Abstract
The classic BCR-ABL-negative myeloproliferative neoplasms (MPNs), a form of chronic malignant hemopathies, have been classified into polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). ET and PMF are two similar disorders in their pathogenesis, which is marked by a key role of the megakaryocyte (MK) lineage. Whereas ET is characterized by MK proliferation, PMF is also associated with aberrant MK differentiation (myelodysplasia), leading to the release of cytokines in the marrow environment, which causes the development of myelofibrosis. Thus, PMF is associated with both myeloproliferation and different levels of myelodysplastic features. MPNs are mostly driven by mutated genes called MPN drivers, which abnormally activate the cytokine receptor/JAK2 pathway and their downstream effectors. The recent discovery of CALR mutations has closed a gap in our knowledge and has shown that this mutated endoplasmic reticulum chaperone activates the thrombopoietin receptor MPL and JAK2. These genetic studies have shown that there are two main types of MPNs: JAK2V617F-MPNs, including ET, PV, and PMF, and the MPL-/CALR-MPNs, which include only ET and PMF. These MPN driver mutations are associated with additional mutations in genes involved in epigenetics, splicing, and signaling, which can precede or follow the acquisition of MPN driver mutations. They are involved in clonal expansion or phenotypic changes or both, leading to myelofibrosis or leukemic transformation or both. Only a few patients with ET exhibit mutations in non-MPN drivers, whereas the great majority of patients with PMF harbor one or several mutations in these genes. However, the entire pathogenesis of ET and PMF may also depend on other factors, such as the patient’s constitutional genetics, the bone marrow microenvironment, the inflammatory response, and age. Recent advances allowed a better stratification of these diseases and new therapeutic approaches with the development of JAK2 inhibitors.
Keywords: myelofibrosis, thrombocythemia, Myeloprolifarative neoplasms
Introduction
Myeloproliferative disorders are characterized by excess proliferation of progenitors belonging to the myeloid lineages (myeloproliferation), leading to an excess of mature functional blood cells 1. They are all clonal disorders of the hematopoietic system deriving from the transformation of a hematopoietic stem cell (HSC). Among the spectrum of myeloid malignancies they lie at one extreme, characterized only in principle by myeloproliferation (without differentiation defects), in contrast to myelodysplastic syndrome (MDS) (predominant differentiation defects) and acute myeloid leukemia (AML) (blockage in differentiation). The classic BCR-ABL-negative myeloproliferative neoplasms (MPNs) have been classified into three entities: polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF). These diseases have common complications: thrombosis or, more rarely, hemorrhages and leukemic transformation. ET is essentially a disorder of the megakaryocyte (MK) lineage with an excess platelet production 2. PMF is defined by the presence of bone marrow fibrosis (excess of collagen fibers) 3. This is also mainly a disorder of the MK/platelet lineage but is also associated with granulocytic proliferation. The typical forms of PV, ET, and PMF are quite different clinically and have different prognosis. ET and PV can progress to secondary myelofibrosis. Certain ET cases are associated with an erythroid hyperplasia and can progress to a true PV or may remain a form “fruste” of PV. Furthermore, boundaries between ET and PMF are not well standardized. A fourth entity has been described, pre-PMF (or early PMF or prefibrotic myelofibrosis), which corresponds to an ET with a high probability of progression to myelofibrosis and a worse prognosis than classic ET 4.
The molecular pathogenesis of BCR-ABL-negative MPNs is now in large part understood because of recent advances in sequencing techniques, particularly with results derived from next-generation sequencing (NGS) techniques. Recently, the discovery of mutations in the calreticulin ( CALR) genes has closed a gap in the knowledge of the physiopathogenesis of these disorders, particularly for ET and myelofibrosis.
In this review, we will focus on the molecular pathogenesis of MPNs, particularly of ET and PMF. However, somatic acquired mutations cannot summarize the entire pathogenesis of these disorders and other factors such as the constitutional genetics, the bone marrow niche environment, the cytokine release, and the inflammatory response, as well as aging, play important roles in the heterogeneity of these disorders.
Discovery of the mutations in exon 9 of the CALR gene in ET and PMF reinforces the hypothesis that BCR-ABL-negative MPNs are driven by an abnormal activation of JAK2
In 2005, a major advance in the understanding of the pathogenesis was the discovery of the somatic acquired recurrent mutation JAK2V617F, which is associated with more than 70% of MPNs; namely 95% of PV, 50% of ET, and 60% of PMF 5– 8. The V617F mutation is located in the pseudokinase domain of JAK2. The V617F mutation appears to prevent the physiologic inhibition and also to directly activate the kinase domain of JAK2 9. JAK2V617F gain-of-function and the constitutive signaling at sufficient expression levels require cytokine receptors, particularly homodimeric type I receptors. The identification of the JAK2V617F mutation has been a cutting-edge discovery in the pathogenesis of MPNs. This has led to the implication of the cytokine receptor/JAK2/STAT5 signaling pathway in their pathologies and the subsequent discovery of other recurrent mutations in this pathway, such as JAK2 exon 12 in 2% of PV 10, and activating mutations in the thrombopoietin receptor MPL. These mutations located in exon 10 of MPL target the W515 residue, which plays a central role in preventing spontaneous activation of the receptor 11. When W515 is substituted by 17 other amino acids—most frequently, Leu and Lys—TpoR/MPL becomes constitutively active and oncogenic 12. These mutations are found only in ET and PMF, with frequencies of approximately 3% and 5–8% in ET and PMF, respectively 13. The somatic MPLS505N is a rare recurrent sporadic mutation in ET and PMF that in certain familial thrombocytosis cases is found in the germline 14. Finally, very rare somatic mutations in LNK, a negative regulator of JAK2 kinase activity, have been described in ET and PMF 15. JAK2V617F and MPL mutations are only very rarely found in the same patient sample and when both are present they are most of the time in different cells, suggesting that they belong to different clones or subclones.
