Abstract
Objective:
Type 2 diabetes is commonly found in schizophrenia and is an important contributor to mortality and morbidity in this condition. Dopamine has been implicated in the aetiology of both diabetes and schizophrenia. It is possible that both disorders share a common genetic susceptibility.
Methods:
In a cross-sectional study, we examined 2 dopamine D2 receptor (DRD2) single-nucleotide polymorphisms (SNPs) previously associated with schizophrenia (C939 T, rs6275 and C957 T, rs6277) along with fasting blood glucose and body mass index (BMI) in 207 antipsychotic-treated patients with schizophrenia. All participants met DSM-IV criteria for schizophrenia, and those with other psychiatric disorders were excluded. Analysis of covariance was used to compare fasting glucose results by DRD2 genotypes, after controlling for known confounds. For significant associations, follow-up Bonferroni post hoc tests examined differences in fasting glucose levels between genotypes. Specific comparisons were also made using analysis of variance and chi-square (Fisher’s exact test).
Results:
The 2 DRD2 risk genotypes were associated with significant increases in blood glucose, after controlling for BMI, age, sex, dosage and type of antipsychotic medication, number of hospitalisations, and negative symptoms (rs6275, F(2, 182) = 5.901, P = 0.003; rs6277 SNP, F(2, 178) = 3.483, P = 0.033).
Conclusions:
These findings support the involvement of DRD2 not only in schizophrenia but also in elevated levels of blood glucose commonly found in antipsychotic-treated patients with schizophrenia. Our data support the notion that diabetes may not merely be a comorbid condition but could be fundamentally associated with the pathogenesis of schizophrenia itself.
Keywords: dopamine 2 receptor, schizophrenia, genetics, type 2 diabetes, hyperglycaemia
Abstract
Objectif:
Le diabète de type 2 s’observe souvent dans la schizophrénie et est un important contributeur à la mortalité et la morbidité de cette affection. La dopamine est impliquée dans l’étiologie du diabète et de la schizophrénie. Il est possible que ces deux troubles aient en commun une susceptibilité génétique.
Méthodes:
Dans une étude transversale, nous avons examiné deux polymorphismes mononucléotidiques (SNP) d’un récepteur de la dopamine D2 (DRD2) précédemment associés à la schizophrénie (C939T, rs6275 et C957T, rs6277) ainsi que la glycémie à jeun et l’indice de masse corporelle (IMC) chez 207 patients souffrant de schizophrénie traités aux antipsychotiques. Tous les participants satisfaisaient aux critères du DSM-IV pour la schizophrénie et ceux qui souffraient d’autres troubles psychiatriques ont été exclus. L’analyse de covariance (ANCOVA) a été utilisée pour comparer les résultats de la glycémie à jeun par génotypes DRD2, après contrôle pour les facteurs de confusion connus. Pour les associations significatives, des tests post hoc de Bonferroni ont examiné les différences des taux de glycémie à jeun entre les génotypes. Des comparaisons spécifiques ont aussi été effectuées à l’aide d’ANOVA et du chi-carré (Fisher’s exact test).
Résultats:
Les deux génotypes à risque DRD2 étaient associés à des augmentations significatives de la glycémie, après contrôle de l’IMC, l’âge, le sexe, le dosage et le type de médicament antipsychotique, le nombre d’hospitalisations et de symptômes négatifs (rs6275, F(2, 182) = 5.901, P = 0,003, rs6277 SNP, F(2, 178) = 3.483, P = 0,033).
Conclusions:
Ces résultats soutiennent la participation de DRD2 non seulement à la schizophrénie mais aussi à des taux élevés de glycémie souvent observés chez les patients souffrant de schizophrénie traités aux antipsychotiques. Nos données appuient la notion que le diabète n’est peut-être pas seulement une affection comorbide, mais qu’il pourrait fondamentalement être associé à la pathogenèse de la schizophrénie même.
