Abstract
IMPORTANCE
Current clinical and immunologic knowledge on cerebellar ataxia (CA) with glutamic acid decarboxylase 65 antibodies (GAD65-Abs) is based on case reports and small series with short-term follow-up data.
OBJECTIVE
To report the symptoms, additional antibodies, prognostic factors, and long-term outcomes in a cohort of patients with CA and GAD65-Abs.
DESIGN, SETTING, AND PARTICIPANTS
Retrospective cohort study and laboratory investigations at a center for autoimmune neurologic disorders among 34 patients with CA and GAD65-Abs, including 25 with long-term follow-up data (median, 5.4 years; interquartile range, 3.1-10.3 years).
MAIN OUTCOMES AND MEASURES
Analysis of clinicoimmunologic features and predictors of response to immunotherapy. Immunochemistry on rat brain, cultured neurons, and human embryonic kidney cells expressing GAD65, GAD67, α1-subunit of the glycine receptor, and a repertoire of known cell surface autoantigens were used to identify additional antibodies. Twenty-eight patients with stiff person syndrome and GAD65-Abs served as controls.
RESULTS
The median age of patients was 58 years (range, 33-80 years); 28 of 34 patients (82%) were women. Nine patients (26%) reported episodes of brainstem and cerebellar dysfunction or persistent vertigo several months before developing CA. The clinical presentation was subacute during a period of weeks in 13 patients (38%). Nine patients (26%) had coexisting stiff person syndrome symptoms. Systemic organ-specific autoimmunities (type 1 diabetes mellitus and others) were present in 29 patients (85%). Twenty of 25 patients with long-term follow-up data received immunotherapy (intravenous immunoglobulin in 10 and corticosteroids and intravenous immunoglobulin or other immunosuppressors in 10), and 7 of them (35%) improved. Predictors of clinical response included subacute onset of CA (odds ratio [OR], 0.50; 95% CI, 0.25-0.99; P = .047) and prompt immunotherapy (OR, 0.98; 95% CI, 0.96-0.99; P = .01). Similar frequencies of serum GAD67-Abs were found in patients with CA (24 of 34 patients [71%]) and in patients with stiff person syndrome (20 of 28 patients [71%]). However, GAD67-Abs were found in all of the cerebrospinal fluid samples examined (22 samples from patients with CA and 17 samples from patients with stiff person syndrome). Glycine receptor antibodies but not other cell surface antibodies were identified in 4 patients with CA. The presence of glycine receptor antibodies did not correlate with any specific clinical feature.
CONCLUSIONS AND RELEVANCE
In patients with CA and GAD65-Abs, subacute onset of symptoms and prompt immunotherapy are associated with good outcome. Persistent vertigo or brainstem and cerebellar episodes can herald CA and should lead to GAD65-Ab testing, particularly in patients with systemic organ-specific autoimmunities.
Autoimmunity is increasingly recognized as a cause of cerebellar dysfunction. Cerebellar ataxia (CA), associated with antibodies against the 65-kDa isoform of glutamic acid decarboxylase (GAD65-Abs), is one of the best-characterized cerebellar syndromes in which autoimmune mechanisms probably have a relevant pathogenic role.1 After stiff person syndrome (SPS), CA is the second most frequent neurologic disorder associated with GAD65-Abs.2 Current knowledge of the clinical and immunologic profile of CA with GAD65-Abs is based on small series and single case reports, and the long-term outcomes after immunotherapy are unknown.1,3
The association of GAD65-Abs with various syndromes such as SPS or CA has led to several hypotheses regarding the possible pathogenic role of these antibodies. The results of experimental investigations using monoclonal antibodies against different GAD65 epitopes suggest that the various neurologic syndromes could be related to the pattern of epitope recognition by human serum.4 Alternatively, the occurrence of CA or SPS could result from additional mechanisms mediated by T cells5 or antibodies against surface antigens such as those described in several autoimmune encephalitis.6 In this regard, γ-aminobutyric acid B receptor and glycine receptor antibodies (GlyR-Abs) have been described in patients with concurrent GAD65-Abs and limbic encephalitis or SPS.7,8
In this study, we retrospectively examined a cohort of patients with CA associated with GAD65-Abs. We aimed to better characterize the clinical presentation, the immunologic profile, the presence of additional antibodies against cell surface antigens, and the long-term response to immunotherapy.
Methods
Patients
The study was approved by the ethics committee of the Hospital Clinic, Barcelona, Spain. Written informed consent was obtained from all patients for the storage and use of serum and cerebrospinal fluid (CSF) samples for research purposes. Patients with CA and GAD65-Abs who were seen at the Hospital Clinic or whose serum or CSF samples were examined at the Institut d’Investigacions Biomèdiques August Pi i Suny, Barcelona, Spain, between December 15, 1994, and April 4, 2013, were included in the study if they met the following criteria: (1) predominant or isolated cerebellar dysfunction at presentation and the absence of another cause that could explain their CA, (2) available clinical information, and (3) the presence of high serum titers of GAD65-Abs confirmed by radioimmunoassay and immunohistochemistry.3 Evidence has shown that positive serum immunoreactivity using rat cerebellar sections is associated with high GAD65-Ab levels on radioimmunoassay (usually >2000 U/mL).2 Overall, 49 potential study patients were identified, 15 of whom were excluded because of a lack of clinical information. Long-term follow-up data were obtained in 25 of 34 patients (74%) (median, 5.4 years; interquartile range, 3.1-10.3 years). Data were obtained from clinical records, and information was collected from referring neurologists using a structured questionnaire mainly focused on the clinical presentation, the presence of neurologic symptoms preceding the cerebellar syndrome, concomitant symptoms of rigidity and spasms, and the response to immunotherapy. The onset of CA was defined as subacute when the cerebellar symptoms reached their nadir or required neurologic assessment within the first 3 months of symptom presentation. Ten patients were examined and followed up by 1 or more of us. Neurologic disability was measured by the modified Rankin Scale (mRS).9 Patients with a history of cancer who met the criteria for definite or possible paraneoplastic neurologic syndrome were excluded from the study.10 Serum and CSF samples used in the study were deposited in the Neuroinmunología collection of biological samples registered in the biobank of the Institut d’Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain.
