Abstract
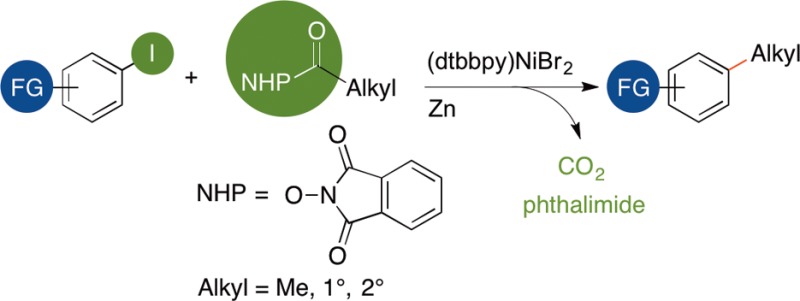
A new method for the decarboxylative coupling of alkyl N-hydroxyphthalimide esters (NHP esters) with aryl iodides is presented. In contrast to previous studies that form alkyl radicals from carboxylic acid derivatives, no photocatalyst, light, or arylmetal reagent is needed, only nickel and a reducing agent (Zn). Methyl, primary, and secondary alkyl groups can all be coupled in good yield (77% ave yield). One coupling with an acid chloride is also presented. Stoichiometric reactions of (dtbbpy)Ni(2-tolyl)I with an NHP ester show for the first time that arylnickel(II) complexes can directly react with NHP esters to form alkylated arenes.
The formation of Csp2–Csp3 bonds by cross-coupling has advanced rapidly in recent years,1 but remains less advanced than the synthesis of Csp2–Csp2 bonds. One challenge for these cross-coupling reactions is the lower availability of alkyl coupling reagents compared to aryl coupling reagents.2 As a potential solution, alkanoic acids are much more abundant than alkyl halides, making them attractive coupling partners. While the use of carboxylic acid derivatives as acyl equivalents in cross-coupling is well-known,3 few decarboxylative couplings of alkanoic acid derivatives have been reported.4
Recently, Doyle and MacMillan reported a method that forms alkylated arenes from α-heteroatom substituted carboxylates and aryl halides (Scheme 1).5,6 The process proceeds by photooxidation of the carboxylate to form an alkyl radical. We show here a complementary approach: coupling of NHP esters with aryl iodides to form alkylated arenes without the need for a cocatalyst or light (Scheme 1).
Scheme 1. Conversion of Alkanoic Acids to Radicals.
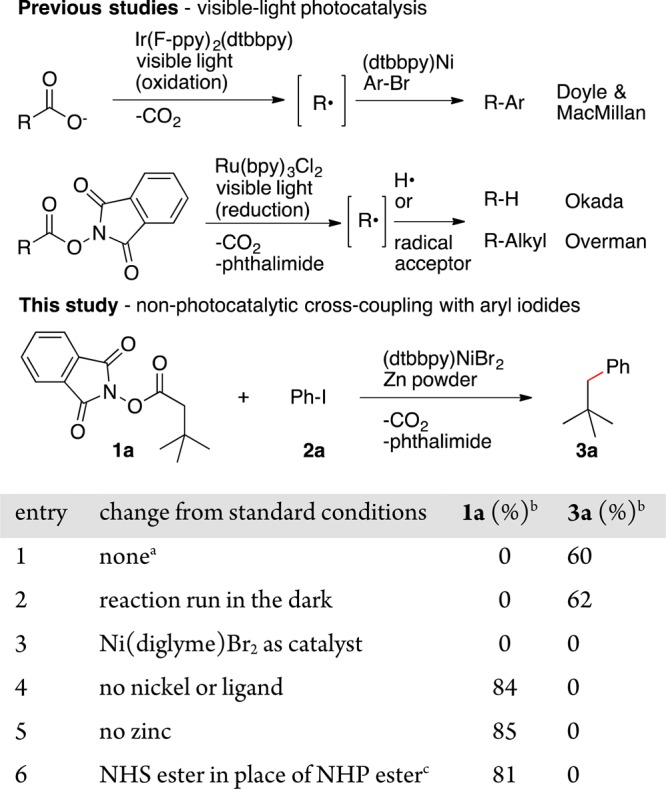
Reactions run with 10 mol % of (dtbbpy)NiBr2 and Zn0 (2 equiv) in DMA (0.8 M) for 20 h. dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine.