Clinical Implications
Schizophrenia and hyperglycaemia may share a common genetic aetiology. Hyperglycaemia is therefore highly likely to be fundamentally associated with schizophrenia. The clinician should have a high index of suspicion regarding detection and treatment of hyperglycaemia in patients with schizophrenia.
Limitations of the Research
The major limitation of this study is the small sample size. Hemoglobin A1c levels were not obtained, and the sample also consisted of a high proportion of males.
Schizophrenia is a serious psychiatric disorder with high heritability afflicting approximately 1% of the worldwide population. Van Rossum1 first postulated the dopamine theory of schizophrenia in 1967: “When the hypothesis of dopamine blockade by neuroleptic agents can be further substantiated, it may have far-reaching consequences for understanding the pathophysiology of schizophrenia. Overstimulation of dopamine receptors could be part of the aetiology.” Many lines of evidence derived from research conducted over almost 50 years support Van Rossum’s hypothesis that schizophrenia is associated with excessive stimulation of striatal dopamine 2 receptors (DRD2).2
Although multiple alleles of small effect contribute to genetic risk for schizophrenia, a recent large genome-wide association study identified DRD2 to be significantly associated with schizophrenia (P = 2.749 × 10–11).3 This association, combined with multiple genes involved in glutamatergic neurotransmission, demonstrated the relevance of molecules previously implicated in aetiological theories of schizophrenia. This study also reported miR137 (microRNA-137) as being highly associated with schizophrenia.3 One of the target genes for miR137 is zinc finger protein 804A (ZNF804A), which is closely associated with schizophrenia.4 ZNF804A regulates the transcription levels of both catechol-O-methyl transferase (COMT) and the DRD2 variant rs6277,5 suggesting that the schizophrenia risk associated with miR137 may be mediated, at least in part, by dopaminergic pathways.
Type 2 diabetes (T2D) is strongly associated with schizophrenia and remains a major contributor to the mortality and morbidity associated with this diagnosis.6 Increased blood glucose is also a major feature of the metabolic syndrome found in antipsychotic-treated individuals with schizophrenia. Other features of this syndrome include dyslipidemia, obesity, and hypertension.7
Dopaminergic pathways are also involved in the regulation of blood glucose. Animal studies have shown that pancreatic dopamine 2 receptors are involved in hyperglycaemia-mediated beta-cell insulin release. DRD2 knockout mice exhibit both a high fasting glucose and blunted insulin secretory response to glucose.8 In humans with Parkinson disease, treatment with L-Dopa, a dopamine precursor, reduces insulin secretion in response to an oral glucose tolerance test.9 Furthermore, bromocriptine, a DRD2 receptor agonist, is approved by the US Food and Drug Administration for the treatment of T2D. Conversely, DRD2 antagonist atypical antipsychotic medication confers an increased risk for T2D.10
Both T2D and schizophrenia result from an interaction between environmental and genetic factors.11,12 Twin and family studies estimate heritability of up to 0.8 in both disorders.11,13 As the DRD2 is likely to be involved in the pathogenesis of both disorders, we examined a clinical sample of patients with schizophrenia for DRD2 variants previously reported to be associated with schizophrenia: C939 T (rs6275)14 and C957 T (rs6277),15 along with fasting blood glucose. The TT genotype of rs6275 has been significantly associated with schizophrenia in 5 studies of Caucasians (meta-analysis odds ratio [OR], 1.15; 95% confidence interval [CI], 1.00 to 1.35, I 2 = 41).16 We have previously reported that the CC genotype of the rs6277 polymorphism is associated with schizophrenia,15 and subsequent studies have confirmed this association.17 Meta-analysis of 9 studies in Caucasians has revealed that rs6277 is significantly associated with schizophrenia (OR, 1.40; 95% CI, 1.17 to 1.68; I 2 = 79).16
Methods
Subjects
We recruited 207 unrelated Caucasian patients older than 18 years who met DSM-IV criteria for schizophrenia (169 males, 38 females). With a predicted medium effect size (0.25), α = 0.05, and power of 90, 207 subjects were required for the focal analyses (analysis of variance [ANOVA], critical F = 3.04).