Autoantibody Assays
All laboratory techniques have been previously reported and are described in detail in the eMethods in the Supplement. These include immunohistochemistry on frozen rat brain, immunoblot of human GAD65 recombinant protein (Diamyd),3 immunocytochemistry on cultures of rat hippocampal and cerebellar granular neurons,3,11,12 and immunocytochemistry on human embryonic kidney 293 cells transfected with the α-1 subunit of the GlyR (obtained by gift) and with GAD65 and GAD67 (OriGene).8
Statistical Analysis
Nonparametric tests were used when the distribution of the analyzed variables differed from normal using the Kolmogorov-Smirnov test. Good outcome was defined as an mRS score of less than 3 at the last follow-up visit, and bad outcome was defined as an mRS score of 3 or higher. In treated patients, improvement was defined as a decrease of at least 1 point on the mRS at the last follow-up visit compared with the score at diagnosis. For the multivariate analysis, a generalized linear model13 was used that included relevant or significant (P ≤ .10 on univariate analysis) factors. As a dependent variable to analyze response to treatment, we used the change in mRS scores from the onset to the last follow-up visit. Odds ratios (95% CIs) were used to measure the effect of predictors on the exponential function of the regression coefficient. Statistical software (SPSS Statistics version 19; IBM) was used for the analyses.
Results
Clinical Characteristics at Diagnosis
The median age of patients was 58 years (range, 33-80 years); 28 of 34 patients (82%) were women. Twenty-nine patients (85%) had concomitant systemic organ-specific autoimmune disorders. Type 1 diabetes mellitus was present at diagnosis in 13 patients (38%), and the incidence increased to 17 of 25 patients (68%) during the follow-up period. Twenty-one patients (62%) had other organ-specific autoimmune disorders, mainly thyroiditis and pernicious anemia (Table 1). Thirteen patients (38%) had subacute presentation of CA lasting for weeks, while the other 21 patients (62%) had a chronic course progressing during months or years. Overall, the demographic features and autoimmune clinical associations of 34 patients with CA were similar to those of 28 patients with SPS and GAD65-Abs.
Table 1.
Clinical and Immunologic Features of Patients Having CA or SPS With GAD65-Abs
| Variable | CA (n = 34) |
SPS (n = 28) |
P Value |
|---|---|---|---|
| Age, median (range), y | 58 (33-80) | 56 (19-77) | .19 |
|
| |||
| Female sex, No. (%) | 28 (82) | 26 (93) | .20 |
|
| |||
| Type 1 diabetes mellitus at diagnosis, No. (%) | 13 (38) | 14 (50) | .25 |
|
| |||
| Other organ-specific autoimmune disorder | |||
|
| |||
| No. (%) | 21 (62) | 13 (46) | .17 |
|
| |||
| Thyroiditis, No. | 18 | 7 | NA |
|
| |||
| Pernicious anemia, No. | 7 | 3 | NA |
|
| |||
| Vitiligo, No. | 2 | 3 | |
|
| |||
| Other, No.a | 3 | 0 | |
|
| |||
| Tumor, No.b | 4 | 1 | .36 |
|
| |||
| Epilepsy, No. (%) | 4 (12) | 3 (11) | .61 |
|
| |||
| GAD65-Ab radioimmunoassay, median (interquartile range), 1000 U/mL | 20.8 (9.5-44.6) | 15.0 (5.7-28.5) | .31 |
|
| |||
| CSF oligoclonal bands, No./total No. (%) | 16/22 (73) | 5/17 (29) | .07 |
|
| |||
| GAD65-Ab intrathecal synthesis, No./total No. (%)c | 13/15 (87) | 9/11 (82) | .57 |
|
| |||
| X-fold of GAD65-Ab intrathecal production, median (range)d | 8.9 (2.2-95.2) | 5.3 (1.1-20.0) | .43 |
|
| |||
| GAD67-Abs | |||
|
| |||
| Serum, No. (%) | 24 (71) | 20 (71) | .45 |
|
| |||
| CSF, No./total No. (%)e | 22/22 (100) | 17/17 (100) | NA |
Abbreviations: CA, cerebellar ataxia; CSF, cerebrospinal fluid; GAD65-Abs, glutamic acid decarboxylase 65 antibodies; NA, not applicable; SPS, stiff person syndrome.
Includes celiac disease, psoriasis, and myasthenia.
Three tumors (thymoma, endometrial carcinoma, and breast cancer) diagnosed at least 7 years (range, 7-7.6 years) before the onset of their CA. The fourth patient manifested a myelodysplastic syndrome 6 years after the diagnosis of CA. The only tumor in patients with SPS was a case of breast cancer diagnosed 20 years after the onset of SPS.