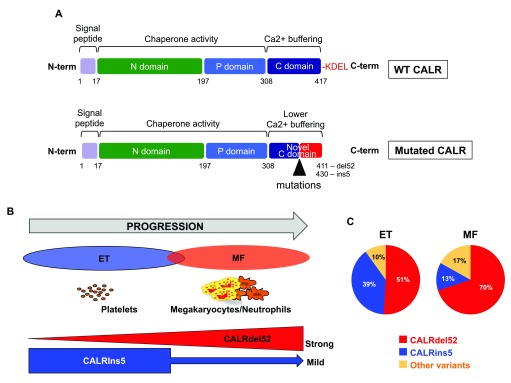
In 2013, it was evident that 55% of ET and 65–70% of PMF cases were linked to JAK2V617F and MPL exon 10 mutations. Activation of the cytokine receptor/JAK2 pathway was a common feature. In approximately 40% of ET and PMF, there were no recurrent mutations in genes involved in signaling. At the end of 2013, the teams of Kralovics 16 and Green 17 discovered mutations (indel) in the CALR gene in 25–30% of ET and PMF that were negative for JAK2 and MPL mutations. More than 50 mutations have been described, but all are in exon 9 and induce a +1 (−1+2) frameshift, leading to a new C-terminal peptide and the absence of the KDEL sequence, a retention sequence for the endoplasmic reticulum (ER) ( Figure 1). The C-terminus is almost identical among mutations with about 30 common amino acids. These new sequences completely change the charge of the molecule. The most frequent mutation, del52 (55% of the mutations), also called type 1, eliminates almost all the negative charges, whereas the ins5 (30%)—also called type 2—eliminates about half of these charges. According to these changes, the other mutations have been classified as type 1- or type 2-like. Physiologically, CALR is not a signaling molecule but an ER chaperone involved in the quality control of N-glycosylated protein and in calcium storage in the ER 18. However, the fact that the CALR mutations were also mutually exclusive with JAK2V617F and MPL mutations in ET and PMF, together with preliminary results showing that del52 mutations could activate STAT5, suggested that the CALR mutants were involved in signaling 16. Recent studies have largely reinforced this hypothesis by showing that CALR mutants activate the MPL receptor after binding to its N-glycosylated residues in the ER 19, 20. This activation required the positive charge of the C-terminus peptide, the lectin binding domain, and the extracellular N-linked sugars of MPL. There is evidence that the CALR mutant associated with MPL traffics to the cell surface in an immature N-glycosylated form 19. In this case, MPL activation can occur anywhere from the ER to the cell surface. Moreover, CALR mutants are secreted proteins, which may be able to activate other cells, especially monocytes, to secrete inflammatory cytokines 21. CALR mutants are not able to activate other cytokine receptors different from MPL—except granulocyte colony-stimulating factor receptor (G-CSF-R). However, this activation is weak and does not allow the autonomous growth of factor-dependent cell lines.
Figure 1. Calreticulin (CALR) and CALR mutation in essential thrombocythemia (ET) and myelofibrosis (MF).
( a) CALR protein structure. CALR includes different domains responsible for the two major activities (chaperone and calcium buffering). The mutations lead to altered C-terminal part with loss of KDEL (retrieval and retention domain in endoplasmic reticulum) and generation of a new tail with low calcium-buffering activity. ( b) Progression from ET to MF with CALR mutants. CALRdel52 induces ET always progressing to MF in mice in contrast to CALRins5. Thus, in vivo modeling of CALRdel52-induced pathologic effects induces a disorder characterized by a continuum between ET and MF. ( c) Pie chart of the different CALR mutations in patients with ET and MF.
Thus, it appears that there are two main types of BCR-ABL-negative MPNs. The first one is the JAK2V617F MPNs (∼70% of MPNs), which includes three disorders: ET, PV, and PMF; the second one consists of the CALR and MPL mutated MPNs (20% of MPNs), which usually includes only ET and PMF although CALR mutations have been described in very rare cases of PV associated with a thrombocytosis. The remaining MPNs are called triple-negative (10%). These appear to be heterogeneous disorders, but a large fraction are associated with increased JAK/STAT signaling 22. Certain triple-negative MPNs are related to atypical MPL or JAK2 mutations 23, 24. A fraction of the so-called triple-negative ET might not be MPNs, but polyclonal disorders, such as hereditary thrombocytosis with germline mutations. Furthermore, the triple-negative PMF, which is of poor prognosis, may not be bona fide MPNs, but more a myelodysplastic syndrome associated with myelofibrosis 25. This underscores the difficulties for classifying myeloid hematological malignancies, which might represent a spectrum of diseases with proliferation and differentiation defects at different levels.
Signaling mutations drive the MPN phenotype
One way to demonstrate that these mutations are really the MPN drivers is to create mouse models. Mutations in JAK2V617F, MPL, and CALR are capable of reproducing the MPN phenotype(s) in mice. JAK2V617F induces a myeloproliferative disorder—usually PV but also ET in some models 26– 29—which may progress to myelofibrosis. The unique models that have been presently described so far for MPLW515 and CALR mutations are bone marrow transplantations after retroviral transfer. In both cases the mice develop thrombocytosis, which progresses to myelofibrosis—quickly in the case of MPLW515L/A and more slowly for CALRdel52 11, 20.
Importantly, in all these models, mice do not develop true PMF, but a secondary myelofibrosis (post-PV or post-ET). Thus, myelofibrosis can be the natural evolution of ET, without requiring other additional genetic abnormalities. Similarly high TPO levels in mice can induce a very severe myelofibrosis 30, 31. Overall, an exaggerated stimulation of the MK lineage can lead to myelofibrosis. These results could suggest that PMF may require other events (genetic/environmental).
One major limitation of the present mouse models is that the disease originates from several hematopoietic stem cells, while human MPNs exhibit a clonal hematopoiesis originating from a single hematopoietic stem cell 32. Therefore, the first part of the human disease (how a single mutated HSC becomes predominant) is not studied in these models 32. Other factors, including oncogenic cooperation, may be necessary for clonal dominance (see below).
Subtle changes in the activation mechanisms of JAK2 among mutants may partially explain the different phenotypes of the MPNs
Although the different mutations induce the activation of JAK2, they do not lead to the same phenotype. For example, JAK2V617F can be associated with ET and PV, inducing hyperplasia of either MK or erythroid cells, depending on the conditions. One determining factor is clearly the number of JAK2V617F gene copies (heterozygous versus homozygous mutation) 28, 33. However, this is just one factor in determining MPN heterogeneity.
Another example is the CALR-mutated and JAK2V617F ET, which display different clinical and biological features, although in both cases the disease is related to the activation of the MPL/JAK2 pathway 20, 34. One obvious difference is related to the fact that JAK2V617F—in contrast to CALR—activates not only the MK cell line but also the erythroid and granulocytic lineages, explaining differences in the hematocrit and polymorphonuclear count. However, among the most marked differences are the higher level of thrombocytosis and the decreased frequency of thrombotic events in the CALR mutated ET 35, 36. The most striking difference concerns the allele frequency of the mutation: in ET, the JAK2V617F variant allele frequency is approximately 15% in granulocytes (30% of mutated cells) but is approximately 40% or more for mutated CALR (80% of the cells) 37. Therefore, a greater clonal advantage at the level of HSCs is conferred by mutated CALR versus JAK2V617F, even if both diseases are dependent on MPL. Subtle differences in signaling pathways downstream of MPL/JAK2 might also be involved. For example, CALR mutants moderately activate the PI3K/AKT pathway, and PI3K inhibitors are not able to synergize with JAK2 inhibitors, contrasting what was observed for JAK2V617F 19, 38, 39. The type of activated STAT could also play a role since MPL/JAK2 can activate STAT1, 2, 3, and 5, which may have markedly different effects on HSC and MK biology 40– 43.
