Abstract
Background—
Cardiac remodeling occurs in response to regular athletic training, and the degree of remodeling is associated with fitness. Understanding the myocardial structural changes in athlete’s heart is important to develop tools that differentiate athletic from cardiomyopathic change. We hypothesized that athletic left ventricular hypertrophy is a consequence of increased myocardial cellular rather than extracellular mass as measured by cardiovascular magnetic resonance.
Methods and Results—
Forty-five males (30 athletes and 15 sedentary age-matched healthy controls) underwent comprehensive cardiovascular magnetic resonance studies, including native and postcontrast T1 mapping for extracellular volume calculation. In addition, the 30 athletes performed a maximal exercise test to assess aerobic capacity and anaerobic threshold. Participants were grouped by athleticism: untrained, low performance, and high performance (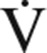 O2max <60 or>60 mL/kg per min, respectively). In athletes, indexed cellular mass was greater in high- than low-performance athletes 60.7±7.5 versus 48.6±6.3 g/m2; P<0.001), whereas extracellular mass was constant (16.3±2.2 versus 15.3±2.2 g/m2; P=0.20). Indexed left ventricular end-diastolic volume and mass correlated with
O2max <60 or>60 mL/kg per min, respectively). In athletes, indexed cellular mass was greater in high- than low-performance athletes 60.7±7.5 versus 48.6±6.3 g/m2; P<0.001), whereas extracellular mass was constant (16.3±2.2 versus 15.3±2.2 g/m2; P=0.20). Indexed left ventricular end-diastolic volume and mass correlated with 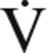 O2max (r=0.45, P=0.01; r=0.55, P=0.002) and differed significantly by group (P=0.01; P<0.001, respectively). Extracellular volume had an inverse correlation with
O2max (r=0.45, P=0.01; r=0.55, P=0.002) and differed significantly by group (P=0.01; P<0.001, respectively). Extracellular volume had an inverse correlation with 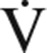 O2max (r=−0.53, P=0.003 and left ventricular mass index (r=-0.44, P=0.02).
O2max (r=−0.53, P=0.003 and left ventricular mass index (r=-0.44, P=0.02).
Conclusions—
Increasing left ventricular mass in athlete’s heart occurs because of an expansion of the cellular compartment while the extracellular volume becomes relatively smaller: a difference which becomes more marked as left ventricular mass increases. Athletic remodeling, both on a macroscopic and cellular level, is associated with the degree of an individual’s fitness. Cardiovascular magnetic resonance ECV quantification may have a future role in differentiating athlete’s heart from change secondary to cardiomyopathy.
Keywords: athlete’s heart, cardiovascular magnetic resonance imaging, ECV, exercise physiology, hypertrophy/remodeling, T1 mapping
Regular exercise training places demands on the heart that may lead to cardiac remodeling. The rare but prominent cases of sudden cardiac death in elite athletes have underlined the importance of understanding the adaptation underlying athlete’s heart (AH).1 Gaining insights into the remodeling process is important to understand the nature of adaptation and to develop tools that distinguish AH from cardiomyopathic change, particularly those of hypertrophic cardiomyopathy (HCM).
See Editorial by Graham-Brown and McCann
See Clinical Perspective
Cardiovascular magnetic resonance (CMR) imaging is particularly well suited to investigate changes of structure and function in athletic cardiac remodeling because of its multiparametric capabilities, high spatial resolution, and lack of ionizing radiation. The excellent reproducibility of the method means that anatomic change can be quantified with greater confidence and smaller sample size than with other methods.2,3 The emerging CMR technique of extracellular volume (ECV) quantification allows the relative volumes of the extracellular and intracellular myocardial compartments to be quantified. This method has been used to quantify extracellular expansion (higher ECV) secondary to interstitial fibrosis in cardiomyopathy4 and conversely greater myocyte volume (lower ECV) in pathologies such as Anderson–Fabry disease.5,6
In this study, we sought to characterize cardiac adaptation in male athletes using CMR to gain insights into the mechanisms underlying athletic remodeling. We hypothesized that in athletes, left ventricular (LV) hypertrophy occurs secondary to an increase in myocardial cellular mass rather than ECV and that changes become increasingly prominent with increasing aerobic capacity (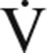 O2max).
O2max).
Methods
Male athletic participants aged between 20 and 45 years were approached via athletic societies and associations. Recruitment was open to athletes competing at regional, national, and international level. Subjects were eligible for inclusion if they took part in regular competition and trained for a minimum of 6 hours per week.
In addition, untrained males who exercised <3 hours per week were recruited from the study institution as a control group. Untrained individuals did not undertake regular athletic training. Subjects were not eligible if they had contraindications to CMR, hypertension (systolic blood pressure >140 mm Hg), or systemic medical illness. All athletes underwent a CMR study before a maximal exercise test, performed on the same day when logistically possible.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee (Research and Ethics Committee reference: 14/YH/0126). All subjects gave informed written consent.
Cardiac Magnetic Resonance Protocol
The CMR study was performed at rest and before exercise testing in athletes. All studies were performed on a 3-Tesla Achieva TX system equipped with a 32-channel cardiac phased array receiver coil and multitransmit technology (Philips Healthcare, Best, The Netherlands). The cardiac long and short axes were determined using standard scout views. Mid-LV native (precontrast) T1 maps were generated using a previously described MOLLI sequence7 planned using the 3 of 5 method8 briefly comprising: ECG triggered 5b(3s)3b MOLLI, flip angle 35°, voxel size of 1.98×1.98×10 mm3. LV mass and volumes were obtained from cine imaging covering the entire LV in the short axis: balanced SSFP, voxel size 1.2×1.2×10 mm3, no gap, 50 cardiac phases. Right ventricular and atrial volumes were obtained from a transaxial stack covering the entire heart: balanced SSFP, voxel size 1.7×1.5×5 mm3, no interslice gap. 0.15 mmol/kg Gadovist (Bayer Schering) was delivered by power injector (Medrad Inc, Warrendale, PA) as a single bolus via a venous cannula placed in the antecubital fossa, followed by a 20 mL saline flush at 5 mL/s. Late gadolinium enhancement (LGE) imaging (inversion recovery-prepared T1-weighted gradient echo, inversion time according to Look-Locker scout, TR/TE/flip angle 3.7 ms/2.0 ms/25°, acquired spatial resolution 1.54×1.75×10 mm) was performed to image the entire LV 7 to 10 minutes after contrast administration. Post-contrast T1 maps were acquired using the same MOLLI scheme 10 minutes after contrast administration.