All patients were being treated either at the Division of Mental Health Services, Royal Brisbane and Women’s Hospital or The Park Centre for Mental Health Treatment, Research and Education. Subjects were recruited from inpatient and outpatient mental health services. Their mean age was 40.41 years (SD = 14.07). The diagnosis of schizophrenia was confirmed on clinical interview by either a psychiatrist (B.R.L., M.B.) or clinical psychologist (R.M.Y.). Exclusion criteria were schizoaffective disorder, bipolar disorder, dementia, organic brain syndrome, major depressive disorder, epilepsy, illicit substance dependence, and pregnancy. All patients were treated with antipsychotic monotherapy at a stable dose for a minimum of 4 weeks; however, most patients were maintained on a stable dose for a substantially longer period than this. No patients were treated with antidepressant or mood-stabilising medications. Furthermore, no patients were diagnosed with diabetes, and none were receiving treatment for diabetes. Participants provided written informed consent and were able to withdraw from the study at any time without prejudice. Institutional ethics approvals were obtained from the clinics and hospitals involved and the Queensland University of Technology.
Assessments
All subjects were diagnosed with schizophrenia by their treating psychiatrist. The diagnosis was further confirmed on clinical interview by either a psychiatrist (B.R.L., M.B.) or clinical psychologist (R.M.Y.).
A clinical history was taken by a consultant psychiatrist (B.R.L., M.B.), a clinical psychologist (R.Y.), or a clinical nurse (K.H.). Demographic details and clinical assessment were undertaken (Table 1). The Positive and Negative Syndrome Scale (PANSS) was administered.18 Information regarding lifetime number of hospital admissions and dosage and type of antipsychotic medication was also obtained. Fasting blood glucose determinations were made for each subject. Blood samples were obtained in the morning before breakfast. Height and weight were measured and body mass index (BMI) was calculated for each subject.
Table 1.
Demographic and Clinical Features of the Sample.a
| Measure | Mean (SD) |
|---|---|
| Years of schizophrenia | 17.27 (12.91) |
| Number of hospital admissions | 11.09 (14.78) |
| Antipsychotic dose: chlorpromazine equivalent (mg) | 493.99 (387.91) |
| Positive symptom scale (PANSS-P) | 17.82 (5.82) |
| Negative symptom scale (PANSS-N) | 18.78 (6.31) |
| Body mass index | 26.68 (5.78) |
| Fasting blood glucose | 5.51 (1.77) |
| Sex (male proportion), % | 81.6 |
PANSS-N, Positive and Negative Syndrome Scale, negative symptoms; PANSS-P, Positive and Negative Syndrome Scale, positive symptoms.
aValues are presented as mean (SD) unless otherwise indicated.
Genotyping
Two DRD2 SNPs were genotyped (rs6275 and rs6277). Either saliva samples (Oragene kits; DNA genotek; Ottowa, ON, Canada) or 10-mL blood samples were obtained from each participant for DNA extraction. DNA was stored at –80°C. Samples were genotyped using a homogeneous MassEXTEND (hME) Sequenom assay performed by the Australian Genome Research Facility, Melbourne, as previously described.19 Genotyping failure rates varied between 1% and 4% for the 2 SNPs. These missing data were not imputed. All laboratory work was conducted blind to the clinical data and vice versa.