Furthermore, among CALR mutated ET, CALRdel52 and CALRins5 may define two different subtypes of diseases characterized by different levels of thrombocytosis and evolution. CALRdel52 ET can progress to secondary myelofibrosis much more frequently than CALRins5 ET 44, with an important predominance of CALRdel52 in PMF ( Figure 1). In the mouse models, CALRdel52-induced thrombocytosis progresses to myelofibrosis, but this progression is rarely observed for CALRins5.
Again, such differences might reflect subtle differences in the activation of the MPL/JAK2 pathway or activation of new signaling pathways. Indeed, the CALRdel52 has nearly lost all its capacity to bind calcium in its C-terminal domain (low affinity, high capacity) in contrast to CALRins5. This may lead to a leak of calcium from the ER to the cytoplasm and a different signaling in MKs and in HSCs 44.
The somatic landscape of acquired mutations demonstrates that additional somatic mutations are present in MPNs but predominantly in PMF
Early studies on JAK2V617F MPNs have suggested that, in certain cases, JAK2V617F is not the initiating event but that it could be preceded by other mutations. With genome-wide approaches, it could be shown that some patients have TET2 mutations (∼15%) 45. Subsequently, mutations in ASXL1 mutations (10–15%) were found in PMF 46.
With the development of whole exome sequencing, it could be demonstrated that mutations in epigenetic regulators (such as TET2, DNMT3A, ASXL1, EZH2, and IDH1/IDH2) and in spliceosome components (such as SRSF2, U2AF1, and SF3B1) were present in BCR-ABL-negative MPNs harboring JAK2/ MPL/ CALR mutations 17, 47, 48. Other mutations were also directly associated with leukemic progression, such as p53, RUNX1, CBL, and deletion in IKAROS 49– 51. These mutations can be associated, and the most frequent co-mutations concern SRSF2 associated with TET2 or ASXL1 or IDH 52.
The additional mutations are mainly phenotypic modifiers discriminating between ET and PMF
In contrast to mutations in signaling genes (MPN driver genes), which are rare in other myeloid malignancies, the additional mutations are not specific to MPNs and are found with a higher frequency in MDS and in mixed MDS/MPN disorders, such as chronic myelomonocytic leukemia 53, 54.
Biological studies and mouse models showed that they may cooperate with MPN drivers to favor clonal dominance ( TET2 or DNMT3A), to modify disease phenotype, or to promote either progression to myelofibrosis or leukemic transformation ( ASXL1, IDH1/2, EZH2, and TP53).
Clonal dominance genes, such as TET2 or DNMT3A, are associated with all types of MPNs with low difference in frequency (∼12% in ET and 18% in PMF). However, all the other mutations are almost exclusively found in PMF 17, 55. In more than 80% of PMF, mutations of epigenetic regulators or spliceosome components are found, but they are identified in less than 25% of ET. Furthermore, in approximately 50% of PMF, two or more of these non-‘MPN driver’ genes are co-mutated. Moreover, CALR is the first mutation in nearly all cases and additional mutations are secondary in disease evolution 16, 17, 47. In contrast, JAK2V617F can be preceded by mutations such as in TET2, DNMT3A, and ASXL1, whereas the inverse can be also observed.
Two non-mutually exclusive explanations can be invoked: (1) CALR mutations have a much higher capacity to provide clonal dominance than JAK2V617F and may not require other associated genetic events for disease initiation. (2) JAK2V617F gives rise to MPNs, which occur approximately 10 years later than CALR mutated MPNs. The genes, which precede JAK2V617F occurrence, are associated with age-related clonal hematopoiesis 56, 57, JAK2V617F MPNs being secondary to aging. Indeed, the order of acquisition of mutations is important in the phenotype of the disease, particularly for TET2 and DNMT3A 58, 59. Moreover, when the JAK2V617F mutation is acquired on an age-related hematopoiesis, leukemia or myelodysplastic syndrome transformation may occur on the initial JAK2V617F-negative clone 60, 61. The fact that the number of acquired mutations allows a good discrimination between ET (one mutation in the MPN driver gene plus eventually another driver mutation) and PMF (one mutation in the MPN driver gene and mutations in one or several other driver genes) is in agreement with the physiopathology of myelofibrosis itself ( Figure 2). Indeed, there is evidence that myelofibrosis mainly results from a stromal reaction to the clonal hematopoiesis 62 as a consequence of the release of profibrotic cytokines 63, 64. MKs are the key cells involved in the myelofibrosis because they can release, in the bone marrow, large amounts of profibrotic (transforming growth factor β1 [TGF-β1], basic fibroblast growth factor, and platelet-derived growth factor), angiogenic (vascular endothelial growth factor) and pro-inflammatory (interleukin-1 [IL-1]) cytokines 62, 65. The role of MKs in myelofibrosis development explains the link between MK hyperplasia and myelofibrosis. Cytokines such as TGF-β1 are stored in specific MK granules called α-granules. However, in PMF, the most important phenomenon is the MK differentiation defect, which may result in defective α-granule storage and in the release of fibrotic cytokines. It explains why morphological features of MK dysplasia are criteria to distinguish ET from early PMF 66. Interestingly, most of the mutations in epigenetic regulators and spliceosome components lead to myeloid differentiation defects, especially in MKs 67, 68. Thus, PMF is not a pure MPN, exhibiting myeloproliferative and myelodysplastic features. The heterogeneity of the disease and its prognosis are dependent on the respective levels of each component, and the prognosis is poor when myelodysplastic features are predominant ( Figure 3). This explains why ultimately the prognosis of PMF is mainly dependent on the type and number of mutations in epigenetic regulators and spliceosome genes 55. Thus, it is expected that the new entity called pre-PMF or prefibrotic myelofibrosis will have a different pattern of acquired mutations from the classic ET, particularly with the presence of mutations in non-MPN driver genes. Otherwise, factors that regulate MPN phenotype and progression (other than acquired somatic mutations) should be identified.
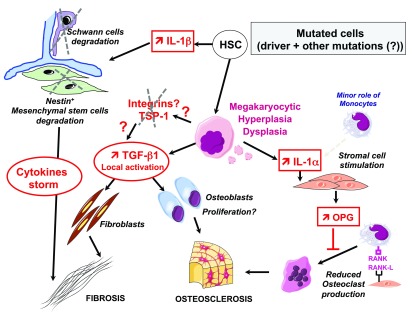
Figure 2. Role of microenvironment in the development of myelofibrosis.
Mutated dysplastic megakaryocytes (MKs) are responsible for the myelofibrosis and osteoclerosis by inducing the release of (i) non-activated transforming growth factor β1 (TGFβ1), which is activated in the bone marrow environment by a so far uncharacterized mechanism, possibly via integrins and matrix such as fibronectin and thrombospondin (TSP). Fibrosis begins around MKs associated with the proliferation of fibroblasts and eventually osteoblasts, (ii) interleukin-1α (IL-1α) is released and induces osteoprotegerin (OPG) by t stromal cells, a decoy receptor that blocks osteoclast production. Mutated hematopoietic stem cells (HSCs) induce the increase in IL-1α and the subsequent degradation of Schwann cells and mesenchymal stem cells, leading to fibrosis and osteosclerosis through cytokine storm and providing a favorable environment for the hematopoietic clone.