Image Analysis
All image analysis was performed using cmr42 software (Circle Cardiovascular Imaging Inc, Calgary, Alberta, Canada). Volumetric and mass analysis was performed in the standard manner from the short-axis stack9 (LV) or long-axis cine images10 (right ventricular, left and right atria). Ventricular and atrial measurements were indexed to body surface area. The presence of focal fibrosis or scar was assessed qualitatively from LGE imaging. T1 values were calculated from source images using manual motion correction, with a region of interest in the mid-inferoseptum as per Rogers et al.11 Partition coefficient (λ) and ECV were calculated using the formulae:
 |
where R1=1/T1 and Hct is hematocrit.
CMR chamber and tissue values were indexed to body surface area and estimated lean body mass (height2.7). Indexed myocyte and extracellular mass were calculated using the formulae: indexed myocyte mass=indexed LV mass×(100−%ECV); indexed extracellular mass=indexed LV mass×%ECV. (The standard deviation of ECV measurement in our center in a cohort of 30 healthy individuals was 2.8%.) All T1, ECV, volumetric, and mass analyses were performed by 2 observers (A. K. McDiarmid and B. Erhayiem) blinded to all subject data, including sporting discipline and aerobic capacity.
Exercise Protocol
Participants were instructed to arrive rested (no strenuous exercise in the preceding 24 hours) and having abstained from any alcohol (preceding 24 hours), food, and caffeine (preceding 3 hours) ingestion. To determine maximal oxygen uptake (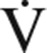 O2max) and ventilatory threshold, participants undertook a ramp-incremental step-exercise test on an electronically braked cycle ergometer (Excalibur Sport, Lode BV, Groningen, the Netherlands), which allows for confirmation of VO2max in a single test.12 Participants wore a nose clip and breathed through a low-dead space, low-resistance mouthpiece which was connected to a bidirectional pitot tube flow sensor and gas sample line assembly, allowing for breath-by-breath measurement of gas volumes and concentrations (O2, Galvanic; CO2 infrared) and subsequent calculation of ventilatory and pulmonary gas exchange variables (Cardio2, Medical Graphics Corporation, St Paul, MN). Before each test, the pitot tube flow sensor was calibrated over a range of flow rates using a 3 L syringe, whereas the gas analysers were calibrated using precision gases that spanned the inspired and expired physiological range. A 12-lead ECG was monitored throughout, and heart rate was measured from the R-R interval. The ramp-incremental step-exercise test was preceded by a rest period (≈2 minutes) and unloaded cycling (20 W; ≈4 minutes) and continued until a steady state was attained, after which work rate increased as a linear function of time at a rate of 20 to 30 W/min (depending on reported training history), with the intention of bringing participants to the limit of tolerance in ≈10 to 12 minutes.13 The ramp increment was then followed by 5 minutes of active recovery (20 W), after which a step exercise (SE) was performed at 95% of the RI work rate peak, with this SE also continued to the limit of tolerance. In both RI and SE parts of the test, the limit of tolerance was defined as the point at which cycling cadence fell below 50 rpm, despite strong verbal encouragement.
O2max) and ventilatory threshold, participants undertook a ramp-incremental step-exercise test on an electronically braked cycle ergometer (Excalibur Sport, Lode BV, Groningen, the Netherlands), which allows for confirmation of VO2max in a single test.12 Participants wore a nose clip and breathed through a low-dead space, low-resistance mouthpiece which was connected to a bidirectional pitot tube flow sensor and gas sample line assembly, allowing for breath-by-breath measurement of gas volumes and concentrations (O2, Galvanic; CO2 infrared) and subsequent calculation of ventilatory and pulmonary gas exchange variables (Cardio2, Medical Graphics Corporation, St Paul, MN). Before each test, the pitot tube flow sensor was calibrated over a range of flow rates using a 3 L syringe, whereas the gas analysers were calibrated using precision gases that spanned the inspired and expired physiological range. A 12-lead ECG was monitored throughout, and heart rate was measured from the R-R interval. The ramp-incremental step-exercise test was preceded by a rest period (≈2 minutes) and unloaded cycling (20 W; ≈4 minutes) and continued until a steady state was attained, after which work rate increased as a linear function of time at a rate of 20 to 30 W/min (depending on reported training history), with the intention of bringing participants to the limit of tolerance in ≈10 to 12 minutes.13 The ramp increment was then followed by 5 minutes of active recovery (20 W), after which a step exercise (SE) was performed at 95% of the RI work rate peak, with this SE also continued to the limit of tolerance. In both RI and SE parts of the test, the limit of tolerance was defined as the point at which cycling cadence fell below 50 rpm, despite strong verbal encouragement.
Breath-by-breath data were edited using the 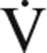 O2 response to eliminate erroneous breaths (occurring outside the local mean 99% prediction limits) that were considered unphysiological.14 Anaerobic threshold was then estimated using the V-slope method15 and supporting ventilatory and pulmonary gas exchange criteria (ie, the fractional end-tidal concentrations of O2 and CO2, and the ventilatory equivalents for O2 and CO216).
O2 response to eliminate erroneous breaths (occurring outside the local mean 99% prediction limits) that were considered unphysiological.14 Anaerobic threshold was then estimated using the V-slope method15 and supporting ventilatory and pulmonary gas exchange criteria (ie, the fractional end-tidal concentrations of O2 and CO2, and the ventilatory equivalents for O2 and CO216). 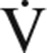 O2peak was identified in both RI and SE phases as the highest 12-breath rolling average (highest mean
O2peak was identified in both RI and SE phases as the highest 12-breath rolling average (highest mean 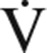 O2 over ≈15–20 s).12 Within subjects, the highest 12-breath rolling average from RI and SE phases were then compared using unpaired t tests, with no difference (P>0.05) between RI and SE
O2 over ≈15–20 s).12 Within subjects, the highest 12-breath rolling average from RI and SE phases were then compared using unpaired t tests, with no difference (P>0.05) between RI and SE 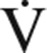 O2peak, and thus the attainment of
O2peak, and thus the attainment of 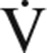 O2max was confirmed in each test.17
O2max was confirmed in each test.17
Statistical Analysis
Statistical analysis was performed using IBM SPSS Statistics 20.0 (IBM Corp, Armonk, NY). Subjects were grouped as follows: untrained, athletes with 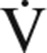 O2max <60 mL/kg per min (low-performance group), and athletes with
O2max <60 mL/kg per min (low-performance group), and athletes with 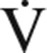 O2max >60 mL/kg per min (high-performance group). Unless otherwise stated, the results are presented as mean±standard deviation (SD). Normality of distribution was determined with Kolmogarov–Smirnov testing. Differences between groups were assessed using the Chi-squared test, independent t test, or 1-way analysis of variance when appropriate. Post hoc analysis was performed with Bonferroni testing. Correlation was assessed with Spearman correlation coefficient. Significance for all tests was assumed with P<0.05.