GC yield, uncorrected.
N-Hydroxysuccinimide ester of 4-phenyl butanoic acid was used in place of NHP ester 1a, along with 20 mol % DMAP.
In searching for a general method to utilize alkanoic acids in cross-electrophile coupling, we found the studies of Overman and Okada particularly interesting.7 Reduction of N-hydroxyphthalimide esters by a strong photogenerated reductant (Ru(bpy)3Cl2) formed alkyl radicals that could be used in a number of transformations. We had recently demonstrated that alkyl radicals generated from alkyl halides,8 benzyl mesylates,9 and epoxides10 could all be coupled with aryl halides under reducing conditions using a simple bipyridine nickel complex as a catalyst.11 By comparison of reduction potentials, it appeared to us that nickel-mediated reduction of an NHP ester to form an alkyl radical, CO2, and phthalimide should be possible without the need for photochemistry.12−15
Concurrent with our study, Baran found that NHP esters could be coupled with an excess of an arylzinc reagent.16 These two approaches are complementary: we use aryl iodides in place of arylzinc reagents, and while we can couple methyl, primary, and secondary radicals, the Baran chemistry uses arylzinc reagents and works with secondary radicals.
The adaptation of NHP esters to cross-electrophile coupling required very little adjustment of our previously published conditions (Scheme 1).8 Consistent with our hypothesis, nickel and zinc were required for activation of the NHP ester (entries 4 and 5), the reaction proceeded equally well in the dark (entries 1 and 2), and a less-easily reduced NHS ester did not react (entry 6).17
Reactions conducted without added ligand did not produce a coupled product, but did consume the NHP ester (Scheme 1, entry 3).18 We found that impure NHP esters resulted in lower yields19 and that currently aryl iodides are required for high yields. Reactions of less reactive substrates (Ar–Br, Ar–Cl) result in consumption of NHP ester but not aryl halide. Finally, for substrates that provided a moderate yield, a slight excess of the NHP ester usually provided a higher yield (Scheme 2).
Scheme 2. Coupling of Alkyl NHP Esters with ArI.
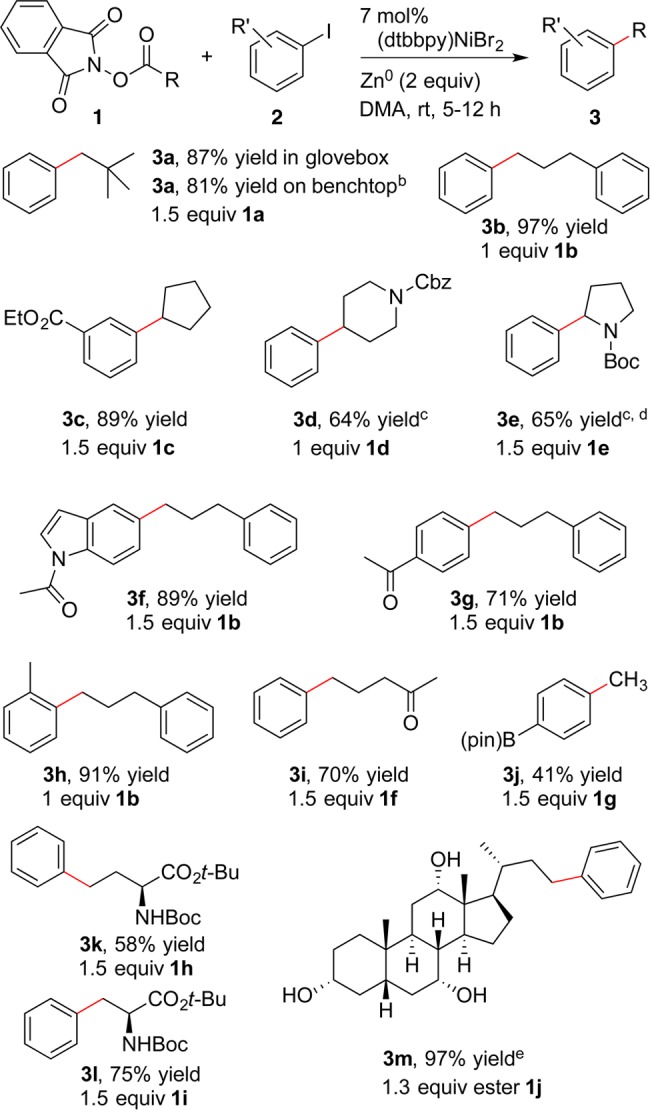
Reactions were run on 0.8 mmol scale in 1 mL of DMA. Yields are after isolation and purification. See Supporting Information.