Medications
At the time of assessment, patients were prescribed the following antipsychotic agents: risperidone (n = 50), olanzapine (n = 47), clozapine (n = 48), quetiapine (n = 8), flupenthixol (n = 12), zuclopenthixol (n = 12), depot risperidone (Risperdal Consta) (n = 11), haloperidol (n = 4), fluphenazine decanoate (n = 5), other typicals (n = 5), aripiprazole (n = 1), amisulpride (n = 2), chlorpromazine (n = 1), missing data (n = 1). Dosage of medication was converted to chlorpromazine equivalents to compare dosage of different pharmacological agents.20
Statistical Analysis
Analysis of variance (ANOVA) was used to compare fasting glucose results by DRD2 genotypes. Further analyses using analysis of covariance (ANCOVA) examined the contribution of possible confounds. This statistical approach adjusted for possible confounds to ensure that any effect observed was independent of known risk factors for elevated fasting blood glucose levels.
For significant associations, follow-up Bonferroni post hoc tests examined differences in fasting glucose levels between genotypes. A P value ≤0.05 was considered significant. Minimal amounts of missing data (<4%) were observed. Three cases were not able to be genotyped for rs6255 and 8 cases for rs6277. These missing data were not imputed. All analyses were conducted with the Statistical Package for the Social Sciences (SPSS, version 22; SPSS, Inc., an IBM Company, Chicago, IL). Genotype frequencies indicated that both polymorphisms were in Hardy-Weinberg equilibrium.
Results
Glucose
Fasting glucose levels ranged from 3 to 24 mmol/L (mean = 5.51, SD = 1.55). In total, 124 patients (60% of the sample) recorded normal fasting glucose levels (<5.6 mmol/L), 54 patients (26% of the sample) had impaired fasting glucose (5.6-6.9 mmol/L), and 29 patients (14% of the sample) had a provisional diagnosis of diabetes based on fasting glucose levels (≥7.0).21
DRD2 SNPs
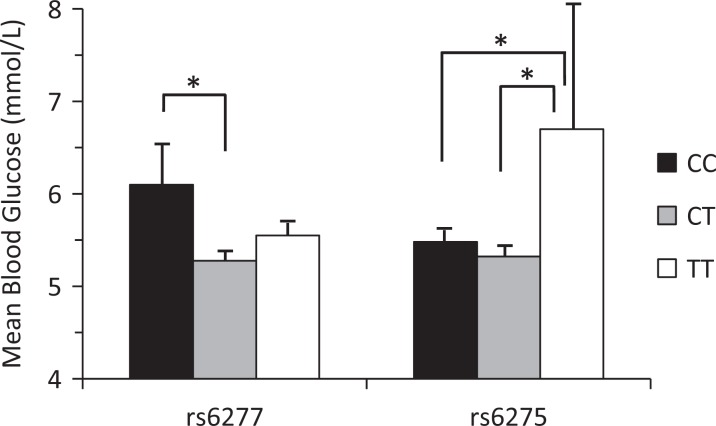
ANOVA examined the relationship between fasting glucose levels and DRD2 SNPs previously associated with schizophrenia. The rs6277 SNP (F(2, 196) = 3.376, P = 0.036) and rs6275 SNP (F(2, 201) = 3.739, P = 0.025) demonstrated significant differences in fasting glucose between genotypes. Glucose levels for patients who carried the C/C genotype (n = 43) of SNP rs6277 were significantly higher (mean = 6.098, SD = 3.066) than the CT (n = 103) genotype (mean = 5.277, SD = 1.14; mean difference = .821, P = 0.03) but not the T/T genotype (n = 61) (mean = 5.550, SD = 1.149; mean difference = –.548, P = 0.388). Glucose levels of patients who carried the T/T genotype (n = 14) of SNP rs6275 were significantly higher (mean = 6.70, SD = 5.056) than both the CT (n = 85) genotype (mean = 5.322, SD = 1.242; mean difference = 1.378, P = 0.021) and the C/C genotype (n = 105) (mean = 5.48, SD = 1.55; mean difference = 1.216, P = 0.046) (Figure 1).
Figure 1.
Mean blood glucose levels in schizophrenia patients for dopamine D2 receptor single-nucleotide polymorphisms rs6277 and rs6275. Error bars are standard error of the mean. *P < 0.05.
