Abstract
Purpose
Detection and treatment of small lung nodules are important in managing pediatric cancer. We studied the effectiveness of preoperative localization of pulmonary nodules by CT-guided needle hook wire placement followed by thoracoscopic resection in children with cancer.
Methods
We reviewed records of patients who underwent thoracoscopic resection of lung nodules localized preoperatively with CT-guided needle and hook wire placement at our hospital between March 1999 and April 2010 for nodule characteristics and outcomes of procedure.
Results
Thirty-seven patients (median age, 14 years) with osteosarcoma or other cancers underwent thoracoscopic resection of needle-localized lung nodules. Lesion (median nodule size, 4mm) location was left lung (n=11), right lung (n=19), and bilateral (n=7). The procedure was successful in 36 (97.3%) patients. Five patients had a pneumothorax after localization but none required chest tube placement before thoracoscopy. All patients underwent thoracoscopy, but 4 required conversion to open thoracotomy. During thoracoscopic inspection, the hook wire slipped out of the lesion in 6 patients, of whom 1 needed thoracotomy to locate nodule. Lesions (malignant in 13 patients) were removed in all patients. Five patients with benign lesions had recurrent malignant lung nodules.
Conclusions
Thoracoscopic resection of preoperatively localized small lung nodules is a safe and effective procedure in children.
Keywords: Pediatric cancer, Lung nodule, Preoperative localization, Hook wire, Thoracoscopy
Technical advances in computed tomography (CT) imaging have led to an increase in the detection of lung nodules smaller than 5mm [1]. Differentiating between benign and malignant lesions can often be challenging. In a review of 41 patients younger than 21 years, McCarville et al. found that well-circumscribed nodules were more often malignant in this age group of patients than in adults, in whom malignant lesions are more likely to be poorly defined, and also lesions smaller than 5mm are likely to be malignant [2]. Thus, results of CT findings in cases of malignancy may vary between children and adults. In a review of 74 children with malignant extrathoracic tumors, Grampp et al. found that nodules that were solitary, less than 5mm, and had ill-defined margins were likely to be benign [3].
As early as 1992, Mack et al. reported CT-guided needle and hook wire localization of pulmonary nodules in 6 patients [4]. In the same year, Plunkett et al. successfully localized 19 peripheral pulmonary nodules in 20 patients by a percutaneous technique, using a conventional mammographic needle localization system with CT guidance. These lesions were then resected thoracoscopically [5]. Since then, numerous studies have reported this technique for resection of pulmonary nodules in adults [6–13], but only few have described its use in children [14–16].
In this retrospective study, we reviewed our experience of children who underwent thoracoscopic resection of small pulmonary nodules that were localized with a needle and hook wire placed preoperatively with CT guidance.
1. Materials and methods
We conducted a retrospective review of medical records of all children who underwent thoracoscopic resection of lung nodules that had been localized preoperatively with a CT-guided needle and hook wire at St. Jude Children’s Research Hospital (St. Jude), Memphis, TN between March 1999 and April 2010. The study was approved by the St. Jude institutional review board. Patients underwent preoperative localization for lung lesions that were either less than or equal to 10mm or if the radiologist considered it to be not amenable to CT-guided biopsy or for lung lesions unlikely to have localizing features visible at thoracoscopic inspection.
Records were reviewed for patient demographics, lung nodule characteristics, procedural details and results of CT-guided localization and thoracoscopy, pathologic diagnosis of lesions, and follow-up.
1.1. Preoperative CT-guided needle and hook wire placement
Preoperative localization of the lung nodule was performed before thoracoscopy under general anesthesia by a pediatric radiologist in the interventional radiology (IR) suite. The patient was placed in a supine, prone, or lateral position, as decided by the radiologist. The nodule was localized with a commercially available Kopans spring hook wire localization needle (Cook Incorporated, Bloomington, IN) under CT guidance after acquiring localizing scout images and scanning images. A 20-gauge localization needle, either 5 cm or 9cm long, was used; the hook wire length was 15 cm. The radiologist selected the shortest transthoracic distance from the target nodule to the pleural surface while avoiding vital intrathoracic structures (Fig. 1). The introducer needle was then introduced sequentially through the skin, and then through the nodule (although this was not always possible) and just beyond it. Sequential CT images were taken after each manipulation of the needle. Then, 0.1–0.2mL of methylene blue (American Regent Incorporated, Shirley, NY) was injected through the needle and the hook wire was then placed through the needle and deployed beyond the nodule and left in place. The needle was then removed over the hook wire. Verification scanning images were taken to confirm that the hook wire was still in continuity with the lesion. The wire was not secured to the chest wall, but a small sterile dressing was placed over the external part of the wire, which was kept loosely coiled on the chest wall. The patient was then transferred to the operating room (OR). The radiologist communicated the result of the localization to the operating surgeon.