Reaction was set up on the benchtop in a round-bottom flask.
12 mol % of catalyst was used on a 0.4 mmol scale reaction.
Yield of 3e adjusted to account for 4 wt % N-Boc pyrrolidine in sample.
Yield of 3m adjusted to account for 14 wt % of phthalimide and diisopropyl urea that we were unable to separate by chromatography.
Overall, NHP esters perform equal to or better than couplings with alkyl iodides and bromides.8 For example, a primary alkanoic ester provided near-quantitative yields (3b and 3f), even when the substrates were reacted in a 1:1 ratio. A more hindered neopentyl group could be coupled in a better yield (3a) than that reported using neopentyl iodide (38%).8a Although these reactions were set up in a glovebox for convenience, the chemistry could be run on preparative scale (4 mmol PhI) without the need for strict exclusion of moisture and air.20 Similarly, cyclopentyl (3c) and piperidine (3d) groups were coupled in yields competitive to the best reported with alkyl halides.10
While 2-halopyrrolidines are not easily accessible starting materials, the NHP ester of proline (1e) was coupled in good yield with iodobenzene to form 3e. This result compares favorably with similar couplings reported using Ni/Ir/photoredox5a and with arylzinc reagents.16
Electron-rich and -poor iodoarenes coupled efficiently (3f and 3g), and some steric hindrance on the iodoarene was tolerated (3h).21 Finally, functional-group compatibility is promising, tolerating ketones (3g and 3i), esters (3c), protected nitrogen (3d, 3e, 3f, 3k, 3l), a boronic acid pinacol ester (3j), and unprotected alcohols (3m).
Several of the substrates demonstrate the value of starting with alkanoic acids because the corresponding alkyl halides are not commercially available or fail to couple. For example, the methylation with O-acetyl N-hydroxyphthalimide represents the first cross-electrophile methylation of an aryl halide.22 Similarly, the NHP esters of aspartic acid and glutamic acid both coupled in high yield without racemization, providing a new route to valuable phenylalanine23 and homophenylalanine24 derivatives. Finally, unprotected cholic acid NHP ester could be coupled in high yield, even with three unprotected alcohols.25
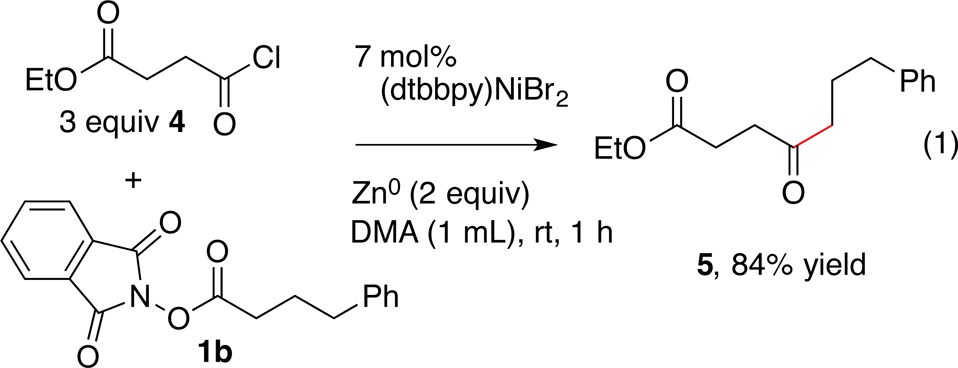
We could also extend this chemistry to the coupling of NHP ester 1b with acid chloride 4 to form dialkyl ketone 5 (eq 1),26 suggesting broad generality for NHP esters in cross-electrophile coupling reactions.
