause k. When considering only two causes of death let k = 1 be cancer death and k = 2 be death due to other causes. At each interval, the conditional probabilities (hazard) of dying of cause k given alive at the beginning of the interval are calculated as
where is the number of people alive at the beginning of the interval. If the actuarial (life table) method is used, is calculated as the number of people alive at the beginning of the interval minus half of individuals censored during the interval, that is, lost to follow-up. Similar to calculation of multiple-decrement life tables, the three quantities are calculated at the end of interval i as:
1. The cumulative overall survival , representing the probability of being alive at the end of interval i. Note is the probability of surviving interval 1, and probabilities of dying of cause 1 and 2, respectively.
2. The cumulative probability of dying of cancer at interval i, .
3. The cumulative probability of dying of other causes at interval i, .
The cumulative probabilities of dying are also referred as cumulative incidence function of cause k.
Other Prognostic Determinants
Age at Diagnosis.
Age at diagnosis was ascertained across SEER registries using standardized coding rules based on hospital medical records and pathology reports. For our analysis, we stratified age into six mutually exclusive groups (20–44, 45–54, 55–64, 65–74, 75–84, and 85+).
Stage at Diagnosis.
Summary staging is the most basic way of categorizing how far a cancer has spread from its origin. It is the most precise clinical and pathological documentation of the extent of disease from the medical record. There are four categories of disease spread: localized to the primary tumor site (localized); tumor with regional spread or metastasis to regional lymph nodes (regional); tumor with distant metastasis (distant); and tumors with unknown stage (14). For our analyses, we excluded patients with unknown stage and used Summary Staging 2000.
Comorbidity Status.
Comorbidities were defined as the presence of any of 16 comorbid conditions in the Charlson index in the year before diagnosis. These conditions include acute myocardial infarction, AIDS, cerebrovascular disease, chronic renal failure, congestive heart failure, chronic obstructive pulmonary disease, dementia, diabetes, moderate/severe liver dysfunction, history of myocardial infarction, paralysis, rheumatologic disease, ulcer disease, cirrhosis and/or chronic hepatitis, and vascular disease.
To ensure that comorbid conditions in the year before diagnosis could be identified, only patients diagnosed at age 66 or older were included because their data were available in the 2012 SEER-Medicare linkage dataset. This cohort consisted of Medicare beneficiaries 66 years of age or older residing in the SEER catchment areas who had received a cancer diagnosis between 1992 and 2009 and were followed until December 31, 2009. We used ICD-9-CM and Current Procedural Terminology (CPT) codes recorded in Medicare claims according to algorithm developed by Klabunde et al. (15,16).
Comorbidities were summarized into a single score. To estimate scores, a Cox proportional hazard model (the dependent variable in the model was time from diagnosis to death due to noncancer causes and censoring events included loss to follow-up or end of study, or cancer death) was used with comorbidity as covariates to estimate weights and estimate comorbidity score (17) while controlling for factors such as age, sex, and race. To illustrate the effect of comorbidities on actual prognosis measures, we focused on two groups: patients with no comorbidities (score = 0) and severe comorbidities (score > 639).
The cut point (score > 639) for classifying scores into severe group was based on weights ranking as well as clinical judgments based on each comorbidity conditions. Cho et al. (4) article contains detail description on the comorbidity variable. Severe comorbidity refers to severe illnesses that frequently lead to organ failure or systemic dysfunction and always require adjusting cancer treatment. The severe comorbidity group included individuals with chronic obstructive pulmonary disease, liver dysfunction, chronic renal failure, dementia, and congestive heart failure. Most individuals with more than one comorbid condition fell into the severe comorbidity group.
All analyses were performed in SEER*Stat software (18). All probabilities were calculated 5 year from cancer diagnosis.