Fig. 1.
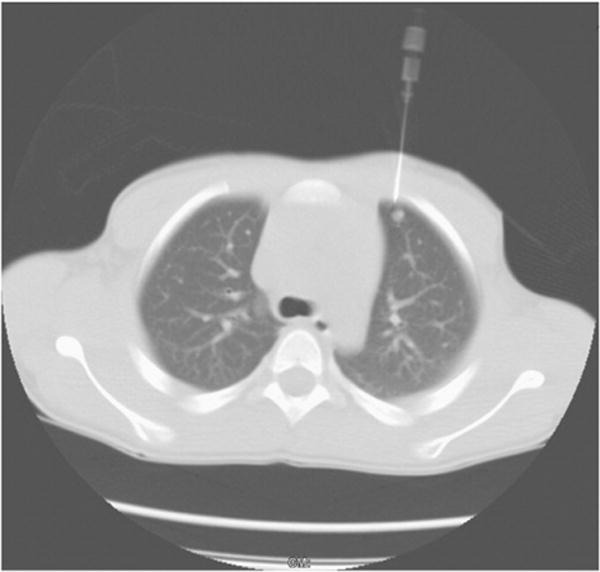
Computed tomography-guided needle and hook wire localization.
1.2. Thoracoscopic resection of localized nodule
One-lung ventilation was established with a bronchial blocker by the anesthesiologist in the OR. The patient was positioned in the lateral decubitus position, with care being taken to not displace the wire. Three entry sites were used for the thoracoscopy in a triangulate manner for optimal visibility and ease of manipulation of the target lesion. In some cases, 2 ports were sufficient, with the wire serving as a “retractor” and elevating the lesion. We did not aggressively pull on the wire and used it only in cases where gentle traction would help in placing the endoscopic stapler; otherwise, we inserted an additional port. Usually, 2 ports of diameter 5mm were inserted in the intercostal space at the level of the anterior and posterior axillary lines and the endoscopic stapler was inserted directly through a small opening in the chest wall usually 2 spaces below the above ports along the midaxillary line; however, the site of port placement could vary in individual cases depending on the location of lesion. The ports were usually placed between the fifth and seventh intercostal spaces. The hook wire marked the site of the lung nodule in the collapsed lung. The wire tented up the lesion, which was excised as a wedge along with a rim of normal-appearing lung parenchyma with an endoscopic linear stapling device (Figs. 2 and 3). If the wire became dislodged, usually a blue discoloration of the pleural surface or a puncture mark due to wire extrusion gave an indication of the site of lesion. The specimen was removed from the chest either through the largest port or directly through the chest wall. If the nodule could not be found at thoracoscopy, a thoracotomy was performed. At the end of the procedure, a chest tube was sometimes placed, which was passed through the midaxillary port incision to drain the pleural space. This was usually removed the next day and the patient was then discharged.
Fig. 2.
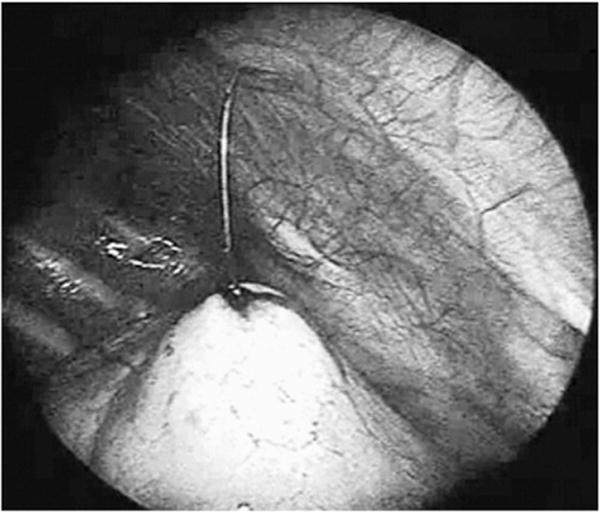
Tenting up of the lesion by the wire.