 |
1 |
Although we have not yet studied the reaction mechanism in detail, it is reasonable to assume that the NHP ester is reduced to form an alkyl radical, CO2, and phthalimide according to the accepted mechanism in the photoreduction chemistry.7 Consistent with this hypothesis, we observed the formation of phthalimide and gas in reactions. We had previously shown how alkyl radicals are a key part of the cross-electrophile coupling of aryl iodides with alkyl iodides.27,28 However, there were no reports of the stoichiometric reactivity of any organonickel complex with an NHP ester.
In order to test the reactivity of an organonickel complex with an NHP ester and its connection to the catalytic reactions, we compared the stoichiometric reactivity of (dtbbpy)NiII(2-tolyl)I (6) with catalytic reactions in the same solvent (eqs 2–4). The catalytic reaction in DMF proceeded in lower yield than the reaction in DMA due to the formation of 1,6-diphenylhexane (eq 2). The stoichiometric reaction of 6 with excess 1b formed cross-product 3h with or without any added reductant (eq 3 and eq 4). However, the reaction with added zinc formed the cross-product in excellent yield (eq 4). Importantly, these are the first known reactions of organonickel intermediates with redox active esters, a key step in both our chemistry and Baran’s arylzinc chemistry.16
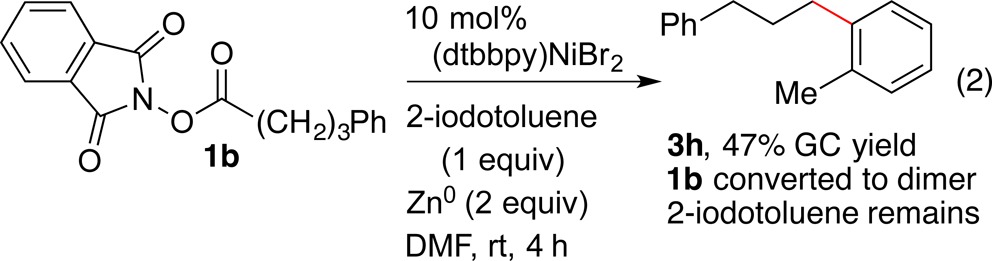 |
2 |
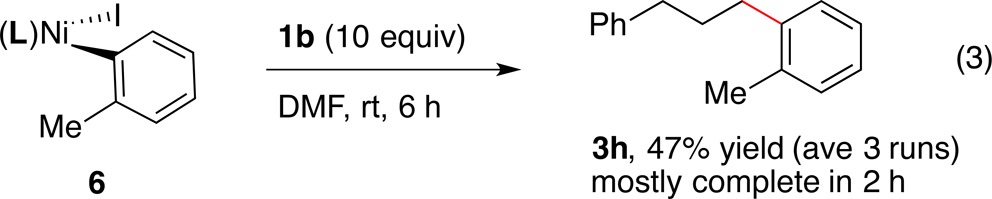 |
3 |
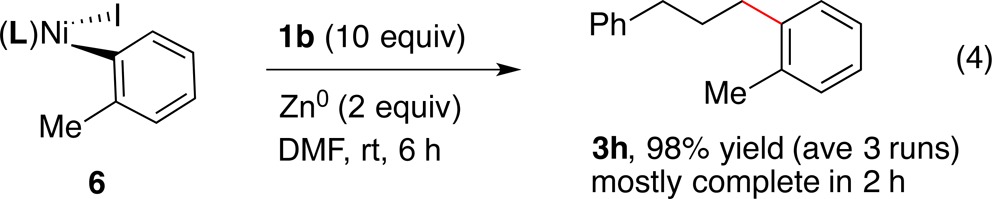 |
4 |
The formation of 3h in yields comparable with the catalytic reaction (eq 2) from reactions that did not contain iodotoluene or zinc (eq 3) argues against the intermediacy of arylzinc reagents, in contrast with Baran’s work.16 Further, we found that a typical aryl iodide, ethyl 3-iodobenzoate, does not directly react with zinc on the time scale of catalytic reactions (see Supporting Information).29
Our current hypothesis is that these reactions proceed by a radical-chain mechanism similar to that proposed for the coupling of aryl iodides with alkyl iodides.27 However, differences between these studies and our previous studies were observed. While the reaction without added zinc formed the product (eq 3), the presence of zinc resulted in improved yields (eq 4). The role of the zinc could be to initiate radical formation by reduction of 6 or 1b, but these results are not conclusive. Further study of the elementary steps is ongoing.