Results
Cancer Versus Actual Prognosis
Figure 1 shows the probabilities of dying from cancer and survival when competing causes of death are not considered (cancer prognosis, ie, “net”) and when they are (actual prognosis, ie, “crude”). For younger and healthier patients, cancer and actual prognosis estimates were quite similar because competing causes of death are rare. For older and sicker patients, these prognosis estimates differed substantially. For example, the five-year survival for an 85-year-old colorectal cancer patient is 54% (cancer prognosis) versus 22% (actual prognosis)—the difference reflecting the patient’s substantial chance of dying from competing causes. The corresponding five-year chances of dying from the patient’s cancer are 46% versus 37%.
Figure 1.
Comparison of cancer prognosis and actual prognosis measures for male and female colorectal cancer patients diagnosed with regional stage.
Actual Prognosis for Four Major Cancers
Figures 2–5 illustrate the chance of surviving, dying from the specific cancer and from competing causes of death in the 5 years after diagnosis for breast, prostate, colorectal, and lung cancers. These chances are stratified by comorbidity (none and severe), age, and stage (localized, regional, and distant). Tables 1 and 2 present the corresponding figure numbers with 95% confidence intervals.
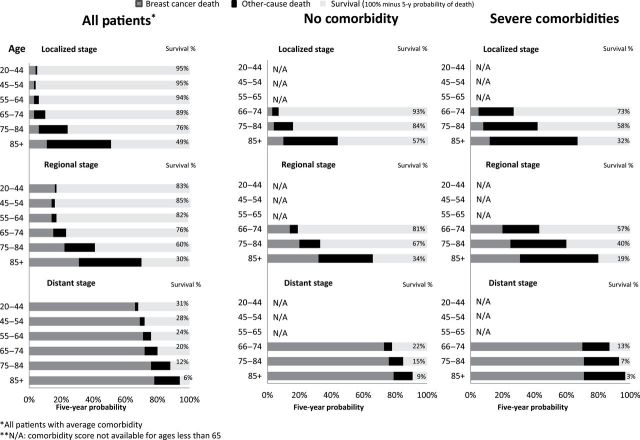
Figure 2.
Actual prognosis—Five-year chance of surviving, dying from breast cancer, or dying from competing causes by age, stage, and comorbidity.
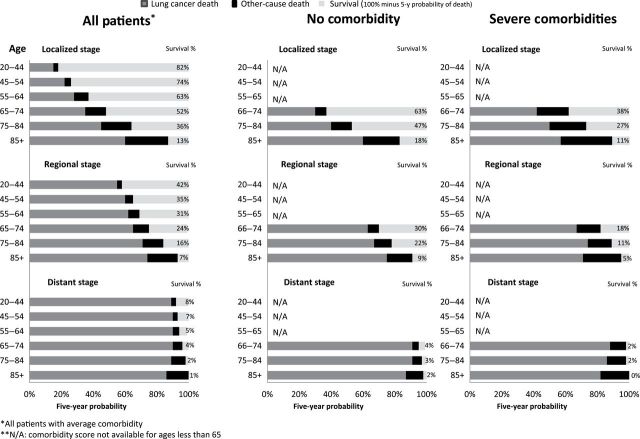
Figure 5.
Actual prognosis—Five-year chance of females surviving, dying from lung cancer, or dying from competing causes by age, stage, and comorbidity.
Figure 3.
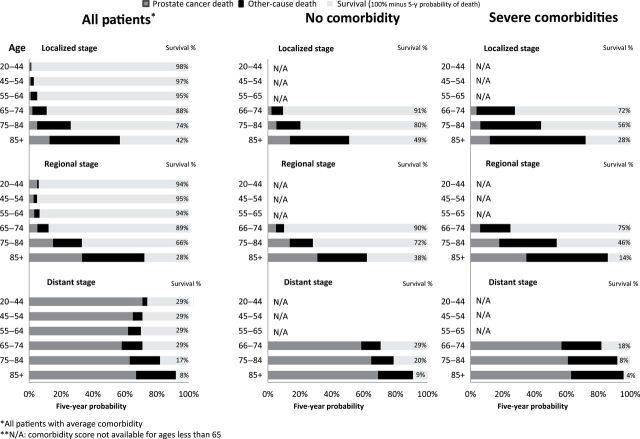
Actual prognosis—Five-year chance of surviving, dying from prostate cancer, or dying from competing causes by age, stage, and comorbidity.
