Abstract
The axoneme genes, their encoded proteins, their functions and the structures they form are largely conserved across species. Much of our knowledge of the function and structure of axoneme proteins in cilia and flagella is derived from studies on model organisms like the green algae, Chlamydomonas reinhardtii. The core structure of cilia and flagella is the axoneme, which in most motile cilia and flagella contains a 9 + 2 configuration of microtubules. The two central microtubules are the scaffold of the central pair complex (CPC). Mutations that disrupt CPC genes in Chlamydomonas and other model organisms result in defects in assembly, stability and function of the axoneme, leading to flagellar motility defects. However, targeted mutations generated in mice in the orthologous CPC genes have revealed significant differences in phenotypes of mutants compared to Chlamydomonas. Here we review observations that support the concept of cell-type specific roles for the CPC genes in mice, and an expanded repertoire of functions for the products of these genes in cilia, including non-motile cilia, and other microtubule-associated cellular functions.
Keywords: cilia, flagella, axoneme, central pair complex
Introduction
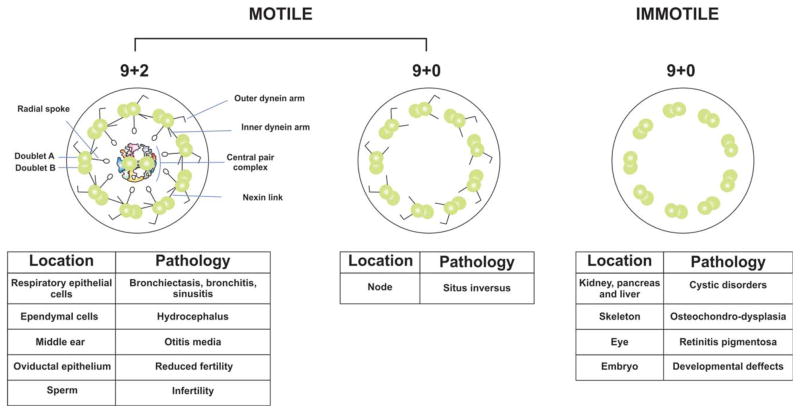
Cilia are specialized hair-like structures, classified as either motile or immotile. Motile cilia have a 9 + 2 organization of microtubules and are present on the respiratory epithelium, ependymal cells, in the female reproductive tract, and in flagellated cells [Brown and Witman, 2014]. Nodal cilia with a 9 + 0 axoneme structure are also a type of motile cilia. Immotile cilia have a 9 + 0 axoneme structure and include primary cilia present on most vertebrate cells [Satir and Christensen, 2007; Brown and Witman, 2014] (Fig. 1).
Fig. 1. Axoneme structures of the different types of cilia.
Cilia and flagella are specialized organelles involved in motility. The structure of both is based on nine pairs of microtubules surrounding two single microtubules, which form the central pair complex in motile cilia. Attached to the nine microtubules are other structures, such as the inner and outer dynein arms, the radial spokes and nexin links. Cilia located in the embryonic node (the nodal cilia) lack the central pair complex but maintain the dynein arms, which confer the capacity for rotary movement. Immotile cilia or primary cilia lack the central pair complex and the other structures around the nine pair of outer microtubules. They are present in almost all vertebrate cells. Ciliopathies encompass a variety of distinct disorders depending upon the organs or tissues affected. The main disorders resulting from defects in the development or function of cilia.
The structure of cilia and flagella has been highly conserved across evolution. The homology between flagellar proteins of unicellular organisms, and mammalian ciliary and flagellar proteins suggests that the main functional role of the genes encoding the axoneme proteins have been preserved [Ibanez-Tallon et al., 2003; Mitchell, 2004; Carvalho-Santos et al., 2011]. Immotile cilia and motile cilia/flagella from organisms in most taxonomic groups share a similar basic structure comprised of an arrangement of nine doublets of microtubules. Motile cilia/flagella have, in addition, associated projections from the 9 doublets including dynein arms, radial spokes, and nexin links surrounding a pair of singlet microtubule known as the central pair complex (CPC) [Lee, 2011] (Fig.1). Most of the genetic, structural, and biochemical work on the central pair has taken advantage of the model organism, the green algae, Chlamydomonas reinhardtii [Mitchell and Smith, 2009].
The two microtubules of the central pair are structurally and biochemically dimorphic, and by convention are given separate designations (i.e., C1 and C2) [Dutcher et al., 1984; Goodenough and Heuser, 1985; Dutcher, 1995; Smith and Lefebvre, 1997a,b]. Several CPC projections, in addition to a sheath partly surrounding the CPC, were identified using classical electron microscopy and 2D averages of CPC cross-sections [Mitchell and Smith, 2009]. Recently, new CPC projections have been identified by cryoelectron tomography and 3D average imaging [Carbajal-González et al., 2013]. This important study also revealed that the sheath is not an independent density connecting adjacent projections, but rather is the outermost part of projections extending from the C1 and C2 microtubules.
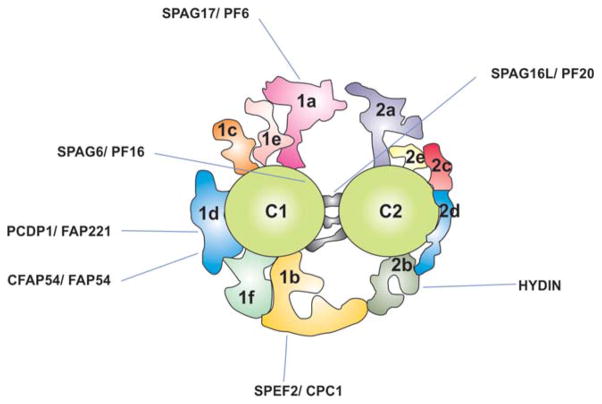
In summary (Fig. 2), the most recently described CPC structure shows that the C1 microtubule has two longer projections (1a and 1b) and four shorter projections (1c, 1d, 1e and 1f). Similar to the C1 microtubule, the C2 microtubule possesses two larger projection (2a and 2b), and three shorter projections (2c, 2d and 2e). There is a bridge linking the C1 and C2 microtubules [Carbajal-González et al., 2013].
Fig. 2.
Schematic representation of the central pair complex based on the cross section of a Chlamydomonas axoneme obtained by cryoelectron tomography. The location of selected CPC proteins is indicated.
The motility and waveforms produced by the beating cilia/flagellum are believed to be the result of coordinated regulation of the dynein arms [Huang et al., 1979; Curry and Rosenbaum, 1993; Porter, 1996; Habermacher and Sale, 1997; Smith and Lefebvre, 1997a,b; Porter and Sale, 2000; Mitchell, 2004]. It has been proposed that the central apparatus and the radial spokes function as a distributor to provide local signals that regulate the activity of specific dynein arms [Smith, 2002; Mitchell, 2004; Lesich et al., 2010]. In this context, mutations in genes encoding CPC proteins (described in detail below) have provided valuable evidence about the important roles that these proteins play in ciliary/flagellar motility.
Ciliopathies encompas a range of human diseases associated with mutations [Lee, 2011; Waters and Beales, 2011; Raidt et al., 2014]. They are characterized by abnormal formation and function of cilia [Lee, 2011]. Because cilia perform diverse biological roles, affected patients have several and variable phenotypes. Primary cilia, serve principally as key coordinators of signaling pathways during development and are also important in tissue homeostasis [Gerdes et al., 2009; Satir and Christensen, 2007; Satir et al., 2010; Oh and Katsanis, 2012]. Thus, defects in primary cilia can lead to a broader set of developmental and adult phenotypes that include polycystic liver and kidney disease, retinal degeneration, skeletal dysplasia and obesity. (Fig. 1). On the other hand, the function of motile cilia and flagella is principally related to movement of fluids over specialized epithelia and swimming of single cells [Silflow and Lefebvre, 2001; Satir and Christensen, 2007; Vincensini et al., 2011]. Motile cilia dysfunction results in bronchiectasis, and patients suffer from rhinitis, sinusitis and otitis media. Other complications include hydrocephalus, situs inversus and infertility [Badano et al., 2006; Brown and Witman, 2014] (Fig. 1). The literature on ciliary dysfunction in animal models and human studies grew significantly during the last decade [Brown and Witman, 2014]. In this review, we have compiled recent information that supports the concepts of cell-type specific roles of the CPC, and an expanded repertoire of functions for genes encoding CPC proteins in mammals.
Ciliary and Flagellar Beat
Ciliary/flagellar beating originates from the sliding of the doublet microtubules driven by the dynein arms in a coordinated fashion [Lee, 2011]. While the molecules involved in the regulatory mechanisms are similar in the mammalian cilia/flagella and Chlamydomonas flagella, there are some important differences [Salathe, 2007; Lee, 2011]. One major difference is that the CPC rotates in Chlamydomonas, but is fixed in position in mammals, which requires a different mechanism to account for asymmetrical activation of dynein arms driving microtubule sliding [Inaba, 2011; Lee, 2011]. The 12 μm Chlamydomonas flagella can beat with two different waveforms at frequencies near to 60 Hz, enabling propulsion in opposite directions [Silflow and Lefebvre, 2001]. Human respiratory cilia from intact epithelia are approximately 7 μm long and beat with a single waveform at much lower frequencies (~10–15 Hz) to transport mucus out of the airways [Wanner et al., 1996; Salathe, 2007]. Motility regulation through the CPC depends on pH, cAMP and calcium signaling for both cilia and flagella [Lee, 2011]. Increases in concentration of cAMP activate protein kinase A (PKA), which then phosphorylates specific target proteins in the axoneme [Salathe, 2007]. In human cilia, [Wanner et al., 1996; Salathe, 2007] and sperm [Turner, 2006], an increase in cAMP stimulates beat frequency, while causing a decrease in Chlamydomonas [Wanner et al., 1996; Lee, 2011]. Similarly, influx of Ca2 + stimulates motility of human cilia [Wanner et al., 1996; Lee, 2011; Inaba, 2015] while causing Chlamydomonas flagella to change from an asymmetric waveform to a symmetric waveform [Wanner et al., 1996; Silflow and Lefebvre, 2001; Lee, 2011; Inaba, 2015]. In sperm, Ca2 + influx promotes flagellar motility [Turner, 2006; Inaba, 2015]. However, flagella are quiescent at high calcium concentrations [Nakano et al., 2003; Turner, 2006; Inaba, 2015]. The effects of calcium on the axoneme are known to be mediated by the interaction with calmodulin (CaM) and proteins present in the CPC and the radial spokes [Wargo et al., 2005; Dymek and Smith, 2007; DiPetrillo and Smith, 2010; Inaba, 2015]. Variations in intracellular pH are also important for ciliary beating [Salathe, 2007]. Bronchial cilia display decreased beat frequency in acidic pH, while alkaline pH produces the opposite effect [Salathe, 2007].
Chlamydomonas and Mouse Mutants
The generation of gene-targeted mice with deficient axonemal components has been critical for the elucidation of differences in phenotypes between mammalian and algal mutants, and in the investigation of numerous ciliary functions, including human pathology [Ibanez-Tallon et al., 2003].
