Abstract
Neuronal communication underlies all brain activity and the genesis of complex behavior. Emerging research has revealed an unexpected role for immune molecules in the development and plasticity of neuronal synapses. Moreover microglia, the resident immune cells of the brain, express and secrete immune-related signaling molecules that alter synaptic transmission and plasticity in the absence of inflammation. When inflammation does occur, microglia modify synaptic connections and synaptic plasticity required for learning and memory. Here we review recent findings demonstrating how the dynamic interactions between neurons and microglia shape the circuitry of the nervous system in the healthy brain and how altered neuron-microglia signaling could contribute to disease.
Introduction
The nervous system and immune system interact on many levels in health and disease. Emerging data reveal that many immune-related molecules have homeostatic and physiological functions in the brain. Surprisingly, several proteins associated with adaptive and innate immunity are localized to synapses where they regulate circuit development and plasticity in the healthy brain. Microglia are the resident immune phagocytes of the central nervous system (CNS) and have profound effects on neuronal circuits. Much like their peripheral counterparts, microglia act as sentinels against damage and disease; however, they also play a crucial role in shaping and maintaining the synaptic network under physiological conditions.
Recent work has revealed that microglia are key regulators of neuronal and synapse function in the healthy brain. Remodeling of neuronal synapses occurs constantly throughout life. During development, neurons must wire together correctly in order to establish the mature CNS circuit, which requires a process of elimination of select synapses, or synapse pruning. Adult neuronal circuits are also highly dynamic, with synaptic connections in many areas constantly undergoing remodeling based on experience, resulting in synaptic plasticity. Decreased neuronal activity at synapses in both the developing and adult nervous system has been linked to physical removal of these less active connections [1–5]. Although there are cell-autonomous mechanisms leading to synapse turnover, recent work has shown that microglia are key regulators of synaptic remodeling during development and in the adult CNS via non-cell-autonomous mechanisms. These mechanisms involve immune-related molecules that regulate the dynamic interactions of microglia with synapses under normal physiological conditions. Here we review recent research in this emerging area of neuroimmunology and discuss mechanisms underlying microglia-synapse interactions and potential implications for neural circuit function, cognition and behavior.
Sculpting Circuits During Development
During brain development, neurons form an excess of synaptic connections, many of which are subsequently removed during synapse pruning, a process necessary for appropriate brain connectivity. Emerging work implicates microglia and immune-related molecules as key regulators of developmental pruning via refinement of immature synapses [6–8]. High resolution imaging studies have established that microglia engulf pre and post-synaptic elements (i.e. axonal terminals and dendritic spines) during developmental periods of refinement in the CNS [9, 10]. Several potential ‘find-me’ and ‘eat-me’ pathways have been identified, most of which are shared with the immune system. CX3CL1 acts as both a membrane tethered chemokine as well as a secreted version [11] and binds its receptor CX3CR1, which is exclusively expressed by microglia in the CNS [12, 13]. In Cx3cr1 knock out (Cx3cr1KO) mice, a transient increase in density of electrically immature dendritic spines was observed in the developing hippocampus [9]. A similar delay in synapse maturation was seen in the barrel cortex of mice when CX3CR1 was lost [14]. Although the underlying mechanisms are not yet clear, one possibility is that neuronal secretion of the CX3CL1 chemokine attracts resident microglia through a ‘find-me’ mechanism, which then affects synaptic maturation through an undiscovered pathway [15].
Another set of immune molecules implicated in developmental synaptic remodeling is the classical complement cascade, an innate immune pathway that functions to eliminate pathogens and apoptotic cells from the periphery. Surprisingly, classical complement proteins C1q and C3 are widely expressed in the healthy postnatal brain and localize to subsets of immature synapses [16, 17], while the complement receptor 3 (CR3) is expressed in resident microglia [17, 18]. In the mouse visual system, C1q, C3, and TGF-beta, the upstream regulator of C1q, are required for proper refinement of the retinogeniculate system [18, 19]. Microglial engulfment of synapses is decreased in C3 and CR3 KO mice [10] (Figure 1), consistent with the hypothesis that complement proteins at developing synapses leads to targeted engulfment by phagocytic microglia. Importantly, microglia-mediated synaptic elimination depends on neuronal activity, as microglia preferentially phagocytose less active presynaptic inputs [10]. This raises the intriguing possibility that C1q and C3 are activity-dependent ‘eat-me’ signals that bind to less active synapses, thus flagging them for removal by microglia. Thus complement proteins have parallel functions in the nervous and immune systems to mark unwanted elements for elimination. A major distinction, however is that complement–mediated synapse elimination occurs in the healthy brain in the absence of neuroinflammation.