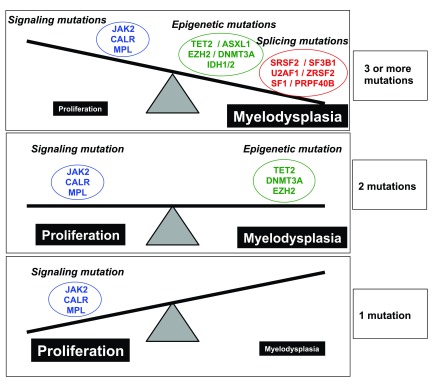
Figure 3. The type and the number of mutations determine the phenotype of the disease.
Boundaries between diseases are not easy to determine and could be dependent on the types or the number of mutations. Proliferation is driven mainly by signaling mutations ( JAK2, CALR, and MPL) while most of the mutations in epigenetic regulators and spliceosome components lead to differentiation defects. Thus, it can be considered that primary myelofibrosis (PMF) is not a pure myeloproliferative neoplasm (MPN) but a disorder with both myeloproliferative and myelodysplastic components. The heterogeneity of the disease and its prognosis are dependent on the respective levels of each component, and prognosis is poor if myelodysplastic features are predominant.
Factors other than acquired somatic mutations are involved in the pathogenesis of MPNs
It is clear that factors other than somatic mutations are involved in the pathogenesis of MPNs, particularly in clinical features. They include different factors.
Germline determinants
Sex-related differences are observed in the distribution of MPNs. ET is predominant in females and PMF in males. There are also differences in the sex ratio between CALR mutated and JAK2V617F ET. The former are slightly more prevalent in men and the latter in women 35. There is no clear explanation for these differences. Hormones could be one explanation. Estrogens can inhibit the JAK2V617F cancer stem cells 69. Iron metabolism could be another determinant, as it plays an important role in red blood cell and platelet production, with inverse effects.
Other genetic determinants predispose to MPNs. The first characterized was the 46/1 haplotype, which involves the JAK2 locus 70–72. This JAK2 haplotype induces a 3- to 5-fold increase in JAK2 V617F MPNs but not in CALR mutated MPNs 73. Other genetic determinants have recently been found, such as TERT, MECOM, and HBS1L/MYB. The SNPs in TERT, MECOM, and JAK2 (other than 46/1) appear to predispose to JAK2V617F-negative MPNs, whereas the HBS1L/MYB SNPs predispose only to JAK2V617F ET 74. An SNP located in the CALR gene could favor CALR mutations 75, but this result remains controversial 76. It is unknown whether other genetic determinants that regulate blood cell levels regulate the phenotype of MPNs.
The importance of these genetic determinants in the initiation and the progression of MPNs has recently been underscored in four families of the same geographical origin that develop hereditary forms of myeloid malignancies. The transmission is autosomal dominant and leads mainly to ET characterized by the same acquired driver mutations as sporadic cases, but with a very poor prognosis due to a rapid evolution to myelofibrosis and leukemia in more than one third of patients 77. A duplication of six genes, two of which are GSKIP and ATG2B, appears to play a key role in this predisposition, implying that the Wnt pathway and autophagy may play important roles in the pathogenesis of MPNs.
Inflammation
The JAK-STAT pathway is central for signaling by the majority of the inflammatory cytokines, which were linked to MPN progression. In a study of 30 cytokine levels in 127 patients with PMF, it was found that circulating IL-8, IL-2R, IL-12, and IL-15 levels independently hold prognostic value in PMF 78. Overall, many cytokines, including the above markers, G-CSF, and type I interferon (IFN), were increased, whereas IFN-γ was decreased 78. Examination of patient-reported outcome and cytokine profiling demonstrated clear associations between MPN symptoms, such as fatigue, abdominal complaints, and microvascular and constitutional symptoms, and high levels of cytokines, particularly IL-1, IL-6, IL-8, and tumor necrosis factor-α (TNF-α) 79.
From the pathophysiology standpoint, some pro-inflammatory cytokines or chemokines may be important by directly promoting an extramedullary hematopoiesis. In addition, by increasing reactive oxygen species (ROS) production, they may contribute to the dominance of the JAK2V617F clone and disease progression by inducing secondary mutations. A special case is represented by TNF-α. Clonal dominance in JAK2V617F-positive MPNs has been associated with TNF-α secretion and signaling 80. TNF-α was also suggested to impair the inhibitory effects of type I IFN on mutated MPN HSCs 81. On the other hand, TNF-α inhibition signaling in one patient with myelofibrosis was associated with leukemia progression 82. TNF-α also deregulates erythropoietin signaling, leading to anemia in AML and MDS 83, 84.
Anemia is also associated with PMF and influences treatment and iron metabolism. Increased levels of both hepcidin and ferritin predicted inferior survival in an independent manner from inflammatory cytokines 85.
Co-morbidities can also be coincident with or induced by MPNs. One could ask whether JAK2 inhibitors would impact co-morbidities, which could act on the MPN clone or on the other cells that participate in production and effects of inflammation 86. An example is STAT3 activation, which plays an important role in the inflammatory state associated with MPNs. However, when STAT3 is activated in hematopoietic cells from the clone, but not in the other hematopoietic and non-hematopoietic cells, it dampens the MPN phenotype, especially the thrombocytosis 42. Chronic inflammation is a driving force for premature atherosclerosis and development of secondary cancer in MPNs 81.
Bone marrow microenvironment: the hematopoietic niche
The anatomical location in which the HSCs reside, the hematopoietic niche, is key for HSC regulation and has been divided into two main compartments: (i) the endosteal niche near the endosteum; and (ii) the perivascular niche near the sinusoids. Many different types of cells compose the niche, mainly derived from mesenchymal stem cells (adipocytes, osteoblasts, and smooth muscle cells) of other origins such as Schwann cells, reticular cells, endothelial cells, and hematopoietic cells such as macrophages, osteoclasts, and MKs. MPN development can be potentially controlled by this bone marrow environment either directly through integrin interactions or indirectly via the production of various chemokines, cytokines, and signaling molecules. Alternatively, mutated HSCs can modify the niche to favor their development and to inhibit normal HSCs to induce clonal expansion. It has been shown that JAK2V617F HSCs secrete IL-1β, which induces the apoptotic death of mesenchymal and Schwann cells, suggesting that the normal but not JAK2V617F HSC is dependent of the niche resulting in a clonal expansion or that JAK2V617F HSCs need to damage the microenvironment to overcome its control. Thus, MPN has been considered a neuropathy that could be controlled by neuroprotective agents 87.