Cpc1/Spef2
Chlamydomonas CPC1 is a 205 kDa protein that possesses a domain with homology to adenylate kinase family members [Zhang and Mitchell, 2004]. The Cpc1 mutants display flagellar motility defects characterized by reduced beat frequency [Mitchell and Sale, 1999; Zhang and Mitchell, 2004; Mitchell and Smith, 2009]. Ultrastructural studies on isolated axonemes revealed absence of the C1b projection [Mitchell and Sale, 1999; Zhang and Mitchell, 2004; Mitchell and Smith, 2009]. The mammalian orthologue of CPC1, SPEF2 also known as KPL2, is expressed in ciliated cell types and testis [Ostrowski et al., 1999; Sironen et al., 2006; Sironen et al., 2010]. There are several transcripts derived from this gene described in public databases (Tables I and III). Spef2-deficient mice develop hydrocephalus, male infertility and sinusitis (Table II). Electron microscopic analysis of epididymal sperm demonstrated absence of recognizable axonemal structures and disorganization of mitochondria and outer dense fibers [Sironen et al., 2011]. Tracheal epithelial cilia beat at lower frequency and possessed a grossly normal 9 + 2 axonemal structure without apparent defects in the dynein arms [Sironen et al., 2011].
Table I.
Transcripts Encoding Humans, Mouse, and Chlamydomonas CPC Proteins
| Gene | Specie | Transcript ID | Protein coding | Length (aa) |
|---|---|---|---|---|
| Spef2/cpc1 | Homo sapiens | ENST00000356031 | Yes | 1822 |
| ENST00000282469 | Yes | 514 | ||
| ENST00000440995 | Yes | 1818 | ||
| ENST00000509059 | Yes | 943 | ||
| ENST00000303129 | Yes | 619 | ||
| ENST00000504054 | Yes | 389 | ||
| ENST00000510777 | Yes | 240 | ||
| ENST00000506526 | Yes | 151 | ||
| ENST00000513078 | Yes | 115 | ||
| ENST00000505847 | Yes | 145 | ||
| ENST00000508817 | Yes | 115 | ||
| ENST00000506759 | Yes | 120 | ||
| ENST00000503074 | Non-coding | - | ||
| ENST00000505088 | Non-coding | - | ||
| ENST00000502454 | Non-coding | - | ||
| Mus musculus | ENSMUST00000041840 | Yes | 875 | |
| ENSMUST00000160236 | Yes | 1724 | ||
| ENSMUST00000159288 | Yes | 1734 | ||
| ENSMUST00000159093 | Yes | 622 | ||
| ENSMUST00000162780 | Yes | 513 | ||
| ENSMUST00000159368 | Yes | 456 | ||
| Chlamydomonas reinhardtii | EDO96779 | Yes | 1929 | |
| Hydin | Homo sapiens | ENST00000393567 | Yes | 5121 |
| ENST00000321489 | Yes | 1017 | ||
| ENST00000538248 | Yes | 950 | ||
| ENST00000541601 | Yes | 940 | ||
| ENST00000288168 | Yes | 725 | ||
| ENST00000538382 | Yes | 204 | ||
| ENST00000545267 | Yes | 36 | ||
| ENST00000539973 | Yes | 39 | ||
| ENST00000542890 | Yes | 127 | ||
| ENST00000316490 | Yes | 5120 | ||
| ENST00000393550 | Yes | 740 | ||
| ENST00000448089 | Yes | 1214 | ||
| ENST00000448691 | Yes | 1017 | ||
| ENST00000539447 | Yes | 151 | ||
| ENST00000545230 | Yes | 528 | ||
| ENST00000393552 | Yes | 833 | ||
| ENST00000378856 | Yes | 49 | ||
| ENST00000538568 | Yes | 113 | ||
| ENST00000546257 | Yes | 43 | ||
| ENST00000543639 | Non-coding | - | ||
| ENST00000542283 | Non-coding | - | ||
| ENST00000540892 | Non-coding | - | ||
| ENST00000543521 | Non-coding | - | ||
| ENST00000309900 | Non-coding | - | ||
| ENST00000537789 | Non-coding | - | ||
| ENST00000536785 | Non-coding | - | ||
| ENST00000546297 | Non-coding | - | ||
| Mus musculus | ENSMUST00000043141 | Yes | 5154 | |
| Chlamydomonas reinhardtii | EDP09735 | Yes | 4524 | |
| Pcdp1/CFAP221 | Homo sapiens | ENST00000602047 | Yes | 554 |
| ENST00000594371 | Yes | 173 | ||
| ENST00000442513 | Yes | 175 | ||
| ENST00000594141 | Yes | 46 | ||
| ENST00000599827 | Yes | 118 | ||
| ENST00000434869 | Yes | 96 | ||
| ENST00000413369 | Yes | 840 | ||
| ENST00000598644 | Yes | 187 | ||
| ENST00000443972 | Yes | 399 | ||
| ENST00000295220 | Yes | 269 | ||
| ENST00000413057 | Yes | 53 | ||
| ENST00000600951 | Yes | 88 | ||
| ENST00000594033 | Yes | 90 | ||
| ENST00000463985 | Yes | 134 | ||
| ENST00000475569 | Non-coding | - | ||
| ENST00000488358 | Non-coding | - | ||
| ENST00000464578 | Non-coding | - | ||
| ENST00000597189 | Non-coding | - | ||
| Mus musculus | ENSMUST00000174370 | Yes | 836 | |
| ENSMUST00000037840 | Yes | 836 | ||
| ENSMUST00000174458 | Yes | 178 | ||
| Chlamydomonas reinhardtii | EDP00231 | Yes | 645 | |
| Cfap54/CFAP54 | Homo sapiens | ENST00000524981 | Yes | 3096 |
| ENST00000550977 | Yes | 265 | ||
| ENST00000553778 | Yes | 644 | ||
| ENST00000554108 | Non-coding | - | ||
| ENST00000556591 | Non-coding | - | ||
| ENST00000342887 | Non-coding | - | ||
| ENST00000554621 | Non-coding | - | ||
| ENST00000554821 | Non-coding | - | ||
| Mus musculus | ENSMUST00000168110 | Yes | 3106 | |
| ENSMUST00000020200 | Yes | 816 | ||
| ENSMUST00000168617 | Yes | 598 | ||
| ENSMUST00000164979 | Yes | 314 | ||
| ENSMUST00000163209 | Yes | 187 | ||
| ENSMUST00000170065 | Yes | 167 | ||
| ENSMUST00000067705 | Non-coding | - | ||
| ENSMUST00000165090 | Non-coding | - | ||
| ENSMUST00000171781 | Non-coding | - | ||
| ENSMUST00000168080 | Non-coding | - | ||
| Chlamydomonas reinhardtii | EDP00642 | Yes | 3081 | |
| Spag6/Pf16 | Homo sapiens | ENST00000313311 | Yes | 458 |
| ENST00000376601 | Yes | 270 | ||
| ENST00000376603 | Yes | 585 | ||
| ENST00000376624 | Yes | 509 | ||
| ENST00000435326 | Yes | 246 | ||
| ENST00000456231 | Yes | 191 | ||
| ENST00000538630 | Yes | 484 | ||
| ENST00000488555 | Non-coding | - | ||
| ENST00000487973 | Non-coding | - | ||
| ENST00000490361 | Non-coding | - | ||
| Mus musculus | ENSMUST00000023468 | Yes | 507 | |
| ENSMUST00000154624 | Non-coding | - | ||
| ENSMUST00000132033 | Non-coding | - | ||
| ENSMUST00000125017 | Non-coding | - | ||
| ENSMUST00000147539 | Non-coding | - | ||
| ENSMUST00000151132 | Non-coding | - | ||
| ENSMUST00000141835 | Non-coding | - | ||
| Chlamydomonas reinhardtii | EDP02264 | Yes | 475 | |
| Spag16/Pf20 | Homo sapiens | ENST00000272898 | Yes | 347 |
| ENST00000331683 | Yes | 631 | ||
| ENST00000374309 | Yes | 537 | ||
| ENST00000413312 | Yes | 251 | ||
| ENST00000447990 | Yes | 374 | ||
| ENST00000432529 | Yes | 183 | ||
| ENST00000451561 | Yes | 255 | ||
| ENST00000406979 | Yes | 57 | ||
| ENST00000452556 | Yes | 64 | ||
| ENST00000440779 | Yes | 94 | ||
| ENST00000420497 | Yes | 98 | ||
| ENST00000480494 | Non-coding | - | ||
| ENST00000414961 | Non-coding | - | ||
| Mus musculus | ENSMUST00000065425 | Yes | 639 | |
| ENSMUST00000113940 | Yes | 405 | ||
| ENSMUST00000161131 | Yes | 253 | ||
| ENSMUST00000161310 | Non-coding | |||
| Chlamydomonas reinhardtii | EDP04185 | Yes | 521 | |
| Spag17/Pf6 | Homo sapiens | ENST00000336338 | Yes | 2223 |
| ENST00000437255 | Yes | 660 | ||
| ENST00000465053 | Non-coding | - | ||
| ENST00000463628 | Non-coding | - | ||
| ENST00000477444 | Non-coding | - | ||
| ENST00000473472 | Non-coding | - | ||
| ENST00000486589 | Non-coding | - | ||
| ENST00000470550 | Non-coding | - | ||
| ENST00000483383 | Non-coding | - | ||
| ENST00000492438 | Non-coding | - | ||
| ENST00000469128 | Non-coding | - | ||
| ENST00000466857 | Non-coding | - | ||
| ENST00000478697 | Non-coding | - | ||
| Mus musculus | ENSMUST00000164539 | Yes | 2320 | |
| ENSMUST00000127518 | Non-coding | - | ||
| ENSMUST00000152586 | Non-coding | - | ||
| ENSMUST00000143050 | Non-coding | - | ||
| Chlamydomonas reinhardtii | EDP06248 | Yes | 2301 |
Data were extracted from Ensembl.
Table III.
