Abstract
Chronic liver injury of many etiologies produces liver fibrosis and may eventually lead to the formation of cirrhosis. Fibrosis is part of a dynamic process associated with the continuous deposition and resorption of extracellular matrix, mainly fibrillar collagen. Studies of fibrogenesis conducted in many organs including the liver demonstrate that the primary source of the extracellular matrix in fibrosis is the myofibroblast. Hepatic myofibroblasts are not present in the normal liver but transdifferentiate from heterogeneous cell populations in response to a variety of fibrogenic stimuli. Debate still exists regarding the origin of hepatic myofibroblasts. It is considered that hepatic stellate cells and portal fibroblasts have fibrogenic potential and are the major origin of hepatic myofibroblasts. Depending on the primary site of injury the fibrosis may be present in the hepatic parenchyma as seen in chronic hepatitis or may be restricted to the portal areas as in most biliary diseases. It is suggested that hepatic injury of different etiology triggers the transdifferentiation to myofibroblasts from distinct cell populations. Here we discuss the origin and fate of myofibroblast in liver fibrosis.
Keywords: hepatic stellate cell, liver fibrosis, myofibroblast, portal fibroblast
Introduction
Liver fibrosis results from continuous injury to the liver, including viral hepatitis, alcohol abuse, metabolic diseases, autoimmune diseases, and cholestatic liver diseases. In other words, fibrosis is a consequence of the excessive healing response triggered by chronic liver injury. The end stage of liver fibrosis, cirrhosis, is histologically characterized by increased deposition and altered composition of the extracellular matrix (ECM) and the appearance of regenerative nodules.1 The destruction of the normal architecture and the loss of hepatocytes prevent the liver from its normal synthetic and metabolic function. Thus, the fibrogenic evolution progresses to cirrhosis, liver failure, and hepatocellular cancer.2 There is increasing evidence that the hepatic fibrosis is reversible if the stimuli are successfully removed.3 However, only subsets of liver diseases are treated effectively, and there are no specific treatments for liver fibrosis. An ideal anti-fibrogenic therapy would be liver-specific and effective in attenuating excessive ECM deposition.4
In all clinical and experimental liver fibrosis, myofibroblasts are the source of the ECM constituting the fibrous scar. Myofibroblasts are only found in the injured, but not the normal, liver. Thus, the activated myofibroblast is a pivotal player in development of liver cirrhosis, and has recently attracted interest as a therapeutic target. However, the origin of the hepatic myofibroblast is still unclear, and perhaps the fibrosis induced by different types of liver injury results from different fibrogenic cells. Hepatic myofibroblasts may originate from bone marrow-derived mesenchymal cells and fibrocytes,5 but only a small contribution of BM derived cells to the myofibroblast population has been detected in experimental liver fibrosis. Another mechanism implicated in fibrogenesis is the epithelial-to-mesenchymal transition (EMT), in which epithelial cells acquire features of mesenchymal cells and may give rise to fully differentiated myofibroblasts.6,7 However, recent cell fate mapping studies have failed to detect any hepatic myofibroblasts originating from hepatocytes, cholangiocytes, or epithelial progenitor cells. Endothelial-to-mesenchymal transition (EndMT), when endothelial cells undergo a similar phenotypic change to myofibroblasts8,9 is a theoretically, but not yet assessed source of liver myofibroblasts. Thus, the major sources of myofibroblasts in liver fibrosis appear to be the endogenous liver mesenchymal cells, the hepatic stellate cells and the portal fibroblasts.