In myelofibrosis, the excessive release of fibrotic factors by the mutated MKs could activate mesenchymal cells, leading to myelofibrosis, but also could modify the properties of mesenchymal stromal cells 88 and their gene expression 89. Some other components of the niche may also belong to the malignant clone. Recently, it has been described that some endothelial cells may also belong to the clone, particularly in the Budd-Chiari syndrome in the liver and the spleen 90. Such mutated endothelial cells could potentially be deregulated to exacerbate cytokine or ROS production and to promote platelet adhesion and thrombosis.
Certain cytokines were shown to contribute to MPN development. FLT3L was found to be increased in samples from patients with PMF. It is produced both by HSCs and stromal cells and was shown to participate through the p38 pathway to the dysmegakaryopoiesis and the migration of CD34 + progenitors 91. IL-33 is overproduced in patients with MPN. It contributes to MPN development through stromal cells by promoting cytokine (granulocyte-macrophage colony-stimulating factor and IL-6) secretion via its receptor ST-2 and by amplifying hematopoietic progenitors 92.
Nevertheless, the role of the niche in the development of the disease remains incompletely understood. The question of whether an initial abnormality in the bone marrow niche can be the initial event in MPNs remains entirely open. Experimentally, engineered mesenchymal cells could induce hematological malignancies. Deletion of Dicer1 in mouse osteoprogenitors led to MDS and leukemia through the acquisition of genetic abnormalities 93. One of the best ways to study this “niche-induced disease” hypothesis will be to evaluate the role of identified genetic predisposing factors responsible for familial forms of MPNs on the microenvironment and HSCs, respectively, by using engineered mouse models.
Aging
MPNs are age-related diseases. Both stromal cells and HSCs are modified during aging. With age, HSCs become myeloid-biased with increased cycling/ROS levels and loss of functional capacities that could be important for disease development 94. Furthermore, these alterations can eventually favor clonal hematopoiesis with selection of mutated HSCs that acquired independence from stromal regulation. It is noteworthy that the most frequently involved somatic mutations ( DNMT3A, TET2, ASXL1, and JAK2) linked to aging are also implicated in myeloid malignant hematological malignancies, including MPNs.
Conclusion
The understanding of the MPN pathogenesis, including ET and PMF, has greatly progressed these 10 last years because of the discovery of the main MPN driver mutations. More than 90% of non- BCR-ABL MPNs are clearly driven by an abnormal JAK2 activation, especially the cytokine receptor/JAK2 pathways and their downstream effectors. Genomic studies demonstrated that PMF is a more advanced form of MPN, but with a molecular redundancy with ET. However, in contrast to classic ET and PV, PMF constantly includes one or several mutations in non-MPN driver genes, which are present also in MDS. This and the cytological features of the disease strongly suggest that PMF is a heterogeneous disorder associating phenotype/genotype features of MPN and MDS, with the latter being crucial for prognosis.
Several important questions remain to be solved:
-
-
What are the mechanisms of disease initiation? Indeed, JAK2V617F can be frequently acquired but rarely gives rise to a disease.
-
-
Why in ET and PMF do JAK2V617F and mutant CALR pathways give rise to close but different diseases, while they both activate MPL/JAK2? A similar question may arise for type 1 and type 2 CALR mutations.
-
-
Why can a mutation like JAK2V617F give rise to several diseases?
-
-
What are the molecular mechanisms of oncogenic cooperation between MPN driver mutations and other acquired somatic mutations? How does this oncogenic cooperation lead to leukemia?
In all cases, one major question remains to be solved: what are the respective roles of the genetic abnormalities, either germline or acquired (intrinsic factors), and of the environment (extrinsic factors) in disease initiation, phenotype, and progression?
Finally, from the therapeutic point of view, new approaches which will preferentially target an oncogenic JAK2 activation versus the physiological JAK2 role in cytokine signaling remain to be identified. In PMF with a high level of myelodysplastic features, this type of approach might not be sufficient and will require novel combined approaches.
Abbreviations
AML, acute myeloid leukemia; CALR, calreticulin; ER, endoplasmic reticulum; ET, essential thrombocythemia; G-CSF, granulocyte colony-stimulating factor; HSC, hematopoietic stem cell; IFN, interferon; IL, interleukin; MDS, myelodysplastic syndrome; MK, megakaryocyte; MPN, myeloproliferative neoplasm; NGS, next-generation sequencing; PMF, primary myelofibrosis; PV, polycythemia vera; ROS, reactive oxygen species; TGF-β1, transforming growth factor β1; TNF-α, tumor necrosis factor-α.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Alessandro M. Vannucchi, Department of Experimental and Clinical Medicine, University of Florence , Florence, Italy
Simon Mendez-Ferrer, Department of Haematology, University of Cambridge, Cambridge, UK
Funding Statement
This work was supported by grants from Ligue Nationale Contre le Cancer (“Equipe labellisée 2016”); Association pour la Recherche sur le Cancer (projet libre 2012 to IP); Agence Nationale de la Recherche, programme Jeunes Chercheuses et Jeunes Chercheurs (ANR-13-JVSV1-GERMPN-01 to IP); Institut National du Cancer (PLBIO2015 to IP); MPN research foundation; and Institut National de la Santé et de la Recherche Médicale (Inserm). The Laboratory of Excellence Globule Rouge-Excellence (IP and WV) is funded by the program “Investissements d’avenir”. SNC has received funding from the Ludwig Institute for Cancer Research, FRS-FNRS, Salus Sanguinis, Fondation contre le cancer, Project Action de Recherche Concertée of the Université catholique de Louvain ARC10/15-027, and the PAI program Belgian Medical Genetics Initiative.
