Abstract
Formation and growth of hydroxyapatite crystals during amelogenesis generate a large number of protons that must be neutralized, presumably by HCO3− ions transported from ameloblasts into the developing enamel matrix. Ameloblasts express a number of transporters and channels known to be involved in HCO3− transport in other epithelia. However, to date, there is no functional evidence for HCO3− transport in these cells. To address questions related to HCO3− export from ameloblasts, we have developed a polarized 2-dimensional culture system for HAT-7 cells, a rat cell line of ameloblast origin. HAT-7 cells were seeded onto Transwell permeable filters. Transepithelial resistance was measured as a function of time, and the expression of transporters and tight junction proteins was investigated by conventional and quantitative reverse transcription polymerase chain reaction. Intracellular pH regulation and HCO3− transport were assessed by microfluorometry. HAT-7 cells formed epithelial layers with measureable transepithelial resistance on Transwell permeable supports and expressed claudin-1, claudin-4, and claudin-8—key proteins for tight junction formation. Transport proteins previously described in maturation ameloblasts were also present in HAT-7 cells. Microfluorometry showed that the HAT-7 cells were polarized with a high apical membrane CO2 permeability and vigorous basolateral HCO3− uptake, which was sensitive to Na+ withdrawal, to the carbonic anhydrase inhibitor acetazolamide and to H2DIDS inhibition. Measurements of transepithelial HCO3− transport showed a marked increase in response to Ca2+- and cAMP-mobilizing stimuli. Collectively, 2-dimensional HAT-7 cell cultures on permeable supports 1) form tight junctions, 2) express typical tight junction proteins and electrolyte transporters, 3) are functionally polarized, and 4) can accumulate HCO3− ions from the basolateral side and secrete them at the apical membrane. These studies provide evidence for a regulated, vectorial, basolateral-to-apical bicarbonate transport in polarized HAT-7 cells. We therefore propose that the HAT-7 cell line is a useful functional model for studying electrolyte transport by ameloblasts.
Keywords: dental enamel, in vitro techniques, ion transport, cytophotometry, fluorescent dyes, tissue engineering
Introduction
Ameloblasts are electrolyte-transporting epithelial cells that transport calcium and phosphate ions, the principal building blocks of hydroxyapatite crystals, into the enamel space. Formation of hydroxyapatite during the maturation stage of amelogenesis generates a large quantity of protons, and to sustain crystal growth, these protons need to be neutralized (Smith 1998; Lacruz et al. 2013; Jalali et al. 2014). To buffer the pH in the enamel space, ameloblasts seem to have the molecular machinery to secrete HCO3− ions into the enamel space. Maturation ameloblasts express carbonic anhydrase 2 and 6 (Car2, Car6), the Cl−-HCO3− exchanger 2 (Slc4a2/Ae2), Na+-HCO3− cotransporter (Slc4a4/Nbce1), Na+-H+ exchanger 1 (Slc9a1/Nhe1), cystic fibrosis transmembrane conductance regulator (Cftr), Slc26a4/pendrin, Slc26A3/Dra, and Slc26a6/Pat1 (Bronckers et al. 2011; Lacruz et al. 2013; Jalali et al. 2014; Jalali et al. 2015). Additional mechanisms probably also participate in extracellular pH control. Recent studies indicated the likely involvement of active proton transport and the importance of tight junction (TJ) proteins in enamel formation (Josephsen et al. 2010; Damkier et al. 2014; Bardet et al. 2016). Studies on loss of function of several of these proteins have indicated their involvement in mineralization (Smith 1998; Lyaruu et al. 2008; Bronckers et al. 2011; Lacruz et al. 2013; Bronckers et al. 2015). At present, all of the available information about pH regulation–related electrolyte transport by ameloblasts is based solely on immunohistochemistry, tracer and staining techniques, and expression studies without any functional corroboration. Consequently, mechanistic models such as these are purely hypothetical, and there is a need for suitable experimental models to enable functional measurements of transport activity.
HAT-7 is a dental epithelial cell line derived from the cervical loop epithelium of a rat incisor, established in 2002 (Kawano et al. 2002). Immunocytochemical studies showed that HAT-7 cells exhibit several ameloblast characteristics, including the expression of amelogenin and ameloblastin (Kawano et al. 2002) and also maturation-stage ameloblast markers such as kallikrein-4 (Klk4) and amelotin. However all of these studies have been restricted to expression profiling (Harada et al. 2006; Yoshizaki et al. 2008; Matsumoto et al. 2011; Zheng et al. 2013). The purpose of the present study was 1) to establish confluent monolayers of HAT-7 cells on permeable supports, 2) to characterize gene expression of TJ and electrolyte transport proteins, and 3) to assess the functional polarization of monolayers and their capacity for basolateral to apical HCO3− transport.
Materials and Methods
Cell Culture
HAT-7 cells were grown on permeable polyester Transwell culture inserts with 0.4-μm pore size and 1.12-cm2 surface area (Corning Inc., Corning, NY, USA) and cultured in 3 media:
C: control medium consisting of DMEM/F12 (Sigma-Aldrich, St. Louis, MO, USA) with 10% HyClone fetal bovine serum (Thermo Scientific, Waltham, MA, USA)
D: differentiation medium, the same medium but supplemented with CaCl2 (final concentration 2.1 mM) and 10-5 mM dexamethasone (Sigma-Aldrich; Arakaki et al. 2012)
H: hepato-STIM medium, a commercially available epithelial selection medium effectively used for primary salivary gland cultures (Szlavik et al. 2008; Hegyesi et al. 2015)
All media contained 100 U/mL of penicillin and 10 µg/mL of streptomycin (Sigma-Aldrich), and cells were grown in a humidified atmosphere containing 5% CO2 at 37 oC.
Measurement of Transepithelial Electrical Resistance, Immunocytochemistry, RT-PCR, RT-qPCR, Microfluorometry, and Statistical Analysis
The following were performed as described in the Appendix: transepithelial electrical resistance (TER) of cells on Transwell, immunocytochemical imaging for identification of cell-specific proteins (Bronckers et al. 2015; Jalali et al. 2015), methods identifying TJ proteins and expected electrolyte transporters by applying standard semiquantitative and quantitative polymerase chain reaction technologies to amplify the mRNA (Hegyesi et al. 2015), microfluorometric measurements, and statistical analyses (Szucs et al. 2006).