Myofibroblasts
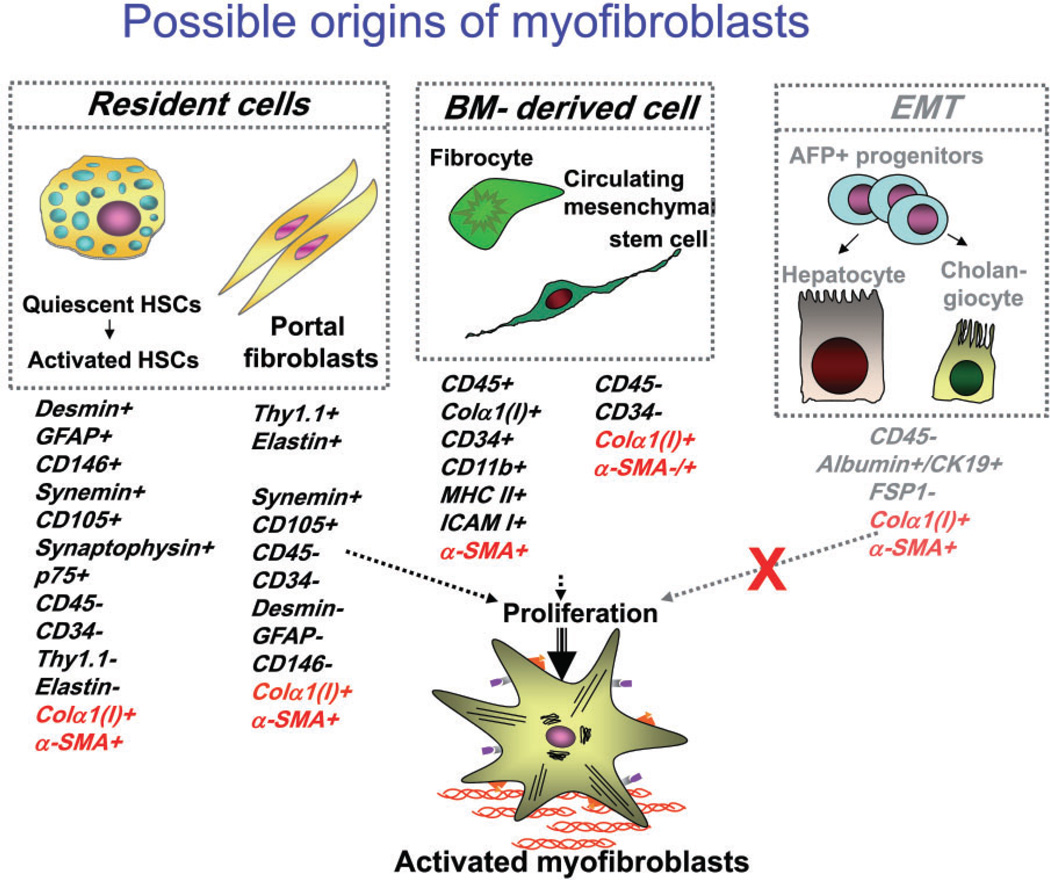
The origin of fibrogenic hepatic myofibroblasts has been intensively discussed and investigated, and several sources of myofibroblasts have been identified10–13 (Fig. 1). In the fibrotic liver, hepatic stellate cells (HSCs) have been reported to mainly contribute to the collagen producing cells.1 Therefore, HSCs are currently considered to be the major, but not the only, source of hepatic myofibroblasts in liver injury.14
Figure 1.
The proposed sources of hepatic myofibroblasts: resident cells (hepatic stellate cells and portal fibroblasts); bone marrow (BM)-derived mesenchymal cells, and cells originated by epithelial-to-mesenchymal transition. Modified from Bataller et al.1
Myofibroblasts are characterized by a stellate shape and expression of specific markers.15 In response to fibrogenic stimuli, pro-fibrogenic cells are converted to myofibroblasts. They express α-smooth muscle actin (α-SMA), secrete ECM (e.g. collagen type I and III, fibronectin) and are highly contractile.14 Classical myofibroblasts differentiate from a mesenchymal lineage and, therefore, lack expression of lymphoid markers such as CD45 or CD34.
The origins of myofibroblasts
Hepatic stellate cells
Hepatic stellate cells (HSCs) are perisinusoidal cells that normally reside in the space of Disse and have stored lipid droplets.2,16 Under normal conditions, HSCs are present in the space of Disse and exhibit a quiescent phenotype. HSCs express neural markers, such as glial fibrilar acidic protein (GFAP), synemin, synaptophysin, 1 and nerve growth factor receptor p75,17,18 desmin, secrete HGF, and store vitamin A in lipid droplets.19 In response to chronic liver injury, quiescent HSCs are activated, release vitamin A and acquire contractility. Upon activation, HSCs change their morphology to become myofibroblasts, migrate to the site of injury, downregulate neural markers and upregulate mesenchymal markers, (e.g. collagen α1(I), α-SMA, and fibronectin).