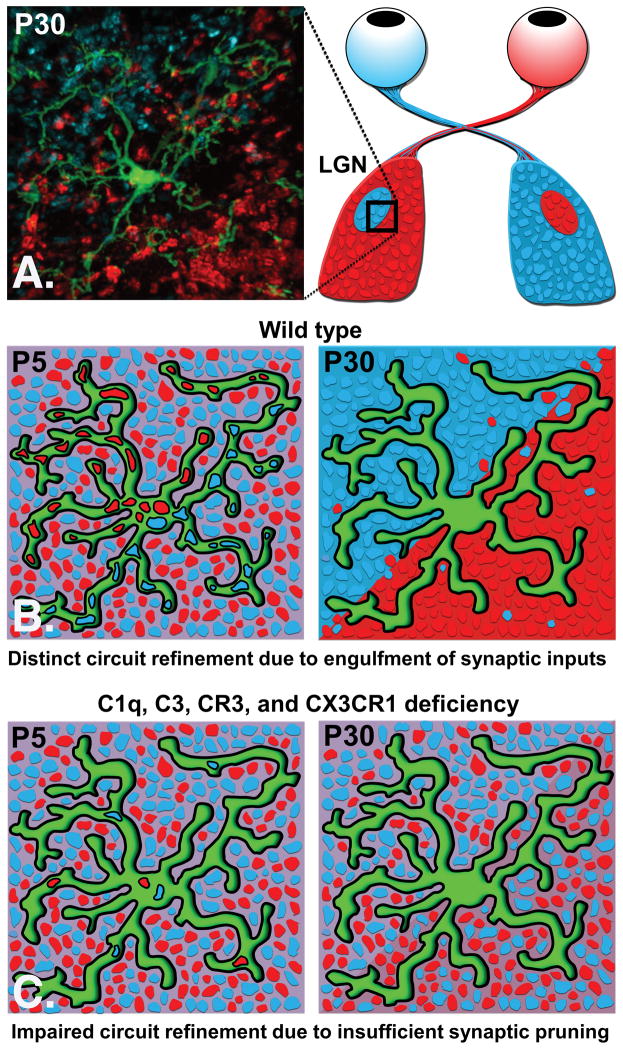
Figure 1. Microglial-mediated Synaptic Pruning.
A. Confocal image of a microglia (green) in the lateral geniculate nucleus (LGN) surrounded by synaptic inputs from both eyes (blue and red) that were visualized by intraocular injections of different colored anterograde tracers. For more details please see [10]. B. In wild type mice, microglial engulfment of synaptic material is observed at postnatal day (P)5 as depicted in the cartoon. Elimination of synapses (referred to as synaptic pruning) is required for circuit refinement, as schematized by segregation of red and blue inputs into distinct territories (P30). C. Insufficient synaptic pruning as observed in C1q, C3, CR3 deficient mice results in impaired circuit refinement. Lack of CX3CR1 has been associated with reduced synaptic pruning in other brain regions [9, 14].
Surveying the Adult Brain
Synaptic connections in the adult CNS are highly dynamic and are constantly undergoing changes in strength and connectivity. Pioneering in vivo imaging studies revealed that the processes of microglia are highly motile and are continually extending, retracting and interacting with synapses [20–23]. It is thought that this dynamic process motility allows microglia to assess or ‘survey’ surrounding synapses and respond accordingly. For example, in the visual cortex 94% of microglial processes touch synaptic elements at any given point in time, and these contacts were found to be correlated with both increased growth and elimination of small spines [22]. Furthermore, decreasing the strength of synaptic input to the visual cortex during dark adaptation causes decreased microglia process motility that was reversed upon re-exposure to light and visual stimulation [22]. Thus, microglia seem to be able to change their surveillance properties in response to changes in neuronal activity.
Intriguingly, several studies have provided evidence that adenosine triphosphate (ATP), which is known as an important purinergic signal molecule for peripheral immune cells, is released during high neuronal activity to promote enhanced surveillance by microglial processes [24–27] (Figure 2). In the retina of adult mice, application of glutamate receptor agonists α-amino-3-hydroxy-5-methyl-isoxazolepropionic acid (AMPA) or kainate triggered an ATP-mediated microglial process outgrowth [25]. More recently, it was discovered that multiple brief applications or prolonged application of N-Methyl-D-aspartic acid (NMDA) also triggered an ATP-dependent outgrowth of microglia processes in the hippocampus and cortex [26, 27]. The outgrowth was characterized by extension of processes away from the cell body and the formation of bulbous tips, which are growth cone-like structures at the leading edge of extending processes (Figure 2). Whole cell patch clamp experiments revealed that activation of dendritic NMDA receptors on single neurons was sufficient to trigger microglia process outgrowth [26], thereby demonstrating a direct link between neuronal activity and microglia process dynamics. In the zebrafish larvae, neuronal activity was reduced by microglia contact while conversely, preventing microglial processes from contacting spontaneously active neurons significantly enhanced neuronal activity [24]. These observations imply an active rather than passive form of surveillance and suggest that neuronal activity itself can be altered by microglial contact. This is further supported by the observation that preventing ATP-mediated microglial process outgrowth exacerbated evoked-seizure activity and mortality [27]. Taken together, ATP-mediated communication between highly active neurons and the brain’s resident immune cells might provide an important but still to be defined feedback to regulate neuronal activity. A major question for future studies is whether or how this form of microglia surveillance impacts synaptic function and plasticity.