We also sought to identify the nickel product of these reactions, with only partial success. Reaction of isolated 6 with 1b in DMF-d7 at rt overnight resulted in consumption of the diamagnetic 6 and formation of new, paramagnetic species (eq 5). These new species are EPR silent, consistent with integer-spin complexes. We tentatively assign these species as a mixture of (dtbbpy)NiII(phthalimide)I, (dtbbpy)NiII(phthalimide)2, and (dtbbpy)NiIII2, in analogy to our previous results with alkyl iodides.27
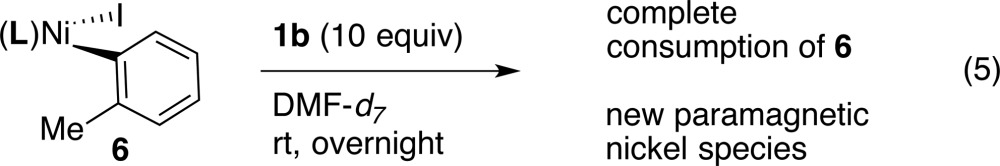 |
5 |
While these results establish the viability of this step for nickel-catalyzed couplings of NHP esters, substantial questions remain. First, the stoichiometric reactions were slower than the catalytic reaction. If a radical chain mechanism is operating and initiation is slow,8,27 this could account for the differences in rate, but this result could also indicate that a different, nonchain mechanism is operative. Second, because zinc alone did not react with an NHP ester (Scheme 1), the higher yield for the stoichiometric reaction of 6 in the presence of zinc suggests that the reduction of 6, or some other nickel species, facilitates the coupling. At this time, it is unclear what this reduced intermediate is, but a nickel(I) species is a possibility.16 Further studies on the elementary reactivity of NHP esters with metals and the mechanism of the present reaction are in progress.
The utilization of carboxylic acid derivatives as alkyl electrophile equivalents in cross-electrophile coupling has been demonstrated for the first time. Our cross-electrophile approach is complementary to photoredox cocatalysis methods to couple carboxylic acids with aryl halides5 because the substrate scope is different and no cocatalysis or light is required. Furthermore, given this study and Baran’s recent report, the use of NHP esters in nickel-catalyzed cross-coupling appears to be a general phenomenon.16 In this context, our stoichiometric studies establish a mechanistic starting point for further work in this area by showing arylnickel complexes react with NHP esters to form the alkylated arene product directly. Studies on the mechanism of this transformation as well as couplings with other electrophiles will be reported in due course.