Portal fibroblasts
Portal fibroblasts are spindle shaped cells that are present in the portal area. Under normal conditions, they participate in physiological ECM turnover14,20–22 and do not express α-SMA. It is induced mostly by cholestatic liver injury,23 that portal fibroblasts proliferate24 and secrete collagen around portal tracts.25 Portal fibroblasts are distinct from HSCs in that they do not have vitamin A droplets, but express elastin and Thy-1.1 (a glycophosphatidylinositol-linked glycoprotein of the outer membrane leaflet described in fibroblasts of several organs).26,27 A proteomics study demonstrated that myofibroblasts derived from portal mesenchymal cells express much higher levels of cofilin-1 than activated HSCs. Portal fibroblasts do not express cytoglobin,28 desmin or GFAP, so that these markers are useful to identify myofibroblasts derived from HSCs. In chronic cholestatic disorders, the fibrosis is initially located around portal tracts (Fig. 2). The histological findings of liver fibrosis combined with immunohistochemistry using these specific markers demonstrate that portal fibroblasts contribute to myofibroblasts in cholestatic liver injury.1
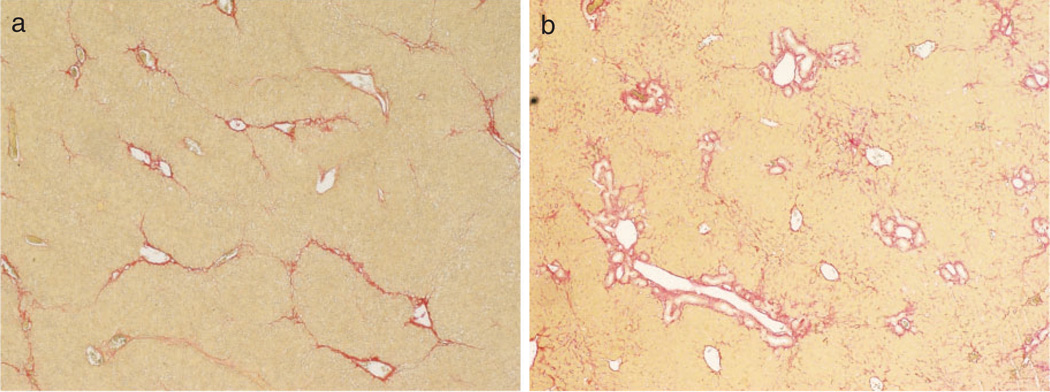
Figure 2.
Liver fibrosis induced by hepatotoxic injury or cholestatic injury in mice. Liver sections were assessed by Sirius red staining. (a) Hepatotoxic injury model; the fibrotic tissue is initially located in pericentral and perisinusoidal areas. In advance, collagen bands to bridging fibrosis to frank cirrhosis occurs. (b) Cholestatic injury model; the fibrotic tissue is initially located around portal tracts.