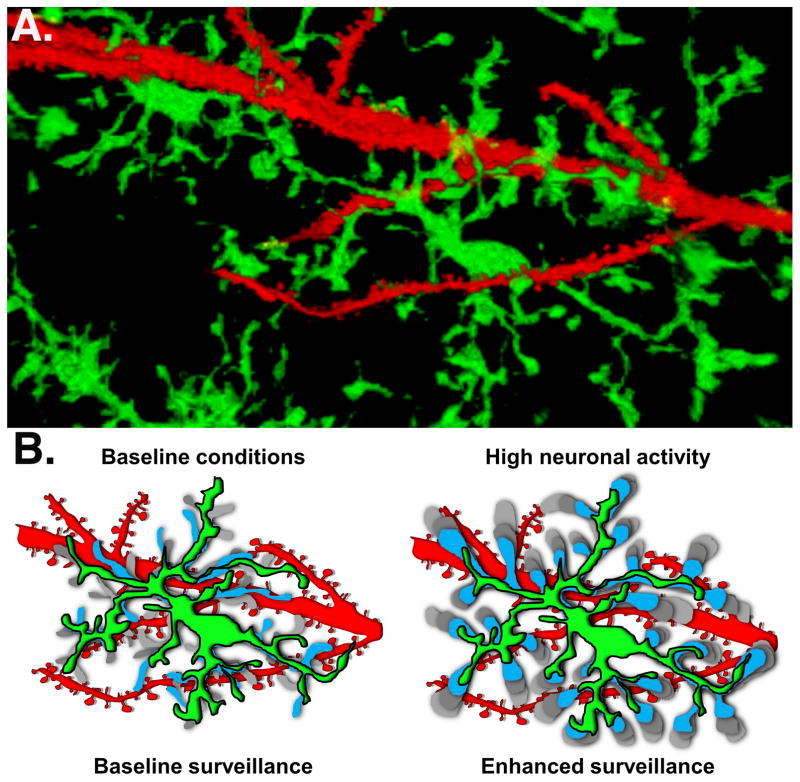
Figure 2. Microglia Surveillance.
A. Two photon image of a microglia (green) and the dendrites of a single patch-filled CA1 pyramidal neuron (red) in a hippocampal brain slice from a CX3CR1-EGFP mouse. The 3D rendering was made using ImageJ. For more details please see [26]. B. The cartoons illustrate the surveillance by microglial processes under baseline conditions. The blue and grey shaded regions represent the positions of microglial processes at different time points. High neuronal activity triggers enhanced surveillance, which is schematized by the movement from blue to light grey over time.
Neural-Immune Interactions Modify Synaptic Plasticity
The activity-dependent modulation of synaptic strength in the adult brain is thought to underlie memory, learning and widespread aspects of adaptive behavior. Both strengthening and weakening of synapses can result from changes in neuronal activity.. Emerging research demonstrate changes in synaptic strength and behavior can arise from signaling in immune-related pathways, particularly those governed by microglia.
The interaction between the microglia chemokine receptor CX3CR1 and its CNS ligand, neuronal CX3CL1, allows for precise communication between neurons and microglia and can result in changes in neuronal activity. Application of CX3CL1 to acutely stimulate microglia in brain slices caused a dose-dependent depression of synaptic transmission that was not observed in the CX3CR1 deficient mouse [28]. This synaptic depression was mediated by the adenosine A3 receptor, as the depression was blocked by A3 (but not A1 or A2) receptor antagonists and was not observed in A3 deficient mice. Thus, a chemokine expressed by neurons acts on microglia to reduce the activity of neurons via a feedback pathway. The mechanisms underlying this feedback loop still remain to be uncovered.