Figure 4.
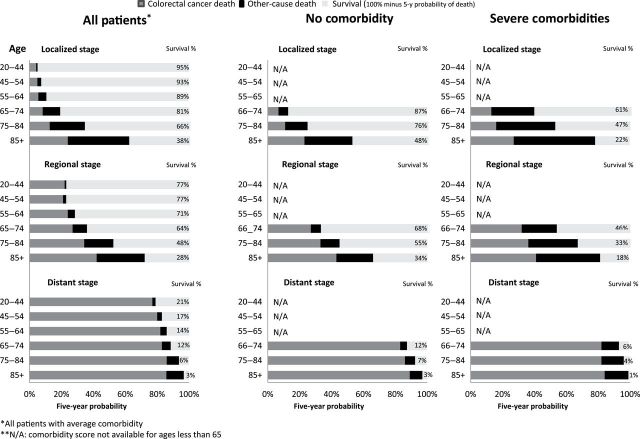
Actual prognosis—Five-year chance of females surviving, dying from colorectal cancer, or dying from competing causes by age, stage, and comorbidity.
Effect of Age
The effect of increasing age is similar across the four cancers. For localized and regional stage disease, older ages had lower survival largely from increasing competing causes of death. For example, survival probability went from being 95% for 20–44-year-old localized breast cancer patient to 49% for 85+-year-old women, due to increasing competing causes of death in the older age group ( Table 1).
Table 1:
Actual prognosis measures for patients with top 4 cancer diagnosis by stage and age: five-year probability of overall survival, dying of cancer, and dying of competing causes*
| Summary stage 2000 | Age at diagnosis, y | N | Survival | Cancer death | Other-cause death | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |||
| Female breast | ||||||||
| Localized | 20–44 | 31076 | 95% | 94%–95% | 4% | 4%–5% | 1% | 1%–1% |
| 45–54 | 62232 | 95% | 95%–96% | 3% | 3%–3% | 1% | 1%–1% | |
| 55–64 | 68071 | 94% | 94%–94% | 3% | 3%–3% | 3% | 3%–3% | |
| 65–74 | 57805 | 89% | 89%–90% | 3% | 3%–4% | 7% | 7%–7% | |
| 75–84 | 42128 | 76% | 76%–77% | 6% | 6%–6% | 18% | 17%–18% | |
| 85+ | 12345 | 49% | 47%–50% | 11% | 11%–12% | 40% | 39%–41% | |
| Regional | 20–44 | 26521 | 83% | 82%–83% | 16% | 16%–17% | 1% | 1%–1% |
| 45–54 | 39759 | 85% | 84%–85% | 14% | 13%–14% | 2% | 2%–2% | |
| 55–64 | 35178 | 82% | 82%–83% | 14% | 14%–15% | 3% | 3%–4% | |
| 65–74 | 24044 | 76% | 76%–77% | 15% | 15%–16% | 8% | 8%–9% | |
| 75–84 | 16706 | 60% | 59%–60% | 22% | 21%–22% | 19% | 18%–20% | |
| 85+ | 5452 | 30% | 28%–31% | 31% | 30%–33% | 39% | 38%–41% | |
| Distant | 20–44 | 2681 | 31% | 29%–34% | 66% | 64%–68% | 2% | 2%–3% |
| 45–54 | 4630 | 28% | 26%–29% | 69% | 68%–71% | 3% | 2%–3% | |
| 55–64 | 5563 | 24% | 23%–26% | 71% | 70%–73% | 5% | 4%–5% | |
| 65–74 | 4504 | 20% | 18%–21% | 72% | 71%–74% | 8% | 7%–9% | |
| 75–84 | 3747 | 12% | 11%–13% | 76% | 74%–77% | 12% | 11%–13% | |
| 85+ | 1563 | 6% | 5%–8% | 78% | 76%–80% | 16% | 14%–18% | |
| Prostate | ||||||||
| Localized | 20–44 | 2514 | 98% | 98%–99% | 1% | 0%–1% | 1% | 0%–1% |
| 45–54 | 37692 | 97% | 97%–97% | 1% | 1%–1% | 2% | 2%–2% | |
| 55–64 | 124644 | 95% | 94%–95% | 1% | 1%–1% | 4% | 4%–4% | |