Born marrow derived mesenchymal cells
Born marrow (BM)-derived mesenchymal cells can also differentiate into myofibroblasts.29,30 Myofbroblasts originated from BM-derived mesenchymal cells are seen in fibrotic lungs31 and liver,30 and contribute to fibrogenesis.2,30,32 By fractionating the BM stem cell compartment, hepatic BM-derived mesenchymal stem cells (MSCs)30,31 may differentiate into hepatic myofibroblasts. MSCs are defined as self-renewable, multipotent progenitor cells with the capacity to differentiate into lineage specific cells that form bone, cartilage, fat, tendon and muscle.33,34 Unlike hematopoietic stem cells, MSCs are more radio-resistant35 and reside mostly in BM stroma, do not express hematopoietic markers and can be isolated as Lin− CD45− CD31− CD34− CD133− Sca-1+ Vitamin A− cells.36,37 Whether BM-derived myofibroblasts contribute to ECM deposition in the course of liver fibrosis is unknown. In experimental liver fibrosis, only a small contribution of BM-derived cells to the myofibroblast population has been detected.38
Epithelial-to-mesenchymal transition
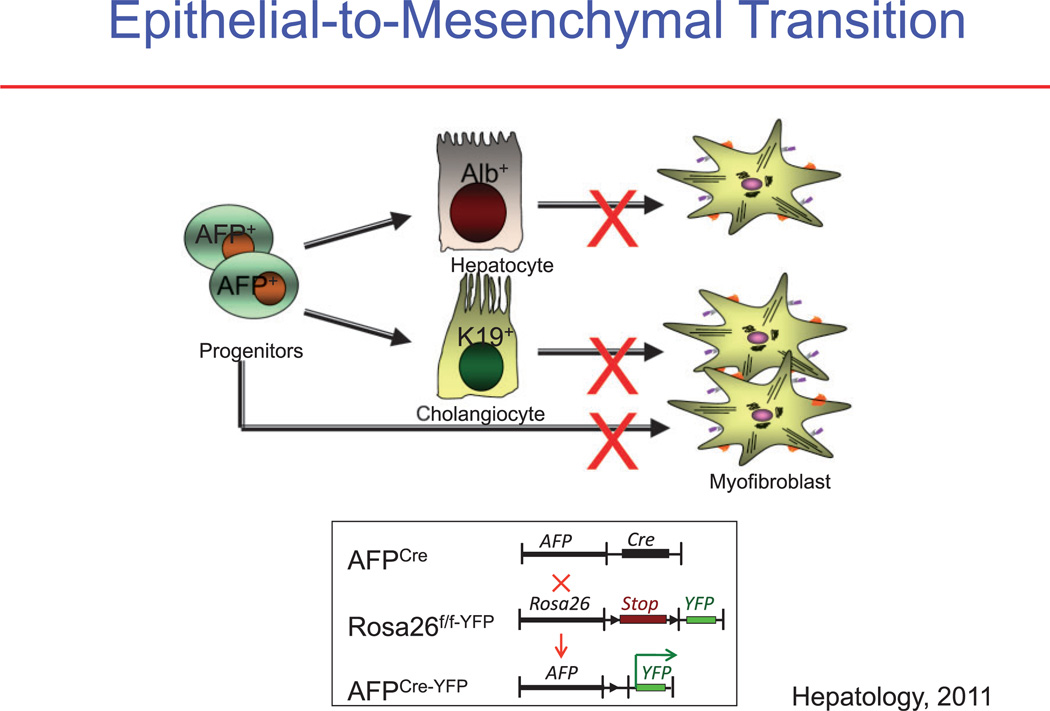
Epithelial-to-mesenchymal transition (EMT) is an important biological concept that describes the reversible transition of differentiated epithelial cells into mesenchymal cells with increased motility and changes in gene expression. Previous studies in the kidney and in the lung proposed that EMT occurs during fibrosis in those organs, and this concept was extrapolated to liver fibrosis.39 Primary cell culture studies have clearly demonstrated that cholangiocytes and hepatocytes undergo a change in the phenotype and gene expression toward a mesenchymal cell, especially after incubation with transforming growth factor (TGF)-β, which is the cytokine most closely associated with EMT.40 However, the more recent reports provide strong evidence against EMT in the liver as a source of myofibroblasts.41 These studies use lineage tracing, such as by marking hepatic epithelial progenitor cells. Such mice are generated by crossing the α-fetoprotein (AFP) Cre mouse with the ROSA26YFP stop mouse to trace the fate of any cell ever expressing AFP. As expected, all cholangiocyutes, hepatocytes, and oval cells were genetically labeled, because they are derived from a common AFP-expressing precursor cell. Furthermore, the critical result was that after inducing liver fibrosis by a variety of methods, none of the resulting myofibroblasts originated from the genetically marked epithelial (AFP+) cells (Fig. 3).
Figure 3.