Human and Mouse SPAG Protein Isoforms
| Specie | Gene | mRNA variant | Predicted protein MW (kDa) | pI | Tissue |
|---|---|---|---|---|---|
| Homo sapiens | SPEF2 | Spef2.aAug10 | 209.8 | 6.1 | Lung, uterus, breast carcinoma, brain, amygdala, other tissues |
| Spef2.bAug10 | 151.0 | 5.6 | Testis, placenta | ||
| Spef2.cAug10 | 108.5 | 6.7 | Amygdala, uterus, breast cancer, eyeball, hippocampus,other tissues | ||
| Spef2.dAug10 | 100.2 | 7.1 | Testis | ||
| Spef2.eAug10 | 70.8 | 6.3 | Lung | ||
| Spef2.fAug10 | 61.2 | 8.3 | Testis, brain, bladder, other tissues | ||
| Spef2.gAug10 | 46.8 | 6.2 | Spinal cord | ||
| Spef2.hAug10 | 27.4 | 9.0 | Brain, embryonic stem cells | ||
| Spef2.iAug10 | 16.5 | 8.8 | Hippocampus | ||
| Spef2.jAug10 | 16.0 | 8.5 | Intestine | ||
| Spef2.kAug10 | 12.9 | 9.1 | Lung | ||
| Spef2.lAug10 | 13.5 | 9.5 | Eye | ||
| Spef2.mAug10 | 12.8 | 9.1 | Kidney | ||
| Spef2.nAug10 | 10.9 | 7.8 | Uterus, fetal heart, pooled melanocyte, pooled tumors | ||
| Spef2.oAug10 | 15.8 | 8.0 | Hippocampus, thalamus | ||
| Spef2.pAug10 | 9.4 | 6.4 | Testis | ||
| Spef2.qAug10- unspliced | 6.2 | 9.6 | Embryonic stem cell | ||
| Homo sapiens | HYDIN | Hydin.aAug10 | 575.8 | 6.3 | Testis, brain, lung, uterus, other tissues |
| Hydin.bAug10 | 123.1 | 6.8 | Testis | ||
| Hydin.cAug10 | 114.2 | 6.5 | Testis | ||
| Hydin.dAug10 | 114.2 | 6.5 | |||
| Hydin.eAug10 | 106.3 | 6.2 | Testis, brain, amygdala | ||
| Hydin.fAug10 | 105.3 | 6.2 | Testis, hippocampus, pancreas | ||
| Hydin.gAug10 | 86.6 | 6.1 | Testis, head, pancreas | ||
| Hydin.hAug10 | 81.6 | 6.6 | Testis, lung | ||
| Hydin.iAug10 | 26.9 | 7.9 | Pancreas, T cells, head, kidney | ||
| Hydin.jAug10 | 25.0 | 5.8 | Eye | ||
| Hydin.kAug10 | 21.5 | 8.1 | Amygdala | ||
| Hydin.lAug10 | 15.8 | 5.2 | |||
| Hydin.mAug 10-unspliced | 14.9 | 5.5 | Carcinoid, lung, head, pancreas, kidney, parathyroid | ||
| Hydin.nAug10 | 7.3 | 7.5 | |||
| Hydin.oAug10 | 26.0 | 5.5 | Testis, trachea | ||
| Hydin.pAug10-unspliced | 10.1 | 6.7 | Eye | ||
| Hydin.qAug10 | 9.2 | 9.1 | |||
| Hydin.rAug10-unspliced | 3.9 | 8.9 | Testis | ||
| Hydin.sAug10-unspliced | 3.2 | 10.7 | Eye | ||
| Hydin.tAug10-unspliced | 3.3 | 11.5 | Skeletal muscle | ||
| Hydin.uAug10-unspliced | 4.2 | 6.8 | Head | ||
| Hydin.vaAug10-unspliced | 4.9 | 9.1 | Placenta | ||
| Homo sapiens | PCDP1 | Pcdp1.aAug10 | 64.4 | 8.0 | Testis, lung, trachea, uterus, cerebellum, other tissues |
| Pcdp1.bAug10 | 60.2 | 8.3 | Lung, kidney, cerebellum, heart, pulled tumors, other tissues | ||
| Pcdp1.cAug10 | 34.9 | 9.0 | Liver and spleen | ||
| Pcdp1.dAug10 | 21.4 | 8.1 | Trachea | ||
| Pcdp1.eAug10 | 17.2 | 8.8 | Amygdala, embryonic stem cells, hyppocampus | ||
| Pcdp1.fAug10 | 13.2 | 5.9 | Brain | ||
| Pcdp1.gAug10 | 13.5 | 5.9 | |||
| Pcdp1.hAug10 | 9.6 | 5.8 | Kidney | ||
| Pcdp1.iAug10 | 8.5 | 5.7 | pooled tumors from uterus, endometrial adenocarcinoma | ||
| Pcdp1.jAug10-unspliced | 2.6 | 8.0 | Testis, brain, blood, carcinoid, eye, heart, other tissues | ||
| Pcdp1.kAug10 | 5.9 | 9.4 | Brain | ||
| Pcdp1.lAug10 | 3.6 | 4.0 | Carcinoid, lung | ||
| Pcdp1.mAug10-unspliced | 4.7 | 9.2 | |||
| Homo sapiens | SPAG6 | Spag6.aAug10 | 55.5 | 6.9 | Testis, lung, pooled germ cell tumors, pancreas, islets of langerhans, other tissues. |
| Spag6.bAug10 | 52.4 | 7.0 | Testis | ||
| Spag6.cAug10 | 52.5 | 6.7 | Testis | ||
| Spag6.dAug10 | 49.7 | 6.8 | Testis, brain, medulla, hippo- campus, trachea, corpus callosum, hypothalamus, other tissue | ||
| Spag6.eAug10 | 38.0 | 8.9 | Lung, mucoepidermoid carcinoma, brain, carcinoid, hippocampus | ||
| Spag6.fAug10 | 30.6 | 9.9 | Brain, medulla | ||
| Spag6.gAug10 | 28.7 | 8.3 | Brain, medulla | ||
| Spag6.hAug10 | 23.1 | 7.2 | Pooled germ cell tumors, pooled lung and spleen | ||
| Spag6.iAug10 | 22.6 | 11.4 | Hippocampus, substantia nigra | ||
| Spag6.jAug10 | 22.6 | 8.6 | Insulinoma, pancreas, brain | ||
| Spag6.kAug10 | 17.6 | 6.2 | |||
| Spag6.lAug10 | 9.3 | 9.3 | Lung from mucoepidermoid carcinoma | ||
| Spag6.mAug10 | 7.6 | 8.6 | Testis | ||
| Spag6.nAug10 | 4.1 | 7.0 | Eye | ||
| Homo sapiens | SPAG16 | Spag16.aAug10 | 70.8 | 6.4 | Testis, brain, hypothalamus, myeloma, pooled colon, kidney, stomach, trachea, embryonic stem, other tissues |
| Spag16.bAug10 | 65.2 | 6.9 | Breast carcinoma, hippocampus, insulinoma, pancreas,testis, brain, hippocampus,other tissues | ||
| Spag16.cAug10 | 61.2 | 8.3 | Testis, lung, spleen | ||
| Spag16.dAug10 | 50.7 | 8.4 | Testis, pancreas | ||
| Spag16.eAug10 | 42.8 | 6.9 | Trachea | ||
| Spag16.fAug10 | 29.1 | 8.2 | Germinal center B cell, hippocampus | ||
| Spag16.gAug10 | 23.6 | 9.3 | Brain, testis | ||
| Spag16.hAug10 | 21.3 | 5.4 | Brain | ||
| Spag16.iAug10 | 20.6 | 4.3 | Lung, germinal center B cell, kidney, pancreas, prostate,brain, neuroblastoma, other tissues | ||
| Spag16.jAug10 | 21.4 | 10.3 | Hippocampus | ||
| Spag16.kAug10 | 20.3 | 5.7 | Pooled germ cell tumors | ||
| Spag16.lAug10 | 17.1 | 5.8 | |||
| Spag16.mAug10 | 16.2 | 6.1 | Insulinoma from pancreas | ||
| Spag16.nAug10 | 11.8 | 5.8 | |||
| Spag16.oAug10 | 12.5 | 8.1 | Liver, spleen | ||
| Spag16.pAug10 | 11.9 | 5.7 | Colon, colon tumor, RER + , eyeball | ||
| Spag16.qAug10 | 9.2 | 9.7 | Pancreas | ||
| Spag16.rAug10-unspliced | 6.0 | 9.2 | Kidney | ||
| Spag16.sAug10-unspliced | 3.2 | 7.7 | |||
| Spag16.tAug10 | 2.9 | 7.4 | Testis | ||
| Spag16.uAug10-unspliced | 2.8 | 4.6 | Gall bladder | ||
| Spag16.vaAug10-unspliced | 3.9 | 3.3 | Uterus tumor | ||
| Homo sapiens | SPAG17 | Spag17.aAug10 | 251.7 | 6.6 | Testis, lung, breast, breast carcinoma, carcinoid, pooled-skin, other tissues |
| Spag17.bAug10 | 128.9 | 6.2 | Kidney tumor, moderately- differentiated endometrial adenocarcinoma, testis, uterus | ||
| Spag17.cAug10 | 121.0 | 6.5 | Trachea | ||
| Spag17.dAug10 | 68.4 | 6.9 | Trachea, eye | ||
| Spag17.eAug10 | 39.7 | 8.3 | Lung | ||
| Spag17.fAug10 | 31.0 | 5.0 | Lung | ||
| Spag17.gAug10 | 27.2 | 8.2 | Lung | ||
| Spag17.hAug10 | 20.3 | 8.4 | |||
| Spag17.iAug10-unspliced | 14.0 | 8.8 | Lung | ||
| Spag17.jAug10 | 13.7 | 8.9 | Liver, spleen | ||
| Spag17.kAug10 | 11.9 | 6.3 | Liver, spleen | ||
| Spag17.lAug10 | 11.6 | 9.3 | |||
| Spag17.mAug10 | 8.7 | 8.5 | |||
| Spag17.nAug10 | 7.5 | 8.6 | |||
| Spag17.oAug10-unspliced | 4.9 | 6.5 | Stomach | ||
| Spag17.pAug10-unspliced | 6.5 | 10.3 | Pooled skin | ||
| Spag17.qAug10-unspliced | 5.0 | 8.0 | Placenta | ||
| Spag17.rAug10 | 11.6 | 9.3 | Kidney | ||
| Spag17.sAug10 | 9.2 | 3.8 | Lung | ||
| Mus musculus | Spef2 (C230086A09Rik) | Spef2.aSep07 | 100.1 | 7.6 | Testis, testicles, brain, thymus, kidney, lung, spleen, liver, heart, ovary, embryo |
| Spef2.bSep07 | 53.9 | 8.4 | Testis, thymus, cerebellum | ||
| Spef2.cSep07 | 21.9 | 8.9 | Testis | ||
| Spef2.dSep07 | 13.4 | 6.4 | |||
| Spef2.eSep07-unspliced | 10.1 | 9.6 | Cerebellum, endoderm, rathke’s pouches | ||
| Mus musculus | Hydin | Hydin.aSep07 | 581.5 | 6.6 | Testis, testicles, 18-day preleptotene spermatocytes, brain,ovary |
| Hydin.bSep07 | 150.0 | 6.5 | Lung | ||
| Hydin.cSep07-unspliced | 54.9 | 6.6 | Cortex, whole body | ||
| Hydin.dSep07 | 8.9 | 12.1 | Testis, unfertilized egg | ||
| Hydin.eSep07-unspliced | 7.1 | 6.7 | |||
| Hydin.fSep07 | 10.3 | 7.6 | Testis | ||
| Hydin.gSep07-unspliced | 6.6 | 10.5 | |||
| Mus musculus | Pcdp1 (Gm101) | Pcdp1.aSep07 | 45.9 | 7.4 | Testis, 18-day preleptotene spermatocytes, round spermatids, mammary gland |
| Mus musculus | Spag6 | Spag6.aSep07 | 55.3 | 6.8 | Testis, cerebellum, spinal cord, visual cortex, hypothalamus, corpora quadrigemina, cortex, oviduct, eye |
| Spag6.bSep07 | 47.4 | 6.7 | Testis, 18-day leptotene and zygotene spermatocytes | ||
| Spag6.cSep07-unspliced | 18.1 | 10.9 | Testis, tongue, embryo, liver, pancreas, hippocampus, small intestine, blood, carcinoma, other tissues | ||
| Spag6.dSep07 | 15.7 | 7.9 | Stomach | ||
| Spag6.eSep07 | 14.4 | 8.1 | |||
| Spag6.fSep07 | 11.1 | 7.5 | Stomach, visual cortex | ||
| Spag6.gSep07 | 8.5 | 8.3 | Cerebellum, cortex | ||
| Spag6.hSep07 | 9.6 | 8.5 | Oviduct, lung | ||
| Mus musculus | Spag16 | Spag16.aSep07 | 71.5 | 6.2 | Testis, brain, thymus, testicle, kidney, spleen, liver, heart, lung, ovary, spermatocytes,spinal cord |
| Spag16.bSep07 | 47.0 | 6.1 | Testis, brain, thymus, testicle, kidney, spleen, liver, heart, lung, ovary, spermatocytes,spinal cord | ||
| Spag16.cSep07 | 45.9 | 7.1 | Testis, embryo, spinal cord, hypothalamus, oocyte, other tissues | ||
| Spag16.dSep07 | 36.4 | 6.3 | |||
| Spag16.eSep07 | 34.1 | 6.4 | Testis, hypothalamus, testicles | ||
| Spag16.fSep07 | 27.0 | 7.0 | In vitro fertilized eggs | ||
| Spag16.gSep07 | 21.0 | 5.1 | Testis | ||
| Mus musculus | Spag17 | Spag17.aSep07 | 249.3 | 6.4 | Testis, lung, cortex, cerebellum, rathke’s pouches, testicles, 18-day leptotene and zygotene spermatocytes |
| Spag17.bSep07 | 112.9 | 6.3 | Testis, 18-day leptotene and zygotene spermatocytes | ||
| Spag17.cSep07 | 33.5 | 5.6 | Testis, 18-day preleptotene spermatocytes, round spermatids | ||
| Spag17.dSep07 | 23.5 | 5.3 | Testis, head, lung, thymus | ||
| Spag17.eSep07 | 18.8 | 7.7 | Cerebellum | ||
| Spag17.fSep07-unspliced | 16.4 | 8.6 | Cerebellum | ||
| Spag17.gSep07 | 15.0 | 7.3 | Testicles |
Data were extracted from the NCBI AceView database.