Potentially Confounding Variables
Abnormal glucose tolerance and diabetes mellitus are associated with increasing age.22 Furthermore, in a recent large cohort, the risk of being diagnosed with T2D was strongly and independently associated with increasing BMI. The size of this association increased significantly with higher BMI values.23 T2D is also more common in adult men than in women.24 Type of antipsychotic medication prescribed is associated with T2D risk. Clozapine can cause elevated fasting blood glucose and, together with olanzapine, is particularly associated with increased risk of T2D.25 Hospitalisation and negative symptoms may result in more sedentary lifestyles and proneness to obesity with consequent insulin resistance. These factors may also increase risk for T2D.26
We examined potential confounds by 2 separate statistical approaches: directly examining potential differences on the basis of genotype and controlling for the same confounds via ANCOVA. Table 2 summarises the relationship between potential confounding variables for significant SNPs rs6277 and rs6275. No significant differences were observed. An ANCOVA was then conducted controlling for BMI, age, sex, dosage and type of antipsychotic medication, number of hospitalisations, and negative symptoms. It showed that rs6275 (F(2, 182) = 5.901, P = 0.003) and rs6277 (F(2, 178) = 3.483, P = 0.033) remained significant after potential confounding factors were accounted for.
Table 2.
Potential Confounds.
| rs6277 | rs6275 | |||
|---|---|---|---|---|
| Measure | F | P | F | P |
| Age | .410 | .664 | 1.464 | .234 |
| Body mass index | .282 | .754 | .389 | .678 |
| Chlorpromazine equivalent (mg) | .756 | .471 | .157 | .855 |
| Number of admissions | .591 | .555 | 1.523 | .221 |
| PANSS-N | 1.015 | .364 | .762 | .468 |
| χ2 | P | χ2 | P | |
| Sex | .183 | .912 | .464 | .793 |
| Antipsychotic medication | 30.034 | .184 | 14.937 | .923 |
PANSS-N, Positive and Negative Syndrome Scale, negative symptoms.
Discussion
Approximately 40% of the clinical sample had either impaired fasting glucose or a provisional diagnosis of diabetes, emphasising the prevalence of elevated glucose levels in patients treated for schizophrenia. This very high frequency of abnormal blood glucose underscores the need to routinely obtain fasting glucose levels in this patient population. This is consistent with the 16% to 25% prevalence of diabetes found in previous studies of patients with schizophrenia.27 The strong association between schizophrenia and elevated blood glucose could account for many of the poor health outcomes observed in patients with this diagnosis.6
The TT genotype of the rs6275 and the CC genotype of the rs6277 have been identified as risk genotypes for schizophrenia. This study found that these genotypes are also associated with increased fasting blood glucose in a sample of patients with schizophrenia. The results of this study lend support to the notion that the comorbidity of T2D and schizophrenia may be at least partly due to shared genetic variants with pleiotropic effects. Two DRD2 SNPs associated with schizophrenia in meta-analyses of multiple studies were associated with increased fasting blood glucose. It is possible that rs6277 and rs6275 may influence dopaminergic pathways in both brain and pancreas, predisposing to both schizophrenia and T2D.