Fig. 3.
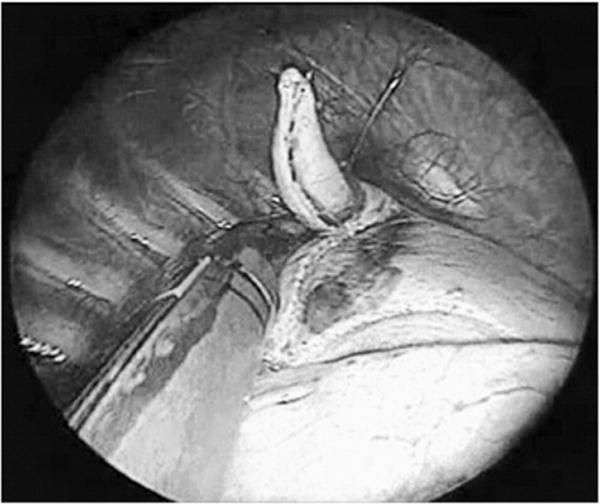
Excision of the lesion as a wedge after the wire tents up the lesion.
2. Results
2.1. Patient demographics
From March 1999 to April 2010, 37 patients [20 females, 17 males; median age at procedure, 14 years (range, 1–21 years)] underwent thoracoscopic resection of needle-localized lung nodules (Tables 1 and 2). A primary cancer diagnosis was known for 27 patients (73%). The remaining 10 patients (27%) presented initially with a lung nodule and an associated extrapulmonary tumor, and they were eventually diagnosed with osteosarcoma (OS). The cancer diagnoses were as follows: OS (n=19), Wilms tumor (n=4), synovial sarcoma (n=3), rhabdomyosarcoma (n=3), dermatofibrosarcoma protuberans (n=1), undifferentiated sarcoma (n=1), Hodgkin lymphoma (n=1), renal cell carcinoma (n=1), melanoma (n=1), primitive neuroectodermal tumor (n= 1), rhabdoid tumor of kidney (n=1), and Ewing sarcoma (n=1). Two patients (patients 15 and 18) with OS and six patients (patients 24, 27, 28, 34, 35, and 36) with non-OS cancers underwent the procedure during their off-therapy period, with a median period of 24 months from the end of therapy (range, 2 months–14 years).
Table 1.
Demographic, lung nodule, and needle hook wire localization data for patients with osteosarcoma (n=19).
| Patient no. | Age (years) | Primary site | Location of localized nodule | U/L or B/L lesion | Size of nodule (mm) | Wire dislodge | Thoracotomy | Pathology |
|---|---|---|---|---|---|---|---|---|
| 1 | 7 | R femur | RUL | U/L | 5 | No | No | Granuloma |
| 2 | 8 | L femur | RUL | U/L | 4 | Yes | No | OS mets |
| 3 | 9 | R femur | LUL | U/L | 5 | Yes | No | Granuloma |
| 4 | 11 | L femur | LUL, LLL | U/L | 4 | No | No | Granuloma |
| 5 | 12 | R tibia | LLL | B/L | 2 | No | Yes | Lymph node |
| 6 | 12 | R femur | LUL | U/L | 4 | Yes | No | Benign fibrovascular nodule |
| 7 | 13 | R tibia | RUL | B/L | 4 | No | No | OS mets |
| 8 | 13 | L tibia | LLL | U/L | 3 | No | Yes | OS mets |
| 9 | 14 | L tibia | RLL | U/L | 3 | No | No | Peribronchiolar scar |
| 10 | 14 | R femur | RUL | U/L | 3 | No | No | Granuloma |
| 11 | 14 | R femur | RML, RLL | U/L | 2 | No | No | Lymph node |
| 12 | 15 | L tibia | RLL | U/L | 4 | No | No | Granuloma |
| 13 | 16 | L femur | LLL | U/L | 5 | No | No | OS mets |
| 14 | 16 | L tibia | LUL | U/L | 3 | No | No | Lymph node |
| 15 | 16 | R femur | LUL | U/L | 7 | No | No | Granuloma |
| 16 | 16 | R humerus | RUL | U/L | 6 | No | No | OS mets |
| 17 | 21 | L femur | LUL | U/L | 3 | No | No | Granuloma |
| 18 | 21 | L femur | RLL | U/L | 5 | No | No | OS mets |
| 19 | 21 | R femur | RUL | U/L | 4 | No | No | Hamartoma |
R, right; L, left; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL; left lower lobe; U/L, unilateral; B/L, bilateral; OS, osteosarcoma; mets, metastasis.