| 65–74 | 154212 | 88% | 88%–89% | 2% | 2%–2% | 9% | 9%–9% | |
| 75–84 | 81942 | 74% | 73%–74% | 5% | 5%–6% | 21% | 21%–21% | |
| 85+ | 12311 | 42% | 42%–43% | 13% | 13%–14% | 44% | 43%–45% | |
| Regional | 20–44 | 543 | 94% | 92%–96% | 5% | 3%–7% | 1% | 0%–2% |
| 45–54 | 8923 | 95% | 95%–96% | 3% | 2%–3% | 2% | 2%–2% | |
| 55–64 | 25476 | 94% | 93%–94% | 3% | 3%–3% | 3% | 3%–4% | |
| 65–74 | 20102 | 89% | 88%–89% | 5% | 4%–5% | 7% | 6%–7% | |
| 75–84 | 3954 | 66% | 65%–68% | 15% | 14%–16% | 18% | 17%–20% | |
| 85+ | 765 | 28% | 24%–31% | 33% | 30%–37% | 39% | 35%–43% | |
| Distant | 20–44 | 147 | 29% | 18%–36% | 71% | 59%–76% | 3% | 1%–6% |
| 45–54 | 1382 | 29% | 26%–32% | 65% | 62%–68% | 6% | 5%–8% | |
| 55–64 | 4337 | 29% | 28%–31% | 62% | 61%–64% | 8% | 7%–9% | |
| 65–74 | 5896 | 29% | 27%–30% | 58% | 57%–60% | 13% | 12%–14% | |
| 75–84 | 6412 | 17% | 16%–18% | 63% | 62%–65% | 19% | 18%–20% | |
| 85+ | 3011 | 8% | 7%–9% | 67% | 66%–69% | 25% | 23%–26% | |
| Female colorectal | ||||||||
| Localized | 20–44 | 2744 | 95% | 94%–96% | 4% | 3%–5% | 1% | 1%–2% |
| 45–54 | 7569 | 93% | 92%–94% | 5% | 4%–5% | 2% | 2%–3% | |
| 55–64 | 10351 | 89% | 89%–90% | 6% | 5%–6% | 5% | 5%–6% | |
| 65–74 | 13922 | 81% | 81%–82% | 8% | 8%–9% | 11% | 10%–11% | |
| 75–84 | 15996 | 66% | 65%–66% | 13% | 12%–13% | 22% | 21%–22% | |
| 85+ | 7483 | 38% | 37%–39% | 24% | 23%–25% | 38% | 37%–39% | |
| Regional | 20–44 | 3152 | 77% | 75%–78% | 22% | 20%–24% | 1% | 1%–2% |
| 45–54 | 6844 | 77% | 76%–78% | 21% | 20%–22% | 2% | 2%–3% | |
| 55–64 | 9761 | 71% | 70%–72% | 24% | 23%–25% | 4% | 4%–5% | |
| 65–74 | 12798 | 64% | 63%–65% | 27% | 26%–28% | 9% | 9%–10% | |
| 75–84 | 15432 | 48% | 47%–49% | 34% | 33%–35% | 18% | 17%–18% | |
| 85+ | 7409 | 28% | 27%–29% | 42% | 41%–43% | 30% | 29%–31% | |
| Distant | 20–44 | 2135 | 21% | 19%–23% | 77% | 75%–79% | 2% | 1%–2% |
| 45–54 | 4177 | 17% | 16%–18% | 80% | 79%–82% | 3% | 2%–3% | |
| 55–64 | 5704 | 14% | 13%–15% | 82% | 81%–83% | 4% | 3%–4% | |
| 65–74 | 6448 | 12% | 11%–13% | 83% | 82%–84% | 6% | 5%–6% | |
| 75–84 | 7168 | 6% | 5%–6% | 86% | 85%–87% | 8% | 8%–9% | |
| 85+ | 3682 | 3% | 2%–3% | 86% | 85%–87% | 11% | 10%–12% | |
| Female lung and bronchus | ||||||||
| Localized | 20–44 | 756 | 82% | 79%–85% | 15% | 12%–18% | 3% | 2%–5% |
| 45–54 | 2444 | 74% | 72%–76% | 22% | 20%–24% | 4% | 4%–5% | |