The lack of association of rs6277 and rs6275 genotypes with the diabetes risk factors of age, sex, BMI, type and dosage of antipsychotic medication, number of hospitalisations, or negative symptoms strongly supports the association of these SNPs with elevated blood glucose in schizophrenia. Furthermore, even after controlling for these confounds by ANCOVA, rs6277 and rs6275 schizophrenia risk genotypes remained associated with elevated blood glucose. This supports the notion that schizophrenia itself may be an independent risk factor for diabetes. In particular, the lack of association with BMI would suggest these variants may not increase the risk for hyperglycaemia via this mechanism. Our findings differ from a previous study that reported rs6277 was associated with increased antipsychotic-induced weight gain.28
One of the DRD2 variants studied, SNP rs6277, has functional effects. It affects DRD2 dopamine binding affinity (K D). Positron emission tomography scans with [11C] raclopride in healthy volunteers indicate that the different genotypes of rs6277 are associated with differing DRD2 K Ds. The K D is highest in C/C genotype carriers, intermediate in C/T genotype carriers, and lowest in T/T genotype carriers. Genotype variants of rs6277 are not associated with differences in DRD2 density (B max).29 Furthermore, in vitro evidence associates rs6277 alleles with differences in DRD2 messenger RNA stability and translation and consequent DRD2 synthesis.30
These differences in DRD2 functioning/synthesis are consistent with variations in plasma prolactin levels between those with diabetes and healthy individuals. Plasma prolactin concentrations vary markedly between lean, insulin-sensitive, glucose-tolerant individuals and those with insulin resistance. In healthy individuals, peak prolactin concentration is nocturnal, in contrast to obese insulin-resistant individuals who have elevated (2-fold) plasma prolactin levels during waking hours.31 This suggests diabetes is associated with reduced dopaminergic tone.
Other evidence linking DRD2 SNPs to T2D has emerged recently, with rs6275 being associated with altered first phase glucose-stimulated insulin secretion in women.32 The CC genotype of rs6277 is also associated with increased consumption of sugars in men and women,33 and increased consumption of sugar-sweetened soft drinks is associated with a modest increased risk for T2D adjusted for BMI.34 Binge eating disorder is also associated with the rs6277 CC genotype.35 There is an important link between binge eating disorder and T2D amongst obese individuals.36
Cognitive impairment is a feature of both schizophrenia and T2D.37,38 In particular, working memory is poorer in both disorders, and hyperglycaemia is associated with impaired performance in T2D alone or in combination with schizophrenia.38 Although the exact pathophysiology linking T2D with cognitive dysfunction is unknown, hyperglycaemia39 and comorbid vascular disease40 are involved. Despite the importance of these factors, cognitive problems exist in the prediabetic41 stage of the illness, suggesting other mechanisms are important. One such factor may be genetic as the DRD2 rs6277 CC genotype is associated with working memory deficits in healthy individuals. In a Word Serial Position Test, CC carriers showed decreased performance.41 Furthermore, a study of healthy individuals found CC genotype carriers exhibited reduced cognitive performance compared with CT/TT carriers when administered the Wisconsin Card Sorting Test.42 CC carriers achieved fewer categories and had more perseverative errors.
This study should be interpreted in light of some limitations. The study design was cross-sectional, and causal relationships cannot be confirmed by association alone. While this clinical population was carefully selected to ensure reliable clinical diagnosis of schizophrenia, these findings cannot be generalised to other ethnic groups. Hemoglobin A1c levels were not obtained. The sample also consisted of a high proportion of males. While our power calculation demonstrated adequate subject numbers to detect meaningful differences, a major limitation of the study was the small sample size. These results should be interpreted as preliminary and exploratory in nature. Further research in larger, more heterogeneous samples is required.
All participants were treated with antipsychotic medication. Further research is needed to investigate whether rs6277 and rs6275 are risk variants for hyperglycaemia in antipsychotic naive persons with schizophrenia. The present results could be due to an interaction between antipsychotic medication and functional differences in DRD2 associated with risk genotypes of rs6277 and rs6275. Candidate gene studies in schizophrenia may reveal further risk SNPs associated with elevated blood glucose. Other genes associated with the dopaminergic system and perhaps schizophrenia, such as COMT Val/Met polymorphism43 or DAT1 VNTR,44 should be investigated for possible effect on glucose level. This would provide more support for schizophrenia being an independent risk factor for diabetes. Further studies of T2D risk genes may also identify novel risk SNPs for schizophrenia. Recently, the T2D susceptibility gene, TCF7L2 (rs12573128), has been associated with schizophrenia.45
The finding that 2 DRD2 risk genotypes for schizophrenia are associated with elevated fasting blood glucose goes some way to explain the high frequency of elevated blood glucose associated with this disorder. Future studies of schizophrenia risk variants may identify further SNPs associated with increased blood glucose commonly found in both drug-naive and antipsychotic-treated patients with this disorder and enable early identification and treatment at those most at risk.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Nicol Foundation and the Queensland State Government. J.P.C. is supported by a National Health and Medical Research Council (NH&MRC) of Australia Career Development Fellowship (1031909).