The index for intrathecal synthesis of GAD-Abs was calculated using the following formula: (CSF GAD-Ab Titer Divided by Serum GAD-Ab Titer) Divided by (CSF Albumin Level in Milligrams per Liter Divided by Serum Albumin Level in Milligrams per Liter).2
Calculated in reference to the general IgG ratio instead of the albumin ratio in 13 patients with CA and 11 patients with SPS.2
Seven patients having CA and 4 patients having SPS with CSF GAD67-Abs did not manifest these antibodies in serum.
Gait ataxia was the most common clinical presentation (31 patients), followed by limb ataxia (25 patients) that was asymmetric in 20, dysarthria (24 patients), and nystagmus (20 patients). Muscle rigidity and spasms were identified in 9 patients (26%), and 4 of them manifested electromyographic features of SPS (Table 2). Muscle rigidity and spasms occurred at the time of their CA in 5 patients and 2 to 5 years later in 3 patients. In 1 patient, leg spasms triggered by emotional stimuli or anxiety were present 2 years before the onset of her CA. Four patients also had epilepsy. In 3 of them, their epilepsy antedated the diagnosis of CA by 15, 13, and 2 years. Two of these patients met the criteria for refractory temporal lobe epilepsy. The fourth patient developed 2 generalized seizures 18 months after the onset of CA.
Table 2.
Symptoms of SPS in 9 Patients With CA and GAD65-Abs Having Muscle Rigidity and Spasms
| Sex/Age at Diagnosis, y |
Distribution of Stiffness |
Other SPS Symptoms |
Neurophysiological Findings |
Temporal Relationship With CA |
Treatment | Clinical Course of SPS and CA |
|---|---|---|---|---|---|---|
| F/53 | Legs | Frequent falls | Compatible with SPSa | Same time | IVIg | No change with SPS and CA |
|
| ||||||
| F/77 | Legs, trunk | Leg spasms, lumbar pain |
Compatible with SPS | 2 y Later | Oral IS, rituximab |
SPS improved and later relapsed, CA improved and then progressed |
|
| ||||||
| F/76 | Right leg | Leg spasms | Compatible with SPS | Same time | IVIg, rituximab, chronic IS |
Remission of SPS after first-line treatment and relapsing at last visit (5 y), slow partial improvement of CA |
|
| ||||||
| F/50 | Legs first, then trunk |
Leg spasms, lumbar hyperlordosis |
Compatible with SPS | 2 y Later | PE, oral IS, rituximab |
SPS improved,CA stable |
|
| ||||||
| M/74 | Leg, trunk | Leg spasms | Sensorimotor polyneuropathy |
Same time | IVIg, oral IS | Improvement of both syndromes after 1 y |
|
| ||||||
| F/40 | Right leg | Leg spasms | Signs of denervation | Same time | IVIg, oral IS | Both syndromes stable |
|
| ||||||
| F/59 | Legs, trunk | Lumbar pain, hyperlordosis |
Normal | Same time | IVIg, oral IS | No response after 4 mo, patient lost to follow-up data |
|
| ||||||
| F/52 | Legs, first asymmetric |
Leg spasms | Not done | 5 y Later | IVIg, PE | Mild improvement of SPS spasms, CA stable |
|
| ||||||
| F/52 | Legs | Leg spasms, agoraphobia |
Normal under benzodiazepine treatment |
2 y Beforeb | IVIg | Clear improvement of SPS after 1 cycle, no CA immediate effect, patient lost to follow-up data |
Abbreviations: CA, cerebellar ataxia; F, female; GAD65-Abs, glutamic acid decarboxylase 65 antibodies; IS, immunosuppression; IVIg, intravenous immunoglobulin; M, male; PE, plasma exchange; SPS, stiff person syndrome.
Continuous motor unit potentials firing at rest and during contraction of antagonist muscles in needle electromyographic recordings.
The patient experienced leg spasms triggered by emotional stimuli and prominent anxiety. She was diagnosed as having conversion disorder until she developed CA.
Neurologic symptoms antedating the diagnosis of CA were reported in 9 patients (26%), with 2 different profiles. Six patients reported fluctuating vertigo 7 to 26 months before developing CA. All manifested exacerbations that lasted days to weeks. During this period, the neurologic examinations showed no signs of CA. The remaining 3 patients had at least 1 episode of transient neurologic deficit, suggesting brainstem or cerebellar involvement, 2 to 24 months before the diagnosis of CA. The first patient had an episode of isolated vertical diplopia that lasted 10 days. The second patient had 2 episodes of dysarthria and gait ataxia that lasted a few days. The third patient developed dysarthria and right arm ataxia that lasted for 2 months and resolved spontaneously. The presence of prodromal symptoms was 3 times more common in men (P = .07).