Acknowledgments
The authors gratefully acknowledge funding from the NIH NIGMS (R01 GM097243). L.K.G.A. acknowledges funding from the University of Rochester (Elon Huntington Hooker Fellowship). K.A.J. acknowledges funding from an NSF GRFP award (DGE-1419118). D.J.W. is a Camille Dreyfus Teacher-Scholar. Additional funding from Novartis, Pfizer, and Boehringer Ingelheim is also gratefully acknowledged. Analytical data were obtained from the CENTC Elemental Analysis Facility at the University of Rochester, funded by NSF CHE-0650456. Prof. Larry Overman is thanked for suggesting that we look at NHP esters for cross-electrophile coupling.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.6b01533.
Detailed experimental procedures, spectral data, and supplementary optimization data (PDF)
Author Present Address
† Department of Chemistry, Princeton University, Princeton, NJ 08544
Author Contributions
‡ K.M.M.H. and J.A.C. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Frisch A. C.; Beller M. Angew. Chem., Int. Ed. 2005, 44, 674–688. 10.1002/anie.200461432. [DOI] [PubMed] [Google Scholar]; b Rudolph A.; Lautens M. Angew. Chem., Int. Ed. 2009, 48, 2656–2670. 10.1002/anie.200803611. [DOI] [PubMed] [Google Scholar]; c Jana R.; Pathak T. P.; Sigman M. S. Chem. Rev. 2011, 111, 1417–1492. 10.1021/cr100327p. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Manolikakes G.3.08 Coupling Reactions Between sp3 and sp2 Carbon Centers A2. In Comprehensive Organic Synthesis II, 2nd ed.; Knochel P., Ed.; Elsevier: Amsterdam, 2014; pp 392–464. [Google Scholar]
- While 318,496 Ar-Br and 1,000,000 Ar-Cl are listed as commercially available in the eMolecules database (accessed via REAXYS 6/22/2015), only 9,282 alkyl-Br and 24,746 alkyl-Cl are listed. In contrast, there are 83,315 alkanoic acids commercially available.
- Dieter R. K. Tetrahedron 1999, 55, 4177–4236. 10.1016/S0040-4020(99)00184-2. [DOI] [Google Scholar]
- The decarbonylative coupling of alkanoic acid derivatives has more precedent; see the following two examples and references therein:; a O’Brien E. M.; Bercot E. A.; Rovis T. J. Am. Chem. Soc. 2003, 125, 10498–10499. 10.1021/ja036290b. [DOI] [PubMed] [Google Scholar]; b Muto K.; Yamaguchi J.; Musaev D. G.; Itami K. Nat. Commun. 2015, 6, 7508. 10.1038/ncomms8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zuo Z.; Ahneman D.; Chu L.; Terrett J.; Doyle A. G.; MacMillan D. W. C. Science 2014, 345, 437–440. 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Noble A.; McCarver S. J.; MacMillan D. W. C. J. Am. Chem. Soc. 2015, 137, 624–627. 10.1021/ja511913h. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Luo J.; Zhang J. ACS Catal. 2016, 6, 873–877. 10.1021/acscatal.5b02204. [DOI] [Google Scholar]
- The coupling of a vinyl bromide with 3 equiv of cyclohexane carboxylic acid (78% yield) and 5-phenylvaleric acid (11% yield) were reported in ref (5b).