In addition to the acute action of CX3CL1-CX3CR1 signaling, chronic reduction of this signaling pathway by permanent deletion or reduction of CX3CR1 leads to elevated levels of the inflammatory cytokine interleukin (IL)-1β, leading to a reduction of long-term potentiation (LTP) in the brain. Ventricular infusion of an IL-1β receptor antagonist for four weeks via osmotic mini pumps reversed the impaired LTP, while infusion of an inactivated antagonist did not [29]. Intact chemokine signaling between neurons and microglia and appropriate levels of CNS cytokines are therefore crucial for maintenance of normal plasticity mechanisms.
The important role of cytokines is further demonstrated by the finding that tumor necrosis factor α (TNFα) can modify synaptic strength via a process termed synaptic scaling [30]. Chronic blockade of synaptic transmission in cultured hippocampal slices increased the ratio of AMPA receptor to NMDA receptor-mediated synaptic current through a TNFα-dependent mechanism [30]. However, in the striatum, TNFα signaling that was upregulated by application of dopamine D2 receptor antagonists, led to a reduction of calcium-permeable AMPA receptors [31, 32]. The opposite effects of TNFα during synaptic scaling in the hippocampus and striatum imply that synaptic modification by cytokine release may have substantially different actions in different regions, and thereby a single factor can impact circuit function in multiple ways.
Interestingly, not all neural-immune pathways implicated in neuronal plasticity involve canonical immune molecules. Recent work has identified a potential role for microglial brain derived neurotrophic factor (BDNF), in maintaining expression of postsynaptic glutamate receptor subtypes, the composition of which is necessary for proper synaptic plasticity. Selective deletion of microglia or genetic removal of microglial-derived BDNF in mice at P30 decreases synaptic expression of two specific glutamate receptor subtypes, GluN2B and GluA2, without affecting the overall density of neurons or synapses in the cortex and hippocampus [33]. Synaptic responses recorded in layer V pyramidal neurons in these mice were also altered compared to controls, with the current responses being dominated by the GluN2A receptor subunit. These results suggest that microglial BDNF may alter synaptic levels of GluN2B, the NMDA receptor subunit associated with immature circuits. As microglia express very low levels of BDNF in the brain, future studies are needed to clarify the roles of neuronal and microglial BDNF, particularly the mechanisms of how microglial BDNF mediates synaptic plasticity.
There is a further link between microglia and the progressive replacement of the NMDA receptor subunit GluN2B by GluN2A that occurs during early brain development [34]. During this developmental period, microglia transiently express the signaling receptor subunit DAP12. The developmental switch from GluN2B to GluN2A is prevented in DAP12 deficient mice but is recapitulated by fetal activation of microglia [35], demonstrating that even early alterations of microglia development and signaling can lead to long-lasting changes in synaptic function.
Inflammation and early changes in neuro-immune interactions alter plasticity
Inflammatory stimuli have been shown to alter synaptic plasticity and depending on the stimulus can result in either aberrant synaptic depression or potentiation. For example, interaction between neuronal CD200, a membrane glycoprotein, and its receptor, CD200R on microglia, contribute to maintaining microglia in their surveillance state, and mice deficient for CD200 exhibit a more inflammatory phenotype with increased levels of TNFα, but not IL-1β. Expression of the pattern recognition receptors, Toll-like receptor (TLR) 2 and TLR4 was also increased in these mutants. The increase in TNFα was associated with a reduction in LTP and application of TNFα to acute hippocampal slices caused a similar reduction in LTP as CD200 deficiency [36]. Moreover, application of TLR2 and TLR4 agonists at concentrations that did not affect LTP in WT mice inhibited LTP in slices prepared from CD200 deficient mice [37]. Chronic CNS inflammation thus has dramatic effects on the expression of cytokines, resulting in deficits in plasticity.
Acute inflammation can also profoundly alter neuronal activity. Treatment of acute hippocampal brain slices with LPS (10 μg/ml) in combination with oxygen deprivation triggered long-term depression (LTD) both in juvenile rats and adult mice [38, 39]. Surprisingly, the effect of LPS was mediated by microglial CR3 and not the canonical LPS receptor, TLR4, as LTD was still observed in TLR4 deficient mice while it was abolished in CR3 deficient mice. The synergistic effect of CR3 activation by LPS and hypoxia promoted NADPH oxidase-mediated production of reactive oxygen species that in turn induced protein phosphatase 2A (PP2A) regulated endocytosis of AMPA receptors, resulting in LTD [39]. Interestingly, blocking PP2A also abolished CX3CL1 evoked AMPA current depression [28]. Together, these observations demonstrate that immune signaling is capable of indirectly modulating synaptic plasticity. Although the functional impact of microglial-dependent LTD remains to be determined, one possibility is that it could protect neurons from excitotoxicity. Alternatively, this microglial-induced LTD could lead to cognitive impairment due to inappropriate weakening of synaptic strength.