Results
Morphology and Immunocytochemistry of HAT-7 Cells
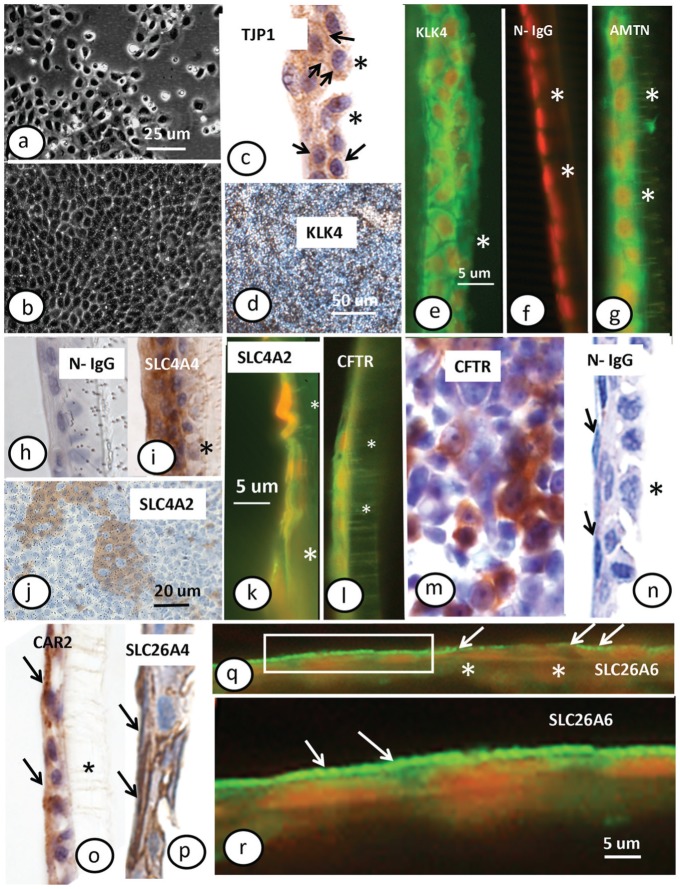
HAT-7 cell cultures showed morphology and growth characteristics similar to their original description (Kawano et al. 2002). The cells covered the Transwell surface and reached confluence in 2 to 3 d regardless of choice of culture medium (Fig. 1a, b). Transverse sections showed that the cells were either very flat or cuboidal and mostly formed a single layer with small regions of ≥2 layers (Fig. 1). Immunostaining for zonula occludens-1 (TJP1/ZO1; Fig. 1c) resulted in widespread positive labeling, while application of normal nonimmune rabbit IgG as control showed no labeling (Fig. 1h). Positive immunostaining for maturation-stage markers such as Klk4 (Fig. 1d, e) and amelotin (Fig. 1g) was observed both en face and in transverse sections, suggesting that HAT-7 cells exhibit a maturation-stage ameloblast phenotype.
Figure 1.
Morphology and immunocytochemistry of HAT-7 cells. HAT-7 cells grown on a plastic culture plate (a) and Transwell membrane (b); phase contrast. Immunocytochemical localization of (c) tight junction protein 1 (TJP1/ZO1, zonula occludens-1; arrows indicate at weakly stained plasma membrane); (d, e) kallikrein 4 (KLK4) with (f) normal IgG control; (g) amelotin (AMTN); (h) normal IgG control with cuboic surface cells; (i) Na+-HCO3− cotransporter-e1 (SLC4A4/NBCe1); (j) anion exchanger 2 (SLC4A2/AE2) in top view and cross section (k); (l, m) cystic fibrosis transmembrane conductance regulator (CFTR) in cross section (l) or top view (m); (n) normal IgG control with flattened surface cells (arrows refer to the apical surface of the cells); (o) carbonic anhydrase type 2 (CAR2; arrows indicate surface cells with positive staining in apical part); (p) SLC26A4/pendrin (arrows point at surface staining); (q, r) SLC26A6/PAT1. Panels a, b, d, j, m: top views; all others: cross sections. Arrows in panels q and r indicate positive reaction in the membrane facing the culture medium. (q) The reaction is interrupted at the right half and continuous at the left half. (r) A higher magnification of boxed area at the left side. Immunofluorescence: green in e to g, k, l, q, r with nuclei in orange; peroxidase: brown in in d, h, j, m to p with nuclei in blue. Asterisks show position of Transwell membrane. Panels a to c, e, h to l, o, p: hepato-STIM culture medium; d to g, m, q, r: differentiation culture medium. Original magnifications: a, b (100×); c to i, k to p, r (400×); d (50×); q (200×). This figure is available in color online at http://jdr.sagepub.com.
Positive staining for SLC4A4/NBCe1, SLC4A2/AE2, SLC26A4/pendrin, SLC26A6/PAT1, CFTR, and CAR2 on transverse sections revealed the presence of all 6 proteins involved in HCO3− secretion (Fig. 1i–r). There were no qualitative differences in the expression patterns of these proteins in HAT-7 cells (data not shown) grown in the D and H media that we used for further experimentation.
TER, TJ Formation, and Transporter Expression
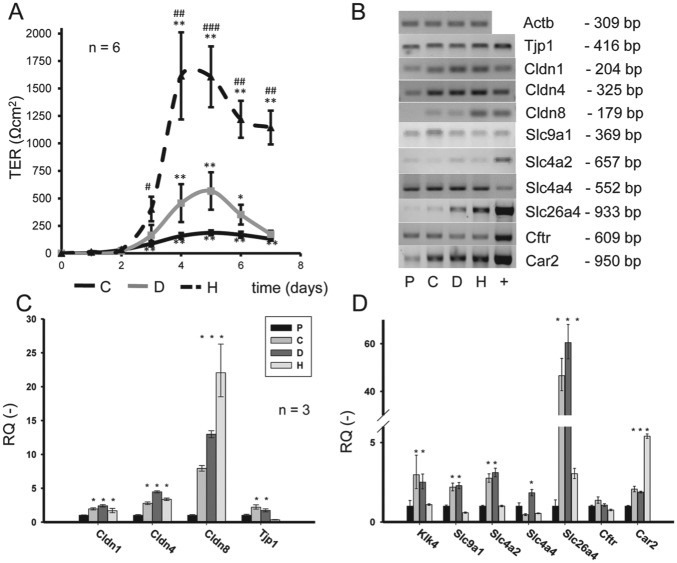
To check for functional polarization of the confluent layers of HAT-7 cells, TER was measured. There were striking differences in the TER value when different media were used. TER values were lowest in cells grown in C medium and highest in H medium (Fig. 2A). Resistance curves typically reached a peak value on the fourth or fifth day and declined to lower plateau phase by the seventh day. The peak values show that the TJs are fully formed, and the lower plateau phase that follows reflects the increasing TJ density as the cell numbers increase.