Table II.
Phenotypes of CPC Gene Mutations in Mice
| Gene | Survival | Airway cilia motility | Hydrocephalus | Sperm motility | Male fertility | Female fertility | Mouse background | References |
|---|---|---|---|---|---|---|---|---|
| Spef2 | Variable, some animals died within a few days after birth and a small number lived longer than 50 days | Reduced CBF | Yes | ? | Infertile | ? | C57BL/6J | Sironen et al., 2011 |
| Survival to adulthood | ? | No | short tail and abnormal flagellar shape | Infertile | ? | mixed C57BL/6J-129S6/SvEvTac | ||
| Hydin | Mostly death by 3 weeks after birth | Reduced CBF Impaired ciliary bending |
Yes | ? | ? | ? | FVB/N |
Mclone et al., 1971 Raimondi et al., 1976 Davy and Robinson,2003 Lechtreck et al., 2008 |
| Pcdp1 | Frequently death within 1 week after birth | ? | Yes | ? | ? | ? | C57BL/6J | Lee et al., 2008 |
| Survival to adulthood | Reduced CBF | Mild or No | No mature sperm | Infertile | ? | mixed C57BL/6J-129S6/SvEvTac | ||
| Cfap54 | Early mortality | ? | Yes | ? | ? | ? | C57BL/6J | McKenzie et al.,2015 |
| Normal | Reduced CBF and cilia-driven flow | Mild | Only few sperm in the epididymis with short tail | Infertile | mixed C57BL/6J-129S6/SvEvTac | |||
| Spag6 | 50% survival to adulthood | Reduced CBF Uncoordinated beat | Yes | Quaking motion | Infertile | Normal | mixed 129/SvJ- C57BL/6J |
Sapiro et al., 2000
Sapiro et al., 2002 Teves et al., 2014 |
| Spag16 | Normal | Grossly normal | No | Quaking motion | Infertile | Normal | mixed 129/SvJ- C57BL/6J |
Zhang et al., 2002 Zhang et al., 2004 |
| Spag17 | Neonatal Death | Mostly immotile Some uncoordinated beat | Yes | ? | ? | ? | mixed C57BL/6J-129S4/SvJ | Teves et al., 2013 |
Hydin
HYDIN is a polypeptide of >500 KDa encoded by a conserved gene present in the genomes of various protists and metazoans. Although, the transcript encoding the full length protein is well known, other smaller mRNAs have been identified (Tables I and Table III). This protein is a component of the C2b projection [Davy and Robinson, 2003; Lechtreck and Witman, 2007] (Fig. 2). Hydin mutant mice develop lethal hydrocephalus with perinatal onset, and early demise before seven weeks of life [Mclone et al., 1971; Raimondi et al., 1976; Davy and Robinson, 2003; Lechtreck et al., 2008] (Table II). Cilia from trachea and ependymal cells lack the C2b central pair projection and have impaired motility with reduced ciliary beat frequency CBF, uncoordinated beat and altered waveform [Lechtreck et al., 2008]. Knockdown of Hydin in Chlamydomonas results in severe flagellar motility defects [Lechtreck and Witman, 2007]. Flagella also are shorter than those of wild type cells, with some cells lacking flagella completely. Ultrastructural studies revealed axonemes lacking C2b and part of C2c projections [Lechtreck and Witman, 2007]. In humans, recessive HYDIN mutations cause primary ciliary dyskinesia (PCD) without randomization of left-right body asymmetry. Respiratory cilia from affected individuals lack the C2b projections as reported in mouse and Chlamydomonas models, and show markedly reduced beating amplitudes [Olbrich et al., 2012].
CFAP221/Pcdp1
The novel murine Pcdp1 gene encodes a 2.5 kb transcript [Lee et al., 2008] and yields a protein ~97 kDa (Pcdp1, primary ciliary dyskinesia protein 1). Mutation of this gene yields several phenotypes commonly associated with primary ciliary dyskinesia. Homozygous mutants on a C57BL/6J background develop severe hydrocephalus and mainly die within the first week of life. However, on other genetic backgrounds (129S6/SvEvTac) mice develop either mild or no hydrocephalus with survival to adulthood (Table II). The male mice are infertile, producing sperm with no visible flagella. Interestingly, the respiratory epithelial cilia have a normal ultrastructure but beat with reduced frequency, and there is an abnormal accumulation of mucus in the mutant sinuses [Lee et al., 2008]. The presence of structurally normal cilia and lack of sperm with mature flagella suggest that the mechanisms regulating the biogenesis of cilia and flagella are likely to be different [Lee, 2011]. FAP221, the orthologue of mammalian Pcdp1, shares 24% amino acid sequence identity when the entire protein sequences are aligned (Table IV). Both Pcdp1 and FAP221 have calmodulin binding sites, and calmodulin preferentially binds to both in the presence of high calcium concentrations [DiPetrillo and Smith, 2010; DiPetrillo and Smith, 2011]. FAP221 together with other proteins (FAP54, FAP46, FAP74 and C1d-87) and calmodulin form a single complex that localizes to the C1d projection [DiPetrillo and Smith, 2010; Brown et al., 2012]. Deficiency of this complex results in severe flagellar motility defects [DiPetrillo and Smith, 2010; DiPetrillo and Smith, 2011; Brown et al., 2012].
Table IV.
Amino Acid Sequence Identity and Similarity of CPC Proteins in Mice and Chlamydomonas (Mouse/Chlamydomonas)
| Proteina | Theoretical Molecular weight (kDa)a | Theoretical Isoelectric Pointa | Identity | Similarity |
|---|---|---|---|---|
| SPEF2/CPC1 | 199/205 | 5.62/5.3 | 24% | 34% |
| HYDIN | 582/488 | 6.33/6.01 | 29% | 44% |
| Pcdp1/FAP221 | 97/104 | 8.66/6.75 | 24% | 34% |
| CFAP54/FAP54 | 353/317 | 8.37/7.79 | 23% | 10% |
| SPAG6/PF16 | 55/60 | 6.76/5.94 | 65% | 81% |
| SPAG16/PF20 | 71/66 | 6.01/7.86 | 35% | 52% |
| SPAG17/PF6 | 263/237 | 6.05/4.84 | 20% | 28% |
Mouse/Chlamydomonas.
Alignments were performed for the full length orthologues, isoforms with MacVector Software, V 12.7.5. The percentage of identity and similarity is based on the entire protein sequence alignment.
CFAP54/Cfap54
The Chlamydomonas CFAP54 gene encodes a protein of 3,081 amino acids (Table I). This protein is important for the normal assembly of the C1d projection into the axoneme [Brown et al., 2012]. It appears to form a heterotrimeric sub-complex with FAP46 and FAP74 proteins. However, it may directly bind to the C1 central pair microtubule and calmodulin [Brown et al., 2012]. The mouse Cfap54 gene is located on chromosome 10. There are several transcripts derived from this gene described in public databases (Table I). Recently, Cfap54 mutant mice were generated and maintained on two different backgrounds. Cfap54 mutant mice on the C57BL/6J background develop severe hydrocephalus which results in early mortality (Table II). In contrast, mutant mice on mixed C57BL/6J-129S6/SvEvTac background develop only mild hydrocephalus and exhibit early mortality. Males are infertile due to defects in spermatogenesis. Flagellar structures are mostly absent in testicular sperm, and few sperm can be found in the epididymis with most possessing short tails. Respiratory epithelial cilia have reduced beat frequency, which results in perturbed cilia-driven flow. Ultrastructural studies reveal disorganized axonemal microtubules in the few flagella present in the testis. While tracheal epithelial cilia have a 9 + 2 structure, they lack the C1d projection. These findings suggest that CFAP54 is required for assembly and function of mammalian flagella and motile cilia [McKenzie et al., 2015].
PF16/Spag6
The Chlamydomonas PF16 gene encodes a protein of ~60 kDa, containing eight contiguous 42 amino acid armadillo repeats, which are important for protein-protein interactions. Therefore, the PF16 gene product has features that promote interactions with other proteins to perform structural or regulatory functions necessary for central microtubule stability and flagellar motility [Smith and Lefebvre, 1997a,b]. Chlamydomonas pf16 mutants show severe flagellar motility defects and the C1 central microtubule is lost after demembranation of the flagella [Smith and Lefebvre, 1996; Mitchell and Smith, 2009]. The murine orthologue of PF16, SPAG6 also contains eight contiguous armadillo repeats [Sapiro et al., 2000]. Several different transcripts encoding mouse SPAG6 are present in public databases (Tables I and III).