In contrast to LPS and hypoxia, lower concentrations of LPS (500 ng/ml) alone can enhance synaptic strength by evoking ATP release from microglia, a process that is abolished in TLR4 deficient mice and microglia deficient mice (PU-1−/−). Similarly, application of the anti-inflammatory drug minocycline and inhibition of P2Y1 receptors also prevented LPS evoked potentiation, while direct stimulation of P2Y1 receptors mimicked this potentiation, even in PU-1−/− mice. Both LPS- and P2Y1-evoked potentiation were found to be mGluR5 dependent [38]. Different levels of inflammation can therefore have opposing effects on plasticity mechanisms.
In addition to CNS inflammation, peripheral inflammation is also associated with changes in synaptic plasticity in the hippocampus. A single intracolonic administration of 2,4,6-trinitrobenzenesulfonic acid in rats resulted in increased synaptic transmission, as measured from acute hippocampal brain slices, while both LTP and LTD were impaired compared to controls. This enhanced synaptic transmission was correlated with a decrease in both GluN2B and GluA2 and could be reversed by blocking GluA2-lacking AMPA receptors or by blocking inflammation with minocycline [40]. It is thus clear that inflammation in the body can affect the function of CNS circuits, likely leading to aberrant neuronal activity and behavior.
Functional and Behavioral Consequences of Altered Immune Signaling in the CNS
The establishment and maintenance of appropriate connectivity within neuronal networks are critical for adult behavior and cognition. Given the evidence that microglia and immune signaling can sculpt neuronal circuits, it is perhaps unsurprising that these same pathways also affect higher brain processes. In Cx3cr1KO mice, both juveniles and adults show decreased social interaction as well as increased repetitive behaviors, traits often linked to autism spectrum disorders. In juveniles this behavior is correlated with the transient synaptic pruning deficits observed in KO animals [41]. Similar to CX3CR1, the immune receptor Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) and its adapter protein DAP12 are expressed exclusively by microglia in the CNS [42, 43]. Mutations in TREM2 or DAP12 in human patients results in Nasu-Hakola disease, which is characterized by bone cysts and ultimately presenile dementia [44–46]. In mice, loss of DAP12 also results in behavioral alterations, such as a reduced startle response following acoustic stimuli and lowered prepulse inhibition [47], which is associated with schizophrenia in humans. Mutations of microglia-specific genes thus have a significant effect on behavior, demonstrating the importance of intact immune mechanisms for normal CNS function.
In addition to the impact of deficits in specific microglial genes, there are several examples of behavioral consequences due to microglial loss. For example, deletion of the Hoxb8 gene results in reduction of microglia and compulsive grooming [48]. This obsessive compulsive disorder-like behavior was rectified following a bone marrow transplant from WT mice. Depletion of microglia via specific expression of diphtheria toxin receptor in these cells led to impaired motor learning and loss of motor learning-dependent synapse formation [33]. Finally, in Mecp2 KO mouse, a mouse model of Rett Syndrome, transplantation of WT bone marrow rescued some deficits of the KO, suggesting microglia play a protective role in Rett Syndrome [49]. It is therefore clear that healthy microglia are required for a functional nervous system. The specifics of how microglia are involved in these diverse circuits and behaviors, particularly how they are communicating with neurons, will serve as important directions for future research. Moreover, a landmark fate mapping study revealed that microglia develop from myeloid progenitors in the yolk sac and colonize the brain early in embryonic development [50], suggesting that microglia have the potential to influence many aspects of brain development and wiring even before synaptic connections are formed.
Microglia-Synapse Interactions in Aging and Disease
As individuals age, their cognitive functioning gradually declines as well. It is now appreciated that the aging immune system may contribute to this decline. The structure of aging microglia changes from a highly ramified morphology to less elaborate arbors, while their numbers increase and their mosaic distribution becomes more irregular [51]. They also become less dynamic and slower to respond to tissue injury [52]. Furthermore, RNA sequencing revealed downregulation of transcripts associated with endogenous ligand recognition and upregulation of those involved in pathogen recognition and neuroprotection in aged microglia compared to healthy adult controls [53]. While these changes have been investigated in the context of neuroinflammation or immune challenge to the CNS [54], the effects on synaptic structure and function in the non-inflamed brain is still relatively mysterious. Further work must be done to elucidate whether and how aging microglia and their secreted factors affect synapses in the CNS.