References
- 1. Van Rossum J. The significance of dopamine-receptor blockade for the action of neuroleptic drugs. Neuropsychopharmacology. 1967;5:321–323. [PubMed] [Google Scholar]
- 2. Laruelle M. Schizophrenia: from dopaminergic to glutamatergic interventions. Curr Opin Pharmacol. 2014;14:97–102. [DOI] [PubMed] [Google Scholar]
- 3. Schizophrenia Working Group of the Psychiatric Genomics Consortium. biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim AH, Parker EK, Williamson V, McMichael GO, Fanous AH, Vladimirov VI. Experimental validation of candidate schizophrenia gene ZNF804A as target for hsa-miR-137. Schizophr Res. 2012;141(1):60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Girgenti MJ, LoTurco JJ, Maher BJ. ZNF804a regulates expression of the schizophrenia-associated genes PRSS16, COMT, PDE4B, and DRD2. PLoS One. 2012;7(2):e32404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bresee LC, Majumdar SR, Patten SB, et al. Prevalence of cardiovascular risk factors and disease in people with schizophrenia: a population-based study. Schizophr Res. 2010;117(1):75–82. [DOI] [PubMed] [Google Scholar]
- 7. Newcomer JW. Metabolic syndrome and mental illness. Am J Manag Care. 2007;13(7, Suppl):S170–S177. [PubMed] [Google Scholar]
- 8. Garcia-Tornadu I, Perez-Millan MI, Recouvreux V, et al. New insights into the endocrine and metabolic roles of dopamine D2 receptors gained from the Drd2 mouse. Neuroendocrinology. 2009;92(4):207–214. [DOI] [PubMed] [Google Scholar]
- 9. Rosati G, Maioli M, Aiello I, et al. Effects of long-term L-dopa therapy on carbohydrate metabolism in patients with Parkinson’s disease. Eur Neurol. 1976;14(3):229–239. [DOI] [PubMed] [Google Scholar]
- 10. Moisan J, Turgeon M, Desjardins O, et al. Comparative safety of antipsychotics: another look at the risk of diabetes. Can J Psychiatry. 2013;58(4):218–224. [DOI] [PubMed] [Google Scholar]
- 11. Ali O. Genetics of type 2 diabetes. World J Diabetes. 2013;4(4):114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wockner L, Noble E, Lawford B, et al. Genome-wide DNA methylation analysis of human brain tissue from schizophrenia patients. Transl Psychiatry. 2014;4(1):e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cardno AG, Marshall EJ, Coid B, et al. Heritability estimates for psychotic disorders: the Maudsley twin psychosis series. Arch Gen Psychiatry. 1999;56(2):162–168. [DOI] [PubMed] [Google Scholar]
- 14. Monakhov M, Golimbet V, Chubabriia K, et al. The association study of the DRD2 gene C939 T polymorphism and schizophrenia. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova. 2006;107(10):58–60. [PubMed] [Google Scholar]
- 15. Lawford BR, Young RM, Swagell CD, et al. The C/C genotype of the C957 T polymorphism of the dopamine D2 receptor is associated with schizophrenia. Schizophr Res. 2005;73(1):31–37. [DOI] [PubMed] [Google Scholar]
- 16.Schizophrenia Research Forum, author. http://www.szgene.org/meta.asp?geneID=93. website [cited 2014 Nov 4]. Available from:
- 17. Betcheva ET, Mushiroda T, Takahashi A, et al. Case-control association study of 59 candidate genes reveals the DRD2 SNP rs6277 (C957 T) as the only susceptibility factor for schizophrenia in the Bulgarian population. J Hum Genet. 2009;54(2):98–107. [DOI] [PubMed] [Google Scholar]