O2max >60 mL/kg per min (high-performance group). Unless otherwise stated, the results are presented as mean±standard deviation (SD). Normality of distribution was determined with Kolmogarov–Smirnov testing. Differences between groups were assessed using the Chi-squared test, independent t test, or 1-way analysis of variance when appropriate. Post hoc analysis was performed with Bonferroni testing. Correlation was assessed with Spearman correlation coefficient. Significance for all tests was assumed with P<0.05.
Results
Study Participant Demographics and Characteristics
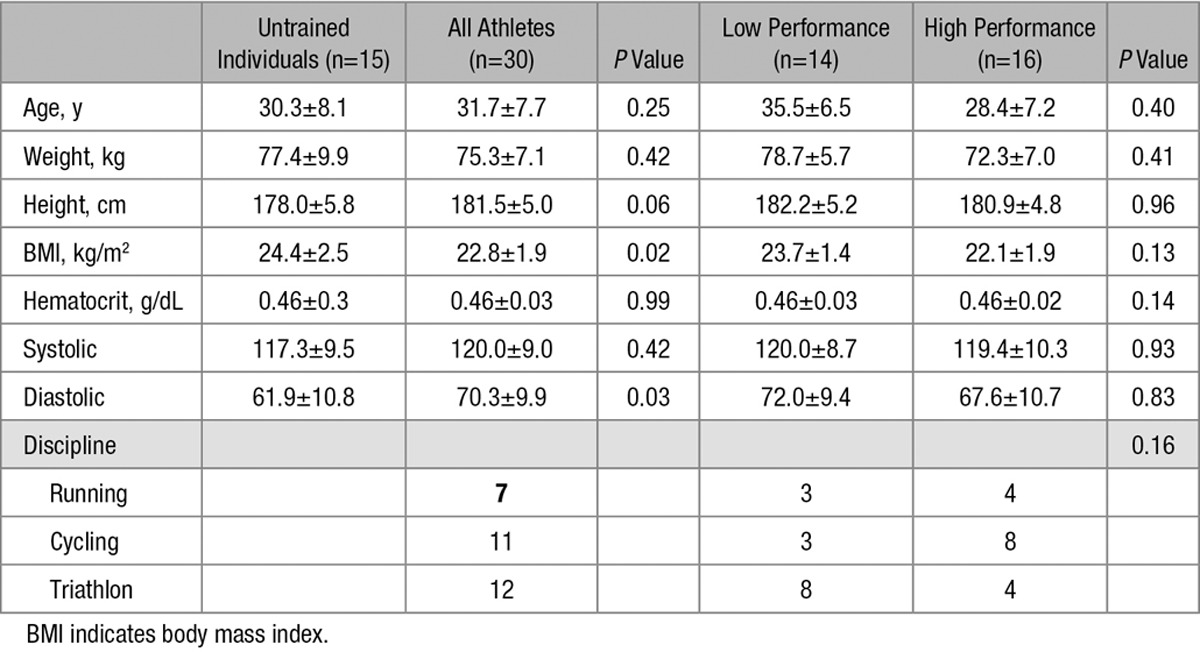
Thirty endurance athletes (7 runners, 11 cyclists, and 12 triathletes) and 15 untrained sedentary controls were recruited. Athletic and untrained participants were prospectively matched for age (31.7±7.7 versus 30.3±8.1 years; P=0.54). Hematocrit was not significantly different between groups (0.46±0.3 versus 0.46±0.3 g/dL; P=0.99), but body mass index was significantly lower in athletes (22.8±1.9 versus 24.4±2.5 kg/m2; P=0.02). CMR study and maximal exercise testing were separated by a median of 0 days (quartile range 1–3). Full study participant information may be seen in Table 1.
Table 1.
Subject Characteristics
Performance Stratification and Cardiopulmonary Exercise Testing
Athletes were divided into high- and low-performance groups at a threshold  O2max of 60 mL/kg per min (n=16 and 14, respectively). Mean
O2max of 60 mL/kg per min (n=16 and 14, respectively). Mean  O2max and anaerobic threshold as a percentage of
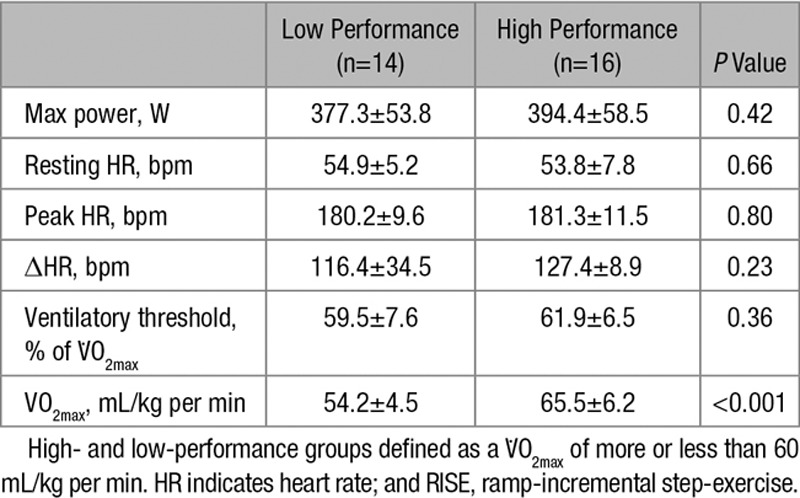
O2max and anaerobic threshold as a percentage of  O2max in high-performance and low-performance athletes, respectively, were 65.5±6.2 versus 54.2±4.5 mL/kg per min (P<0.001) and 61.9±6.5% versus 59.5±7.6% (P=0.36). No significant atrial or ventricular ectopy was recorded in the exercise or recovery phase of the exercise protocol. None of resting, peak, or Δheart rate differed between groups. Maximal exercise test results are summarized in Table 2.