Microglia have been extensively studied in the context of the neuroinflammation that is associated with many neurodegenerative disorders. However, as discussed above, microglia play a vital role in the maintenance and development of normal, healthy brains. The response of microglia to a variety of neuronal signals, the release of critical factors from microglia and the phagocytosis of synapses that have to be eliminated perhaps due to inactivity, for example, are now known to be part of the normal sculpting and refinement of CNS circuitry during development and in the adult brain. This raises the possibility that misregulation of these neural-immune interactions, rather than activation of a separate inflammatory cascade, can directly contribute to cognitive decline in aging and pathological changes in disorders of the CNS. For example, mutations in the microglia specific immune receptor TREM2 has been identified as a significant risk factor for neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease [55, 56]. These forms of neurodegeneration are known to involve early synapse loss and dysfunction. Future studies are needed to investigate whether immune dysregulation can lead directly to synapse and cognitive dysfunction.
Concluding Remarks
The roles for microglia, the resident immune cells of the brain, and the signaling pathways of the immune system, have been found to be important for normal brain development, circuit refinement and synaptic plasticity in ways that were previously unsuspected. The longstanding concept of an immune-privileged brain has been transformed so that now it is known that the immune system has a unique and privileged relationship with the brain that goes well beyond merely responding to inflammation. Elements of the immune system, including microglia and immune molecules, are required for proper development of synaptic contacts and wiring of the neuronal network during development. Furthermore, throughout life microglia respond to immune signals from the periphery or to stimulation of immune receptors and can change the strength of synaptic connections between neurons, even altering activity-dependent changes called plasticity that are required for learning and memory.. Interestingly, microglia-neuronal communication involves immunological ‘find-me’ and ‘eat-me’ signals that allow microglia to target specific synapses both in development and during adult surveillance. Furthermore, the evidence that neuronal activity itself alters microglia motility and that microglia in turn can affect behavior indicates that understanding neuron-microglia communication will undoubtedly lead to important insights about all aspects of the CNS, from development to behavior to disease.
Table 1.
Immune alterations resulting in synaptic modifications or behavioural changes
| Immune alteration | Synaptic modification | Behavior |
|---|---|---|
| CD200 KO (Increased levels of T NFα, TLR2 and TLR4) | Reduced LTP in acute mouse hippocampal slices [37] | |
| Elevated TNFα (Evoked by inhibition of synaptic transmission) | Synaptic scaling in mouse hippocampal slices [30] | |
| Elevated TNFα (Evoked by blockage of D2 receptors) | Reduction of calcium-permeable AMPA receptors in striatum [31, 32] | |
| LPS (10 μg/ml) + hypoxia (CR3 and NADPH oxidase-dependent production of ROS) | Triggers LTD by PP2A-dependent endocytosis of AMPA receptors in rat and mouse hippocampal slices [38, 39] | |
| LPS (500 ng/ml) (TLR4-mediated ATP release and activation of P2Y1) | Triggers LTP by activation of mGluR5 in mouse hippocampal slices [38] | |
| Peripheral inflammation (colonic inflammation for 4 days) | Increased synaptic transmission and impaired LTP and LTD in rat hippocampal slices [40] | |
| Microglia-neuron contact (evoked by visual stimulation) | Reduced neuronal activity in the optic tectum in zebrafish larvea [24] | |
| MHCI (H2-Db) KO | Deficits in synaptic pruning in the mouse retinogeniculate system [57] | |
| Activation of CX3CR1 (by bath application of CX3CL1) | Depression of synaptic transmission in rat and mouse hippocampal slices [28, 58] | |
| C1q KO (Failure to prune excessive excitatory synapses) | Structural modification of dendritic spines of cortical neurons [16] and enhanced LTP in the dentate gyrus of adult mice [59] | Less age-related cognitive and memory decline in hippocampus dependent behavior test [59] |
| CX3CR1 KO (elevated IL-1β ) | Reduced LTP (reversed by IL-1β receptor antagonist) [29] | Decreased social interaction and increased repetitive behaviors [41] |
| Depletion of microglia (with diphtheria toxin) and depletion of microglial-derived BDNF | Decreases learning-dependent formation of dendritic spines and altered mEPSC in mouse cortical neurons [33] | Reduced motor learning and decreased fear response [33] |
| DAP12 KO | Delayed switch from GluN2B to GluN2A and enhanced LTP in mouse hippocampal slices [35, 60] | Reduced startle response and lowered prepulse inhibition [47] |
| P2Y12KO (selective microglial receptor activated by ATP/ADP) | Exacerbate evoked-seizure activity and mortality [27] | |
| Hoxb8 KO | Compulsive grooming which was reversed by bone marrow transplant from WT mice [48] | |
| Mecp2 KO | Rett Syndrome-like phenotype, recued by bone marrow transplant from WT mice [49] |
Trends Box.