Table 2.
Demographic, lung nodule, and needle hook wire localization data for patients with cancers other than osteosarcoma (n=18).
| Patient no. | Age (years) | Primary site diagnosis | Location of localized nodule | U/L or B/L lesion | Size of nodule (mm) | Whether wire dislodge | Thoracotomy | Pathology |
|---|---|---|---|---|---|---|---|---|
| 20 | 1 | R Wilms tumor | RML | U/L | 2 | No | Yes | Granuloma |
| 21 | 1 | L renal rhabdoid | RLL | U/L | 4 | No | No | Rhabdoid mets |
| 22 | 3 | R Wilms tumor | LUL | U/L | 4 | No | No | Granuloma |
| 23 | 5 | R leg RMS | RUL | U/L | 5 | No | No | RMS mets |
| 24 | 5 | R pterygopalatine Ewing’s sarcoma | RLL | U/L | 5 | Yes | No | Granuloma |
| 25 | 6 | L Wilms tumor | LLL | B/L | 4 | No | No | Granuloma |
| 26 | 6 | R pinna RMS | LUL | U/L | 5 | No | No | RMS mets |
| 27 | 8 | L Wilms tumor | RLL | B/L | 4 | No | No | Granuloma |
| 28 | 9 | R RCC | RUL/RML | B/L | 3 | No | No | RCC mets |
| 29 | 11 | R thigh undifferentiated sarcoma | LLL | B/L | 5 | No | No | Metastasis |
| 30 | 14 | L elbow synovial sarcoma | RLL | U/L | 3 | No | No | Granuloma |
| 31 | 14 | Paratesticular RMS | RML | U/L | 5 | No | No | Lymph node |
| 32 | 18 | R tonsil synovial sarcoma | LLL | U/L | 5 | No | No | Metastasis |
| 33 | 18 | L scapula melanoma | RML | U/L | 2 | No | No | Granuloma |
| 34 | 18 | L foot DFSP | RML | U/L | 3 | Yes | No | Lymph node |
| 35 | 19 | R inguinal synovial sarcoma | RLL | U/L | 8 | Yes | Yes | Granuloma |
| 36 | 19 | Hodgkin’s disease | LLL | B/L | 10 | No | No | BOOP |
| 37 | 20 | R fibula PNET | RLL | U/L | 3 | No | No | PNET mets |
R, right; L, left; RMS, rhabdomyosarcoma; RCC, renal cell carcinoma; DFSP, dermatofibrosarcoma protuberans; PNET, primitive neuroectodermal tumor; RUL, right upper lobe; RML, right middle lobe; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe; U/L, unilateral/; B/L, bilateral; BOOP, bronchiolitis obliterans with organizing pneumonia; mets, metastasis.
2.2. Lung nodule characteristics
Lesions were located in the left lung (n=11), right lung (n=19), and bilaterally (n=7). In cases of bilateral nodules, the radiologist selected the nodule that had the shortest transthoracic distance to the pleural surface and with the least chance of injury to vital intrathoracic structures. The localized nodule was present in the left upper lobe (n=7), left lower lobe (n=6), right upper lobe (n=7), right middle lobe (n=3), right lower lobe (n=9); of the 5 remaining patients, 4 had at least 1 nodule in 2 adjacent lung lobes and 1 patient had multiple nodules in 1 lobe.
2.3. Needle and hook wire localization
None of the patients had previously undergone CT-guided biopsy for the lung lesion or had a prior negative thoracoscopy. The needle and hook wire preoperative localization was successful in 36 patients (97.3%), but not in 1 patient who had a nodule in the left lower lobe. This lesion was near the scapula and beneath a rib and only methylene blue was injected into the overlying pleura. Patients’ position during needle localization was supine (n=17), prone (n=11), or lateral (n=4); this information was not available for 5 patients. Methylene blue was injected in 33 patients, and information on injection was not available for 4 patients. No blood was injected in any patient. Thirty patients had only 1 nodule localized whereas 7 patients had 2 nodules localized before thoracoscopy. The radiologist reported that the nodule was pierced by the needle and hook wire in 14 patients, the wire was placed adjacent to the lesion in 22 patients, and only methylene blue was injected in 1 patient. CT scan revealed a small pneumothorax after the localization procedure in 5 patients, but none of them required a chest tube placement in the radiology suite prior to thoracoscopy.