[version 1; referees: 2 approved]
References
- 1. Spivak JL: The chronic myeloproliferative disorders: clonality and clinical heterogeneity. Semin Hematol. 2004;41(2 Suppl 3):1–5. 10.1053/j.seminhematol.2004.02.011 [DOI] [PubMed] [Google Scholar]
- 2. Tefferi A, Vardiman JW: Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;22(1):14–22. 10.1038/sj.leu.2404955 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Barosi G, Ambrosetti A, Finelli C, et al. : The Italian Consensus Conference on Diagnostic Criteria for Myelofibrosis with Myeloid Metaplasia. Br J Haematol. 1999;104(4):730–7. 10.1046/j.1365-2141.1999.01262.x [DOI] [PubMed] [Google Scholar]
- 4. Tefferi A, Barbui T: Personalized management of essential thrombocythemia-application of recent evidence to clinical practice. Leukemia. 2013;27(8):1617–20. 10.1038/leu.2013.99 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Baxter EJ, Scott LM, Campbell PJ, et al. : Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. 10.1016/S0140-6736(05)71142-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. James C, Ugo V, Le Couédic JP, et al. : A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434(7037):1144–8. 10.1038/nature03546 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Kralovics R, Passamonti F, Buser AS, et al. : A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352(17):1779–90. 10.1056/NEJMoa051113 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Levine RL, Wadleigh M, Cools J, et al. : Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell. 2005;7(4):387–97. 10.1016/j.ccr.2005.03.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Shan Y, Gnanasambandan K, Ungureanu D, et al. : Molecular basis for pseudokinase-dependent autoinhibition of JAK2 tyrosine kinase. Nat Struct Mol Biol. 2014;21(7):579–84. 10.1038/nsmb.2849 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Scott LM, Tong W, Levine RL, et al. : JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356(5):459–68. 10.1056/NEJMoa065202 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Pikman Y, Lee BH, Mercher T, et al. : MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3(7):e270. 10.1371/journal.pmed.0030270 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Defour JP, Chachoua I, Pecquet C, et al. : Oncogenic activation of MPL/thrombopoietin receptor by 17 mutations at W515: implications for myeloproliferative neoplasms. Leukemia. 2015. 10.1038/leu.2015.271 [DOI] [PubMed] [Google Scholar]
- 13. Pardanani AD, Levine RL, Lasho T, et al. : MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood. 2006;108(10):3472–6. 10.1182/blood-2006-04-018879 [DOI] [PubMed] [Google Scholar]
- 14. Ding J, Komatsu H, Wakita A, et al. : Familial essential thrombocythemia associated with a dominant-positive activating mutation of the c-MPL gene, which encodes for the receptor for thrombopoietin. Blood. 2004;103(11):4198–200. 10.1182/blood-2003-10-3471 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Oh ST, Simonds EF, Jones C, et al. : Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116(6):988–92. 10.1182/blood-2010-02-270108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klampfl T, Gisslinger H, Harutyunyan AS, et al. : Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369(25):2379–90. 10.1056/NEJMoa1311347 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Nangalia J, Massie CE, Baxter EJ, et al. : Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med. 2013;369(25):2391–405. 10.1056/NEJMoa1312542 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Michalak M, Groenendyk J, Szabo E, et al. : Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J. 2009;417(3):651–66. 10.1042/BJ20081847 [DOI] [PubMed] [Google Scholar]
- 19. Chachoua I, Pecquet C, El-Khoury M, et al. : Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood. 2016;127(10):1325–1335. 10.1182/blood-2015-11-681932 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Marty C, Pecquet C, Nivarthi H, et al. : Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood. 2016;127(10):1317–24. 10.1182/blood-2015-11-679571 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Garbati MR, Welgan CA, Landefeld SH, et al. : Mutant calreticulin-expressing cells induce monocyte hyperreactivity through a paracrine mechanism. Am J Hematol. 2016;91(2):211–9. 10.1002/ajh.24245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rampal R, Al-Shahrour F, Abdel-Wahab O, et al. : Integrated genomic analysis illustrates the central role of JAK-STAT pathway activation in myeloproliferative neoplasm pathogenesis. Blood. 2014;123(22):e123–33. 10.1182/blood-2014-02-554634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cabagnols X, Favale F, Pasquier F, et al. : Presence of atypical thrombopoietin receptor (MPL) mutations in triple-negative essential thrombocythemia patients. Blood. 2016;127(3):333–42. 10.1182/blood-2015-07-661983 [DOI] [PubMed] [Google Scholar]
- 24. Milosevic Feenstra JD, Nivarthi H, Gisslinger H, et al. : Whole-exome sequencing identifies novel MPL and JAK2 mutations in triple-negative myeloproliferative neoplasms. Blood. 2016;127(3):325–32. 10.1182/blood-2015-07-661835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sultan C, Sigaux F, Imbert M, et al. : Acute myelodysplasia with myelofibrosis: a report of eight cases. Br J Haematol. 1981;49(1):11–6. 10.1111/j.1365-2141.1981.tb07191.x [DOI] [PubMed] [Google Scholar]
- 26. Hasan S, Lacout C, Marty C, et al. : JAK2 V617F expression in mice amplifies early hematopoietic cells and gives them a competitive advantage that is hampered by IFNα. Blood. 2013;122(8):1464–77. 10.1182/blood-2013-04-498956 [DOI] [PubMed] [Google Scholar]
- 27. Lacout C, Pisani DF, Tulliez M, et al. : JAK2 V617F expression in murine hematopoietic cells leads to MPD mimicking human PV with secondary myelofibrosis. Blood. 2006;108(5):1652–60. 10.1182/blood-2006-02-002030 [DOI] [PubMed] [Google Scholar]
- 28. Li J, Kent DG, Godfrey AL, et al. : JAK2V617F homozygosity drives a phenotypic switch in myeloproliferative neoplasms, but is insufficient to sustain disease. Blood. 2014;123(20):3139–51. 10.1182/blood-2013-06-510222 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Tiedt R, Coers J, Ziegler S, et al. : Pronounced thrombocytosis in transgenic mice expressing reduced levels of Mpl in platelets and terminally differentiated megakaryocytes. Blood. 2009;113(8):1768–77. 10.1182/blood-2008-03-146084 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Villeval JL, Cohen-Solal K, Tulliez M, et al. : High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood. 1997;90(11):4369–83. [PubMed] [Google Scholar]
- 31. Yan XQ, Lacey D, Hill D, et al. : A model of myelofibrosis and osteosclerosis in mice induced by overexpressing thrombopoietin (mpl ligand): reversal of disease by bone marrow transplantation. Blood. 1996;88(2):402–9. [PubMed] [Google Scholar]
- 32. Lundberg P, Takizawa H, Kubovcakova L, et al. : Myeloproliferative neoplasms can be initiated from a single hematopoietic stem cell expressing JAK2-V617F. J Exp Med. 2014;211(11):2213–30. 10.1084/jem.20131371 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Dupont S, Massé A, James C, et al. : The JAK2 617V>F mutation triggers erythropoietin hypersensitivity and terminal erythroid amplification in primary cells from patients with polycythemia vera. Blood. 2007;110(3):1013–21. 10.1182/blood-2006-10-054940 [DOI] [PubMed] [Google Scholar]