During brain development, complement-mediated engulfment of synapses by microglia is required for activity-dependent refinement of neuronal circuits.
Microglia processes constantly move as they survey the surrounding environment in response to neuronal activity. Increased neuronal activity increases process extension enhancing surveillance.
Microglia can modify activity - dependent changes in synaptic strength between neurons that underlie memory and learning using classical immunological signaling pathways involving cytokine release and NADPH oxidase activation.
Altered immune system function in the brain triggered by inflammatory responses or immune dysregulation can lead to cognitive dysfunction and behavioural abnormalities.
Glossary
- Dark adaptation
refers to the deprivation of visual experiences. This is generally achieved by housing juvenile mice in complete darkness, which increases dendritic spine turnover in the visual cortex.
- Dendritic spines
are postsynaptic neuronal structures that are correlated with neuronal synapses. Physically they appear as a protrusion along neuronal dendrites.
- Excitotoxicity
refers to the toxic effect that certain neurotransmitters (e.g., glutamate) can exert on excitatory cells (e.g., neurons) and eventually leads to cell death.
- Neuronal synapses
are connections between neurons that allow for rapid communication via release of neurotransmitters (e.g. glutamate) from a presynaptic neuron and consequently activation of receptors on a postsynaptic neuron. In this review ‘synapse’ will refer only to neuronal synapses and not immunological synapses.
- The retinogeniculate system
refers to the neurons of the retina (retinal ganglion cells) projecting to and synapsing with postsynaptic relay neurons in the dorsal lateral geniculate nucleus of the visual thalamus. This system has played a crucial role in elucidating mechanisms of synaptic pruning due to its stereotyped development and relative ease of manipulation.
- Synaptic plasticity
is the process by which synapses increase or decrease in strength following alterations in neuronal activity. For example, long-term potentiation (LTP) and long-term depression (LTD) are persistent changes in synaptic strength following high frequency stimulation or a prolonged patterned stimulus, respectively. LTP leads to strengthened synapses, while LTD results in synaptic weakening. Synaptic plasticity mechanisms are thought to underlie learning and memory.
- Synaptic pruning
is the developmental process by which immature or extraneous synapses are eliminated. This process is essential in sculpting of a functional and mature neuronal circuit. Low activity synapses are preferentially pruned over highly active synapses.
- Synaptic scaling
refers to a feedback mechanism whereby postsynaptic AMPA receptor insertion or deletion is used to scale synaptic strength up or down to ensure consistent electrical output from synaptic inputs.
- Whole cell patch clamp
is a technique that allows access to the intracellular environment of a cell through a glass micropipette and thereby enables the possibility of controlling and recording the cells electrical properties.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balice-Gordon RJ, Lichtman JW. Long-term synapse loss induced by focal blockade of postsynaptic receptors. Nature. 1994;372(6506):519–24. doi: 10.1038/372519a0. [DOI] [PubMed] [Google Scholar]
- 2.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424(6947):430–4. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 3.Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52(2):281–91. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci. 2004;7(4):327–32. doi: 10.1038/nn1218. [DOI] [PubMed] [Google Scholar]
- 5.Wilbrecht L, et al. Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J Neurosci. 2010;30(14):4927–32. doi: 10.1523/JNEUROSCI.6403-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schafer DP, Lehrman EK, Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol. 2013;23(6):1034–40. doi: 10.1016/j.conb.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sierra A, et al. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front Cell Neurosci. 2013;7:6. doi: 10.3389/fncel.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paolicelli RC, et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 10.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauro C, et al. Fractalkine in the nervous system: neuroprotective or neurotoxic molecule? Ann N Y Acad Sci. 2015 doi: 10.1111/nyas.12805. [DOI] [PubMed] [Google Scholar]
- 12.Harrison JK, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95(18):10896–901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoshiko M, et al. Deficiency of the Microglial Receptor CX3CR1 Impairs Postnatal Functional Development of Thalamocortical Synapses in the Barrel Cortex. Journal of Neuroscience. 2012;32(43):15106–15111. doi: 10.1523/JNEUROSCI.1167-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ransohoff RM, Stevens B. Neuroscience. How many cell types does it take to wire a brain? Science. 2011;333(6048):1391–2. doi: 10.1126/science.1212112. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, et al. Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy. Epilepsia. 2013;54(7):1232–9. doi: 10.1111/epi.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369–89. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 18.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131(6):1164–78. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Bialas AR, Stevens B. TGF-beta signaling regulates neuronal Clq expression and developmental synaptic refinement. Nature Neuroscience. 2013;16(12):1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Davalos D, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 21.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 22.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8(11):e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wake H, et al. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, et al. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23(6):1189–202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Fontainhas AM, et al. Microglial morphology and dynamic behavior is regulated by ionotropic glutamatergic and GABAergic neurotransmission. PLoS One. 2011;6(1):e15973. doi: 10.1371/journal.pone.0015973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dissing-Olesen L, et al. Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J Neurosci. 2014;34(32):10511–27. doi: 10.1523/JNEUROSCI.0405-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eyo UB, et al. Neuronal Hyperactivity Recruits Microglial Processes via Neuronal NMDA Receptors and Microglial P2Y12 Receptors after Status Epilepticus. J Neurosci. 2014;34(32):10528–40. doi: 10.1523/JNEUROSCI.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ragozzino D, et al. Chemokine fractalkine/CX3CL1 negatively modulates active glutamatergic synapses in rat hippocampal neurons. J Neurosci. 2006;26(41):10488–98. doi: 10.1523/JNEUROSCI.3192-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers JT, et al. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31(45):16241–50. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440(7087):1054–9. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 31.Lewitus GM, et al. An adaptive role of TNFalpha in the regulation of striatal synapses. J Neurosci. 2014;34(18):6146–55. doi: 10.1523/JNEUROSCI.3481-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stellwagen D, et al. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25(12):3219–28. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parkhurst CN, et al. Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monyer H, et al. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12(3):529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 35.Roumier A, et al. Prenatal activation of microglia induces delayed impairment of glutamatergic synaptic function. PLoS One. 2008;3(7):e2595. doi: 10.1371/journal.pone.0002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santello M, Volterra A. TNFalpha in synaptic function: switching gears. Trends Neurosci. 2012;35(10):638–47. doi: 10.1016/j.tins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Costello DA, et al. Long Term Potentiation Is Impaired in Membrane Glycoprotein CD200-deficient Mice A ROLE FOR Toll-LIKE RECEPTOR ACTIVATION. Journal of Biological Chemistry. 2011;286(40):34722–34732. doi: 10.1074/jbc.M111.280826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascual O, et al. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109(4):E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J, et al. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014;82(1):195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]
- 40.Riazi K, et al. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci. 2015;35(12):4942–52. doi: 10.1523/JNEUROSCI.4485-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhan Y, et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17(3):400–6. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 42.Bechade C, Cantaut-Belarif Y, Bessis A. Microglial control of neuronal activity. Front Cell Neurosci. 2013;7:32. doi: 10.3389/fncel.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chertoff M, et al. Differential modulation of TREM2 protein during postnatal brain development in mice. PLoS One. 2013;8(8):e72083. doi: 10.1371/journal.pone.0072083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klunemann HH, et al. The genetic causes of basal ganglia calcification, dementia, and bone cysts: DAP12 and TREM2. Neurology. 2005;64(9):1502–7. doi: 10.1212/01.WNL.0000160304.00003.CA. [DOI] [PubMed] [Google Scholar]
- 45.Paloneva J, et al. CNS manifestations of Nasu-Hakola disease - A frontal dementia with bone cysts. Neurology. 2001;56(11):1552–1558. doi: 10.1212/wnl.56.11.1552. [DOI] [PubMed] [Google Scholar]
- 46.Paloneva J, et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000;25(3):357–61. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 47.Kaifu T, et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J Clin Invest. 2003;111(3):323–32. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen SK, et al. Hematopoietic Origin of Pathological Grooming in Hoxb8 Mutant Mice. Cell. 2010;141(5):775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derecki NC, et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature. 2012;484(7392):105-+. doi: 10.1038/nature10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong WT. Microglial aging in the healthy CNS: phenotypes, drivers, and rejuvenation. Frontiers in Cellular Neuroscience. 2013;7 doi: 10.3389/fncel.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hefendehl JK, et al. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell. 2014;13(1):60–9. doi: 10.1111/acel.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hickman SE, et al. The microglial sensome revealed by direct RNA sequencing. Nature Neuroscience. 2013;16(12):1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norden DM, Godbout JP. Review: Microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and Applied Neurobiology. 2013;39(1):19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guerreiro R, et al. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–27. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rayaprolu S, et al. TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol Neurodegener. 2013;8:19. doi: 10.1186/1750-1326-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee H, et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509(7499):195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piccinin S, et al. CX3CL1-induced modulation at CA1 synapses reveals multiple mechanisms of EPSC modulation involving adenosine receptor subtypes. J Neuroimmunol. 2010;224(1–2):85–92. doi: 10.1016/j.jneuroim.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Stephan AH, et al. A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013;33(33):13460–74. doi: 10.1523/JNEUROSCI.1333-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roumier A, et al. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. Journal of Neuroscience. 2004;24(50):11421–11428. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]