| 55–64 | 6039 | 63% | 62%–65% | 28% | 26%–29% | 9% | 8%–10% | |
| 65–74 | 9908 | 52% | 51%–53% | 35% | 34%–36% | 13% | 12%–14% | |
| 75–84 | 8396 | 36% | 35%–37% | 45% | 44%–47% | 19% | 18%–20% | |
| 85+ | 1908 | 13% | 11%–15% | 60% | 58%–63% | 27% | 24%–29% | |
| Regional | 20–44 | 912 | 42% | 38%–45% | 55% | 51%–58% | 3% | 2%–5% |
| 45–54 | 4062 | 35% | 34%–37% | 60% | 58%–61% | 5% | 4%–6% | |
| 55–64 | 8757 | 31% | 30%–32% | 62% | 61%–63% | 7% | 6%–7% | |
| 65–74 | 12725 | 24% | 23%–25% | 65% | 65%–66% | 10% | 10%–11% | |
| 75–84 | 9947 | 16% | 15%–16% | 71% | 70%–72% | 13% | 12%–14% | |
| 85+ | 2075 | 7% | 5%–8% | 74% | 72%–76% | 19% | 17%–21% | |
| Distant | 20–44 | 2681 | 8% | 7%–9% | 89% | 87%–90% | 3% | 2%–4% |
| 45–54 | 10421 | 7% | 6%–7% | 90% | 89%–91% | 3% | 3%–4% | |
| 55–64 | 21059 | 5% | 5%–5% | 90% | 90%–91% | 4% | 4%–5% | |
| 65–74 | 28486 | 4% | 4%–4% | 90% | 89%–90% | 6% | 6%–7% | |
| 75–84 | 24917 | 2% | 2%–3% | 89% | 89%–89% | 9% | 8%–9% | |
| 85+ | 7482 | 1% | 1%–1% | 86% | 85%–86% | 14% | 13%–14% | |
* We utilized Surveillance, Epidemiology, and End Results-18 registries; diagnosis year includes 2000–2009 and patients were followed until December 31, 2010. CI = confidence interval.
For distant stage, age had much less of an effect—the vast majority died from their cancer: for example, the chance of dying from distant lung cancer ranged from 86% to 90% depending on age at diagnosis.
Effect of Comorbidity
Comorbidity also had a similar effect across the four cancers. For localized and regional stage disease, increased comorbidity had lower survival largely from increasing competing causes of death. However, for distant stage, comorbidity had much less of an effect—the vast majority died from their cancer: for example, colorectal cancer death ranged 82%–89% depending on comorbidity condition (see Supplementary Table 1, available online).
Finally, we noticed comorbidity effected both cancer and competing causes of deaths, but the effect was greater for competing causes. For example, for 66–74-year-old prostate cancer patient diagnosed with localized disease, probability of death from cancer changed little (2%-4%); whereas, probability of competing cause death changed more drastically (7%–24%) due to comorbidity (see Table 2). For more cancer sites see Supplementary Table 1. ).