Long-term Outcomes
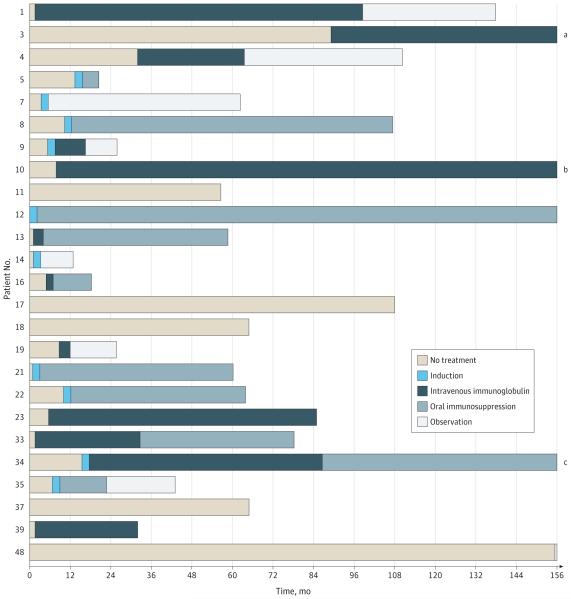
Long-term follow-up data were available for 25 patients. Figure 1 shows the type and duration of treatment during the course of the disease. Five patients received no immunotherapy, and 2 patients were untreated for more than 2 years. None of them improved, and the condition in 3 patients slowly deteriorated. Twenty patients received immunotherapy, which in 10 patients included intravenous immunoglobulin (IVIg), while 9 patients received intravenous methylprednisolone alone (4 patients) or in combination with IVIg (4 patients) or rituximab (1 patient). One patient was treated with oral prednisone (1 mg/kg/d). Among treated patients, 17 received various types of maintenance therapy: 6 were treated with IVIg during a median of 56.2 months (interquartile range, 24.4-121.5 months), and 11 received 1 or more regimen of oral corticosteroids, azathioprine, or mycophenolate mofetil.
Figure 1. Individual Therapy Regimens in 25 Patients With Long-term Follow-up Data.
For visual purposes, periods of treatment or between-periods are approximate, and therapeutic schemes are simplified. See the Long-term Outcomes subsection of the Results section for a description of the types of induction therapy and oral immunosuppression.
aTotal follow-up: 283 months. Beyond 156 months, patient 3 was treated with oral immunosuppression for 63 months and then observation.
bTotal follow-up: 209 months. Beyond 156 months, patient 10 continued treatment with intravenous immunoglobulins.
cTotal follow-up: 197 months. Beyond 156 months, patient 34 continued treatment with oral immunosuppression.
The eFigure in the Supplement shows the degree of disability at diagnosis, at the end of the first treatment (up to 6 months), and at the last follow-up visit. At the last follow-up visit, 11 patients had an mRS score of less than 3 (good outcome), and 14 patients had an mRS score of 3 or higher (bad outcome). Patients with good outcome had a better mRS score at diagnosis than patients with bad outcome (median, 2 vs 3; P = .01) and responded to immunotherapy more frequently (6 patients [55%] vs 1 patient [7%], P = .01) (Table 3).
Table 3.
Clinical Features of 25 Patients With Long-term Follow-up Data by Good Outcome (mRS Score, <3) and Bad Outcome (mRS Score, ≥3)
| Variable | Good Outcome (n = 11) |
Bad Outcome (n = 14) |
P Value |
|---|---|---|---|
| Age, median (range), y | 69 (33-76) | 54 (36-79) | .58 |
|
| |||
| Female sex, No. | 9 | 13 | .41 |
|
| |||
| Type 1 diabetes mellitus at diagnosis, No. | 7 | 10 | .50 |
|
| |||
| Associated SPS or epilepsy, No. | 5 | 5 | .46 |
|
| |||
| Subacute onset, No. | 6 | 3 | .09 |
|
| |||
| mRS score at diagnosis, median (range) | 2 (1-4) | 3 (2-5) | .01 |
|
| |||
| GAD65-Ab radioimmunoassay, median (interquartile range), 1000 U/mL |
11.5 (6.6-20.8) | 20.1 (9.3-31.5) | .28 |
|
| |||
| Time to treatment, median (range), moa | 5.88 (0.99-64.95) | 6.70 (0.13-165.40) | .49 |
|
| |||
| Treated, No. | 9 | 11 | .62 |
|
| |||
| Induction therapyb | 3 | 5 | .49 |
|
| |||
| Maintenance therapy | 7 | 10 | .50 |
|
| |||
| IVIg as chronic therapyc | 3 | 4 | .64 |
|
| |||
| Improvement after therapyd | 6 | 1 | .01 |
|
| |||
| Total duration of disease, median (range), y | 5.02 (1.52-9.15) | 8.01 (0.98-23.61) | .08 |
Abbreviations: GAD65-Ab, glutamic acid decarboxylase 65 antibody; IVIg, intravenous immunoglobulin; mRS, modified Rankin Scale; SPS, stiff person syndrome.
From the onset of symptoms to immunotherapy.
Includes high dose of corticosteroids, rituximab, or cyclophosphamide.
The other patients received immunossupressors (corticosteroids, azathioprine, or mycophenolate mofetil).
Defined as sustained decrease in mRS score of at least 1 point in treated patients at the last follow-up visit compared with mRS score at diagnosis.
Among 20 patients who were treated, 10 showed clinical improvement (≥1 point on the mRS during the first 6 months of treatment), which in 7 patients (35%) persisted at the last follow-up visit. Four of them were treated with pulses of intravenous methylprednisolone, and 3 were treated with IVIg. Four patients also received maintenance immunotherapy (for 1.1-4.9 years) with oral immunosuppressors (3 patients) or IVIg (1 patient). Improvement after immunotherapy was more common in patients with subacute onset (5 of 7 subacute patients [71%] vs2 of 13 chronic patients [15%], P = .02). Age, sex, mRS score at diagnosis, the presence of type 1 diabetes mellitus or other autoimmune disorders, and evidence of cerebellar atrophy on magnetic resonance imaging at diagnosis were similar in responders and nonresponders. On multivariate analysis, favorable predictors of clinical response included subacute onset of CA (odds ratio, 0.50; 95% CI, 0.25-0.99; P = .047) and prompt immunotherapy (odds ratio, 0.98; 95% CI, 0.96-0.99; P = .01).