Figure 2.
Transepithelial resistance, tight junction formation in HAT-7 cells cultured in different media. (A) Transepithelial resistance (TER) of HAT-7 cells cultured on Transwell membranes for 7 d. Cells were cultured in control (C), differentiation (D), or Hepato-STIM (H) medium (n = 6). Significant changes in TER compared with day 0: *P < 0.05, **P < 0.01, ***P < 0.005. Significant differences in TER compared with same-day control: #P < 0.05, ##P < 0.01, ###P < 0.005. (B) Conventional RT-PCR (reverse transcription polymerase chain reaction), data showing mRNA expression of Tjp1, Cldn1, Cldn2, Cldn8, Slc9a1/Nhe1, Slc4a2/Ae2, Slc4a4/Nbce1, Slc26a4/pendrin, Cftr, and Car2 genes in HAT-7 cells cultured on Petri dishes in control medium (plastic [P]) and on Transwells in C, D, and H media. Kidney and ileum mRNAs were used as positive controls (+). (C) Quantitative RT-PCR data showing expression of tight junction–specific Cldn1, Cldn2, Cldn8, and Tjp1/Zo1 genes in HAT-7 cells grown on Transwells in C, D, and H media, normalized to their expression in cells grown on plastic in C medium (n = 3). (D) Quantitative RT-PCR data showing expression of maturation phase ameloblast marker gene Klk4; electrolyte transporters Slc9a1/Nhe1, Slc4a2/Ae2, Slc4a4/Nbce1, and Slc26a4/pendrin; and Cftr and Car2 genes in HAT-7 cells treated as described above (n = 3). Significant changes in expression compared with cells grown on P surface: *P < 0.05.
Using RT-PCR (reverse transcription polymerase chain reaction), we found expression of Tjp1/Zo1 and claudins (Cldn1, Cldn4, and Cldn8) at mRNA level in every HAT-7 sample, regardless of whether they were grown on plastic or on porous Transwell membranes (Fig. 2B). Quantitative polymerase chain reaction data normalized to Rplpo (Fig. 2C) revealed that claudins were at least doubled in the Transwell groups (C, D, and H media) as compared with their expression on plastic. The culture medium also influenced the expression pattern. The greatest difference was observed in Cldn8 expression, where the relative quantity (normalized to the plastic group samples) ranged from 7.9 ± 0.4 in C medium to 22.1 ± 4.2 in H. In the case of Cldn1 and Cldn4, expression primarily depended on the surface used but not on the culture medium (Fig. 2C). The expression of Tjp1/Zo1 was increased in C and D media but not in H medium. Likewise, the expression of maturation ameloblast-specific Klk4 considerably increased in HAT-7 cells cultivated on Transwells in C and D media but not in H medium (Fig. 2D).
Key electrolyte transporters/channels such as Slc9a1/Nhe1, Slc4 a2/Ae2, Slc4a4/Nbce1, Slc26a4/pendrin, and Cftr were all expressed in HAT-7 cells (Fig. 2D), although at variable levels. Similar to this, the cytoplasmic carbonic anhydrase Car2 isoform highly expressed in maturation ameloblasts in situ could be also detected (Fig. 2D). Quantitative polymerase chain reaction experiments showed that the expression of Slc 9a1/Nhe1, Slc4a2/Ae2, Slc4a4/Nbce1, Slc26a4/pendrin significantly increased in cells cultivated on Transwells both in C and D media but not in H medium (Fig. 2D). No significant changes were observed for Cftr, while Car2 expression increased only in cells cultivated in H medium on Transwells (Fig. 2D).
Functional Polarization of HAT-7 Cells
Although culture in H medium yielded the highest TER values and the highest levels of expression of TJ proteins, preliminary functional experiments indicated that in this medium, HAT-7 cells produced more variable and less consistent results (data not shown). Therefore, we continued our studies with HAT-7 cells cultivated in D medium, which produced moderate TER values and behaved consistently in microfluorometric experiments.
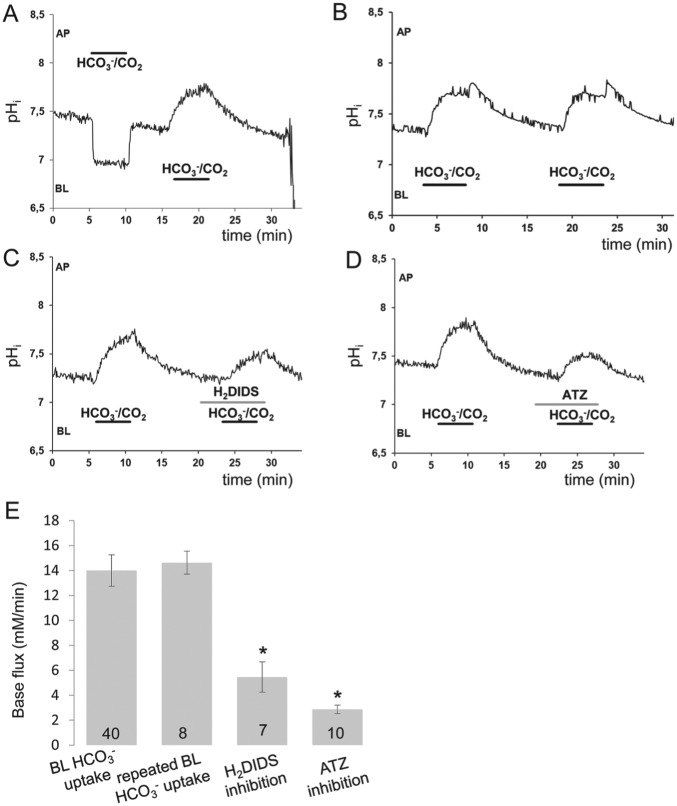
Bicarbonate and CO2 membrane permeabilities were investigated by unilaterally exposing the HAT-7 cells to HCO3−/CO2 (Fig. 3). After perfusing the apical and basolateral sides of the cells with the HCO3−-free HEPES-buffered solution, apical perfusion was switched to the HCO3−/CO2 solution. A rapid acidification of intracellular pH (pHi) occurred as a result of CO2 diffusion into the cells (Fig. 3A). Following this, pHi reached a new level and remained there until the HEPES solution was restored at the apical side. This induced a rapid alkalinization of pHi toward the resting value as a result of the diffusion of CO2 out of the cells (Fig. 3A). When the same change, from HEPES to HCO3−/CO2, was performed on the basolateral side, pHi increased rapidly and reversibly showing the uptake of HCO3− ions (Fig. 3A).