O2max in high-performance and low-performance athletes, respectively, were 65.5±6.2 versus 54.2±4.5 mL/kg per min (P<0.001) and 61.9±6.5% versus 59.5±7.6% (P=0.36). No significant atrial or ventricular ectopy was recorded in the exercise or recovery phase of the exercise protocol. None of resting, peak, or Δheart rate differed between groups. Maximal exercise test results are summarized in Table 2.
Table 2.
RISE Maximal Exercise Test Results
Myocardial Remodeling
Indexed cellular mass was greater in high-performance athletes than in low-performance athletes (60.7±7.5 versus 48.6±6.3 g/m2; P<0.001); however, extracellular mass remained constant (16.3±2.2 versus 15.3±2.2 g/m2; P=0.20; Figure 1). Myocardial ECV and native T1 in athletes were lower than in untrained controls (22.5±2.6% versus 24.5±2.2%, P=0.02; 1178±32 versus 1202±33 ms, P=0.02). Although both the cellular and extracellular masses were higher in athletes than in controls (15.8±2.2 versus 13.6±1.7, P<0.001; 55.1±9.2 versus 42.3±5.7, P<0.001), the relative expansion of the cellular compartment was greater than the increase seen of the extracellular compartment (130% and 116%, respectively).
Figure 1.
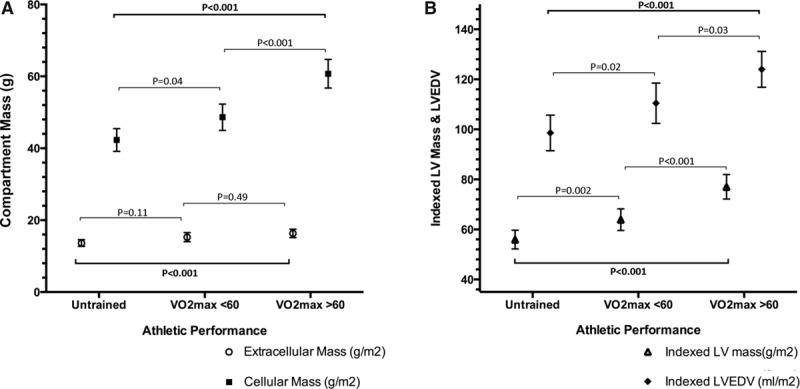
Myocardial remodeling by performance group. A, Cellular mass increases out of proportion to increase in extracellular mass. B, Indexed left ventricular end-diastolic volume (LVEDV) and left ventricular (LV) mass increases stepwise as athletic grouping changes (P<0.001 for both).
Chamber Remodeling in High-Performance and Low-Performance Athletes
Typical findings may be seen in Figure 2, and full CMR data are given in Table 3. Both left ventricular mass index and left ventricular end-diastolic volume index differed significantly between athletes and untrained controls and between athletic groups (P<0.001 for all; Figure 2). Significant correlations were observed between cardiac chamber remodeling and  O2max: both left ventricular mass index and left ventricular end-diastolic volume index correlated significantly with each other (r=0.71, P<0.001) and with
O2max: both left ventricular mass index and left ventricular end-diastolic volume index correlated significantly with each other (r=0.71, P<0.001) and with  O2max (r=0.55, P=0.002 and r=0.45, P=0.01, respectively; Figure 3). In athletes, CMR measures of tissue composition (ECV and partition coefficient) differed between athletic groups and correlated significantly with
O2max (r=0.55, P=0.002 and r=0.45, P=0.01, respectively; Figure 3). In athletes, CMR measures of tissue composition (ECV and partition coefficient) differed between athletic groups and correlated significantly with 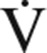 O2max: ECV r=−0.53, P=0.003; partition coefficient r=−0.41, P=0.02. Native T1 did not differ between athletic groups (low versus high performance; 1185±36 versus 1171±28 ms; P=0.26). LGE was seen in one athlete only (3%) and was in a typical myocarditis pattern. A significant inverse correlation was seen between ECV and left ventricular mass index (r=−0.44, P=0.02; Figure 3).
O2max: ECV r=−0.53, P=0.003; partition coefficient r=−0.41, P=0.02. Native T1 did not differ between athletic groups (low versus high performance; 1185±36 versus 1171±28 ms; P=0.26). LGE was seen in one athlete only (3%) and was in a typical myocarditis pattern. A significant inverse correlation was seen between ECV and left ventricular mass index (r=−0.44, P=0.02; Figure 3).
Figure 2.
Typical cardiac magnetic resonance (CMR) appearances in low-performance and high-performance athletes. Left column shows an athlete with a  O2max of 50 mL/kg per in, and right column shows a high-performance athlete with a
O2max of 50 mL/kg per in, and right column shows a high-performance athlete with a  O2max of 75 mL/kg per min. A and D, Short-axis images of left and right ventricle. B and E, extracellular volume (ECV) color maps showing lower ECV in the high performance athletes throughout the myocardium. C and F, Pie charts scaled to overall indexed LV mass displaying the relative indexed masses of the cellular and extracellular compartments (not short axis images). ECMI indicates indexed extracellular mass; and ICMI, indexed intracellular mass.
O2max of 75 mL/kg per min. A and D, Short-axis images of left and right ventricle. B and E, extracellular volume (ECV) color maps showing lower ECV in the high performance athletes throughout the myocardium. C and F, Pie charts scaled to overall indexed LV mass displaying the relative indexed masses of the cellular and extracellular compartments (not short axis images). ECMI indicates indexed extracellular mass; and ICMI, indexed intracellular mass.
Table 3.