Table 2.
Actual prognosis measure for patients diagnosed with prostate cancer by comorbidity, stage, and age: five-year probability of overall survival, dying of cancer, and dying of competing causes*
| Summary stage 2000 | Age at diagnosis | Comorbidity | N | Survival | Cancer death | Other-cause death | |||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | ||||
| Localized | 66–74 | No comorbidity | 56,500 | 91% | 91%–91% | 2% | 2%–2% | 7% | 6%–7% |
| Severe comorbidity | 13,650 | 72% | 71%–73% | 4% | 3%–4% | 24% | 24%–25% | ||
| 75–84 | No comorbidity | 32,355 | 80% | 80%–80% | 5% | 5%–5% | 15% | 15%–15% | |
| Severe comorbidity | 12,915 | 56% | 55%–57% | 6% | 6%–7% | 38% | 37%–39% | ||
| 85+ | No comorbidity | 4,434 | 49% | 47%–50% | 14% | 13%–15% | 37% | 36%–39% | |
| Severe comorbidity | 2,798 | 28% | 26%–30% | 12% | 11%–14% | 60% | 58%–62% | ||
| Regional | 66–74 | No comorbidity | 7,641 | 90% | 89%–91% | 5% | 4%–5% | 5% | 5%–6% |
| Severe comorbidity | 1,091 | 75% | 72%–78% | 6% | 4%–8% | 19% | 16%–22% | ||
| 75–84 | No comorbidity | 1,742 | 72% | 70%–74% | 14% | 12%–16% | 14% | 12%–16% | |
| Severe comorbidity | 490 | 46% | 41%–51% | 18% | 14%–22% | 36% | 31%–41% | ||
| 85+ | No comorbidity | 255 | 38% | 31%–45% | 31% | 25%–37% | 31% | 25%–38% | |
| Severe comorbidity | 180 | 14% | 8%–20% | 35% | 28%–42% | 51% | 43%–59% | ||
| Distant | 66–74 | No comorbidity | 2,080 | 29% | 27%–31% | 59% | 57%–61% | 12% | 10%–13% |
| Severe comorbidity | 616 | 18% | 15%–21% | 57% | 53%–61% | 25% | 22%–29% | ||
| 75–84 | No comorbidity | 2,334 | 20% | 19%–22% | 65% | 63%–67% | 14% | 13%–16% | |
| Severe comorbidity | 1,120 | 8% | 6%–10% | 61% | 58%–64% | 31% | 28%–33% | ||
| 85+ | No comorbidity | 955 | 9% | 7%–11% | 69% | 66%–72% | 22% | 19%–24% | |
| Severe comorbidity | 688 | 4% | 3%–6% | 63% | 59%–67% | 33% | 29%–36% | ||
* We utilized Surveillance, Epidemiology, and End Results (SEER)-18 registries; diagnosis year includes 2000–2009 and patients were followed until December 31, 2010. CI = confidence interval.
Effect of Stage
Although age and comorbidity lowered actual prognosis, stage at diagnosis was the most powerful factor. For example, a 55-year-old woman’s probability of dying from breast cancer was 3% for localized stage, 14% for regional stage, and 71% for distant stage; for lung cancer, the corresponding probabilities are 28% for localized, 62% for regional, and 90% for distant.
Within each stage, the chance of dying from cancer did not vary much by age; for example, a 20-year- and 74-year-old man diagnosed with regional disease experienced a small chance of dying from prostate; however, 85+-year-old man diagnosed with the same disease had a 39% probability of competing causes of death.
Discussion
Traditional cancer prognosis statistics (eg, five-year relative survival) are useful for tracking progress against cancer over time because they isolate the effect of cancer on survival by removing the effects of competing causes. Competing causes of death provide fundamental context for prognosis for cancer patients. The patients need actual prognosis measures to understand what their chance of dying from the cancer and from competing causes after a period of time.