Figure 3.
Functional polarization and bicarbonate uptake by HAT-7 cells. (A) Changes in intracellular pH (pHi) in HAT-7 cells grown on Transwells in differentiation medium and bathed initially in HCO3−/CO2-free, HEPES-buffered solution. Apical (AP) and basolateral (BL) surfaces were separately exposed to HCO3−/CO2-buffered perfusate for 5 min. (B, C) Changes in pHi evoked by basolateral exposure to HCO3−/CO2 in the absence and presence of H2DIDS (500 µM). (D) Changes in pHi evoked by basolateral exposure to HCO3−/CO2 in response to acetazolamide (ATZ; 100 µM). (E) Mean base fluxes (± SEM) calculated from the initial rates of increase in pHi following basolateral exposure to HCO3−/CO2 in the presence and absence of the inhibitors (n = 7 to 40). *P < 0.05 compared with control.
Inhibition of Basolateral HCO3− Uptake by HAT-7 Cells
To test whether anion transporters are responsible for HCO3− uptake, we repeated the switch from HEPES to HCO3−/CO2 in the absence and the presence of H2DIDS, an inhibitor of anion transport. Repeated switch from HEPES to HCO3−/CO2 without inhibitor application resulted in no change of alkalinization dynamics (Fig. 3B, E). When H2DIDS was added, the alkalinization was smaller and slower than in controls (Fig. 3C, E), indicating that a basolateral HCO3− transporter, most probably NBCe1/SLC4A4, has an important role in HCO3− uptake. To investigate the role of carbonic anhydrase in intracellular HCO3− accumulation, we used acetazolamide (100 µM), a membrane-permeable carbonic anhydrase inhibitor. In the presence of acetazolamide, the alkalinization was again inhibited, suggesting that carbonic anhydrase also contributes to HCO3− accumulation (Fig. 3D, E).
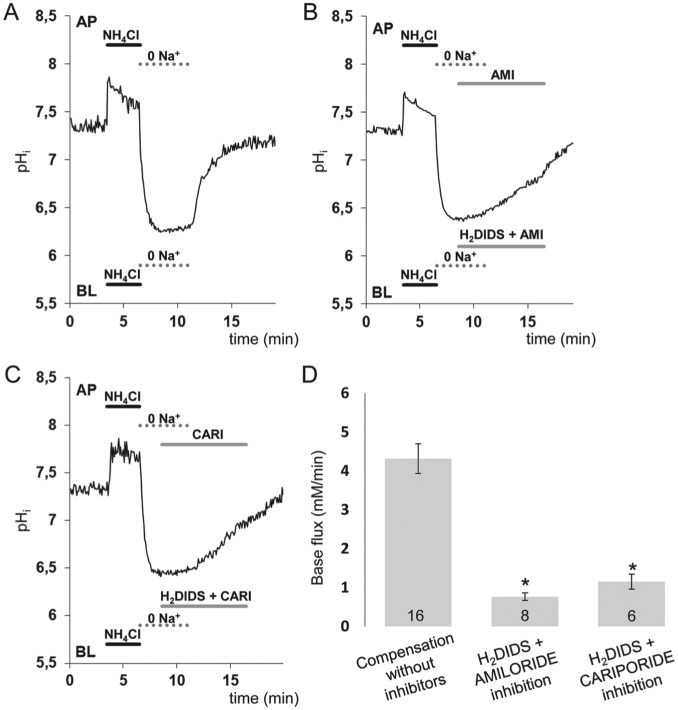
To identify the mechanism of HCO3− transport across the basolateral membrane, we next used the NH4+ prepulse technique. As expected, bilateral application of a 20mM NH4+ pulse caused a transient intracellular alkalinization, followed by a marked acidification (Fig. 4). In the standard HCO3−/CO2 solution, pHi recovered gradually from the acidification, most probably as a result of H+ extrusion by Na+-H+ exchange and HCO3− uptake by Na+-HCO3− cotransport (data not shown). However, when the NH4+ pulse was immediately followed by bilateral substitution of Na+ with the nontransported cation NMDG+, the recovery of pHi was completely abolished (Fig. 4A). Restoration of Na+ led to a rapid recovery in pHi. This indicates that the basolateral transport mechanism is Na+ dependent and does not involve proton pump activity. It is therefore most likely to be mediated by NBCe1/SLC4A4 and/or NHE/SLC9A1 (Fig. 4A, B). To test this hypothesis, 300μM amiloride and 500μM H2DIDS were applied to suppress basolateral NHE1/SLC9A1 and NBCe1/SLC4A4 activities, respectively, immediately after Na+ restoration (Fig. 4B). The recovery of pHi following the NH4+ pulse was reduced by about 85% in the presence of the inhibitors (Fig. 4B, C) in support of our hypothesis. To further confirm the identity of Na+-H+ exchange cariporide, a highly selective NHE1/SLC9A1 inhibitor was used instead of amiloride (Harguindey et al. 2013). These experiments revealed that 10µM cariporide was as effective as amiloride (Fig. 4D).
Figure 4.