2.4. Thoracoscopy
Thoracoscopy was performed in all patients, but 4 patients (10.8%) required conversion to open thoracotomy. Patients’ position during surgery was right lateral (n=16) and left lateral (n=21) decubitus. The number of ports used was 1 (n=1), 2 (n=8), or 3 (n=28). Concomitant surgical procedures were done in 17 patients. These included placement of tunneled central venous catheters (n=16), bone biopsy (n=5), retroperitoneal lymph node dissection (n=1), and laparoscopic cholecystectomy (n=1).
The hook wire slipped out of the lesion in 6 patients (patients 2, 3, 6, 24, 34, and 35). Patients 24 and 34 had their lesions near a pulmonary fissure and patients 3 and 35 had a small pneumothorax during the localization procedure. Methylene blue staining of the pleura helped in successful thoracoscopic wedge resection in patients 3, 6, 24, and 34, whereas a puncture mark on the pleural surface in patient 2 helped in successful thoracoscopy. In patient 35 in whom the wire had slipped out, there was neither methylene blue staining nor a puncture point. Hence, a decision to convert to thoracotomy was taken and a right lower lobe nodule was resected. Patient 2 had metastasis in the lesion, whereas the remaining 5 patients had a benign pathology in their lung lesions. One of the wires slipped out in 2 patients (patients 11 and 37) who had 2 nodules each localized. The wire was in place in 28 patients, and methylene blue dye without any needle and hook wire localization was injected in 1 patient (patient 8).
In 4 patients (patients 5, 8, 20, and 35), conversion to thoracotomy was required. Patient 5 was a 12-year-old male with right tibia OS with bilateral lung nodules, of which a left lower lobe nodule was localized. Analysis of frozen sections of the thoracoscopic specimen did not reveal any malignancy. However, due to high suspicion, a thoracotomy was done and a second specimen showed evidence of metastatic OS. Patient 8 was a 13-year-old male with a left tibia OS and a left lower lobe nodule. A hook wire could not be deployed as the lesion was near the scapula and beneath a rib and only methylene blue was injected into the overlying pleura. A thoracotomy was done after the nodule could not be identified after initial thoracoscopy. Patient 20 was a 1-year-old female with Wilms tumor who had a right middle lobe nodule near the confluence of the major and minor fissures. Since the resected wedge did not contain any nodule, a thoracotomy was done, which resulted in detection of a nodule in the right lower lobe, and it was reported as a granuloma. Patient 35 was a 19-year-old female with a right groin synovial sarcoma who had a successful hook wire localization of a right lower lobe nodule. However, a thoracotomy had to be done because the wire had dislodged from the lesion, which could not be localized. This patient also had an asymptomatic pneumothorax in the IR suite.
There was no visible hemorrhage at the pleural surface in all 37 patients. There were no allergic reactions to the blue dye. There was local diffusion of the blue dye in 7 patients. The nodule was palpable ex vivo in 12 patients, not palpable in 6 patients, there was no mention of palpability in records of 18 patients, and the localized nodule was not resected in 1 patient. A chest tube was placed electively in 30 patients; 7 patients did not have chest tube placement. The surgeon decided on the need for chest tube placement on the basis of factors such as lung expansion at end of surgery, extent of pulmonary wedge resection, and hemostasis.
2.5. Pathology
Nodule sizes ranged from 2 to 10mm. The pathology of the excised nodule was metastatic disease in 13 patients and benign histology in 24 patients. Benign pathology included granuloma (n=15), lymph node (n=5), hamartoma (n=1), peribronchiolar scar (n=1), benign fibrovascular nodule (n=1), and bronchiolitis obliterans with organizing pneumonia (n=1). Of the metastatic nodules, 53.9% (7/13) were equal to or more than 5mm and 29.2% (7/24) of the benign nodules were equal to or more than 5mm. Intraoperative frozen section pathology was performed for 19 patients.