Immunologic Studies
All serum and CSF samples from patients with CA and SPS included in the study showed reactivity to human embryonic kidney cells transfected with GAD65. Similarly, all but one CA serum samples were positive in immunoblots of GAD65 recombinant protein, suggesting that the antibodies from patients with CA and SPS recognize linear epitopes. The assessment of GAD65-Abs by immunohistochemistry revealed that in all cases the predominant IgG isotype was IgG1. Additional isotypes were detected in a small proportion of patients, including IgG3 in 2 of 34 patients with CA and 2 of 28 patients with SPS and IgG2 in 2 of 34 patients with CA and 6 of 28 patients with SPS.
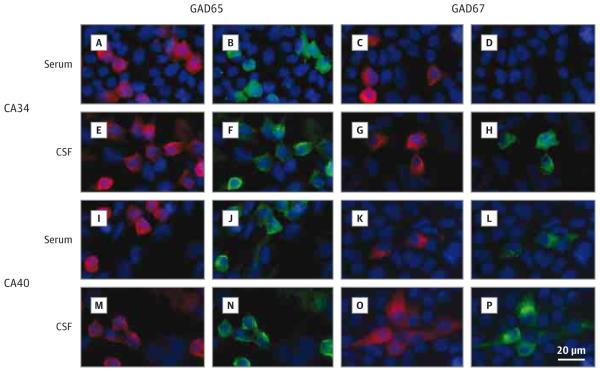
The frequencies of serum GAD67-Abs were similar in patients with CA (24 of 34 patients [71%]) and in patients with SPS (20 of 28 patients [71%]). GAD67-Abs were found in all CSF samples available from 22 patients with CA and from 17 patients with SPS. GAD67-Abs were present in CSF samples but not in serum samples of 7 patients with CA and 4 patients with SPS having paired serum and CSF samples (Figure 2). Serum and CSF samples from patients with CA did not immunoreact with live hippocampal and granular cerebellar neurons, and none manifested antibodies against N-methyl-D-aspartate, γ-aminobutyric acid B, and α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors or leucine-rich glioma-inactivated protein 1, dipeptidyl peptidase–like protein 6, and contactin-associated protein 2 antigens. In contrast, 4 patients with CA had GlyR-Abs in serum samples but not in the 2 CSF samples available. The presence of GlyR-Abs did not correlate with any specific clinical feature, no patient had associated symptoms of SPS, and only one patient responded to immunotherapy.
Figure 2.
Reactivity of Serum and Cerebrospinal Fluid (CSF) Samples to Fixed Human Embryonic Kidney Cells Expressing Glutamic Acid Decarboxylase 65 (GAD65) and GAD67
Discussion
To our knowledge, this is the first study to describe the long-term outcomes in a series of patients with CA and GAD65-Abs. Our findings indicate that patients with subacute presentation of CA are more likely to respond to immunotherapy and achieve good functional status (mRS score, <3) and confirm that a shorter delay in the initiation of immunotherapy predicts clinical improvement.14
Previous autopsy studies15,16 of patients with CA and GAD65-Abs revealed selective loss of Purkinje cells. However, the clinical improvement observed in some of our patients indicates that part of the cerebellar dysfunction at the time of diagnosis may be due to functional impairment that can be reversed by early onset of immunotherapy. Although the pathogenic role of GAD65-Abs is unclear, studies17,18 have shown that they interfere with the γ-aminobutyric acid–ergic synaptic transmission in tissue culture systems and that these effects are reversible after removing the GAD65-Abs. In addition, intracerebellar injection of GAD65-Abs induces an increase in glutamate levels that may lead to glutamate excitotoxic effects.4 These neurochemical and neurophysiological abnormalities could initially affect the function of Purkinje cells without causing irreversible damage but may cause their death in the long run.
The retrospective design of this study and the multi-center locations of the patients make it difficult to recommend a particular type of immunotherapy. The effect of various immunotherapies has been described in single case reports (summarized in the eTable in the Supplement).19-29 As in the present series, the most common treatments used were cycles of IVIg or methylprednisolone. Most of the described patients had a chronic presentation of symptoms, and treatment was started after a median delay of 12 months (range, 2-120 months). Improvement was reported in 13 of 16 patients (81%) compared with 7 of 20 patients (35%) in this series. However, the figures are not comparable because the degree of improvement reported in many of these case reports would not fulfill the required decrease of at least 1 point on the mRS as used herein (eTable in the Supplement).18-28
As in SPS,30,31 we observed that patients with CA who responded to immunotherapy did so early, during the first 6 months. A lack of improvement in this short period should be an indication for switching to a second-line immunotherapy or stopping treatment.32 Our study does not clarify whether maintenance immunotherapy is useful: 3 of 7 patients who improved with the initial treatment remained stable without subsequent immunotherapy.