Recovery of intracellular pH (pHi) in HAT-7 cells exposed to an acid load in the presence of HCO3−/ CO2. HAT-7 cells grown on Transwell supports in differentiation medium were exposed bilaterally to 20mM NH4+, followed by bilateral substitution of Na+ with NMDG+. (A) Recovery of pHi following bilateral restoration of extracellular Na+. (B) Inhibition of pHi recovery following restoration of Na+ in the presence of basolateral (BL) H2DIDS (500 μM) and amiloride (300 μM). Amiloride was also included in the apical (AP) perfusate to inhibit any apical NHE activity. (C) Similar inhibitory experiment is shown as in panel B, but amiloride was replaced by the specific inhibitor cariporide (10 µM) to selectively block the NHE1/SLC9A1 antiport. (D) Mean base fluxes (± SEM) calculated from the initial rates of increase in pHi following restoration of Na+ in the presence and absence of the inhibitors (n = 8 to 10). *P < 0.05 compared with control.
Bicarbonate Secretion
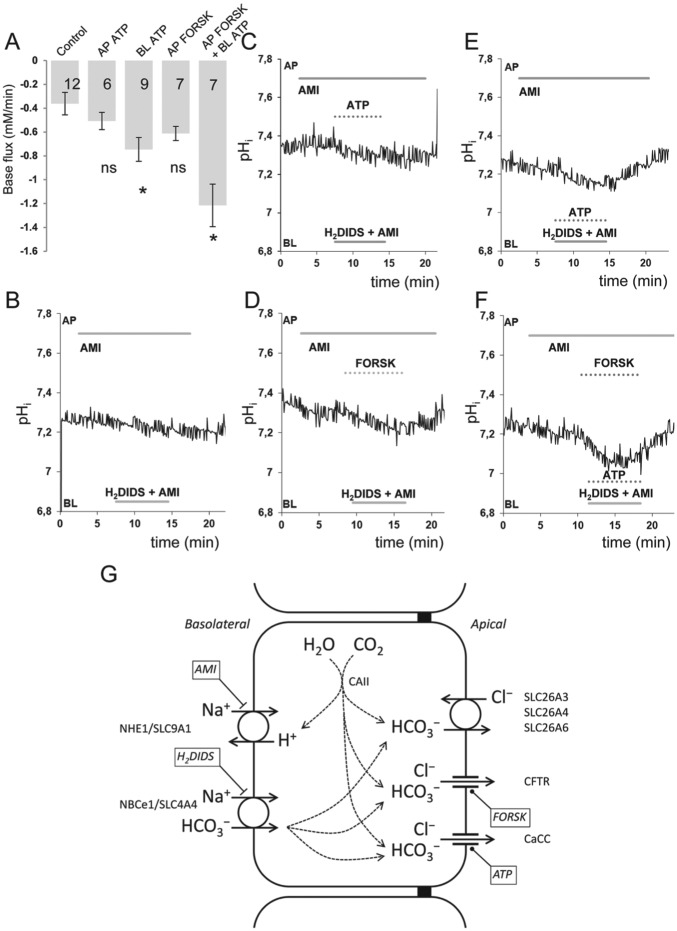
The ability to block HCO3− uptake across the basolateral membrane allows us to estimate the rate of HCO3− secretion across the apical membrane. Following basolateral blockade, HCO3− secretion continues at the apical membrane, and pHi falls as a result. The initial acidification rate, which is a measure of HCO3− secretion, was estimated with unstimulated HAT-7 cells (Fig. 5A, B) and also during stimulation with ATP (50 µM) to mobilize intracellular Ca2+ (Fig. 5C, E) or forskolin (10 µM) and IBMX (500 µM) to elevate intracellular cAMP (Fig. 5D) or the 3 combined (Fig. 5F). The initial acidification rates, expressed as base fluxes, are summarized in Figure 5A. In the absence of stimulation, the base flux was very low (Fig. 5B), suggesting only a low level of basal HCO3− secretion. Apical application of ATP had no significant effect (Fig. 5A, C), but basolateral ATP caused a small but significant increase (Fig. 5C), as did apical forskolin and IBMX (Fig. 5E). The largest response was seen when basolateral ATP and apical forskolin and IBMX were applied simultaneously (Fig. 5F), providing clear evidence that HAT-7 cells are capable of vectorial HCO3− secretion in a basal to apical direction. When amiloride was replaced by the selective NHE1/SLC9A1 antagonist cariporide, simultaneous ATP, forskolin, and IBMX administration yielded an initial base flux of 1.77 ± 0.24 mM/min, a similar value found during amiloride application.
Figure 5.
Intracellular acidification evoked in HAT-7 cells by inhibition of basolateral HCO3− uptake in the presence and absence of ATP and forskolin. Basolateral HCO3− uptake in HAT-7 cells grown on Transwells in differentiation medium was inhibited by simultaneous basolateral (BL) application of 500μM H2DIDS and 300μM amiloride (AMI). AMI was also included in the apical (AP) perfusate to inhibit any apical NHE1/SLC9A1 activity. (A) Mean base fluxes (± SEM) calculated from the initial rates of decrease in intracellular pH (pHi) following application of basolateral H2DIDS and AMI in the presence and absence of ATP and/or forskolin and IBMX (FORSK). *P < 0.05 compared with control. Representative pHi traces obtained in unstimulated control conditions (B) and in the presence of (C) apical ATP (50 µM), (D) apical forskolin (10 µM) and IBMX (500 µM), (E) basolateral ATP (50 µM), and (F) apical forskolin (10 µM) and IBMX (500 µM) in combination with basolateral ATP (50 µM). (G) Schematic depiction of the proposed mechanism of vectorial bicarbonate transport of HAT-7 cells, exhibiting the major transporters and channels involved in the process.