Mice deficient in SPAG6 develop hydrocephalus with early demise of 50% of the animals on a mixed 129/SvJ-C57BL/6J background [Sapiro et al., 2002] (Table II). The surviving mutants are infertile due to functional and structural defects in the sperm flagellar axoneme. The epididymal sperm show a twitching motion, some lack heads and/or have a truncated flagellum and an abnormal midpiece. Ultrastructural studies revealed missing central pairs in the axonemes and disorganized outer dense fibers [Sapiro et al., 2000]. These phenotypes are consistent with the expected function of a protein present in the CPC. However, further characterization of the Spag6 mutant mice revealed reduced and uncoordinated cilia beat, low cilia density, random basal feet orientation, and reduced epithelial cell polarity [Teves et al., 2014]. Mutation of the Spag6 gene also results in disorganization of ciliary basal feet in the middle ear, and disrupted planar cell polarity in the middle ear and Eustachian tubes [Li et al., 2014]. Although, these phenotypes may be a consequence of a primary defect in ciliary beating, they may also reflect unique functions of SPAG6 outside of the expected CPC structure, as described below. Studies have shown that SPAG6 is present along the cylindrical outer hair cells (OHCs) in addition to the expected kinocilium localization [Wang et al., 2015]. Its expression in OHC cells appears to be indispensable for the stability and mechanosensory function of these cells [Wang et al., 2015]. Moreover, expression of Spag6 in Chinese Hamster Ovary (CHO) cells (cells without 9 + 2 axoneme structure) revealed that SPAG6 decorates microtubule structures [Sapiro et al., 2000; Zhang et al., 2002; Zhang et al., 2005]. The association with microtubules and the armadillo repeat domains of SPAG6 suggest that SPAG6 plays a role in the function of tubulin-containing organelles, besides the central pair of microtubules of motile cilia and flagella [Li et al., 2015a,b]. We have recently shown that mutation of the Spag6 gene disturbs cytoskeleton components and affects cell division, morphology, adhesion, and migration. It also reduced acetylation of α-tubulin [Li et al., 2015a,b], which is one of the more important micro-tubule post-translational modifications. Conversely, overexpression of SPAG6 in CHO and COS-1 cells increases acetylated-tubulin. Similarly, overexpression of Spag6 in Spag6 mutant mouse embryonic fibroblast (MEFs) increases acetylated-tubulin [Li et al., 2015a,b]. Recognizing that SPAG6 is a microtubule-associated protein not exclusively confined to the CPC microtubules, these findings may explain a number of the phenotypes observed in Spag6 mutant mouse cells that are not related to tissues with motile cilia. In summary, mammalian SPAG6 may not only regulate ciliary/flagellar motility (functions related to its localization in the CPC), it also plays a role in ciliogenesis, the control of axoneme orientation, cell polarity, division, morphology, adhesion and migration.
A duplication of the Spag6 gene (Spag6-BC061194 or Spag6l), located on chromosome 2qA3 (chr2:18,620,650-18,672,040), was reported in the mouse and rat [Qiu et al., 2013]. The first characterized Spag6 mutant mouse had a targeted Spag6 mutation located on chromosome 16. This mutant retains the Spag6-BC061194 gene [Teves et al., 2014]. The amino acid sequences of the two SPAG6 proteins are 97% identical, but the nucleotide sequences of the two Spag6 genes are significantly different [Teves et al., 2014]. It is not clear yet, whether Spag6-BC061194 is functional or it is a pseudogene.
PF20/Spag16
The algae PF20 gene encodes a protein of ~66 kDa which contain five contiguous WD repeats in the carboxyl-terminal half of the protein [Mitchell and Smith, 2009]. These WD repeats are important for interaction with other proteins [Neer et al., 1994]. PF20 also contains regions rich in basic and acidic amino acids as well as alanine-rich regions [Smith and Lefebvre, 1997a,b; Mitchell and Smith, 2009]. PF20 localizes along the length of the C2 microtubule on the intermicrotubule bridges connecting the two CPC microtubules (Fig. 2). The flagella of Chlamydomonas pf20 mutants have severe motility defects, and upon isolation of flagella and demembranation to prepare axonemes, the mutant axonemes usually lack the entire CPC [Smith and Lefebvre, 1997a,b; Mitchell and Smith, 2009]. The murine orthologue of pf20 is Spag16 which encodes two major transcripts of 1.4 and 2.5 kb [Zhang et al., 2002] and yields proteins of 35 (SPAG16S) and 71 (SPAG16L) kDa, respectively [Zhang et al., 2004]. SPAG16L is found in all murine cells with motile cilia and flagella, and SPAG16S is expressed only in male germ cells, where it is predominantly found in specific regions within the nucleus known as nuclear speckles [Zhang et al., 2004; Nagarkatti-Gude et al., 2011]. The 71 kDa isoform is the orthologue of PF20 and contains a coiled-coil region followed by up to seven contiguous WD repeats in the C-terminus [Zhang et al., 2004; Zhang et al., 2007a,2007b; Nagarkatti-Gude et al., 2011]. Several CPC proteins were shown to interact with SPAG16. SPAG6 co-immunoprecipitates with SPAG16 in extracts of CHO cells expressing both proteins [Zhang et al., 2002]. Similarly, the kinase, Stk36, physically associates with SPAG16 after expression of both proteins in HEK 293T cells [Wilson et al., 2009; Nozawa et al., 2013]. However, Stk36 does not interact with SPAG6 [Wilson et al., 2009]. Recently, it has been shown that the manchette (a unique organelle involved in the assembly of the sperm flagella) localization of SPAG16L in elongating spermatids is dependent upon MEIG1 and PACRG proteins [Li et al., 2015a,b]. Targeted disruption of Spag16 exon 11 affects the expression of both SPAG16L and SPAG16S, and results in spermatogenesis defects in chimeric mice [Zhang et al., 2004]. In contrast, a targeted mutation that deletes exon 2 and 3 of the Spag16 gene affects only SPAG16L expression. The resulting null mice show no evidence of primary ciliary dyskinesia phenotypes including hydrocephalus, or sinusitis, [Zhang et al., 2006], and display tracheal epithelial cells with motile cilia [Teves et al., 2013]. Males have normal spermatogenesis but are infertile due to severe sperm motility defects, even though the sperm have a normal axoneme ultrastructure [Zhang et al., 2006] (Table II).
Functional studies have been carried out on mouse sperm lacking SPAG16L [Lesich et al., 2010]. Spag16L-null sperm exhibited much less bending of the flagellum during the beat, and the amount of microtubule sliding in the reverse bend direction was selectively restricted. This suggests that there is limited activation of the dyneins on one side of the axoneme. Additionally, the flagellar response to calcium was greatly reduced in this model. This response requires activation of the dyneins on specific outer microtubule doublets; the same dyneins required for reverse bend formation. In axonemes prepared to disintegrate in vitro as a result of microtubule sliding, little or no extrusion of specific microtubule doublets was observed, a finding consistent with reduced activity of their dyneins. This deficit in motor function can explain the observed motility characteristics of the Spag16L-null sperm [Lesich et al., 2010].
A heterozygous mutation disrupting the human SPAG16 gene results in biochemical instability of the interacting proteins of the central apparatus in response to a thermal challenge [Zhang et al., 2007a,b]. Freezing-boiling of isolated sperm from two male heterozygotes for the SPAG16 mutation, resulted in the loss of the SPAG16L protein, SPAG6, and the 28-kDa fragment of SPAG17 [Zhang et al., 2007a,2007b]. Ultrastructure of sperm from these subjects revealed axonemes that are missing the central pair, and the outer double microtubules [Zhang et al., 2007a,b].
Double knockouts of Spag6 and Spag16 have been created revealing synergistic interactions of the two genes on cilia-related phenotypes [Zhang et al., 2007a,b]. The double mutant mice displayed more profound growth retardation and hydrocephalus compared to mice nullizygous for SPAG6. They also died younger, and mortality was significantly greater. The double mutant mice developed pneumonia and its complications, including hemorrhage, edema, and atelectasis [Zhang et al., 2007a,b]. These lung pathologies were not observed in mice nullizygous for a mutation in either single gene. In addition, the ultrastructure of cilia in both brain and lung of the double mutant mice appeared to be grossly normal [Zhang et al., 2007a,b].
PF6/Spag17
The Chlamydomonas PF6 gene encodes a large polypeptide (2301 amino acids) that contains alanine-rich (18%), proline-rich (12%), and highly basic domains [Mitchell and Smith, 2009]. Domains near the C-terminus of PF6 are essential for flagellar motility and assembly of C1a projection [Goduti and Smith, 2012]. Meanwhile, the N-terminal half is not required for the assembly but it is important for stability of the C1a complex [Goduti and Smith, 2012]. Flagella from pf6 mutants twitch ineffectively, and lack the C1a projection, and some polypeptides that co-sediment and co-immunoprecipitate with PF6 protein [Rupp et al., 2001; Mitchell and Smith, 2009]. SPAG17 is the mammalian orthologue of PF6. The murine Spag17 gene encodes a full-length 250 kDa protein found in testis and tissues with motile cilia, and a 97 kDa protein present in testis that derived from an alternatively spliced variant. The 97 kDa protein is cleaved to generate 72 kDa (N-terminus) and 28 kDa (C-terminus) fragments in epididymal and ejaculated sperm [Zhang et al., 2005]. Moreover, there is a maturation-dependent pattern of SPAG17 isoform expression in fractionated germ cells with the 97 kDa isoform appearing prior to the 250 kDa isoform, and the appearance of 72 and 28 kDa fragments of the 97 kDa isoform in epididymal sperm. The 28 kDa SPAG17 isoform is missing in SPAG6-deficient mice, supporting the hypothesis that the C-terminus of the 97 kDa SPAG17 isoform, of which the 28 kDa form is a fragment, is the interacting partner with SPAG6 in the CPC [Zhang et al., 2005]. Spag17-null mice have an expected primary ciliary dyskinesia phenotype characterized by immotile nasal and tracheal cilia, reduced clearance of nasal mucus, profound respiratory distress associated with lung fluid accumulation and disruption of the alveolar epithelium, hydrocephalus, and neonatal demise within 12 hours of birth (Table II). Electron microscopy analysis revealed the loss of one CP microtubule in approximately one quarter of tracheal cilia axonemes, the absence of a C1 microtubule projection, and other less frequent CPC structural abnormalities [Teves et al., 2013]. However, further characterization of Spag17 mutant revealed unexpected functions of Spag17 in regulation of skeletal growth and mineralization. Mutants show shorter hind limbs due to increased bone formation and mineralization in the femur, and reduced growth plate and bone formation in the tibia. Other skeletal malformations include fused sternebrae, reduced mineralization in the skull, medial and metacarpal phalanges. Primary cilia from chondrocytes, osteoblasts, and embryonic fibroblasts (MEFs) are shorter and fewer cells have primary cilia in comparison to cells isolated from wild-type mice [Teves et al., 2015]. Interestingly, an antibody against the C-terminal domain of SPAG17 detected a ~75 kDa protein expressed in MEFs, suggesting that it is an isoform of the 250 kDa full length protein. This ~75 kDa protein may be derived from alternative splicing or post-translational processing. These findings reveal an unexpected expression pattern and function of the Spag17 gene. In addition, they point out several differences between the mammalian gene and its orthologue in algae. Interestingly, single-nucleotide polymorphisms (SNPs) in the SPAG17 gene have been associated with human height [Weedon and Frayling, 2008; Weedon et al., 2008; Takeuchi et al., 2009; Zhao et al., 2010; N’Diaye et al., 2011; van der Valk et al., 2014; Wood et al., 2014] and idiopathic short stature in a Korean population [Kim et al., 2010]. These data are consistent with the shorter hind limbs observed in Spag17 mutant mice [Teves et al., 2015].