- 34. Sangkhae V, Etheridge SL, Kaushansky K, et al. : The thrombopoietin receptor, MPL, is critical for development of a JAK2V 617F-induced myeloproliferative neoplasm. Blood. 2014;124(26):3956–63. 10.1182/blood-2014-07-587238 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Cabagnols X, Defour JP, Ugo V, et al. : Differential association of calreticulin type 1 and type 2 mutations with myelofibrosis and essential thrombocytemia: relevance for disease evolution. Leukemia. 2015;29(1):249–52. 10.1038/leu.2014.270 [DOI] [PubMed] [Google Scholar]
- 36. Tefferi A, Pardanani A: Genetics: CALR mutations and a new diagnostic algorithm for MPN. Nat Rev Clin Oncol. 2014;11(3):125–6. 10.1038/nrclinonc.2014.16 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Rumi E, Pietra D, Ferretti V, et al. : JAK2 or CALR mutation status defines subtypes of essential thrombocythemia with substantially different clinical course and outcomes. Blood. 2014;123(10):1544–51. 10.1182/blood-2013-11-539098 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Bartalucci N, Tozzi L, Bogani C, et al. : Co-targeting the PI3K/mTOR and JAK2 signalling pathways produces synergistic activity against myeloproliferative neoplasms. J Cell Mol Med. 2013;17(11):1385–96. 10.1111/jcmm.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Choong ML, Pecquet C, Pendharkar V, et al. : Combination treatment for myeloproliferative neoplasms using JAK and pan-class I PI3K inhibitors. J Cell Mol Med. 2013;17(11):1397–409. 10.1111/jcmm.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen E, Beer PA, Godfrey AL, et al. : Distinct clinical phenotypes associated with JAK2V617F reflect differential STAT1 signaling. Cancer Cell. 2010;18(5):524–35. 10.1016/j.ccr.2010.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Duek A, Lundberg P, Shimizu T, et al. : Loss of Stat1 decreases megakaryopoiesis and favors erythropoiesis in a JAK2-V617F-driven mouse model of MPNs. Blood. 2014;123(25):3943–50. 10.1182/blood-2013-07-514208 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Grisouard J, Shimizu T, Duek A, et al. : Deletion of Stat3 in hematopoietic cells enhances thrombocytosis and shortens survival in a JAK2-V617F mouse model of MPN. Blood. 2015;125(13):2131–40. 10.1182/blood-2014-08-594572 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Yan D, Hutchison RE, Mohi G: Critical requirement for Stat5 in a mouse model of polycythemia vera. Blood. 2012;119(15):3539–49. 10.1182/blood-2011-03-345215 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Pietra D, Rumi E, Ferretti VV, et al. : Differential clinical effects of different mutation subtypes in CALR-mutant myeloproliferative neoplasms. Leukemia. 2016;30(2):431–8. 10.1038/leu.2015.277 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Delhommeau F, Dupont S, Della Valle V, et al. : Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360(22):2289–301. 10.1056/NEJMoa0810069 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Gelsi-Boyer V, Trouplin V, Adélaïde J, et al. : Mutations of polycomb-associated gene ASXL1 in myelodysplastic syndromes and chronic myelomonocytic leukaemia. Br J Haematol. 2009;145(6):788–800. 10.1111/j.1365-2141.2009.07697.x [DOI] [PubMed] [Google Scholar]
- 47. Lundberg P, Karow A, Nienhold R, et al. : Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood. 2014;123(14):2220–8. 10.1182/blood-2013-11-537167 [DOI] [PubMed] [Google Scholar]
- 48. Yoshida K, Sanada M, Shiraishi Y, et al. : Frequent pathway mutations of splicing machinery in myelodysplasia. Nature. 2011;478(7367):64–9. 10.1038/nature10496 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Abdel-Wahab O, Manshouri T, Patel J, et al. : Genetic analysis of transforming events that convert chronic myeloproliferative neoplasms to leukemias. Cancer Res. 2010;70(2):447–52. 10.1158/0008-5472.CAN-09-3783 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Harutyunyan A, Klampfl T, Cazzola M, et al. : p53 lesions in leukemic transformation. N Engl J Med. 2011;364(5):488–90. 10.1056/NEJMc1012718 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Klampfl T, Harutyunyan A, Berg T, et al. : Genome integrity of myeloproliferative neoplasms in chronic phase and during disease progression. Blood. 2011;118(1):167–76. 10.1182/blood-2011-01-331678 [DOI] [PubMed] [Google Scholar]
- 52. Zhang SJ, Rampal R, Manshouri T, et al. : Genetic analysis of patients with leukemic transformation of myeloproliferative neoplasms shows recurrent SRSF2 mutations that are associated with adverse outcome. Blood. 2012;119(19):4480–5. 10.1182/blood-2011-11-390252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Itzykson R, Kosmider O, Renneville A, et al. : Prognostic score including gene mutations in chronic myelomonocytic leukemia. J Clin Oncol. 2013;31(19):2428–36. 10.1200/JCO.2012.47.3314 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Kosmider O, Gelsi-Boyer V, Cheok M, et al. : TET2 mutation is an independent favorable prognostic factor in myelodysplastic syndromes (MDSs). Blood. 2009;114(15):3285–91. 10.1182/blood-2009-04-215814 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Vannucchi AM, Lasho TL, Guglielmelli P, et al. : Mutations and prognosis in primary myelofibrosis. Leukemia. 2013;27(9):1861–9. 10.1038/leu.2013.119 [DOI] [PubMed] [Google Scholar]
- 56. Jaiswal S, Fontanillas P, Flannick J, et al. : Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371(26):2488–98. 10.1056/NEJMoa1408617 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Xie M, Lu C, Wang J, et al. : Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20(12):1472–8. 10.1038/nm.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Nangalia J, Nice FL, Wedge DC, et al. : DNMT3A mutations occur early or late in patients with myeloproliferative neoplasms and mutation order influences phenotype. Haematologica. 2015;100(11):e438–42. 10.3324/haematol.2015.129510 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Ortmann CA, Kent DG, Nangalia J, et al. : Effect of mutation order on myeloproliferative neoplasms. N Engl J Med. 2015;372(7):601–12. 10.1056/NEJMoa1412098 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Beer PA, Delhommeau F, LeCouédic JP, et al. : Two routes to leukemic transformation after a JAK2 mutation-positive myeloproliferative neoplasm. Blood. 2010;115(14):2891–900. 10.1182/blood-2009-08-236596 [DOI] [PubMed] [Google Scholar]
- 61. Theocharides A, Boissinot M, Girodon F, et al. : Leukemic blasts in transformed JAK2-V617F-positive myeloproliferative disorders are frequently negative for the JAK2-V617F mutation. Blood. 2007;110(1):375–9. 10.1182/blood-2006-12-062125 [DOI] [PubMed] [Google Scholar]
- 62. Castro-Malaspina H, Rabellino EM, Yen A, et al. : Human megakaryocyte stimulation of proliferation of bone marrow fibroblasts. Blood. 1981;57(4):781–7. [PubMed] [Google Scholar]
- 63. Wagner-Ballon O, Chagraoui H, Prina E, et al. : Monocyte/macrophage dysfunctions do not impair the promotion of myelofibrosis by high levels of thrombopoietin. J Immunol. 2006;176(11):6425–33. 10.4049/jimmunol.176.11.6425 [DOI] [PubMed] [Google Scholar]