Discussion
A major finding of the present work is that ameloblast-derived HAT-7 cells are able to form polarized confluent monolayers on permeable supports and develop measurable TER. We also found expression of Cldn1, Cldn4, Cldn8, and Tjp1/Zo1, which indicates the presence of mature TJs (Figs. 1, 2). These are a prerequisite for vectorial electrolyte secretion by restricting free transepithelial ion movements but permitting passage of certain ions between the cells (Melvin et al. 2005; Steward et al. 2005; Hou 2014). The higher level of Cldn8 expression in the H medium compared with the D and C media is in line with our observation that TER was highest in the H medium, modest in the D medium, and very low in the C medium (Amasheh et al. 2009). The data are also in accordance with previous studies (Colegio et al. 2002; Amasheh et al. 2009; Lal-Nag and Morin 2009) showing the expression of Cldn1, Cldn4, and Cldn8 in maturation-stage ameloblasts (Inai et al. 2008; Hata et al. 2010). In spite of the fact that H medium induced the highest TER and the highest expression of claudins, functional measurements of electrolyte transport with H medium were erratic and inconsistent, and transporter expression was diminished as compared with cells in C or D medium. In many Cl−- and HCO3−-secreting epithelia (e.g., salivary acini and pancreatic ducts), the TJs have to be relatively “leaky” to support the necessary paracellular transport of Na+ ions (Melvin et al. 2005; Steward et al. 2005; Hou 2014). If HCO3− secretion by ameloblasts is accompanied by paracellular Na+ transport, we would anticipate that a relatively leaky junctional phenotype would be more likely than the very high resistances observed in the cells grown in H medium—hence, our choice of the D medium for all subsequent studies.
Previous studies indicated that Slc9a1/Nhe1, Slc4a2/Ae2, Slc4a4/Nbce1, Slc26a4/pendrin, and Cftr are expressed by ameloblasts and are necessary for intracellular and extracellular pH regulation (Bronckers et al. 2011; Lacruz et al. 2013; Jalali et al. 2014). Among carbonic anhydrases, the cytoplasmic Car2 isoform is dominant, although others have been described (Lacruz, Smith, Moffatt, et al. 2012; Reibring et al. 2014). The fact that HAT-7 cells grown on Transwell filters express maturation-stage ameloblast-specific markers (including Slc9a1/Nhe1, Slc4a2/Ae2, Slc4a4/Nbce1, Slc26a4/pendrin, Cftr, and Car2) suggests that this cell line is suitable as an experimental model for studying ameloblast acid/base transport (Fig. 2).
Our results demonstrate that HAT-7 cells are functionally polarized with 1) an apical membrane that is highly permeable to CO2 but does not take up HCO3− and 2) a basolateral membrane that has a lower permeability to CO2 but is capable of vigorous HCO3− uptake (Fig. 3). This is similar to other HCO3−-secreting epithelia, such as guinea pig pancreatic duct (Ishiguro et al. 2000) and the human CFPAC cells (Rakonczay et al. 2006). It is also consistent with the suggestion that maturation-stage ameloblasts are equipped to secrete HCO3− to neutralize the acidity generated at the apical border of ameloblasts during hydroxyapatite formation (Smith 1998; Lacruz et al. 2013; Jalali et al. 2014).
The 2 most likely pathways for basolateral HCO3− uptake in ameloblasts are by Na+-HCO3− cotransport and by CO2 diffusion into the cells, with carbonic anhydrase catalyzing its conversion into HCO3− ions and protons (Lacruz et al. 2013; Jalali et al. 2014). In our conditions, a substantial proportion of the basolateral HCO3− uptake was Na+ dependent and inhibited by H2DIDS and therefore most likely due to the action of the ubiquitously expressed NBCe1/SLC4A4 cotransporter. Again there are parallels with the pancreatic duct epithelium, where NBCe1/SLC4A4 makes a major contribution to the basolateral uptake of HCO3− (Ishiguro et al. 1996). The application of the membrane-permeable carbonic anhydrase inhibitor acetazolamide also partially inhibited the basolateral base flux in HAT-7 cells, suggesting that the alternative mechanism is present and presumably dependent on H+ extrusion via a basolateral Na+-H+ exchanger (Fig. 3). Among the large number of different isoenzymes in the carbonic anhydrase gene family, the dominant isoform in ameloblasts is Car2 (Lacruz et al. 2010; Reibring et al. 2014), the isoform that we found to be expressed in HAT-7 cells.
Our NH4+ pulse experiments showed that removal of Na+ from the bathing solution fully prevented the pHi recovery of the HAT-7 cells following acidification (Fig. 4), similar to other HCO3−-secreting epithelia (Ishiguro et al. 1996; Szucs et al. 2006; Demeter et al. 2009). Application of 300μM amiloride and 500μM H2DIDS to inhibit NHE1/SLC9A1 and NBCe1/SLC4A4 (Ishiguro et al. 1996; Demeter et al. 2009; Lee et al. 2012) resulted in an approximately 85% inhibition of the recovery rate from the acid load, suggesting that NHE1/SLC9A1 and NBCe1/SLC4A4 transporters are jointly responsible for most of the basolateral HCO3− uptake. This was further confirmed when amiloride was replaced by the NHE1/SLC9A1 selective cariporide (Harguindey et al. 2013).
As H2DIDS and amiloride blocked most of the basolateral uptake of HCO3−, we could use a relatively simple fluorometric method to test whether HAT-7 cells are able to achieve vectorial HCO3− secretion. In secretory epithelia, HCO3− entry across the basolateral membrane is closely coupled to HCO3− efflux across the luminal membrane (Ishiguro et al. 1996; Szucs et al. 2006; Demeter et al. 2009). Therefore, when HCO3− entry is blocked by transport inhibitors, the continuing efflux of HCO3− across the luminal membrane leads to a fall in pHi. The initial rate of fall in pHi therefore serves as an index of instantaneous HCO3− efflux across the apical membrane. When we applied a combination of NHE1/SLC9A1 and NBCe1/ SLC4A4 inhibitors to unstimulated HAT-7 cells, we observed a slow acidification due to apical HCO3− secretion, but this was more pronounced when the cells were stimulated (Fig. 5). Extracellular ATP, a bioactive molecule acting through purinergic receptors to raise intracellular Ca2+ (Schwiebert and Zsembery 2003), stimulated HCO3− transport when applied to the basolateral but not to the apical side. In other secretory epithelia, the differing effects of apical and basolateral ATP on HCO3− secretion are well documented (Schwiebert and Zsembery 2003; Szucs et al. 2006; Baggaley et al. 2007; Demeter et al. 2009). The present work raises the possibility that extracellular ATP could be an important regulator of ameloblast function, most probably acting via calcium-activated chloride channels recently identified in maturation-stage ameloblasts using protein expression assays (Lacruz, Smith, Bringas, et al. 2012). Forskolin, which activates the cAMP/ protein kinase A pathway, strongly potentiated the effect of ATP, most probably by opening CFTR chloride channels (Lacruz et al. 2013; Bronckers et al. 2015).