Radial Spoke Mutations
The radial spokes play an essential role in the control of dynein arm activity by transmitting signals from the central pair complex to the dynein arms. Defects in the CPC, including loss of one or both CPC microtubule have been reported in families with mutations that disrupt genes encoding radial spoke head (RSPH) proteins [Castleman et al., 2009]. The abnormalities in the CPC in individuals with mutations in the radial spoke head genes RSPH1 [Kott et al., 2013; Onoufriadis et al., 2014], RSPH3 [Jeanson et al., 2015], RSPH9 and RSPH4A [Castleman et al., 2009; Zie˛tkiewicz et al., 2012] suggest that the nexus between the CPC and radial spokes is critical for stabilizing the human cilia axoneme, as in Chlamydomonas [Mitchell and Smith, 2009].
Positive Selection of CPC Genes
The positive and negative selection of genes has played a key role in the generation of the enormous diversity of species observed in the nature. Mutations in coding regions of genes that change the resulting amino acid sequence of the protein have been positively selected and there are numerous studies documenting positive selection in a variety of genes and organisms, including immune-response-related genes, viral genes, genes involved in reproduction, and genes related to sensory perception and olfaction in humans [Nielsen, 2005]. Evidence suggests that genes involved in reproduction are evolving rapidly, especially proteins present in the sperm [Torgerson et al., 2002; Vicens et al., 2014]. Several CPC proteins were shown to be positively selected in mammals. For example, SPAG17 was reported to be one of a small number of genes that distinguishes humans from Neanderthals [Green et al., 2010], and its encoding protein was suggested to be positively selected [Torgerson et al., 2002]. Nucleotide substitutions were detected at amino acid positions 431 (humans have a Tyr and Neanderthals and mice have a Asp), and 1415 (humans have a Thr, Neanderthals have an Ala and mice have a Lys) [Green et al., 2010]. SPAG6 is another CPC gene that has undergone positive selection [Williamson et al., 2007], although its selection is more likely to be related to transcriptional control than to a change in the protein sequence [Hu et al., 2012]. As mentioned earlier, the Spag6 gene was recently discovered to be duplicated in rodents. The newly identified, highly divergent Spag6-BC061194 gene is the parental gene [Qiu et al., 2013]. It has been proposed that the Spag6 gene represents a rare exception from the general rule of rapid evolution of derived rather than parental genes following gene duplication [Qiu et al., 2013].
Central Pair Complex Isoforms
The orthologous CPC proteins present in mouse and Chlamydomonas have variable amino acid sequence identity and similarity that presumably reflect conserved functions as well as evolving roles for these molecules (Table IV). SPAG6 is highly conserved whereas SPAG17 shows the least amino acid sequence similarity and identity. In mice and humans, the CPC genes code for a variety of isoforms that are generated by either an alternative promoter, alternative splicing, or post-translational processing [Zhang et al., 2002; Zhang et al., 2004; Zhang et al., 2006; Nagarkatti-Gude et al., 2011] (Table I and III). The expression of isoforms appears to be tissue/cell specific. Although most of the different isoforms were cloned from the same tissues, some of the isoforms appear to be present only in one tissue (e.g., in mus musculus, Spag17.eSep07 and Spag17.fSep07 are only present in the cerebellum; in homo sapiens Spag6.nAug10 is only present in the eye) (Table III). In addition, some of the isoforms appear to be putative proteins (e.g., in homo sapiens Spag6.kAug10; Spag16.lAug10 and Spag16.sAug10; and Spag17.hAug10, Spag17.lAug10, Spag17.mAug10 and Spag17.nAug10 were not observed in any tissue). The fact that these genes encode several isoforms allows for greater complexity in expression pattern and function. However, little is known about the function of these protein isoforms. Most of our knowledge comes from mutants where the full length protein isoform have been disrupted. The exceptions are SPAG17 and SPAG16, where different isoforms or truncated proteins appear to be related to different tissue/cell functions [Zhang et al., 2005; Nagarkatti-Gude et al., 2011; Teves et al., 2015].
Tissue/cell-Specific Roles
Defects in cilia or flagella motility/function caused by mutations in CPC genes appear to depend upon the cellular context and genetic background in mammals. For instance, the presence of structurally normal cilia and lack of sperm with mature flagella in the Pcdp1 knockout mice suggests that the mechanisms regulating the biogenesis of motile cilia and flagella are different [Lee, 2011]. As noted above, Spef2 deficient mice have tracheal epithelial cilia with a normal 9 + 2 axonemal structure without apparent defects in the dynein arms, but epididymal sperm lack recognizable axonemal structures [Sironen et al., 2011]. SPAG16L-null mice show no evidence of cilia dysfunction such as hydrocephalus, sinusitis, and bronchial infection [Zhang et al., 2006], and have tracheal epithelial cells with motile cilia [Teves et al., 2013]. However, males are infertile due to severe sperm motility defects, even though the sperm have a normal axoneme ultrastructure [Zhang et al., 2006]. In the Spag17 mutant mouse, the rapid neonatal demise is associated with a profound respiratory phenotype characterized by immotile cilia that is not observed in knockouts of the Spag6 and Spag16 genes [Teves et al., 2013]. Although the genetic background of mutant mice may significantly influence the phenotypes in knockout mice, the observations summarized above suggest that due to the complexity of sperm flagellar development, the assembly of the sperm flagellar axoneme may be particularly sensitive to mutations in genes encoding axonemal proteins.
Although, the CPC Spag genes were initially analyzed in the context of male reproduction, new studies suggest that these genes play unique roles in different cell types. Overexpression of SPAG6 was identified in acute myeloid leukemia compared to healthy bone marrow [Steinbach et al., 2006]. The levels of expression of this gene have been used to assess minimal residual disease [Steinbach et al., 2015]. In addition, SPAG6 has been shown to be important for microtubule stability in MEFs[Li et al., 2015a,b]. SPAG6 also appears to control neuronal migration as well as neurite branching and elongation in mice [Yan et al., 2015]. Expression of SPAG16 in synovium and fibroblast-like synoviocytes has been suggested to protect against joint destruction in autoantibody-positive rheumatoid arthritis [Knevel et al., 2014]. This gene is also associated with bipolar disorder in Canadian and UK populations [Xu et al., 2014]. In addition, SPAG16 is reported to be upregulated in astrocytes in multiple sclerosis patients and encephalo-myelitis spinal cord lesions [de Bock et al., 2014]. Expression of SPAG16L, SPAG6 and SPAG17 is associated with several cancers [Silina et al., 2011]. However, the seemingly abnormal expression of these genes needs to be further studied to determine whether these observations are related to novel functions and disease pathophysiology. On the other hand, expression of Spag17 by mouse chondrocytes and osteoblasts appears to play a role in skeletal development, linear growth and bone mineralization [Teves et al., 2015]. This extends the role of Spag17 gene outside tissues with motile 9 + 2 cilia. It is not clear yet if this new function is directly associated to bone and cartilage physiology or it is mediated through defects in development and function of primary cilia, or a combination of both.
Conclusions
Ciliary and flagellar genes, proteins and functions are believed to be conserved throughout evolution from protists to humans [Ibanez-Tallon et al., 2003; Mitchell, 2004; Carvalho-Santos et al., 2011]. Most of the studies of CPC proteins have been performed in Chlamydomonas. However, during the past decade, the field of ciliary/flagellar biology has grown tremendously due to advances in technology and gene-targeted models. These studies have pointed out significant differences in the roles of the CPC proteins in Chlamydomonas and mammals, including variability in the architecture, function, and biogenesis of the axoneme components [Wanner et al., 1996; Silflow and Lefebvre, 2001; Carvalho-Santos et al., 2011]. Evidence also suggests that in mammals, the CPC proteins (SPAG6, SPAG16 and SPAG17) have functions outside of the CPC. They also interact with each other [Zhang et al., 2002; Zhang et al., 2005] and might work as a complex. These interactions have not been identified or evaluated in Chlamydomonas [Neilson et al., 1999; Sapiro et al., 2000; Sapiro et al., 2002; Zhang et al., 2002; Zhang et al., 2005]. To date, no evidence for functions of SPEF2, HYDIN, Pcdp1 and CFAP54 outside the CPC have been reported. In Chlamydomonas, the CPC genes encoding orthologues of mammalian proteins yield only a single protein product, while in mice and humans, these genes encode a variety of isoforms that are generated either by an alternative promoter, alternative splicing, or post-translational processing [Zhang et al., 2002; Zhang et al., 2004; Zhang et al., 2006; Nagarkatti-Gude et al., 2011]. However, little is known about these protein isoforms, and further studies are required to determine whether they have distinct functional roles. That being said, we do have clear indication that different isoforms of SPAG17 and SPAG16, have different tissue/cellular locations than their full length sisters and different functions [Zhang et al., 2005; Nagarkatti-Gude et al., 2011; Teves et al., 2015]. The greater complexity of mammalian CPC proteins and functions provides a foundation for the elucidation of the mechanisms underlying ciliary/flagellar motility, and a better understanding of the molecular and structural basis of the genetically and phenotypically heterogeneous disorder of primary ciliary dyskinesia.
Acknowledgments
DRN-G was supported by National Institutes of Health-NICHD grant 5F31HD062314. ZZ is supported by National Institutes of Health grant R01HD076257. JFS was supported by a National Institutes of Health-NICHD grant R01HD037416.