We have identified a previously unrecognized feature to date of patients with CA and GAD65-Abs. Three patients (9%) reported episodes of diplopia or combinations of dysarthria and ataxia of unclear etiology months before the development of full-blown CA. In addition, 6 patients (18%) reported isolated vertigo in the absence of other symptoms of cerebellar dysfunction. We consider that vertigo in these patients was heralding the development of more widespread involvement of the cerebellum. This feature has been observed in other cerebellar syndromes. In patients with spinocerebellar ataxia, episodic vertigo antedated the development of gait ataxia by several years in 4% of patients.33 Investigations of selective ischemic infarcts of the cerebellum indicate that patients who were seen with isolated vertigo more frequently had the infarct in the caudal vermis.34 Taken together, these data suggest that in some patients GAD65 autoimmunity may result in subtle, focal, or transient symptoms, likely representing involvement of selective brainstem or cerebellar regions. In some patients, particularly those with diseases that are associated with GAD autoimmunity (eg, type 1 diabetes mellitus), the development of persistent vertigo of unknown etiology or episodes of diplopia, dysarthria, or ataxia, should lead to GAD65-Ab testing. In that clinical scenario, the detection of high-titer GAD65-Abs (usually >2000 U/mL) should raise concern about impending CA.
In our patients with CA, the immunologic response against GAD did not differ from that observed in patients with SPS. We found no significant increase in intrathecal production of GAD65-Abs in patients having CA compared with those having SPS, as previously suggested.35 A relevant observation was that all patients in whom CSF samples could be analyzed manifested GAD67-Abs, despite that some of them did not have these antibodies in serum samples. The presence of GAD67-Abs only in CSF, along with previous demonstration of intrathecal synthesis of GAD65-Abs35 and a different epitope repertoire noted between paired serum and CSF samples,36 strongly supports the presence of GAD-specific B cells in the central nervous system and emphasizes the importance of examining the CSF in autoimmune disorders of the central nervous system.
Except for 4 patients with CA who had concomitant GlyR-Abs in serum samples, we did not find (as suggested in SPS37) other antibodies against neuronal surface antigens. Glycine receptor antibodies were initially described in patients having progressive encephalomyelitis with rigidity and myoclonus.38 More recently, GlyR-Abs were reported in 12% to 15% of patients with SPS (with or without GAD65-Abs) and in 3% of patients with epilepsy.8,39,40 In our patients, the significance of GlyR-Abs is unknown because the clinical course and outcome did not differ in patients without this antibody. In 2 of these 4 patients, CSF samples were available and were negative for GlyR-Abs in both of them. Although earlier described patients having progressive encephalomyelitis with rigidity and myoclonus showed GlyR-Abs in serum and CSF samples,41,42 in patients with SPS or epilepsy GlyR-Abs were usually studied based on serum samples only.8,39,40 Therefore, it is unclear whether the presence or absence of GlyR-Abs in CSF is associated with different clinical phenotypes as recently reported in other autoimmune encephalitis.43 Future studies should determine the degree of syndrome specificity of GlyR-Abs, comparing paired serum and CSF samples in larger groups of patients and control subjects.
Conclusions
Our findings reveal that patients with CA and GAD65-Abs may respond to immunotherapy and maintain good functional status for many years. The retrospective design of the study prevents our making definite recommendations, but in our experience the use of IVIg or corticosteroids should be considered in all patients with CA and GAD65-Abs, particularly those with subacute presentation. Early initiation of treatment likely offers a greater chance of improvement.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grants R01NS077851 and R01MH094741 from the US National Institutes of Health (Dr Dalmau), by grants 11/01780 (Dr Dalmau) and 12/00611 (Dr Graus) from the Spanish Fondo Investigaciones Sanitarias, and by Fundació la Marató TV3 (Dr Dalmau). Dr Ariño is supported by a postresidency grant from the Hospital Clinic, Barcelona, Spain, and Dr Martínez-Hernández is supported by grant CM12/00055 from Instituto Carlos III, Madrid, Spain.
Footnotes
Author Contributions: Dr Graus had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Drs Ariño and Gresa-Arribas contributed equally to this work.
Study concept and design: Ariño, Saiz, Graus. Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Ariño, Blanco, Dalmau, Saiz, Graus.
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: Ariño, Dalmau, Graus.
Study supervision: Dalmau, Saiz, Graus.
Conflict of Interest Disclosures: Dr Dalmau reported receiving a research grant from Euroimmun and royalties from patents for the use of Ma2 and N-methyl-D-aspartate receptor as autoantibody tests. No other disclosures were reported.
Role of the Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: Mercedes Alba (Neuroimmunology Laboratory, Institut d’Investigacions Biomèdiques August Pi i Sunyer, Barcelona, Spain) provided excellent technical support. We thank the physicians for the clinical information on their patients, and we thank the patients for their generous contribution to research.