2.6. Follow up
In most cases, follow-up was done within 2 years. Of the 24 patients with benign pathology in their lesions, 5 (patients 3, 5, 15, 20, and 36) had tumor recurrence in the lung. Patients 15 and 20 had relapse on the lung on the same side as the previous localization and patients 3, 5, and 36 had relapse on the lung opposite to the previous localization.
3. Discussion
The diagnosis and treatment of small lung nodules are important in the management of pediatric cancer. Our study shows that thoracoscopic resection of small pulmonary nodules that were localized preoperatively by CT-guided needle hook wire is a safe and effective method to diagnose these nodules. The technique requires good coordination between the IR and surgical teams. The coordination extends beyond just the communication of the result of the localization procedure. It includes preoperative discussion about the feasibility of doing the localization, informing the surgeon of any development of pneumothorax; and application of loose dressing on the chest wall at the exit site of the wire rather than strapping it firmly after the localization is done. The role of the anesthesiologist is equally important, and involves providing adequate anesthesia during the localization procedure, patient transfer, and establishment of one-lung ventilation prior to thoracoscopy. This minimally invasive technique reduces the physical and psychologic burden on children with cancer or suspected cancer while yielding important diagnostic information on lung nodules.
We do not use this technique for lesions near the lung hilum, as the radiologist may not be comfortable inserting a needle into an area having important anatomic structures. For such lesions, a thoracotomy is performed. However, we use this technique even if the lesion is near a fissure. We use the hook wire localization technique for small lung nodules that are subpleural in location or a few centimeters beyond the pleural surface. The nodules are localized preoperatively with CT-guided needle and hook wire to facilitate thoracoscopic wedge resection. We also inject a small volume of methylene blue to stain the area around the nodule, the needle pathway, and the visceral pleura.
Suzuki et al. have reported that failure to localize subpleural nodules during thoracoscopy can be responsible for 46% of conversions to thoracotomy [17]. Dendo et al. classified localization techniques into 3 types [7], which were later described in detail in other studies: (a) localization with imaging modalities during thoracoscopy, which includes intraoperative ultrasonography and CT fluoroscopy [18–20]; (b) preoperative localization with injection of dyes, contrast media, radionuclides, or colored adhesive agents [21–24]; and (c) preoperative localization with hook wire placement.
The median age of patients in our study was 14 years (range, 1–21 years). There have been few pediatric studies on the preoperative localization of lung nodules. Partrick et al. reported successful thoracoscopic lung biopsy in 12 children with a mean age of 12 years (range, 4–19 years) [14] by using preoperative methylene blue injection under CT guidance for nodule localization; they did not use an additional hook wire for localization. Waldhausen et al. used preoperative hook wire localization under CT guidance in 3 children with a median age of 2.5 years (range, 2–11 years) [15]. Martin et al. used a combination of methylene blue and low osmolar contrast injection along with hook wire placement in 4 patients with a median age of 13.5 years (range, 6–17 years) for preoperative localization before thoracoscopy [16].
The success rate of preoperative localization of nodules in our study was 97.3% (36 of 37 patients). Other studies have reported localization rates of 90% to 100% [5–9,13]. The rate of conversion from thoracoscopy to thoracotomy in our study was 10.8% (4/37), which is higher than the rate of 4%–7.5% reported for adults for a similar technique [8–10,12,13]. These studies have reported wire dislodgement, pleural adhesions, and deep location of the localized nodule (which prevents an endoscopic stapler to be applied) as reasons for conversion to thoracotomy.
The main disadvantage of hook wire localization is the risk of dislodgement of the wire from the lesion. In our study, the hook wire was dislodged in 6 (16.2%) patients. Previous studies have reported wire dislodgement rates of 2.4%–22% [6–8,10–13]. In their study of 101 patients, Thaete et al. reported wire dislodgement in 22 patients (21.7%), but methylene blue staining allowed localization in 13 of these 22 patients [11]. Further, 16 of these 22 patients had a pneumothorax during localization. In our study, 2 of the 6 patients who were eventually found to have a dislodged wire at thoracoscopy had a pneumothorax during localization. The dislodgement of the hook wire can occur while the patient is being transferred from the IR suite to the OR or when the lung is collapsed on introduction of iatrogenic pneumothorax at the start of thoracoscopy. We, therefore, do not strap it firmly to the chest wall and the wire will slide in easily with the collapsing lung.