Cardiac Magnetic Resonance Findings
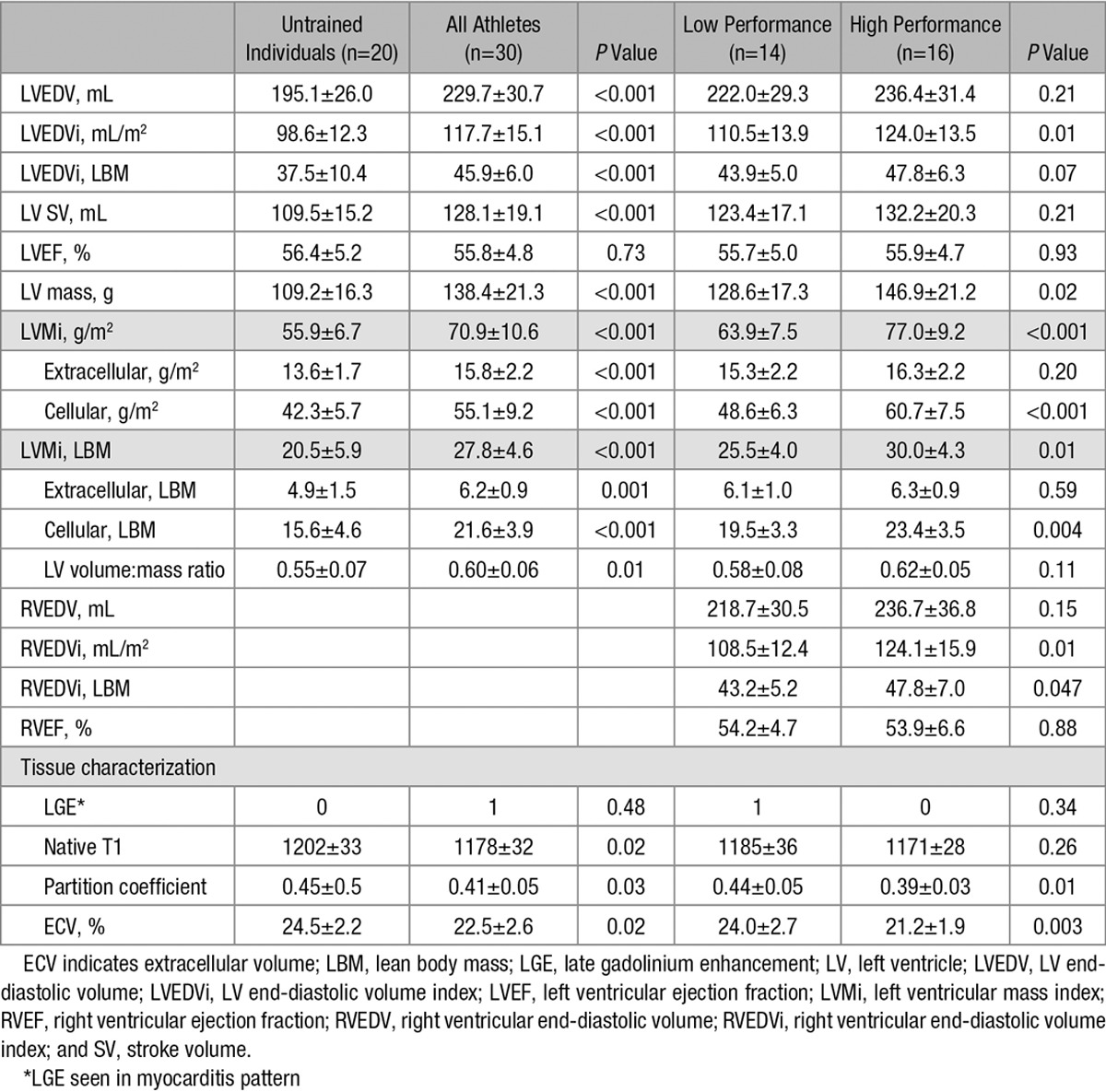
Figure 3.
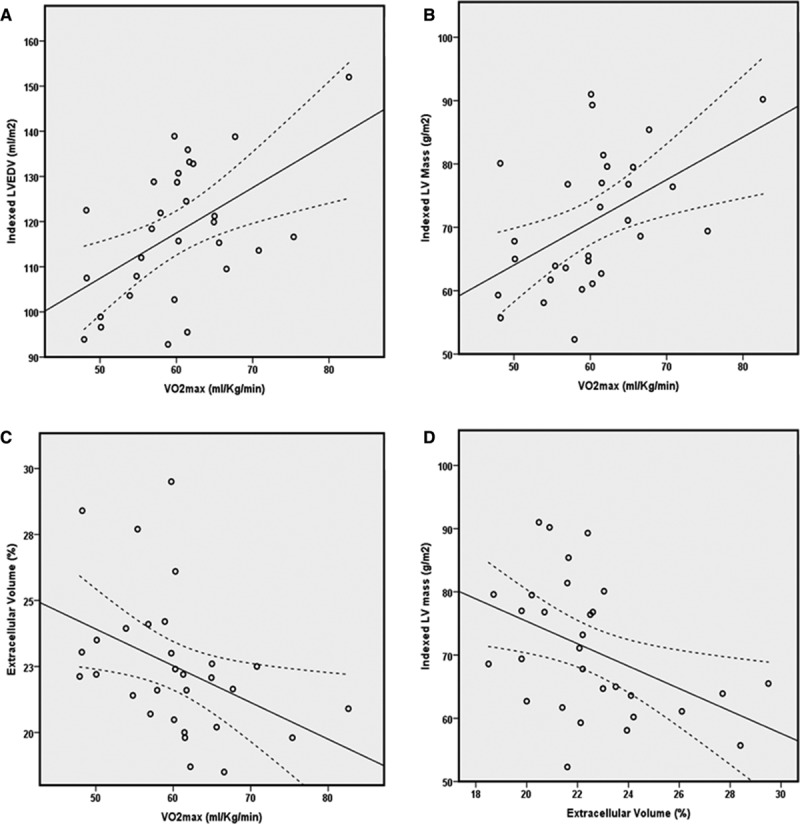
Relationship between exercise capacity and left ventricular (LV) remodeling and remodeling and tissue composition (R and P values determined with Spearman correlation coefficient for all). A, LVEDVi and VO2max, r=0.45, P=0.01; B, LVMi and VO2max, r=0.55, P=0.002; C, extracellular volume and VO2max, r=−0.53, P=0.003; D, significant inverse relationship between extracellular volume (ECV) and indexed LV mass (r=−0.44, P=0.02). LVEDVi indicates LV end-diastolic volume index; and LVMi, left ventricular mass index.
Discussion
This study shows for the first time that LV hypertrophy in AH occurs as a consequence of differences in the relative composition of myocardium and that athletic hypertrophy is driven by an expansion of the cellular compartment with a relative decrease of the extracellular compartment. Furthermore, we have provided evidence of a potential relationship between increasing fitness and expansion of the cellular compartment. This finding provides novel insight into the physiological change underpinning the poorly understood phenomenon of athletic LVH. We have further demonstrated that the extent of cellular volume expansion is linearly related to aerobic capacity in this cohort of endurance athletes.