- a Okada K.; Okamoto K.; Oda M. J. Am. Chem. Soc. 1988, 110, 8736–8738. 10.1021/ja00234a047. [DOI] [Google Scholar]; b Okada K.; Okamoto K.; Oda M. J. Chem. Soc., Chem. Commun. 1989, 1636–1637. 10.1039/c39890001636. [DOI] [Google Scholar]; c Okada K.; Okamoto K.; Morita N.; Okubo K.; Oda M. J. Am. Chem. Soc. 1991, 113, 9401–9402. 10.1021/ja00024a074. [DOI] [Google Scholar]; d Okada K.; Okubo K.; Morita N.; Oda M. Tetrahedron Lett. 1992, 33, 7377–7380. 10.1016/S0040-4039(00)60192-2. [DOI] [Google Scholar]; e Okada K.; Okubo K.; Morita N.; Oda M. Chem. Lett. 1993, 22, 2021–2024. 10.1246/cl.1993.2021. [DOI] [Google Scholar]; f Schnermann M. J.; Overman L. E. Angew. Chem., Int. Ed. 2012, 51, 9576–9580. 10.1002/anie.201204977. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Pratsch G.; Lackner G. L.; Overman L. E. J. Org. Chem. 2015, 80, 6025–6036. 10.1021/acs.joc.5b00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Everson D. A.; Shrestha R.; Weix D. J. J. Am. Chem. Soc. 2010, 132, 920–921. 10.1021/ja9093956. [DOI] [PubMed] [Google Scholar]; b Everson D. A.; Jones B. A.; Weix D. J. J. Am. Chem. Soc. 2012, 134, 6146–6159. 10.1021/ja301769r. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang S.; Qian Q.; Gong H. Org. Lett. 2012, 14, 3352–3355. 10.1021/ol3013342. [DOI] [PubMed] [Google Scholar]
- Ackerman L. K. G.; Anka-Lufford L. L.; Naodovic M.; Weix D. J. Chem. Sci. 2015, 6, 1115–1119. 10.1039/C4SC03106G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zhao Y.; Weix D. J. J. Am. Chem. Soc. 2014, 136, 48–51. 10.1021/ja410704d. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhao Y.; Weix D. J. J. Am. Chem. Soc. 2015, 137, 3237–3240. 10.1021/jacs.5b01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weix D. J. Acc. Chem. Res. 2015, 48, 1767–1775. 10.1021/acs.accounts.5b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHP esters are reported to have E1/2 −1.56 to −1.66 V vs Ag/Ag+ (in MeCN, refs (7a) and (13), converted using ref (15)), and (bpy)NiBr2 is reported to have a two-electron E1/2 of −1.52 V vs Ag/Ag+ (in DMF, ref (14)).
- Lackner G. L.; Quasdorf K. W.; Pratsch G.; Overman L. E. J. Org. Chem. 2015, 80, 6012–6024. 10.1021/acs.joc.5b00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhaylov D.; Gryaznova T.; Dudkina Y.; Khrizanphorov M.; Latypov S.; Kataeva O.; Vicic D. A.; Sinyashin O. G.; Budnikova Y. Dalton Trans. 2012, 41, 165–172. 10.1039/C1DT11299F. [DOI] [PubMed] [Google Scholar]
- Pavlishchuk V. V.; Addison A. W. Inorg. Chim. Acta 2000, 298, 97–102. 10.1016/S0020-1693(99)00407-7. [DOI] [Google Scholar]
- Cornella J.; Edwards J. T.; Qin T.; Kawamura S.; Wang J.; Pan C.-M.; Gianatassio R.; Schmidt M. A.; Eastgate M. D.; Baran P. S. J. Am. Chem. Soc. 2016, 138, 2174–2177. 10.1021/jacs.6b00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The NHS ester of acetic acid has an E1/2 of −2.05 V while the NHP ester of acetic acid has an E1/2 of −1.04 V vs Ag/Ag+ in methanol. See:Horner L.; Jordan M. Justus Liebigs Ann. Chem. 1978, 1978, 1518–1525. 10.1002/jlac.197819780916. [DOI] [Google Scholar]
- A variety of ligands (2,2′-biphenyl, 1,10-phenanthroline, 4,4′-dimethoxy-2,2′-biphenyl, bathophenanthroline) and nickel sources (NiBr2(diglyme), NiBr2·3H2O) were effective.
- NHP esters synthesized with DIC according to the method by Overman7g consistently performed better than esters synthesized with other coupling agents (DCC, EDC). Spiking experiments with possible contaminants have not yet revealed the reason for this discrepancy.