Analysis of epithelial-to-mesenchymal transition (EMT). (a) It is proposed that in EMT the myofibroblasts in liver fibrosis originate from hepatic epithelial cells, consisting of hepatocytes (albumin+ [Alb+] cells), cholangiocytes (cytokeratin-19+ [K19+] cells), or progenitor cells (AFP+ cells). (b) Determining the origin of myofibroblasts using cell fate mapping. If a cell expressed α-fetoprotein (AFP), it will be irreversibly genetically labeled. AFP-driven Cre labeled epithelial progenitor cells, cholangiocytes, and hepatocytes, but failed to label any hepatic stellate cells (HSCs) or myofibroblasts. YFP, yellow fluorescent protein.41
Conclusions
Myofibroblasts are the source of the fibrous scar in liver fibrosis. Hepatic myofibroblasts are transdifferentiated from heterogeneous cell populations in response to variety fibrogenic stimuli. According to the most recent studies, the major sources of hepatic myofibroblasts in experimental liver fibrosis are hepatic stellate cells and portal fibroblasts. The role of EMT in liver fibrosis has been recently questioned. As a first approximation, myofibroblasts generated in hepatotoxic liver injury appear to originate from HSCs and myofibroblasts generated in cholestatic liver injury may originate from portal fibroblasts (Fig. 2).
Acknowledgments
This study was supported by grants from the National Institute of Health (NIH RO1 DK090962-01, NIH RO1 DK072237-06, NIH RO1 GM041804-24, and NIH P50 AA011999-13) from David A. Brenner.
Biography

David A Brenner
Footnotes
Conflict of Interests: There are no conflicts of interests.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J. Clin. Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J. Clin. Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schnabl B, Scholten D, Brenner DA. What is the potential role of antifibrotic agents for the treatment of liver disease? Nat. Clin. Pract. Gastroenterol. Hepatol. 2008;5:496–497. doi: 10.1038/ncpgasthep1200. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL, Bansal MB. Reversal of hepatic fibrosis—fact or fantasy? Hepatology. 2006;43:S82–S88. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 5.Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc. Am. Thorac. Soc. 2008;5:338–342. doi: 10.1513/pats.200711-168DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;13:565–580. doi: 10.1002/hep.23196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 9.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2008;19:2282–2287. doi: 10.1681/ASN.2008050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J. Gastroenterol. Hepatol. 2006;21(Suppl. 3):S84–S87. doi: 10.1111/j.1440-1746.2006.04584.x. [DOI] [PubMed] [Google Scholar]
- 11.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J. Clin. Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J. Leukoc. Biol. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 13.Fallowfield JA, Mizuno M, Kendall TJ, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J. Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 14.Parola M, Marra F, Pinzani M. Myofibroblast—like cells and liver fibrogenesis: emerging concepts in a rapidly moving scenario. Mol. Aspects Med. 2008;29:58–66. doi: 10.1016/j.mam.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Eyden B. The myofibroblast: phenotypic characterization as a prerequisite to understanding its functions in translational medicine. J. Cell. Mol. Med. 2008;12:22–37. doi: 10.1111/j.1582-4934.2007.00213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geerts A. History, heterogeneity, developmental biology, and functions of quiescent hepatic stellate cells. Semin. Liver Dis. 2001;21:311–335. doi: 10.1055/s-2001-17550. [DOI] [PubMed] [Google Scholar]
- 17.Sachs BD, Baillie GS, McCall JR, et al. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J. Cell Biol. 2007;177:1119–1132. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendall TJ, Hennedige S, Aucott RL, et al. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49:901–910. doi: 10.1002/hep.22701. [DOI] [PubMed] [Google Scholar]
- 19.Senoo H, Kojima N, Sato M. Vitamin a-storing cells (stellate cells) Vitam. Horm. 2007;75:131–159. doi: 10.1016/S0083-6729(06)75006-3. [DOI] [PubMed] [Google Scholar]