- 64. Chagraoui H, Komura E, Tulliez M, et al. : Prominent role of TGF-beta 1 in thrombopoietin-induced myelofibrosis in mice. Blood. 2002;100(10):3495–503. 10.1182/blood-2002-04-1133 [DOI] [PubMed] [Google Scholar]
- 65. Le Bousse-Kerdilès MC, Chevillard S, Charpentier A, et al. : Differential expression of transforming growth factor-beta, basic fibroblast growth factor, and their receptors in CD34+ hematopoietic progenitor cells from patients with myelofibrosis and myeloid metaplasia. Blood. 1996;88(12):4534–46. [PubMed] [Google Scholar]
- 66. Barosi G: Essential thrombocythemia vs. early/prefibrotic myelofibrosis: why does it matter. Best Pract Res Clin Haematol. 2014;27(2):129–40. 10.1016/j.beha.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 67. Quivoron C, Couronné L, Della Valle V, et al. : TET2 inactivation results in pleiotropic hematopoietic abnormalities in mouse and is a recurrent event during human lymphomagenesis. Cancer Cell. 2011;20(1):25–38. 10.1016/j.ccr.2011.06.003 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 68. Tefferi A, Finke CM, Lasho TL, et al. : U2AF1 mutations in primary myelofibrosis are strongly associated with anemia and thrombocytopenia despite clustering with JAK2V617F and normal karyotype. Leukemia. 2014;28(2):431–3. 10.1038/leu.2013.286 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Sánchez-Aguilera A, Arranz L, Martín-Pérez D, et al. : Estrogen signaling selectively induces apoptosis of hematopoietic progenitors and myeloid neoplasms without harming steady-state hematopoiesis. Cell Stem Cell. 2014;15(6):791–804. 10.1016/j.stem.2014.11.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Campbell PJ: Somatic and germline genetics at the JAK2 locus. Nat Genet. 2009;41(4):385–6. 10.1038/ng0409-385 [DOI] [PubMed] [Google Scholar]
- 71. Jones AV, Chase A, Silver RT, et al. : JAK2 haplotype is a major risk factor for the development of myeloproliferative neoplasms. Nat Genet. 2009;41(4):446–9. 10.1038/ng.334 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Olcaydu D, Harutyunyan A, Jäger R, et al. : A common JAK2 haplotype confers susceptibility to myeloproliferative neoplasms. Nat Genet. 2009;41(4):450–4. 10.1038/ng.341 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. Soler G, Bernal-Vicente A, Antón AI, et al. : The JAK2 46/1 haplotype does not predispose to CALR-mutated myeloproliferative neoplasms. Ann Hematol. 2015;94(5):789–94. 10.1007/s00277-014-2266-y [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Tapper W, Jones AV, Kralovics R, et al. : Genetic variation at MECOM, TERT, JAK2 and HBS1L-MYB predisposes to myeloproliferative neoplasms. Nat Commun. 2015;6:6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Harutyunyan AS, Jäger R, Chen D, et al. : Allelic imbalance in CALR somatic mutagenesis. Leukemia. 2015;29(6):1431–5. 10.1038/leu.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Eder-Azanza L, Evans P, Wickham C, et al. : Constitutional genetic association with CALR mutations? Leukemia. 2015;29(12):2410–1. 10.1038/leu.2015.186 [DOI] [PubMed] [Google Scholar]
- 77. Saliba J, Saint-Martin C, Di Stefano A, et al. : Germline duplication of ATG2B and GSKIP predisposes to familial myeloid malignancies. Nat Genet. 2015;47(10):1131–40. 10.1038/ng.3380 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Tefferi A, Vaidya R, Caramazza D, et al. : Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29(10):1356–63. 10.1200/JCO.2010.32.9490 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 79. Geyer HL, Dueck AC, Scherber RM, et al. : Impact of Inflammation on Myeloproliferative Neoplasm Symptom Development. Mediators Inflamm. 2015;2015: 284706. 10.1155/2015/284706 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Fleischman AG, Aichberger KJ, Luty SB, et al. : TNFα facilitates clonal expansion of JAK2 V617F positive cells in myeloproliferative neoplasms. Blood. 2011;118(24):6392–8. 10.1182/blood-2011-04-348144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hasselbalch HC: Perspectives on the impact of JAK-inhibitor therapy upon inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Expert Rev Hematol. 2014;7(2):203–16. 10.1586/17474086.2013.876356 [DOI] [PubMed] [Google Scholar]
- 82. Ferrer-Marín F, Amigo ML, Vicente V: Leukaemic transformation in patients with haematological disease receiving tumour necrosis factor inhibitors. Clin Drug Investig. 2012;32(6):423–6. 10.2165/11599850-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 83. Kurzrock R: The role of cytokines in cancer-related fatigue. Cancer. 2001;92(6 Suppl):1684–8. [DOI] [PubMed] [Google Scholar]
- 84. Meyers CA, Albitar M, Estey E: Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104(4):788–93. 10.1002/cncr.21234 [DOI] [PubMed] [Google Scholar]
- 85. Pardanani A, Finke C, Abdelrahman RA, et al. : Associations and prognostic interactions between circulating levels of hepcidin, ferritin and inflammatory cytokines in primary myelofibrosis. Am J Hematol. 2013;88(4):312–6. 10.1002/ajh.23406 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 86. Kleppe M, Kwak M, Koppikar P, et al. : JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response. Cancer Discov. 2015;5(3):316–31. 10.1158/2159-8290.CD-14-0736 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Arranz L, Sánchez-Aguilera A, Martín-Pérez D, et al. : Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512(7512):78–81. 10.1038/nature13383 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Martinaud C, Desterke C, Konopacki J, et al. : Osteogenic Potential of Mesenchymal Stromal Cells Contributes to Primary Myelofibrosis. Cancer Res. 2015;75(22):4753–65. 10.1158/0008-5472.CAN-14-3696 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Martinaud C, Desterke C, Konopacki J, et al. : Transcriptome analysis of bone marrow mesenchymal stromal cells from patients with primary myelofibrosis. Genom Data. 2015;5:1–2. 10.1016/j.gdata.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 90. Sozer S, Fiel MI, Schiano T, et al. : The presence of JAK2V617F mutation in the liver endothelial cells of patients with Budd-Chiari syndrome. Blood. 2009;113(21):5246–9. 10.1182/blood-2008-11-191544 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 91. Desterke C, Bilhou-Nabéra C, Guerton B, et al. : FLT3-mediated p38-MAPK activation participates in the control of megakaryopoiesis in primary myelofibrosis. Cancer Res. 2011;71(8):2901–15. 10.1158/0008-5472.CAN-10-1731 [DOI] [PubMed] [Google Scholar]
- 92. Mager LF, Riether C, Schürch CM, et al. : IL-33 signaling contributes to the pathogenesis of myeloproliferative neoplasms. J Clin Invest. 2015;125(7):2579–91. 10.1172/JCI77347 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 93. Raaijmakers MH, Mukherjee S, Guo S, et al. : Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–7. 10.1038/nature08851 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 94. Marty C, Lacout C, Droin N, et al. : A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression. Leukemia. 2013;27(11):2187–95. 10.1038/leu.2013.102 [DOI] [PubMed] [Google Scholar]