Abbreviations used
- ARM
armadillo
- CaM
calmodulin
- CBF
Ciliary beat frequency
- CHO
Chinese hamster ovary
- CPC
central pair complex
- PCD
primary ciliary dyskinesia
- RSPH
radial spoke head
References
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annual Rev Genom Hum Gene. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Brown JM, Witman GB. Cilia and diseases. Bioscience. 2014;64:1126–1137. doi: 10.1093/biosci/biu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JM, DiPetrillo CG, Smith EF, Witman GB. A FAP46 mutant provides new insights into the function and assembly of the C1d complex of the ciliary central apparatus. J Cell Sci. 2012;125:3904–3913. doi: 10.1242/jcs.107151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajal-González BI, Heuser T, Fu X, Lin J, Smith BW, Mitchell DR, Nicastro D. Conserved structural motifs in the central pair complex of eukaryotic flagella. Cytoskeleton. 2013;70:101–120. doi: 10.1002/cm.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Santos Z, Azimzadeh J, Pereira-Leal JB, Bettencourt-Dias M. Evolution: tracing the origins of centrioles, cilia, and flagella. J Cell Biol. 2011;194:165–175. doi: 10.1083/jcb.201011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleman V, Romio L, Chodhari R, Hirst R, de Castro S, Parker K, Ybot-Gonzalez P, et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet. 2009;84:197–209. doi: 10.1016/j.ajhg.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry A, Rosenbaum J. Flagellar radial spoke: a model molecular genetic system for studying organelle assembly. Cell Motil Cytoskeleton. 1993;24:224–232. doi: 10.1002/cm.970240403. [DOI] [PubMed] [Google Scholar]
- Davy BE, Robinson ML. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum Mol Genet. 2003;12:1163–1170. doi: 10.1093/hmg/ddg122. [DOI] [PubMed] [Google Scholar]
- de Bock L, Somers K, Fraussen J, Hendriks JJA, van Horssen J, Rouwette M, Hellings N, et al. Sperm-associated antigen 16 is a novel target of the humoral autoimmune response in multiple sclerosis. J Immunol. 2014;193:2147–2156. doi: 10.4049/jimmunol.1401166. [DOI] [PubMed] [Google Scholar]
- DiPetrillo CG, Smith EF. Pcdp1 is a central apparatus protein that binds Ca2 +-calmodulin and regulates ciliary motility. J Cell Biol. 2010;189:601–612. doi: 10.1083/jcb.200912009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPetrillo CG, Smith EF. The Pcdp1 complex coordinates the activity of dynein isoforms to produce wild-type ciliary motility. Mol Biol Cell. 2011;22:4527–4538. doi: 10.1091/mbc.E11-08-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher S. Flagellar assembly in two hundred and fifty easy-to-follow steps. Trends Genet. 1995;11:398–404. doi: 10.1016/s0168-9525(00)89123-4. [DOI] [PubMed] [Google Scholar]
- Dutcher S, Huang B, Luck D. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J Cell Biol. 1984;98:229–236. doi: 10.1083/jcb.98.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek EE, Smith EF. A conserved CaM- and radial spoke–associated complex mediates regulation of flagellar dynein activity. J Cell Biol. 2007;179:515–526. doi: 10.1083/jcb.200703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, hHomeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goduti D, Smith E. Analyses of functional domains within the PF6 protein of the central apparatus reveal a role for PF6 sub-complex members in regulating flagellar beat frequency. Cytoskeleton (Hoboken) 2012;69:179–194. doi: 10.1002/cm.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough U, Heuser J. Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J Cell Biol. 1985;100:2008–2018. doi: 10.1083/jcb.100.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermacher G, Sale WS. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J Cell Biol. 1997;136:167–176. doi: 10.1083/jcb.136.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Ayub Q, Guerra-Assuncao J, Long Q, Ning Z, Huang N, Romero I, et al. Exploration of signals of positive selection derived from genotype-based human genome scans using re-sequencing data. Hum Genet. 2012;131:665–674. doi: 10.1007/s00439-011-1111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Piperno G, Luck DJ. Paralyzed flagella mutants of Chlamydomonas reinhardtii. Defective for axonemal doublet micro-tubule arms. J Biol Chem. 1979;254:3091–3099. [PubMed] [Google Scholar]
- Ibanez-Tallon I, Heintz N, Omran H. To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet. 2003;12:R27–R35. doi: 10.1093/hmg/ddg061. [DOI] [PubMed] [Google Scholar]
- Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod. 2011;17:524–538. doi: 10.1093/molehr/gar034. [DOI] [PubMed] [Google Scholar]
- Inaba K. Calcium sensors of ciliary outer arm dynein: functions and phylogenetic considerations for eukaryotic evolution. Cilia. 2015;4:6. doi: 10.1186/s13630-015-0015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanson L, Copin B, Papon JF, Dastot-Le Moal F, Duquesnoy P, Montantin G, Cadranel J, et al. RSPH3 mutations cause primary ciliary dyskinesia with central-complex defects and a near absence of radial spokes. Am J Hum Gene. 2015;97:153–162. doi: 10.1016/j.ajhg.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee H, Park T, Kim K, Lee J, Cho N, Shin C, et al. Identification of 15 loci influencing height in a Korean population. J Hum Genet. 2010;55:27–31. doi: 10.1038/jhg.2009.116. [DOI] [PubMed] [Google Scholar]
- Knevel R, Klein K, Somers K, Ospelt C, Houwing-Duistermaat JJ, van Nies JAB, de Rooy DPC, et al. Identification of a genetic variant for joint damage progression in autoantibody-positive rheumatoid arthritis. Annals Rheumatic Dis. 2014;73:2038–2046. doi: 10.1136/annrheumdis-2013-204050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kott E, Legendre M, Copin B, Papon JF, Dastot-Le Moal F, Montantin G, Duquesnoy P, et al. Loss-of-function mutations in RSPH1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am J Hum Gene. 2013;93:561–570. doi: 10.1016/j.ajhg.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Witman GB. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J Cell Biol. 2007;176:473–482. doi: 10.1083/jcb.200611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Delmotte P, Robinson ML, Sanderson MJ, Witman GB. Mutations in Hydin impair ciliary motility in mice. J Cell Biol. 2008;180:633–643. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. Mechanisms of mammalian ciliary motility: insights from primary ciliary dyskinesia genetics. Gene. 2011;473:57–66. doi: 10.1016/j.gene.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Lee L, Campagna DR, Pinkus JL, Mulhern H, Wyatt TA, Sisson JH, Pavlik JA, et al. Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol Cell Biol. 2008;28:949–957. doi: 10.1128/MCB.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesich KA, Zhang Z, Kelsch CB, Ponichter KL, Strauss JF, III, Lindemann CB. Functional deficiencies and a reduced response to calcium in the glagellum of mouse sperm lacking SPAG16L. Biol Reprod. 2010;82:736–744. doi: 10.1095/biolreprod.109.080143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xu L, Li J, Li B, Bai X, Strauss JF, III, Zhang Z, et al. Otitis media in sperm-associated antigen 6 (Spag6)-deficient mice. PLoS One. 2014;9:e112879. doi: 10.1371/journal.pone.0112879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mukherjee A, Wu J, Zhang L, Teves ME, Li H, Nambiar S, et al. Sperm associated antigen 6 (SPAG6) regulates fibroblast cell growth, morphology, migration and ciliogenesis. Sci Rep. 2015a;5:16506. doi: 10.1038/srep16506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Tang W, Teves ME, Zhang Z, Zhang L, Li H, Archer KJ, et al. A MEIG1/PACRG complex in the manchette is essential for building the sperm flagella. Development. 2015b;142:921–930. doi: 10.1242/dev.119834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie CW, Craige B, Kroeger TV, Finn R, Wyatt TA, Sisson JH, Pavlik JA, et al. CFAP54 is required for proper ciliary motility and assembly of the central pair apparatus in mice. Mol Biol Cell. 2015;26:3140–3149. doi: 10.1091/mbc.E15-02-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclone DG, Bondareff W, Raimondi AJ. Brain edema in the hydrocephalic hy-3 mouse: submicroscopic morphology. J Neuropathol Exp Neurol. 1971;30:627–637. doi: 10.1097/00005072-197110000-00007. [DOI] [PubMed] [Google Scholar]
- Mitchell D. Speculations on the evolution of 9 + 2 organelles and the role of central pair microtubules. Biol Cell. 2004;96:691–696. doi: 10.1016/j.biolcel.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D, Smith B. Analysis of the central pair microtubule complex in Chlamydomonas reinhardtii. Methods Cell Biol. 2009;92:197–213. doi: 10.1016/S0091-679X(08)92013-6. [DOI] [PubMed] [Google Scholar]
- Mitchell DR, Sale WS. Characterization of a chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol. 1999;144:293–304. doi: 10.1083/jcb.144.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Diaye A, Chen G, Palmer C, Ge B, Tayo B, Mathias R, Ding J, et al. Identification, replication, and fine-mapping of Loci associated with adult height in individuals of african ancestry. PLoS Genet. 2011;7:e1002298. doi: 10.1371/journal.pgen.1002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkatti-Gude D, Jaimez R, Henderson S, Teves M, Zhang Z, Strauss J. Spag16, an axonemal central apparatus gene, encodes a male germ cell nuclear speckle protein that regulates SPAG16 mRNA expression. PLoS One. 2011;6:e20625. doi: 10.1371/journal.pone.0020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano I, Kobayashi T, Yoshimura M, Shingyoji C. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J Cell Sci. 2003;116:1627–1636. doi: 10.1242/jcs.00336. [DOI] [PubMed] [Google Scholar]
- Neer E, Schmidt C, Nambudripad R, Smith T. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994;371:297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Neilson L, Schneider P, Van Deerlin P, Kiriakidou M, Driscoll D, Pellegrini M, Millinder S, et al. cDNA cloning and characterization of a human sperm antigen (SPAG6) with homology to the product of the Chlamydomonas PF16 locus. Genomics. 1999;60:272–280. doi: 10.1006/geno.1999.5914. [DOI] [PubMed] [Google Scholar]
- Nielsen R. Molecular signatures of natural selection. Annu Rev Genet. 2005;39:197–218. doi: 10.1146/annurev.genet.39.073003.112420. [DOI] [PubMed] [Google Scholar]
- Nozawa YI, Yao E, Lin C, Yang JH, Wilson CW, Gacayan R, Chuang PT. Fused (Stk36) is a ciliary protein required for central pair assembly and motile cilia orientation in the mammalian oviduct. Developmental Dynamics. 2013;242:1307–1319. doi: 10.1002/dvdy.24024. [DOI] [PubMed] [Google Scholar]
- Oh EC, Katsanis N. Cilia in vertebrate development and disease. Development. 2012;139:443–448. doi: 10.1242/dev.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich H, Schmidts M, Werner C, Onoufriadis A, Loges N, Raidt J, Banki N, et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet. 2012;91:672–684. doi: 10.1016/j.ajhg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoufriadis A, Shoemark A, Schmidts M, Patel M, Jimenez G, Liu H, Thomas B, et al. Targeted NGS gene panel identifies mutations in RSPH1 causing primary ciliary dyskinesia and a common mechanism for ciliary central pair agenesis due to radial spoke defects. Hum Mol Genet. 2014;23:3362–3374. doi: 10.1093/hmg/ddu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski L, Andrews K, Potdar P, Matsuura H, Jetten A, Nettesheim P. Cloning and characterization of KPL2, a novel gene induced during ciliogenesis of tracheal epithelial cells. Am J Respir Cell Mol Biol. 1999;20:675–683. doi: 10.1165/ajrcmb.20.4.3496. [DOI] [PubMed] [Google Scholar]
- Porter M. Axonemal dyneins: assembly, organization, and regulation. Curr Opin Cell Biol. 1996;8:10–17. doi: 10.1016/s0955-0674(96)80042-1. [DOI] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–F42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Golstroke;as A, Grzmil P, Wojnowski L. Lineage-specific duplications of muroidea faim and Spag6 genes and atypical accelerated evolution of the parental Spag6 gene. J Mol Evol. 2013:1–11. doi: 10.1007/s00239-013-9585-9. [DOI] [PubMed]