- 20.Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab. Invest. 1996;74:265–278. [PubMed] [Google Scholar]
- 21.Guyot C, Lepreux S, Combe C, et al. Hepatic fibrosis and cirrhosis: the (myo) fibroblastic cell subpopulations involved. Int. J. Biochem. Cell Biol. 2006;38:135–151. doi: 10.1016/j.biocel.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Kinnman N, Francoz C, Barbu V, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab. Invest. 2003;83:163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 23.Beaussier M, Wendum D, Schiffer E, et al. Prominent contribution of portal mesenchymal cells to liver fibrosis in ischemic and obstructive cholestatic injuries. Lab. Invest. 2007;87:292–303. doi: 10.1038/labinvest.3700513. [DOI] [PubMed] [Google Scholar]
- 24.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–110. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 25.Knittel T, Kobold D, Saile B, et al. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205–1221. doi: 10.1016/s0016-5085(99)70407-5. [DOI] [PubMed] [Google Scholar]
- 26.Dudas J, Mansuroglu T, Batusic D, Saile B, Ramadori G. Thy-1 is an in vivo and in vitro marker of liver myofibroblasts. Cell Tissue Res. 2007;329:503–514. doi: 10.1007/s00441-007-0437-z. [DOI] [PubMed] [Google Scholar]
- 27.Yovchev MI, Zhang J, Neufeld DS, Grozdanov PN, Dabeva MD. Thymus cell antigen-1-expressing cells in the oval cell compartment. Hepatology. 2009;50:601–611. doi: 10.1002/hep.23012. [DOI] [PubMed] [Google Scholar]
- 28.Bosselut N, Housset C, Marcelo P, et al. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics. 2010;10:1017–1028. doi: 10.1002/pmic.200900257. [DOI] [PubMed] [Google Scholar]
- 29.Forbes SJ, Russo FP, Rey V, et al. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955–963. doi: 10.1053/j.gastro.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Russo FP, Alison MR, Bigger BW, et al. The bone marrow functionally contributes to liver fibrosis. Gastroenterology. 2006;130:1807–1821. doi: 10.1053/j.gastro.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J. Clin. Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp. Biol. Med. (Maywood) 2008;233:109–122. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- 33.Kallis YN, Forbes SJ. The bone marrow and liver fibrosis: friend or foe? Gastroenterology. 2009;137:1218–1221. doi: 10.1053/j.gastro.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 34.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–982. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 35.Bartsch K, Al-Ali H, Reinhardt A, et al. Mesenchymal stem cells remain host-derived independent of the source of the stem-cell graft and conditioning regimen used. Transplantation. 2009;87:217–221. doi: 10.1097/TP.0b013e3181938998. [DOI] [PubMed] [Google Scholar]
- 36.Short BJ, Brouard N, Simmons PJ. Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol. Biol. 2009;482:259–268. doi: 10.1007/978-1-59745-060-7_16. [DOI] [PubMed] [Google Scholar]
- 37.Simmons PJ, Przepiorka D, Thomas ED, Torok-Storb B. Host origin of marrow stromal cells following allogeneic bone marrow transplantation. Nature. 1987;328:429–432. doi: 10.1038/328429a0. [DOI] [PubMed] [Google Scholar]
- 38.Kisseleva T, Uchinami H, Feirt N, et al. Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J. Hepatol. 2006;45:429–438. doi: 10.1016/j.jhep.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Zeisberg M, Duffield JS. Resolved: EMT produces fibroblasts in the kidney. J. Am. Soc. Nephrol. 2010;21:1247–1253. doi: 10.1681/ASN.2010060616. [DOI] [PubMed] [Google Scholar]
- 40.Taura K, Miura K, Iwaisako K, et al. Hepatocytes do not undergo epithelial-mesenchymal transition in liver fibrosis in mice. Hepatology. 2010;51:1027–1036. doi: 10.1002/hep.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu AS, Diaz R, Hui JJ, et al. Lineage tracing demonstrates no evidence of cholangiocyte epithelial-to-mesenchymal transition in murine models of hepatic fibrosis. Hepatology. 2011;53:1685–1695. doi: 10.1002/hep.24206. [DOI] [PMC free article] [PubMed] [Google Scholar]