In conclusion, we have identified the conditions required to obtain an optimally polarized layer of HAT-7 cells, and we have shown that these express the most relevant transport proteins described in maturation ameloblasts in vivo, as depicted in Figure 5G. Our microfluorometric pHi measurements provided evidence for the presence of the basolateral elements responsible for intracellular bicarbonate accumulation and for regulated basal-to-apical HCO3− transport in polarized HAT-7 cells (Fig. 5G). The outcome is therefore a novel experimental model for studying these electrolyte transport processes that are essential for dental enamel formation. However, we must note the limitations of the model. First, as HAT-7 cells are derived from the cervical loop, they exhibit maturation-stage ameloblast markers but alone cannot sufficiently serve as an optimal maturation ameloblast model. Second, besides bicarbonate transport, additional mechanisms have to be identified, such as active proton transport and TJ functionality, as well as their unknown coordinating mechanisms. Therefore, more complex cell culture models need to be developed (Bhatia and Ingber 2014) in the future for better morphologic and functional modeling of maturation ameloblast function.
Author Contributions
E. Bori contributed to conception and design, data acquisition, analysis, and interpretation, drafted and critically revised manuscript; J. Guo, R. Rácz, B. Burghardt contributed to data acquisition and analysis, and critically revised manuscript; A. Földes contributed to data acquisition, analysis, and interpretation, and drafted and critically revised manuscript; B. Kerémi contributed to data analysis and interpretation, and critically revised manuscript; H. Harada contributed to conception and to data interpretation, and critically revised manuscript; M.C. Steward contributed to design, data analysis and interpretation, and critically revised manuscript; P. Den Besten contributed to conception and design, data interpretation, and critically revised manuscript; A.L.J.J. Bronckers contributed to conception, design, data acquisition, analysis, and interpretation, and drafted and critically revised manuscript; G. Varga, contributed to conception, design, data analysis and interpretation, and drafted and critically revised manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was supported by the National Institutes of Health (National Institute of Dental and Craniofacial Research grant 5R01DE013508, subaward 7743sc), the Hungarian National Development Agency (TÁMOP-4.2.1/B-09/1/KMR-2010-0001, TÁMOP-4.2.2/B-10/1-2010-0013), and the Hungarian Scientific Research Fund (OTKA-NKTH CK-80928).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Amasheh S, Milatz S, Krug SM, Bergs M, Amasheh M, Schulzke JD, Fromm M. 2009. Na+ absorption defends from paracellular back-leakage by claudin-8 upregulation. Biochem Biophys Res Commun. 378(1):45–50. [DOI] [PubMed] [Google Scholar]
- Arakaki M, Ishikawa M, Nakamura T, Iwamoto T, Yamada A, Fukumoto E, Saito M, Otsu K, Harada H, Yamada Y, et al. 2012. Role of epithelial-stem cell interactions during dental cell differentiation. J Biol Chem. 287(13):10590–10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggaley E, McLarnon S, Demeter I, Varga G, Bruce JI. 2007. Differential regulation of the apical plasma membrane Ca(2+) -ATPase by protein kinase A in parotid acinar cells. J Biol Chem. 282(52):37678–37693. [DOI] [PubMed] [Google Scholar]
- Bardet C, Courson F, Wu Y, Khaddam M, Salmon B, Ribes S, Thumfart J, Yamaguti PM, Rochefort GY, Figueres ML, et al. 2016. Claudin-16 deficiency impairs tight junction function in ameloblasts, leading to abnormal enamel formation. J Bone Miner Res. 31(3):498–513. [DOI] [PubMed] [Google Scholar]
- Bhatia SN, Ingber DE. 2014. Microfluidic organs-on-chips. Nat Biotechnol. 32(8):760–772. [DOI] [PubMed] [Google Scholar]
- Bronckers AL, Guo J, Zandieh-Doulabi B, Bervoets TJ, Lyaruu DM, Li X, Wangemann P, DenBesten P. 2011. Developmental expression of solute carrier family 26A member 4 (SLC26A4/pendrin) during amelogenesis in developing rodent teeth. Eur J Oral Sci. 119 Suppl 1:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronckers AL, Lyaruu DM, Guo J, Bijvelds MJ, Bervoets TJ, Zandieh-Doulabi B, Medina JF, Li Z, Zhang Y, DenBesten PK. 2015. Composition of mineralizing incisor enamel in cystic fibrosis transmembrane conductance regulator-deficient mice. Eur J Oral Sci. 123(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. 2002. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 283(1):C142–C147. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Josephsen K, Takano Y, Zahn D, Fejerskov O, Frische S. 2014. Fluctuations in surface pH of maturing rat incisor enamel are a result of cycles of H(+)-secretion by ameloblasts and variations in enamel buffer characteristics. Bone. 60:227–234. [DOI] [PubMed] [Google Scholar]
- Demeter I, Hegyesi O, Nagy AK, Case MR, Steward MC, Varga G, Burghardt B. 2009. Bicarbonate transport by the human pancreatic ductal cell line HPAF. Pancreas. 38(8):913–920. [DOI] [PubMed] [Google Scholar]
- Harada H, Ichimori Y, Yokohama-Tamaki T, Ohshima H, Kawano S, Katsube K, Wakisaka S. 2006. Stratum intermedium lineage diverges from ameloblast lineage via Notch signaling. Biochem Biophys Res Commun. 340(2):611–616. [DOI] [PubMed] [Google Scholar]
- Harguindey S, Arranz JL, Polo Orozco JD, Rauch C, Fais S, Cardone RA, Reshkin SJ. 2013. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs: an integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. J Transl Med. 11:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata M, Kawamoto T, Kawai M, Yamamoto T. 2010. Differential expression patterns of the tight junction-associated proteins occludin and claudins in secretory and mature ameloblasts in mouse incisor. Med Mol Morphol. 43(2):102–106. [DOI] [PubMed] [Google Scholar]