Supplemental content at jamaneurology.com
REFERENCES
- 1.Honnorat J, Saiz A, Giometto B, et al. Cerebellar ataxia with anti–glutamic acid decarboxylase antibodies: study of 14 patients. Arch Neurol. 2001;58(2):225–230. doi: 10.1001/archneur.58.2.225. [DOI] [PubMed] [Google Scholar]
- 2.Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131(pt 10):2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 3.Saiz A, Arpa J, Sagasta A, Casamitjana R, Zarranz JJ, Tolosa E. Autoantibodies to glutamic acid decarboxylase in three patients with dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology. 1997;49(4):1026–1030. doi: 10.1212/wnl.49.4.1026. [DOI] [PubMed] [Google Scholar]
- 4.Manto MU, Hampe CS, Rogemond V, Honnorat J. Respective implications of glutamate decarboxylase antibodies in stiff person syndrome and cerebellar ataxia. Orphanet J Rare Dis. 2011;6(1):3. doi: 10.1186/1750-1172-6-3. doi:10.1186/1750-1172-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton AR, Baquet Z, Eisenbarth GS, et al. Central nervous system destruction mediated by glutamic acid decarboxylase–specific CD4+ T cells. J Immunol. 2010;184(9):4863–4870. doi: 10.4049/jimmunol.0903728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lancaster E, Dalmau J. Neuronal autoantigens: pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8(7):380–390. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABAB receptor antibodies in limbic encephalitis and anti-GAD–associated neurologic disorders. Neurology. 2011;76(9):795–800. doi: 10.1212/WNL.0b013e31820e7b8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKeon A, Martinez-Hernandez E, Lancaster E, et al. Glycine receptor autoimmune spectrum with stiff-man syndrome phenotype. JAMA Neurol. 2013;70(1):44–50. doi: 10.1001/jamaneurol.2013.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 10.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75(8):1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancaster E, Lai M, Peng X, et al. Antibodies to the GABAB receptor in limbic encephalitis with seizures: case series and characterisation of the antigen. Lancet Neurol. 2010;9(1):67–76. doi: 10.1016/S1474-4422(09)70324-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabater L, Höftberger R, Boronat A, Saiz A, Dalmau J, Graus F. Antibody repertoire in paraneoplastic cerebellar degeneration and small cell lung cancer. PLoS One. 2013;8(3):e60438. doi: 10.1371/journal.pone.0060438. doi:10.1371/journal.pone.0060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullagh P. Generalized linear models. Eur J Oper Res. 1984;16(3):285–292. [Google Scholar]
- 14.Pittock SJ, Yoshikawa H, Ahlskog JE, et al. Glutamic acid decarboxylase autoimmunity with brainstem, extrapyramidal, and spinal cord dysfunction. Mayo Clin Proc. 2006;81(9):1207–1214. doi: 10.4065/81.9.1207. [DOI] [PubMed] [Google Scholar]
- 15.Ishida K, Mitoma H, Wada Y, et al. Selective loss of Purkinje cells in a patient with anti–glutamic acid decarboxylase antibody–associated cerebellar ataxia. J Neurol Neurosurg Psychiatry. 2007;78(2):190–192. doi: 10.1136/jnnp.2006.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccolo G, Tavazzi E, Cavallaro T, Romani A, Scelsi R, Martino G. Clinico-pathological findings in a patient with progressive cerebellar ataxia, autoimmune polyendocrine syndrome, hepatocellular carcinoma and anti-GAD autoantibodies. J Neurol Sci. 2010;290(1-2):148–149. doi: 10.1016/j.jns.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Ishida K, Mitoma H, Song SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol. 1999;46(2):263–267. [PubMed] [Google Scholar]
- 18.Ishida K, Mitoma H, Mizusawa H. Reversibility of cerebellar GABAergic synapse impairment induced by anti–glutamic acid decarboxylase autoantibodies. J Neurol Sci. 2008;271(1-2):186–190. doi: 10.1016/j.jns.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Virgilio R, Corti S, Agazzi P, et al. Effect of steroid treatment in cerebellar ataxia associated with anti–glutamic acid decarboxylase antibodies. J Neurol Neurosurg Psychiatry. 2009;80(1):95–96. doi: 10.1136/jnnp.2007.142745. [DOI] [PubMed] [Google Scholar]
- 20.Planche V, Marques A, Ulla M, Ruivard M, Durif F. Intravenous immunoglobulin and rituximab for cerebellar ataxia with glutamic acid decarboxylase autoantibodies [published online November 12, 2013] Cerebellum. doi: 10.1007/s12311-013-0534-3. [DOI] [PubMed] [Google Scholar]
- 21.Pedroso JL, Braga-Neto P, Dutra LA, Barsottini OG. Cerebellar ataxia associated to anti–glutamic acid decarboxylase autoantibody (anti-GAD): partial improvement with intravenous immunoglobulin therapy [letter] Arq Neuropsiquiatr. 2011;69(6):993. doi: 10.1590/s0004-282x2011000700030. http://dx.doi.org/10.1590/S0004-282X2011000700030. [DOI] [PubMed] [Google Scholar]