Estimates of prognosis presented here make it fairly easy to understand that cancer is biggest concern for healthy, young, and distant disease patients. For example, 20-year-old woman diagnosed with localized breast cancer has a 5% chance of dying in the next 5 years; however, most of the risk comes from dying from breast cancer (4%) rather than competing causes (1%). For older patients diagnosed with early stage disease, competing causes of death pose a much greater threat. For example, a 75-year-old woman diagnosed with localized breast cancer has a 24% chance of dying in the next 5 years: most of the risk arises from competing causes (18%) rather than breast cancer (6%). Similar pattern was observed for prostate cancer patients in our study as well as other studies (19,20).
Actual prognosis measures have been unavailable for older patients and those with multiple comorbidities because population-based registries do not routinely report comorbidity conditions on cancer patients and clinical trials generally under-represent or exclude these patients. But this trend is changing. The SEER program recently developed nomograms for prostate, colorectal, breast, and head and neck cancer as part of a Web-based Cancer Survival Calculator SEER*CSC, formerly known as CSQS, which will include actual prognosis for a broad array of patients (21) (22).
Our results presented here have several limitations. Actual prognosis calculation based on cause of death may be fraught with inaccuracies due to misclassification error of cause of death from the death certificates (23,24). We estimated actual prognosis using US life tables, but found that this approach did not accurately estimate the probability of cancer-specific deaths in some subgroups (eg, screen-detected cancers such as breast or prostate, cancer patients with serve comorbidity conditions). In addition, we were able to calculate effect of comorbidity only available for 65 and older patients. Lastly, we were not able to include prognostic estimates by other important factors (eg, cancer subtypes, grade, or treatment).
Finally, although actual prognosis measures may be most relevant to patients, it is not known whether or how patients would use this information. The SEER*CSC (21, 22) are being tested in clinical settings, to understand the usability of actual prognosis measures for both patients and clinicians. More information regarding the results of the feasibility test usability of actual prognosis measures using SEER*CSC tool maybe found in articles by Rabin et al. (25) and Feuer et al. (22) in this monograph. It is also important to note that the SEER*CSC tool is much more personalized to the individual (eg, individual age, American Joint Committee on Cancer (AJCC) substage, specific levels of comorbidity) than the estimates presented in this paper. There are also other tools besides SEER*CSC that present actual prognosis (26,27) for cancer patients.
Finally, both cancer and actual prognosis measures are important. We provided a format that can serve as a template for summarizing both measures according to very specific age and health status categories and that can be easily used in the routine reporting of such statistics (1). Cancer prognosis measures can uniquely answer questions for policy makers and researchers about cancer survival across different countries or across time (11,12) because these measures control for difference in competing causes (although these comparisons need to be interpreted cautiously when early detection [eg, screening] differs across place or time). On the other hand, actual prognosis measures can help clinicians and patients approach treatment decisions because they reflect clinical reality: patients can die from cancer or competing causes, and competing causes increase with age and comorbidity.
Cancer registries should routinely report both cancer and actual prognosis to help clinicians and researchers understand the difference between these measures and what question they can and cannot answer. We encourage them to use formats like the ones presented in this paper to communicate them clearly.
Funding
Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute, National Institutes of Health contracts with the SEER registries.
Disclaimer
Findings and conclusions are the authors’ and do not necessarily represent the official positions of their affiliations, or those of the National Cancer Institute, the National Institutes of Health, the US Department of Health and Human Services or the Department of Veterans Affairs.