Mechanism of Myocardial Adaption and Remodeling
Cardiovascular adaptation occurs in response to hemodynamic challenge and prolonged endurance training and even in previously untrained individuals leads to marked changes in cardiac geometry.18 It has been shown in previous longitudinal studies that early adaption is characterized by an increase in LV mass and as a consequence change in LV mass:volume ratio, later followed by LV dilation, normalization of the ratio, and eccentric hypertrophy.18 LV geometry and particularly LV mass have previously been shown to be related to 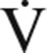 O2max.19–21
O2max.19–21
CMR has provided new insights into the mechanisms that underpin cardiac remodeling with exercise training. The multiparametric assessment of the human heart and high reproducibility provided by the technique allow both accurate functional and anatomic assessment.22–24 Native T1 and ECV measurement are robust and validated techniques for tissue characterization,25 and the correlation of ECV in particular with histological tissue specimens is excellent,22,23,26 though has not previously been applied to athletes with known 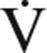 O2max. ECV increases in myocardial fibrosis, edema, and expansion of the extracellular space, with subsequent relative decrease in myocyte mass.27 Conversely, an expanded cellular mass reduces ECV as the distribution volume for conventional extracellular contrast agents is reduced.
O2max. ECV increases in myocardial fibrosis, edema, and expansion of the extracellular space, with subsequent relative decrease in myocyte mass.27 Conversely, an expanded cellular mass reduces ECV as the distribution volume for conventional extracellular contrast agents is reduced.
In this study, we have demonstrated that indexed LV mass is correlated with aerobic capacity and that indexed LV mass and ECV are inversely related. Participants with a higher 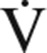 O2max had a similar extracellular mass as those with a lower
O2max had a similar extracellular mass as those with a lower 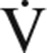 O2max, but a significantly higher intracellular mass.
O2max, but a significantly higher intracellular mass.
These data allow postulation of the mechanism underlying the development of AH that is consistent with known concepts: After rapid division in fetal life, cardiac myocytes are terminally differentiated shortly after delivery. As a result, any increase in overall myocardial mass is secondary to myocyte hypertrophy or extracellular matrix expansion rather than cell hyperplasia.28 CMR allows in vivo quantification of the 2 tissue compartments, and our data show that in AH, the overall extracellular compartment volume is similar to previously reported normal ranges,29 although there is marked cellular expansion.
Important differential diagnoses of AH include dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM), which may both display increased LV mass or left ventricular end-diastolic volume. The role of CMR in the detection of these myocardial diseases is established, and both DCM and HCM display characteristic morphological abnormalities on CMR. At a microscopic level, HCM is characterized by myocyte disarray and interstitial fibrosis30 and, thus, higher ECV. LGE CMR allows detection of focal replacement fibrosis and infarction in both HCM31 and DCM32; however, diffuse processes are poorly detected with this technique. T1 mapping and ECV measurement have shown expansion of the extracellular space in HCM, occurring in both hypertrophic23,29 and nonhypertrophic segments, as well as in DCM.33,34 Our observation that hypertrophy in AH is cellular without, or with relatively less, ECV expansion suggests that T1 mapping CMR may be used to distinguish between athletic LV adaptation and pathological hypertrophy or remodeling.
Both side-by-side sarcomere addition in concentric remodeling and end-to-end addition in eccentric remodeling result in myocardial hypertrophy,35 with an increase of muscle mass without increase in myocyte number.36 In this study, conducted without tissue biopsy, we are unable to determine the intracellular sarcomere arrangement; however, other CMR techniques that allow myocyte size to be quantified, as investigated by Coelho-Filho et al,37 may allow this to be determined in the future.
LGE and Myocardial Scar
LGE has previously been demonstrated in ≤13% of elite38 and 50% of veteran athletes.39 In this cohort of athletes, LGE was seen in only 3% (n=1). This difference may be as a consequence of the relatively young age of athletes studied.
Remodeling and Relationship With Performance
The relationship between aerobic capacity and LV remodeling is known.19,20 In this study, we have demonstrated that left ventricular end-diastolic volume index and left ventricular mass index correlate with 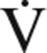 O2max, confirming that the degree of remodeling is related to fitness, and our data are consistent with the literature.
O2max, confirming that the degree of remodeling is related to fitness, and our data are consistent with the literature.
Limitations
There was relatively little diversity in the range of athletic discipline pursued; however, this has previously been shown not to affect the phenotype of cardiac remodeling.40 Native T1 differed significantly between athletes and controls, but not athletic groups. Native T1 is increased in myocardial edema and inflammation, but also in increased extracelluar volume; consequently, these findings are consistent with ECV in this study. The reason for a lack of difference between athletic groups is not clear, however, and merits further study. Furthermore, the assumptions made regarding the mode of myocardial hypertrophy in AH in this study have not been validated by histological sample. We have not studied female athletes in this study in order to have a homogenous study sample, but it is possible that athletic adaption differs compared with men. Finally, the participants were all of white origin, and this study should be repeated in a range of ethnic origins in an effort to understand AH in all athletes because risk and remodeling may differ between ethnicities.41
Conclusions
Cardiac remodeling and LVH in AH occurs as a consequence of greater myocyte mass, with an associated relative decrease of the extracellular space. This is unlike HCM or DCM, where CMR tissue characterization detects marked expansion of the extracellular space, in the presence or absence of LVH. Athletic remodeling, both on a macroscopic and microscopic level, is associated with the degree of aerobic capacity. ECV quantification with CMR may have a future role in differentiating AH from change secondary to cardiomyopathy, especially HCM, and warrants further study.
Acknowledgments
We are grateful for the support and assistance of the radiographers (Gavin Bainbridge, Margaret Saysell, Caroline Richmond, and Stephen Mhiribidi) assistants (Deborah Scarlett and Ann Heald) during this project.
Sources of Funding
Dr McDiarmid is funded by a British Heart Foundation (BHF) Project Grant (PG/14/10/30641). Dr Swoboda is funded by BHF Clinical Fellowship (FS/12/88/29474). Dr Plein is funded by BHF Senior Research Fellowship (FS/10/62/28409).
Disclosures
Drs Greenwood and Plein received a research grant from Philips Healthcare. The other authors report no conflicts.
Footnotes
Drs McDiarmid and Swoboda contributed equally to this work.