- 22.Nociti V, Frisullo G, Tartaglione T, et al. Refractory generalized seizures and cerebellar ataxia associated with anti-GAD antibodies responsive to immunosuppressive treatment. Eur J Neurol. 2010;17(1):e5. doi: 10.1111/j.1468-1331.2009.02839.x. doi:10.1111/j.1468-1331.2009.02839.x. [DOI] [PubMed] [Google Scholar]
- 23.Nanri K, Okita M, Takeguchi M, et al. Intravenous immunoglobulin therapy for autoantibody-positive cerebellar ataxia. Intern Med. 2009;48(10):783–790. doi: 10.2169/internalmedicine.48.1802. [DOI] [PubMed] [Google Scholar]
- 24.Lauria G, Pareyson D, Pitzolu MG, Bazzigaluppi E. Excellent response to steroid treatment in anti-GAD cerebellar ataxia. Lancet Neurol. 2003;2(10):634–635. doi: 10.1016/s1474-4422(03)00534-9. [DOI] [PubMed] [Google Scholar]
- 25.Bonnan M, Cabre P, Olindo S, Signate A, Saint-Vil M, Smadja D. Steroid treatment in four cases of anti-GAD cerebellar ataxia [in French] Rev Neurol (Paris) 2008;164(5):427–433. doi: 10.1016/j.neurol.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Bayreuther C, Hieronimus S, Ferrari P, Thomas P, Lebrun C. Auto-immune cerebellar ataxia with anti-GAD antibodies accompanied by de novo late-onset type 1 diabetes mellitus. Diabetes Metab. 2008;34(4, pt 1):386–388. doi: 10.1016/j.diabet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Vulliemoz S, Vanini G, Truffert A, Chizzolini C, Seeck M. Epilepsy and cerebellar ataxia associated with anti–glutamic acid decarboxylase antibodies. J Neurol Neurosurg Psychiatry. 2007;78(2):187–189. doi: 10.1136/jnnp.2006.089268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abele M, Weller M, Mescheriakov S, Bürk K, Dichgans J, Klockgether T. Cerebellar ataxia with glutamic acid decarboxylase autoantibodies. Neurology. 1999;52(4):857–859. doi: 10.1212/wnl.52.4.857. [DOI] [PubMed] [Google Scholar]
- 29.McFarland NR, Login IS, Vernon S, Burns TM. Improvement with corticosteroids and azathioprine in GAD65-associated cerebellar ataxia. Neurology. 2006;67(7):1308–1309. doi: 10.1212/01.wnl.0000238389.83574.be. [DOI] [PubMed] [Google Scholar]
- 30.McKeon A, Robinson MT, McEvoy KM, et al. Stiff-man syndrome and variants: clinical course, treatments, and outcomes. Arch Neurol. 2012;69(2):230–238. doi: 10.1001/archneurol.2011.991. [DOI] [PubMed] [Google Scholar]
- 31.Dalakas MC, Fujii M, Li M, Lutfi B, Kyhos J, McElroy B. High-dose intravenous immune globulin for stiff-person syndrome. N Engl J Med. 2001;345(26):1870–1876. doi: 10.1056/NEJMoa01167. [DOI] [PubMed] [Google Scholar]
- 32.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Globas C, du Montcel ST, Baliko L, et al. Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6. Mov Disord. 2008;23(15):2232–2238. doi: 10.1002/mds.22288. [DOI] [PubMed] [Google Scholar]
- 34.Ye BS, Kim YD, Nam HS, Lee HS, Nam CM, Heo JH. Clinical manifestations of cerebellar infarction according to specific lobular involvement. Cerebellum. 2010;9(4):571–579. doi: 10.1007/s12311-010-0200-y. [DOI] [PubMed] [Google Scholar]
- 35.Rakocevic G, Raju R, Semino-Mora C, Dalakas MC. Stiff person syndrome with cerebellar disease and high-titer anti-GAD antibodies. Neurology. 2006;67(6):1068–1070. doi: 10.1212/01.wnl.0000237558.83349.d0. [DOI] [PubMed] [Google Scholar]
- 36.Raju R, Foote J, Banga JP, et al. Analysis of GAD65 autoantibodies in stiff-person syndrome patients. J Immunol. 2005;175(11):7755–7762. doi: 10.4049/jimmunol.175.11.7755. [DOI] [PubMed] [Google Scholar]
- 37.Chang T, Alexopoulos H, McMenamin M, et al. Neuronal surface and glutamic acid decarboxylase autoantibodies in nonparaneoplastic stiff person syndrome. JAMA Neurol. 2013;70(9):1140–1149. doi: 10.1001/jamaneurol.2013.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutchinson M, Waters P, McHugh J, et al. Progressive encephalomyelitis, rigidity, and myoclonus: a novel glycine receptor antibody. Neurology. 2008;71(16):1291–1292. doi: 10.1212/01.wnl.0000327606.50322.f0. [DOI] [PubMed] [Google Scholar]
- 39.Alexopoulos H, Akrivou S, Dalakas MC. Glycine receptor antibodies in stiff-person syndrome and other GAD-positive CNS disorders. Neurology. 2013;81(22):1962–1964. doi: 10.1212/01.wnl.0000436617.40779.65. [DOI] [PubMed] [Google Scholar]
- 40.Brenner T, Sills GJ, Hart Y, et al. Prevalence of neurologic autoantibodies in cohorts of patients with new and established epilepsy. Epilepsia. 2013;54(6):1028–1035. doi: 10.1111/epi.12127. [DOI] [PubMed] [Google Scholar]
- 41.Piotrowicz A, Thümen A, Leite MI, Vincent A, Moser A. A case of glycine-receptor antibody–associated encephalomyelitis with rigidity and myoclonus (PERM): clinical course, treatment and CSF findings. J Neurol. 2011;258(12):2268–2270. doi: 10.1007/s00415-011-6078-x. [DOI] [PubMed] [Google Scholar]
- 42.Mas N, Saiz A, Leite MI, et al. Antiglycine-receptor encephalomyelitis with rigidity. J Neurol Neurosurg Psychiatry. 2011;82(12):1399–1401. doi: 10.1136/jnnp.2010.229104. [DOI] [PubMed] [Google Scholar]
- 43.Petit-Pedrol M, Armangue T, Peng X, et al. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13(3):276–286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.