- Hegyesi O, Foldes A, Bori E, Nemeth Z, Barabas J, Steward MC, Varga G. 2015. Evidence for active electrolyte transport by two-dimensional monolayers of human salivary epithelial cells. Tissue Eng Part C Methods. 21(12):1226–1236. [DOI] [PubMed] [Google Scholar]
- Hou J. 2014. The kidney tight junction (review). Int J Mol Med. 34(6):1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inai T, Sengoku A, Hirose E, Iida H, Shibata Y. 2008. Differential expression of the tight junction proteins, claudin-1, claudin-4, occludin, ZO-1, and PAR3, in the ameloblasts of rat upper incisors. Anat Rec (Hoboken). 291(5):577–585. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Naruse S, Kitagawa M, Suzuki A, Yamamoto A, Hayakawa T, Case RM, Steward MC. 2000. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J Physiol. 528(Pt 2):305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Steward MC, Lindsay AR, Case RM. 1996. Accumulation of intracellular HCO3– by Na(+)-HCO3– cotransport in interlobular ducts from guinea-pig pancreas. J Physiol. 495(Pt 1):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali R, Guo J, Zandieh-Doulabi B, Bervoets TJ, Paine ML, Boron WF, Parker MD, Bijvelds MJ, Medina JF, DenBesten PK, et al. 2014. NBCe1 (SLC4A4) a potential pH regulator in enamel organ cells during enamel development in the mouse. Cell Tissue Res. 358(2):433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali R, Zandieh-Doulabi B, DenBesten PK, Seidler U, Riederer B, Wedenoja S, Micha D, Bronckers AL. 2015. Slc26a3/Dra and Slc26a6 in murine ameloblasts. J Dent Res. 94(12):1732–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephsen K, Takano Y, Frische S, Praetorius J, Nielsen S, Aoba T, Fejerskov O. 2010. Ion transporters in secretory and cyclically modulating ameloblasts: a new hypothesis for cellular control of preeruptive enamel maturation. Am J Physiol Cell Physiol. 299(6):C1299–C1307. [DOI] [PubMed] [Google Scholar]
- Kawano S, Morotomi T, Toyono T, Nakamura N, Uchida T, Ohishi M, Toyoshima K, Harada H. 2002. Establishment of dental epithelial cell line (HAT-7) and the cell differentiation dependent on Notch signaling pathway. Connect Tissue Res. 43(2–3):409–412. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Hilvo M, Kurtz I, Paine ML. 2010. A survey of carbonic anhydrase mRNA expression in enamel cells. Biochem Biophys Res Commun. 393(4):883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Bringas P, Jr, Chen YB, Smith SM, Snead ML, Kurtz I, Hacia JG, Hubbard MJ, Paine ML. 2012. Identification of novel candidate genes involved in mineralization of dental enamel by genome-wide transcript profiling. J Cell Physiol. 227(5):2264–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Kurtz I, Hubbard MJ, Paine ML. 2013. New paradigms on the transport functions of maturation-stage ameloblasts. J Dent Res. 92(2):122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacruz RS, Smith CE, Moffatt P, Chang EH, Bromage TG, Bringas P, Jr, Nanci A, Baniwal SK, Zabner J, Welsh MJ, et al. 2012. Requirements for ion and solute transport, and pH regulation during enamel maturation. J Cell Physiol. 227(4):1776–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal-Nag M, Morin PJ. 2009. The claudins. Genome Biol. 10(8):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Ohana E, Park HW, Yang D, Muallem S. 2012. Molecular mechanism of pancreatic and salivary gland fluid and HCO3 secretion. Physiol Rev. 92(1):39–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, Oude Elferink RP, Everts V. 2008. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol. 27(2):119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Harada H, Saito M, Taniguchi A. 2011. Induction of enamel matrix protein expression in an ameloblast cell line co-cultured with a mesenchymal cell line in vitro. In Vitro Cell Dev Biol Anim. 47(1):39–44. [DOI] [PubMed] [Google Scholar]
- Melvin JE, Yule D, Shuttleworth T, Begenisich T. 2005. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 67:445–469. [DOI] [PubMed] [Google Scholar]
- Rakonczay Z, Jr, Fearn A, Hegyi P, Boros I, Gray MA, Argent BE. 2006. Characterization of H+ and HCO3– transporters in CFPAC-1 human pancreatic duct cells. World J Gastroenterol. 12(6):885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reibring CG, El Shahawy M, Hallberg K, Kannius-Janson M, Nilsson J, Parkkila S, Sly WS, Waheed A, Linde A, Gritli-Linde A. 2014. Expression patterns and subcellular localization of carbonic anhydrases are developmentally regulated during tooth formation. PLoS One. 9(5):e96007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert EM, Zsembery A. 2003. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 1615(1–2):7–32. [DOI] [PubMed] [Google Scholar]
- Smith CE. 1998. Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med. 9(2):128–161. [DOI] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H, Case RM. 2005. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 67:377–409. [DOI] [PubMed] [Google Scholar]
- Szlavik V, Szabo B, Vicsek T, Barabas J, Bogdan S, Gresz V, Varga G, O’Connell B, Vag J. 2008. Differentiation of primary human submandibular gland cells cultured on basement membrane extract. Tissue Eng Part A. 14(11):1915–1926. [DOI] [PubMed] [Google Scholar]
- Szucs A, Demeter I, Burghardt B, Ovari G, Case RM, Steward MC, Varga G. 2006. Vectorial bicarbonate transport by Capan-1 cells: a model for human pancreatic ductal secretion. Cell Physiol Biochem. 18(4–5):253–264. [DOI] [PubMed] [Google Scholar]
- Yoshizaki K, Yamamoto S, Yamada A, Yuasa K, Iwamoto T, Fukumoto E, Harada H, Saito M, Nakasima A, Nonaka K, et al. 2008. Neurotrophic factor neurotrophin-4 regulates ameloblastin expression via full-length TrkB. J Biol Chem. 283(6):3385–3391. [DOI] [PubMed] [Google Scholar]
- Zheng L, Seon YJ, Mourao MA, Schnell S, Kim D, Harada H, Papagerakis S, Papagerakis P. 2013. Circadian rhythms regulate amelogenesis. Bone. 55(1):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.