CLINICAL PERSPECTIVE
The need for reliable methods to differentiate physiological and pathological left ventricular hypertrophy are brought into focus by the rare but prominent cases of sudden death in elite athletes and the young. However, the myocardial changes that occur in an athlete’s heart are poorly understood, and current assessment is dependent on a constellation of findings, but may be limited when significant abnormalities are not present. Consequently, differentiating between physiological and pathological left ventricular hypertrophy can be difficult in borderline cases. Cardiac magnetic resonance provides highly reproducible assessment of cardiac structure and function. The developing field of cardiac magnetic resonance T1 mapping is now allowing insights into tissue composition with quantification of the extracellular space, not previously possible with contrast-enhanced cardiac magnetic resonance. In this study, we have used T1 mapping to demonstrate that increased left ventricular mass in physiological hypertrophy is predominantly because of expansion of the cellular, rather than the extracellular compartment. Furthermore, the degree of myocardial adaptation and cellular expansion is related to aerobic capacity, with fitter individuals displaying more expansion of the cellular compartment. Relatively lower extracellular volume is a crucial difference from many cardiomyopathies that are characterized by myocardial fibrosis and expansion of the extracellular space. The relatively lower extracellular volume in physiological left ventricular hypertrophy may provide a foundation for future studies to test whether extracellular volume quantification can differentiate the underlying nature of left ventricular hypertrophy.
References
- 1.Harmon KG, Drezner JA, Wilson MG, Sharma S. Incidence of sudden cardiac death in athletes: a state-of-the-art review. Br J Sports Med. 2014;48:1185–1192. doi: 10.1136/bjsports-2014-093872. doi: 10.1136/bjsports-2014-093872. [DOI] [PubMed] [Google Scholar]
- 2.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 3.Myerson SG, Bellenger NG, Pennell DJ. Assessment of left ventricular mass by cardiovascular magnetic resonance. Hypertension. 2002;39:750–755. doi: 10.1161/hy0302.104674. [DOI] [PubMed] [Google Scholar]
- 4.Sado DM, Flett AS, Banypersad SM, White SK, Maestrini V, Quarta G, Lachmann RH, Murphy E, Mehta A, Hughes DA, McKenna WJ, Taylor AM, Hausenloy DJ, Hawkins PN, Elliott PM, Moon JC. Cardiovascular magnetic resonance measurement of myocardial extracellular volume in health and disease. Heart. 2012;98:1436–1441. doi: 10.1136/heartjnl-2012-302346. doi: 10.1136/heartjnl-2012-302346. [DOI] [PubMed] [Google Scholar]
- 5.Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G, Fontana M, Maestrini V, Flett AS, Robson MD, Lachmann RH, Murphy E, Mehta A, Hughes D, Neubauer S, Elliott PM, Moon JC. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging. 2013;6:392–398. doi: 10.1161/CIRCIMAGING.112.000070. doi: 10.1161/CIRCIMAGING.112.000070. [DOI] [PubMed] [Google Scholar]
- 6.Neilan TG, Coelho-Filho OR, Shah RV, Abbasi SA, Heydari B, Watanabe E, Chen Y, Mandry D, Pierre-Mongeon F, Blankstein R, Kwong RY, Jerosch-Herold M. Myocardial extracellular volume fraction from T1 measurements in healthy volunteers and mice: relationship to aging and cardiac dimensions. JACC Cardiovasc Imaging. 2013;6:672–683. doi: 10.1016/j.jcmg.2012.09.020. doi: 10.1016/j.jcmg.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDiarmid AK, Swoboda PP, Erhayiem B, Ripley DP, Kidambi A, Broadbent DA, Higgins DM, Greenwood JP, Plein S. Single bolus versus split dose gadolinium administration in extra-cellular volume calculation at 3 Tesla. J Cardiovasc Magn Reson. 2015;17:6. doi: 10.1186/s12968-015-0112-6. doi: 10.1186/s12968-015-0112-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messroghli DR, Bainbridge GJ, Alfakih K, Jones TR, Plein S, Ridgway JP, Sivananthan MU. Assessment of regional left ventricular function: accuracy and reproducibility of positioning standard short-axis sections in cardiac MR imaging. Radiology. 2005;235:229–236. doi: 10.1148/radiol.2351040249. doi: 10.1148/radiol.2351040249. [DOI] [PubMed] [Google Scholar]
- 9.Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. doi: 10.1002/jmri.10262. doi: 10.1002/jmri.10262. [DOI] [PubMed] [Google Scholar]
- 10.Alfakih K, Plein S, Bloomer T, Jones T, Ridgway J, Sivananthan M. Comparison of right ventricular volume measurements between axial and short axis orientation using steady-state free precession magnetic resonance imaging. J Magn Reson Imaging. 2003;18:25–32. doi: 10.1002/jmri.10329. doi: 10.1002/jmri.10329. [DOI] [PubMed] [Google Scholar]
- 11.Rogers T, Dabir D, Mahmoud I, Voigt T, Schaeffter T, Nagel E, Puntmann VO. Standardization of T1 measurements with MOLLI in differentiation between health and disease–the ConSept study. J Cardiovasc Magn Reson. 2013;15:78. doi: 10.1186/1532-429X-15-78. doi: 10.1186/1532-429X-15-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossiter HB, Kowalchuk JM, Whipp BJ. A test to establish maximum O2 uptake despite no plateau in the O2 uptake response to ramp incremental exercise. J Appl Physiol (1985) 2006;100:764–770. doi: 10.1152/japplphysiol.00932.2005. doi: 10.1152/japplphysiol.00932.2005. [DOI] [PubMed] [Google Scholar]
- 13.Buchfuhrer MJ, Hansen JE, Robinson TE, Sue DY, Wasserman K, Whipp BJ. Optimizing the exercise protocol for cardiopulmonary assessment. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1558–1564. doi: 10.1152/jappl.1983.55.5.1558. [DOI] [PubMed] [Google Scholar]
- 14.Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol (1985) 1987;62:2003–2012. doi: 10.1152/jappl.1987.62.5.2003. [DOI] [PubMed] [Google Scholar]
- 15.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol (1985) 1986;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 16.Whipp BJ, Ward SA, Wasserman K. Respiratory markers of the anaerobic threshold. Adv Cardiol. 1986;35:47–64. doi: 10.1159/000413438. [DOI] [PubMed] [Google Scholar]