Pneumothorax occurred in 5 of 37 (13.5%) patients in our study after localization in the IR suite, but none of them required any intervention. The reported rate of asymptomatic pneumothorax after localization is 7.5%–50% [5,7,8,11–13]. One patient in our study had an upper lobe atelectasis. Other complications such as pleuritic pain, lung hemorrhage, hemothorax, prolonged air leak, pneumonia, and retained wire fragment have been reported [6–9,11,13].
In our study, the pathologist-reported nodule size of lesions ranged from 2mm to 10mm. Nodules ranging from 2mm to 5mm were found in 6 patients in whom the nodule was not palpable at the end of surgery, indicating that these tiny nodules can escape tactile discrimination despite being present in the resected wedge. The majority of our patients (89.2%) did not have to undergo a thoracotomy with its associated morbidity, but obtained a diagnosis for their small lung lesions. In our study, 70.8% of benign nodules were less than 5mm whereas 46.1% with those of metastatic nodules were less than 5mm. Our findings conform to Grampp et al. [3] who had determined that nodules less than 5mm tend to be benign, but probably a larger series would be needed to determine the statistical significance.
In our study, the rate of asymptomatic pneumothorax and wire slippage was within the range reported in previous studies. The rate of conversion to open thoracotomy in our study was higher than that observed in adults for a similar technique, but we are reporting for a relatively large pediatric population. Our study was limited by its retrospective design as well as the relatively small number and heterogenous population of patients. However, to the best of our knowledge, this is the largest study of this technique in children. We recommend this technique for small pulmonary nodules that are subpleural in location. We do not advocate the use of thoracoscopy for the therapeutic management of OS lung nodules. We conclude that preoperative localization of small lung nodules with CT-guided hook wire placement followed by thoracoscopic resection is a safe and effective procedure in the pediatric population, provided there is close cooperation between the surgeon and radiologist. A majority of small pulmonary nodules can be identified at the time of thoracoscopy by using this elegant localizing technique.
Acknowledgments
We thank Vani Shanker, Department of Scientific Editing, St. Jude Children’s Research Hospital, for editing this manuscript. This work is supported in part by a grant from Cancer Center Support (CORE) grant 21765 and grant 23099 from the National Cancer Institute and the American Lebanese Syrian Associated Charities (ALSAC).
References
- 1.Silva CT, Amaral JG, Moineddin R, et al. CT characteristics of lung nodules present at diagnosis of extrapulmonary malignancy in children. AJR Am J Roentgenol. 2010;194(3):772–8. doi: 10.2214/AJR.09.2490. [DOI] [PubMed] [Google Scholar]
- 2.McCarville MB, Lederman HM, Santana VM, et al. Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology. 2006;239(2):514–20. doi: 10.1148/radiol.2392050631. [DOI] [PubMed] [Google Scholar]
- 3.Grampp S, Bankier AA, Zoubek A, et al. Spiral CT of the lung in children with malignant extra-thoracic tumors: distribution of benign vs malignant pulmonary nodules. Eur Radiol. 2000;10(8):1318–22. doi: 10.1007/s003300000359. [DOI] [PubMed] [Google Scholar]
- 4.Mack MJ, Gordon MJ, Postma TW, et al. Percutaneous localization of pulmonary nodules for thoracoscopic lung resection. Ann Thorac Surg. 1992;53(6):1123–4. doi: 10.1016/0003-4975(92)90407-u. [DOI] [PubMed] [Google Scholar]
- 5.Plunkett MB, Peterson MS, Landreneau RJ, et al. Peripheral pulmonary nodules: preoperative percutaneous needle localization with CT guidance. Radiology. 1992;185(1):274–6. doi: 10.1148/radiology.185.1.1523323. [DOI] [PubMed] [Google Scholar]