Supplementary Material
References
- 1. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2010. Bethesda, MD: National Cancer Institute; 2013. http://seer.cancer.gov/csr/1975_2010/ Published April 2013. Accessed June 17, 2013. [Google Scholar]
- 2. Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 3. Marubini E VM. Analysing Survival Data From Clinical Trials and Observational Studies. Chichester, UK: John Wiley & Sons; 1995. [Google Scholar]
- 4. Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol. 2013;178(3):339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Esserman L, Shieh Y, Thompson I. Rethinking screening for breast cancer and prostate cancer. JAMA. 2009;302(15):1685–1692. [DOI] [PubMed] [Google Scholar]
- 6. Welch HG, Albertsen PC, Nease RF, Bubolz TA, Wasson JH. Estimating treatment benefits for the elderly: the effect of competing risks. Ann Intern Med. 1996;124(6):577–584. [DOI] [PubMed] [Google Scholar]
- 7. Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120(9):1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cronin KA, Feuer EJ. Cumulative cause-specific mortality for cancer patients in the presence of other causes: a crude analogue of relative survival. Stat Med. 2000;19(13):1729–1740. [DOI] [PubMed] [Google Scholar]
- 9. Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96(17):1311–1321. [DOI] [PubMed] [Google Scholar]
- 10. Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baili P, Micheli A, De Angelis R, et al. Life tables for world-wide comparison of relative survival for cancer (CONCORD study). Tumori. 2008;94(5):658–668. [DOI] [PubMed] [Google Scholar]
- 12. Coleman MP, Quaresma M, Berrino F, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol. 2008;9(8):730–756. [DOI] [PubMed] [Google Scholar]
- 13. SEER cause-specific death classification. Surveillance, Epidemiology, and End Results Program Web site http://seer.cancer.gov/causespecific/ Accessed June 17, 2013.
- 14. Localized/regional/distant stage adjustments: for 1973-2009 SEER research data (November 2011 submission) and later releases. Surveillance, Epidemiology, and End Results Program Web site http://seer.cancer.gov/seerstat/variables/seer/yr1973_2009/lrd_stage/index.html Accessed June 17, 2013.
- 15. Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. [DOI] [PubMed] [Google Scholar]
- 16. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. [DOI] [PubMed] [Google Scholar]
- 17. Mariotto AB, Wang Z, Klabunde CN, Cho H, Das B, Feuer EJ. Life tables adjusted for comorbidity more accurately estimate noncancer survival for recently diagnosed cancer patients. J Clin Epidemiol. 2013;66(12):1376–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. SEER*Stat software [computer program]. Version 7.0.4. Bethesda, MD: Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute; 2010. [Google Scholar]
- 19. Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daskivich TJ, Fan KH, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158(10):709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feuer EJ, Lee M, Mariotto AB, et al. The Cancer Survival Query System: making survival estimates from the Surveillance, Epidemiology, and End Results program more timely and relevant for recently diagnosed patients. Cancer. 2012;118(22):5652–5662. [DOI] [PubMed] [Google Scholar]
- 22. Feuer EJ, Rabin B, Zou Z, et al. The Surveillance, Epidemiology, and End Results Cancer Survival Calculator SEER*CSC: validation in a managed care setting. J Natl Cancer Inst Monogr. 2014;49:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Begg CB, Schrag D. Attribution of deaths following cancer treatment. J Natl Cancer Inst. 2002;94(14):1044–1045. [DOI] [PubMed] [Google Scholar]
- 24. Percy C, Stanek E, 3rd, Gloeckler L. Accuracy of cancer death certificates and its effect on cancer mortality statistics. Am J Public Health. 1981;71(3):242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rabin BA, Ellis JL, Steiner JF, et al. Health-care utilization by prognosis profile in a managed care setting: using the Surveillance, Epidemiology, and End Results Cancer Survival Calculator SEER*CSC. J Natl Cancer Inst Monogr. 2014;49:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rabin BA, Gaglio B, Sanders T, et al. Predicting cancer prognosis using interactive online tools: a systematic review and implications for cancer care providers. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1645–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gulati R, Inoue LYT, Gore JL, et al. Individualized estimates of overdiagnosis in screen-detected prostate cancer. J Natl Cancer Inst. (2014);106(2)djt367. 10.1093/jnci/djt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.