- 17.Bowen TS, Cannon DT, Begg G, Baliga V, Witte KK, Rossiter HB. A novel cardiopulmonary exercise test protocol and criterion to determine maximal oxygen uptake in chronic heart failure. J Appl Physiol (1985) 2012;113:451–458. doi: 10.1152/japplphysiol.01416.2011. doi: 10.1152/japplphysiol.01416.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arbab-Zadeh A, Perhonen M, Howden E, Peshock RM, Zhang R, Adams-Huet B, Haykowsky MJ, Levine BD. Cardiac remodeling in response to 1 year of intensive endurance training. Circulation. 2014;130:2152–2161. doi: 10.1161/CIRCULATIONAHA.114.010775. doi: 10.1161/CIRCULATIONAHA.114.010775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scharhag J, Schneider G, Urhausen A, Rochette V, Kramann B, Kindermann W. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. J Am Coll Cardiol. 2002;40:1856–1863. doi: 10.1016/s0735-1097(02)02478-6. [DOI] [PubMed] [Google Scholar]
- 20.Steding K, Engblom H, Buhre T, Carlsson M, Mosén H, Wohlfart B, Arheden H. Relation between cardiac dimensions and peak oxygen uptake. J Cardiovasc Magn Reson. 2010;12:8. doi: 10.1186/1532-429X-12-8. doi: 10.1186/1532-429X-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milliken MC, Stray-Gundersen J, Peshock RM, Katz J, Mitchell JH. Left ventricular mass as determined by magnetic resonance imaging in male endurance athletes. Am J Cardiol. 1988;62:301–305. doi: 10.1016/0002-9149(88)90228-7. [DOI] [PubMed] [Google Scholar]
- 22.Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- 23.White SK, Sado DM, Fontana M, Banypersad SM, Maestrini V, Flett AS, Piechnik SK, Robson MD, Hausenloy DJ, Sheikh AM, Hawkins PN, Moon JC. T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging. 2013;6:955–962. doi: 10.1016/j.jcmg.2013.01.011. doi: 10.1016/j.jcmg.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Lockie T, Nagel E, Redwood S, Plein S. Use of cardiovascular magnetic resonance imaging in acute coronary syndromes. Circulation. 2009;119:1671–1681. doi: 10.1161/CIRCULATIONAHA.108.816512. doi: 10.1161/CIRCULATIONAHA.108.816512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dabir D, Child N, Kalra A, Rogers T, Gebker R, Jabbour A, Plein S, Yu CY, Otton J, Kidambi A, McDiarmid A, Broadbent D, Higgins DM, Schnackenburg B, Foote L, Cummins C, Nagel E, Puntmann VO. Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2014;16:69. doi: 10.1186/s12968-014-0069-x. doi: 10.1186/s12968-014-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kehr E, Sono M, Chugh SS, Jerosch-Herold M. Gadolinium-enhanced magnetic resonance imaging for detection and quantification of fibrosis in human myocardium in vitro. Int J Cardiovasc Imaging. 2008;24:61–68. doi: 10.1007/s10554-007-9223-y. doi: 10.1007/s10554-007-9223-y. [DOI] [PubMed] [Google Scholar]
- 27.Treibel TA, White SK, Moon JC. Myocardial tissue characterization: histological and pathophysiological correlation. Curr Cardiovasc Imaging Rep. 2014;7:9254. doi: 10.1007/s12410-013-9254-9. doi: 10.1007/s12410-013-9254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation. 2000;102:470–479. doi: 10.1161/01.cir.102.4.470. [DOI] [PubMed] [Google Scholar]
- 29.Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E. Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:475–484. doi: 10.1016/j.jcmg.2012.08.019. doi: 10.1016/j.jcmg.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001;104:1380–1384. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 31.Maron MS. Contrast-enhanced CMR in HCM: what lies behind the bright light of LGE and why it now matters. JACC Cardiovasc Imaging. 2013;6:597–599. doi: 10.1016/j.jcmg.2012.10.028. doi: 10.1016/j.jcmg.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 32.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 33.Ho CY, Abbasi SA, Neilan TG, Shah RV, Chen Y, Heydari B, Cirino AL, Lakdawala NK, Orav EJ, González A, López B, Díez J, Jerosch-Herold M, Kwong RY. T1 measurements identify extracellular volume expansion in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:415–422. doi: 10.1161/CIRCIMAGING.112.000333. doi: 10.1161/CIRCIMAGING.112.000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.aus dem Siepen F, Buss SJ, Messroghli D, Andre F, Lossnitzer D, Seitz S, Keller M, Schnabel PA, Giannitsis E, Korosoglou G, Katus HA, Steen H. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur Heart J Cardiovasc Imaging. 2015;16:210–216. doi: 10.1093/ehjci/jeu183. doi: 10.1093/ehjci/jeu183. [DOI] [PubMed] [Google Scholar]
- 35.Wakatsuki T, Schlessinger J, Elson EL. The biochemical response of the heart to hypertension and exercise. Trends Biochem Sci. 2004;29:609–617. doi: 10.1016/j.tibs.2004.09.002. doi: 10.1016/j.tibs.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Hart G. Exercise-induced cardiac hypertrophy: a substrate for sudden death in athletes? Exp Physiol. 2003;88:639–644. doi: 10.1113/eph8802619. [DOI] [PubMed] [Google Scholar]
- 37.Coelho-Filho OR, Shah RV, Mitchell R, Neilan TG, Moreno H, Jr, Simonson B, Kwong R, Rosenzweig A, Das S, Jerosch-Herold M. Quantification of cardiomyocyte hypertrophy by cardiac magnetic resonance: implications for early cardiac remodeling. Circulation. 2013;128:1225–1233. doi: 10.1161/CIRCULATIONAHA.112.000438. doi: 10.1161/CIRCULATIONAHA.112.000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbüchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998–1006. doi: 10.1093/eurheartj/ehr397. doi: 10.1093/eurheartj/ehr397. [DOI] [PubMed] [Google Scholar]
- 39.Wilson M, O’Hanlon R, Prasad S, Deighan A, Macmillan P, Oxborough D, Godfrey R, Smith G, Maceira A, Sharma S, George K, Whyte G. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J Appl Physiol (1985) 2011;110:1622–1626. doi: 10.1152/japplphysiol.01280.2010. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Utomi V, Oxborough D, Ashley E, Lord R, Fletcher S, Stembridge M, Shave R, Hoffman MD, Whyte G, Somauroo J, Sharma S, George K. Predominance of normal left ventricular geometry in the male ‘athlete’s heart’. Heart. 2014;100:1264–1271. doi: 10.1136/heartjnl-2014-305904. doi: 10.1136/heartjnl-2014-305904. [DOI] [PubMed] [Google Scholar]
- 41.Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123:1594–1600. doi: 10.1161/CIRCULATIONAHA.110.004622. doi: 10.1161/CIRCULATIONAHA.110.004622. [DOI] [PubMed] [Google Scholar]