- 6.Shah RM, Spirn PW, Salazar AM, et al. Localization of peripheral pulmonary nodules for thoracoscopic excision: value of CT-guided wire placement. AJR Am J Roentgenol. 1993;161(2):279–83. doi: 10.2214/ajr.161.2.8333361. [DOI] [PubMed] [Google Scholar]
- 7.Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology. 2002;225(2):511–8. doi: 10.1148/radiol.2252011025. [DOI] [PubMed] [Google Scholar]
- 8.Chen YR, Yeow KM, Lee JY, et al. CT-guided hook wire localization of subpleural lung lesions for video-assisted thoracoscopic surgery (VATS) J Formos Med Assoc. 2007;106(11):911–8. doi: 10.1016/S0929-6646(08)60061-3. [DOI] [PubMed] [Google Scholar]
- 9.Pittet O, Christodoulou M, Pezzetta E, et al. Video-assisted thoracoscopic resection of a small pulmonary nodule after computed tomography-guided localization with a hook-wire system. Experience in 45 consecutive patients. World J Surg. 2007;31(3):575–8. doi: 10.1007/s00268-006-0343-7. [DOI] [PubMed] [Google Scholar]
- 10.Gossot D, Miaux Y, Guermazi A, et al. The hook-wire technique for localization of pulmonary nodules during thoracoscopic resection. Chest. 1994;105(5):1467–9. doi: 10.1378/chest.105.5.1467. [DOI] [PubMed] [Google Scholar]
- 11.Thaete FL, Peterson MS, Plunkett MB, et al. Computed tomography-guided wire localization of pulmonary lesions before thoracoscopic resection: results in 101 cases. J Thorac Imag. 1999;14(2):90–8. doi: 10.1097/00005382-199904000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg. 2004;25(3):429–33. doi: 10.1016/j.ejcts.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 13.Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc. 2011;25(6):1723–9. doi: 10.1007/s00464-010-1502-3. [DOI] [PubMed] [Google Scholar]
- 14.Partrick DA, Bensard DD, Teitelbaum DH, et al. Successful thoracoscopic lung biopsy in children utilizing preoperative CT-guided localization. J Pediatr Surg. 2002;37(7):970–3. doi: 10.1053/jpsu.2002.33820. [discussion 970–3] [DOI] [PubMed] [Google Scholar]
- 15.Waldhausen JH, Shaw DW, Hall DG, et al. Needle localization for thoracoscopic resection of small pulmonary nodules in children. J Pediatr Surg. 1997;32(11):1624–5. doi: 10.1016/s0022-3468(97)90468-1. [DOI] [PubMed] [Google Scholar]
- 16.Martin AE, Chen JY, Muratore CS, et al. Dual localization technique for thoracoscopic resection of lung lesions in children. J Laparoendosc Adv Surg Tech A. 2009;19(Suppl 1):S161–4. doi: 10.1089/lap.2008.0143.supp. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest. 1999;115(2):563–8. doi: 10.1378/chest.115.2.563. [DOI] [PubMed] [Google Scholar]
- 18.Piolanti M, Coppola F, Papa S, et al. Ultrasonographic localization of occult pulmonary nodules during video-assisted thoracic surgery. Eur Radiol. 2003;13(10):2358–64. doi: 10.1007/s00330-003-1916-6. [DOI] [PubMed] [Google Scholar]
- 19.Santambrogio R, Montorsi M, Bianchi P, et al. Intraoperative ultrasound during thoracoscopic procedures for solitary pulmonary nodules. Ann Thorac Surg. 1999;68(1):218–22. doi: 10.1016/s0003-4975(99)00459-2. [DOI] [PubMed] [Google Scholar]
- 20.Bladt O, De Wever W. Additional value of CT-fluoroscopic biopsy of pulmonary lesions: a retrospective study of 69 patients. JBR-BTR. 2006;89(6):298–302. [PubMed] [Google Scholar]
- 21.Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol. 1994;163(2):297–300. doi: 10.2214/ajr.163.2.7518642. [DOI] [PubMed] [Google Scholar]
- 22.Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg. 1998;14(3):265–70. doi: 10.1016/s1010-7940(98)00160-2. [DOI] [PubMed] [Google Scholar]
- 23.Grogan EL, Jones DR, Kozower BD, et al. Identification of small lung nodules: technique of radiotracer-guided thoracoscopic biopsy. Ann Thorac Surg. 2008;85(2):S772–7. doi: 10.1016/j.athoracsur.2007.10.105. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida J, Nagai K, Nishimura M, et al. Computed tomography-fluoroscopy guided injection of cyanoacrylate to mark a pulmonary nodule for thoracoscopic resection. Jpn J Thorac Cardiovasc Surg. 1999;47(5):210–3. doi: 10.1007/BF03217996. [DOI] [PubMed] [Google Scholar]