- The coupling of alkyl halides with aryl halides has been scaled to multigram scale using standard equipment:Everson D. A.; George D. T.; Weix D. J.; Buergler J. F.; Wood J. L. Org. Synth. 2014, 90, 200–214. 10.1002/0471264229.os090.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The reaction with 2-iodocumene failed to produce the cross-coupled product, in contrast to reactions with alkyl iodides reported in ref (27).
- Methyl tosylate was reported to methylate alkyl halides and acid chlorides, but not aryl halides:Liang Z.; Xue W.; Lin K.; Gong H. Org. Lett. 2014, 16, 5620–5623. 10.1021/ol502682q. [DOI] [PubMed] [Google Scholar]
- The coupling of β-iodoalanine or the corresponding organozinc reagent with aryl halides is another viable route to phenylalanine derivatives:; a Jackson R. F. W.; Perez-Gonzalez M.; Danheiser R. L.; Crombie A. L. Org. Synth. 2005, 81, 77–88. 10.15227/orgsyn.081.0077. [DOI] [Google Scholar]; b Lu X.; Yi J.; Zhang Z.-Q.; Dai J.-J.; Liu J.-H.; Xiao B.; Fu Y.; Liu L. Chem. - Eur. J. 2014, 20, 15339–15343. 10.1002/chem.201405296. [DOI] [PubMed] [Google Scholar]
- Even simple homophenylalanine is an important starting material for the pharmaceutical industry.Ahmad A. L.; Oh P. C.; Abd Shukor S. R. Biotechnol. Adv. 2009, 27, 286–296. 10.1016/j.biotechadv.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Although the corresponding alkyl iodide is known, it has not been used in a cross-coupling:; a Kenji K.; Takahiro M.; Seiichiro I.; Akira O.; Michiko Y.; Shigeo M.; Mosbach E. H.; Takahiko H. Steroids 1992, 57, 193–198. 10.1016/0039-128X(92)90008-W. [DOI] [PubMed] [Google Scholar]; b Banerjee S.; Vidya V. M.; Savyasachi A. J.; Maitra U. J. Mater. Chem. 2011, 21, 14693–14705. 10.1039/c1jm11912e. [DOI] [Google Scholar]
- a Wotal A. C.; Weix D. J. Org. Lett. 2012, 14, 1476–1479. 10.1021/ol300217x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wu F.; Lu W.; Qian Q.; Ren Q.; Gong H. Org. Lett. 2012, 14, 3044–3047. 10.1021/ol3011198. [DOI] [PubMed] [Google Scholar]; c Yin H.; Zhao C.; You H.; Lin K.; Gong H. Chem. Commun. 2012, 48, 7034–7036. 10.1039/c2cc33232a. [DOI] [PubMed] [Google Scholar]; d Zhao C.; Jia X.; Wang X.; Gong H. J. Am. Chem. Soc. 2014, 136, 17645–17651. 10.1021/ja510653n. [DOI] [PubMed] [Google Scholar]; e Cherney A. H.; Kadunce N. T.; Reisman S. E. J. Am. Chem. Soc. 2013, 135, 7442–7445. 10.1021/ja402922w. [DOI] [PubMed] [Google Scholar]
- Biswas S.; Weix D. J. J. Am. Chem. Soc. 2013, 135, 16192–16197. 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For related studies suggesting similar mechanisms at play in different reactions, see:; a Schley N. D.; Fu G. C. J. Am. Chem. Soc. 2014, 136, 16588–16593. 10.1021/ja508718m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhao C.; Jia X.; Wang X.; Gong H. J. Am. Chem. Soc. 2014, 136, 17645–17651. 10.1021/ja510653n. [DOI] [PubMed] [Google Scholar]
- A reaction conducted with tetrakis(dimethylamino)ethylene in place of zinc did not turnover at rt. At elevated temperatures (40–60 °C), reactions run with either TDAE or zinc resulted in decomposition of the NHP ester and no consumption of aryl iodide.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