- Raidt J, Wallmeier J, Hjeij R, Onnebrink JGe, Pennekamp P, Loges NT, Olbrich H, et al. Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respiratory J. 2014;44:1579–1588. doi: 10.1183/09031936.00052014. [DOI] [PubMed] [Google Scholar]
- Raimondi AJ, Clark SJ, McLone DG. Pathogenesis of aqueductal occlusion in congenital murine hydrocephalus. J Neurosurg. 1976;45:66–77. doi: 10.3171/jns.1976.45.1.0066. [DOI] [PubMed] [Google Scholar]
- Rupp G, O’Toole E, Porter ME. The chlamydomonas PF6 locus encodes a large alanine/proline-rich polypeptide that is required for assembly of a central pair projection and regulates flagellar motility. Mol Biol Cell. 2001;12:739–751. doi: 10.1091/mbc.12.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathe M. Regulation of mammalian ciliary beating. Annual Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- Sapiro R, Tarantino LM, Velazquez F, Kiriakidou M, Hecht NB, Bucan M, Strauss JF., III Sperm antigen 6 is the murine homologue of the chlamydomonas reinhardtii central apparatus protein encoded by the PF16 locus. Biol Reprod. 2000;62:511–518. doi: 10.1095/biolreprod62.3.511. [DOI] [PubMed] [Google Scholar]
- Sapiro R, Kostetskii I, Olds-Clarke P, Gerton GL, Radice GL, Strauss JF., III Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol. 2002;22:6298–6305. doi: 10.1128/MCB.22.17.6298-6305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annual Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silflow CD, Lefebvre PA. Assembly and motility of eukaryotic cilia and flagella. Lessons from Chlamydomonas reinhardtii. Plant Physiol. 2001;127:1500–1507. [PMC free article] [PubMed] [Google Scholar]
- Silina K, Zayakin P, Kalnina Z, Ivanova L, Meistere I, Endzelinš E, Abols A, et al. Sperm-associated antigens as targets for cancer immunotherapy: expression pattern and humoral immune response in cancer patients. J Immunotherapy. 2011;34:28–44. doi: 10.1097/CJI.1090b1013e3181fb1064fa. [DOI] [PubMed] [Google Scholar]
- Sironen A, Thomsen B, Andersson M, Ahola V, Vilkki J. An intronic insertion in KPL2 results in aberrant splicing and causes the immotile short-tail sperm defect in the pig. Proc Natl Acad Sci USA. 2006;103:5006–5011. doi: 10.1073/pnas.0506318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironen A, Hansen J, Thomsen B, Andersson M, Vilkki J, Toppari J, Kotaja N. Expression of SPEF2 during mouse spermatogenesis and identification of IFT20 as an interacting protein. Biol Reprod. 2010;82:580–590. doi: 10.1095/biolreprod.108.074971. [DOI] [PubMed] [Google Scholar]
- Sironen A, Kotaja N, Mulhern H, Wyatt TA, Sisson JH, Pavlik JA, Miiluniemi M, et al. Loss of SPEF2 function in mice results in spermatogenesis defects and primary ciliary dyskinesia. Biol Reprod. 2011;85:690–701. doi: 10.1095/biolreprod.111.091132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. Regulation of flagellar dynein by the axonemal central apparatus. Cell Motil Cytoskeleton. 2002;52:33–42. doi: 10.1002/cm.10031. [DOI] [PubMed] [Google Scholar]
- Smith E, Lefebvre P. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J Cell Biol. 1996;132:359–370. doi: 10.1083/jcb.132.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Lefebvre P. PF20 gene product contains WD repeats and localizes to the intermicrotubule bridges in Chlamydomonas flagella. Mol Biol Cell. 1997a;8:455–467. doi: 10.1091/mbc.8.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E, Lefebvre P. The role of central apparatus components in flagellar motility and microtubule assembly. Cell Motil Cytoskeleton. 1997b;38:1–8. doi: 10.1002/(SICI)1097-0169(1997)38:1<1::AID-CM1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Steinbach D, Bader P, Willasch A, Bartholomae S, Debatin KM, Zimmermann M, Creutzig U, et al. Prospective validation of a new method of monitoring minimal residual disease in childhood acute myelogenous leukemia. Clin Cancer Res. 2015;21:1353–1359. doi: 10.1158/1078-0432.CCR-14-1999. [DOI] [PubMed] [Google Scholar]
- Steinbach D, Schramm A, Eggert A, Onda M, Dawczynski K, Rump A, Pastan I, et al. Identification of a set of seven genes for the monitoring of minimal residual disease in pediatric acute myeloid leukemia. Clin Cancer Res. 2006;12:2434–2441. doi: 10.1158/1078-0432.CCR-05-2552. [DOI] [PubMed] [Google Scholar]
- Takeuchi F, Nabika T, Isono M, Katsuya T, Sugiyama T, Yamaguchi S, Kobayashi S, et al. Evaluation of genetic loci influencing adult height in the Japanese population. J Hum Genet. 2009;54:749–752. doi: 10.1038/jhg.2009.99. [DOI] [PubMed] [Google Scholar]
- Teves ME, Sears PR, Li W, Zhang Z, Tang W, van Reesema L, Costanzo RM, et al. Sperm-associated antigen 6 (SPAG6) deficiency and defects in ciliogenesis and cilia function: polarity, density, and beat. PLoS One. 2014;9:e107271. doi: 10.1371/journal.pone.0107271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves ME, Sundaresan G, Cohen DJ, Hyzy SL, Kajan I, Maczis M, Zhang Z, et al. Spag17 Deficiency results in skeletal malformations and bone abnormalities. PLoS One. 2015;10:e0125936. doi: 10.1371/journal.pone.0125936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teves ME, Zhang Z, Costanzo RM, Henderson SC, Corwin FD, Zweit J, Sundaresan G, et al. Sperm-associated antigen-17 gene is essential for motile cilia function and neonatal survival. Am J Respir Cell Mol Biol. 2013;48:765–772. doi: 10.1165/rcmb.2012-0362OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgerson DG, Kulathinal RJ, Singh RS. Mammalian sperm proteins are rapidly evolving: evidence of positive selection in functionally diverse genes. Mol Biol E. 2002;19:1973–1980. doi: 10.1093/oxfordjournals.molbev.a004021. [DOI] [PubMed] [Google Scholar]
- Turner R. Moving to the beat: a review of mammalian sperm motility regulation. Reprod Fertil Dev. 2006;18:25–38. doi: 10.1071/rd05120. [DOI] [PubMed] [Google Scholar]
- van der Valk RJP, Kreiner-Møller E, Kooijman MN, Guxens M, Stergiakouli E, Saaf A, Bradfield JP, et al. A novel common variant in DCST2 is associated with length in early life and height in adulthood. Hum Mol Gene. 2014;24:1155–1168. doi: 10.1093/hmg/ddu510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicens A, Luke L, Roldan ERS. Proteins involved in motility and sperm-egg interaction evolve more rapidly in mouse spermatozoa. PLoS One. 2014;9:e91302. doi: 10.1371/journal.pone.0091302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincensini L, Blisnick T, Bastin P. 1001 model organisms to study cilia and flagella. Biol Cell. 2011;103:109–130. doi: 10.1042/BC20100104. [DOI] [PubMed] [Google Scholar]
- Wang J, Li X, Zhang Z, Wang H, Li J. Expression of prestin in OHCs is reduced in Spag6 gene knockout mice. Neurosci Lett. 2015;592:42–47. doi: 10.1016/j.neulet.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner A, Salathe M, O’Riordan T. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Wargo MJ, Dymek EE, Smith EF. Calmodulin and PF6 are components of a complex that localizes to the C1 microtubule of the flagellar central apparatus. J Cell Sci. 2005;118:4655–4665. doi: 10.1242/jcs.02585. [DOI] [PubMed] [Google Scholar]
- Waters AM, Beales PL. Ciliopathies: an expanding disease spectrum. Pediatric Nephrology (Berlin, Germany) 2011;26:1039–1056. doi: 10.1007/s00467-010-1731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weedon MN, Frayling TM. Reaching new heights: insights into the genetics of human stature. Trends Genet. 2008;24:595–603. doi: 10.1016/j.tig.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Weedon MN, Lango H, Lindgren CM, Wallace C, Evans DM, Mangino M, Freathy RM, et al. Genome-wide association analysis identifies 20 loci that influence adult height. Nat Genet. 2008;40:575–583. doi: 10.1038/ng.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson S, Hubisz M, Clark A, Payseur B, Bustamante C, Nielsen R. Localizing recent adaptive evolution in the human genome. PLoS Genet. 2007;3:e90. doi: 10.1371/journal.pgen.0030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Nguyen C, Chen M, Yang J, Gacayan R, Huang J, Chen J, et al. Fused has evolved divergent roles in vertebrate Hedgehog signalling and motile ciliogenesis. Nature. 2009;459:98–102. doi: 10.1038/nature07883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AR, Esko T, Yang J, Vedantam S, Pers TH, Gustafsson S, Chu AY, et al. Defining the role of common variation in the genomic and biological architecture of adult human height. Nat Genet. 2014;46:1173–1186. doi: 10.1038/ng.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Cohen-Woods S, Chen Q, Noor A, Knight J, Hosang G, Parikh SV, et al. Genome-wide association study of bipolar disorder in Canadian and UK populations corroborates disease loci including SYNE1 and CSMD1. BMC Med Gene. 2014;15:2–2. doi: 10.1186/1471-2350-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Hu X, Zhang Q, Song L, Zhang M, Zhang Y, Zhao S. Spag6 negatively regulates neuronal migration during mouse brain development. J Mol Neurosci. 2015:1–7. doi: 10.1007/s12031-015-0608-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Mitchell DR. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci. 2004;117:4179–4188. doi: 10.1242/jcs.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Sapiro R, Kapfhamer D, Bucan M, Bray J, Chennathukuzhi V, McNamara P, et al. A sperm-associated WD repeat protein orthologous to chlamydomonas PF20 associates with Spag6, the mammalian orthologue of chlamydomonas PF16. Mol Cell Biol. 2002;22:7993–8004. doi: 10.1128/MCB.22.22.7993-8004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kostetskii I, Moss SB, Jones BH, Ho C, Wang H, Kishida T, et al. Haploinsufficiency for the murine orthologue of Chlamydomonas PF20 disrupts spermatogenesis. Proc Natl Acad Sci USA. 2004;101:12946–12951. doi: 10.1073/pnas.0404280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jones BH, Tang W, Moss SB, Wei Z, Ho C, Pollack M, et al. Dissecting the axoneme interactome: the mammalian orthologue of chlamydomonas PF6 interacts with sperm-associated antigen 6, the mammalian orthologue of chlamydomonas PF16. Mol Cell Proteomics. 2005;4:914–923. doi: 10.1074/mcp.M400177-MCP200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, Patel AM, et al. Deficiency of SPAG16L Causes Male Infertility Associated with Impaired Sperm Motility. Biol Reprod. 2006;74:751–759. doi: 10.1095/biolreprod.105.049254. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tang W, Zhou R, Shen X, Wei Z, Patel A, Povlishock J, et al. Accelerated mortality from hydrocephalus and pneumonia in mice with a combined deficiency of SPAG6 and SPAG16L reveals a functional interrelationship between the two central apparatus proteins. Cell Motil Cytoskeleton. 2007a;64:360–376. doi: 10.1002/cm.20189. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zariwala MA, Mahadevan MM, Caballero-Campo P, Shen X, Escudier E, Duriez B, et al. A Heterozygous Mutation Disrupting the SPAG16 Gene Results in Biochemical Instability of Central Apparatus Components of the Human Sperm Axoneme. Biol Reprod. 2007b;77:864–871. doi: 10.1095/biolreprod.107.063206. [DOI] [PubMed] [Google Scholar]
- Zhao J, Li M, Bradfield J, Zhang H, Mentch F, Wang K, Sleiman P, et al. The role of height-associated loci identified in genome wide association studies in the determination of pediatric stature. BMC Med Genet. 2010;11:96. doi: 10.1186/1471-2350-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zie˛tkiewicz E, Bukowy-Bieryłło Z, Voelkel K, Klimek B, Dmeńska H, Pogorzelski A, Sulikowska-Rowińska A, et al. Mutations in radial spoke head genes and ultrastructural cilia defects in East-European cohort of primary ciliary dyskinesia patients. PLoS One. 2012;7:e33667. doi: 10.1371/journal.pone.0033667. [DOI] [PMC free article] [PubMed] [Google Scholar]