- 18. Kay SR, Flszbein A, Opfer LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 19. Voisey J, Swagell C, Hughes IP, Lawford BR, Young R, Morris C. Analysis of HapMap tag-SNPs in dysbindin (DTNBP1) reveals evidence of consistent association with schizophrenia. Eur Psychiatry. 2010;25(6):314–319. [DOI] [PubMed] [Google Scholar]
- 20. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the US population National Health and Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29(6):1263–1268. [DOI] [PubMed] [Google Scholar]
- 23. Ganz ML, Wintfeld N, Li Q, et al. The association of body mass index with the risk of type 2 diabetes: a case-control study nested in an electronic health records system in the United States. Diabetol Metab Syndr. 2014;6(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sattar N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Prac Res Clin Endocrinol Metab. 2013;27(4):501–507. [DOI] [PubMed] [Google Scholar]
- 25. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects. CNS Drugs. 2005;19(Suppl 1):S1–S93. [DOI] [PubMed] [Google Scholar]
- 26. Gury C. Schizophrenia, diabetes mellitus and antipsychotics. Encéphale. 2004;30(4):382. [DOI] [PubMed] [Google Scholar]
- 27. Mukherjee S, Decina P, Bocola V, et al. Diabetes mellitus in schizophrenic patients. Compr Psychiatry. 1996;37(1):68–73. [DOI] [PubMed] [Google Scholar]
- 28. Müller D, Zai C, Sicard M, et al. Systematic analysis of dopamine receptor genes (DRD1-DRD5) in antipsychotic-induced weight gain. Pharmacogenomics J. 2012;12(2):156–164. [DOI] [PubMed] [Google Scholar]
- 29. Hirvonen MM, Laakso A, Någren K, et al. C957 T polymorphism of dopamine D2 receptor gene affects striatal DRD2 in vivo availability by changing the receptor affinity. Synapse. 2009;63(10):907–912. [DOI] [PubMed] [Google Scholar]
- 30. Duan J, Wainwright MS, Comeron JM, et al. Synonymous mutations in the human dopamine receptor D2 (DRD2) affect mRNA stability and synthesis of the receptor. Hum Mol Gen. 2003;12(3):205–216. [DOI] [PubMed] [Google Scholar]
- 31. Cincotta AH, Meier AH, Cincotta M., Jr Bromocriptine improves glycaemic control and serum lipid profile in obese type 2 diabetic subjects: a new approach in the treatment of diabetes. Expert Opin Investig Drugs. 1999;8(10):1683–1707. [DOI] [PubMed] [Google Scholar]
- 32. Guigas B, Leeuw van Weenen J, Leeuwen N, et al. Sex-specific effects of naturally occurring variants in the dopamine receptor D2 locus on insulin secretion and type 2 diabetes susceptibility. Diabetes Med. 2014;31(8):1001–1008. [DOI] [PubMed] [Google Scholar]
- 33. Eny KM, Corey PN, El-Sohemy A. Dopamine D2 receptor genotype (C957 T) and habitual consumption of sugars in a free-living population of men and women. J Nutrigenet Nurtrigenomics. 2008;2(4-5):235–242. [DOI] [PubMed] [Google Scholar]
- 34. Greenwood D, Threapleton D, Evans C, et al. Association between sugar-sweetened and artificially sweetened soft drinks and type 2 diabetes: systematic review and dose-response meta-analysis of prospective studies. Br J Nutr. 2014;112(5):725–734. [DOI] [PubMed] [Google Scholar]
- 35. Davis C, Levitan RD, Yilmaz Z, et al. Binge eating disorder and the dopamine D2 receptor: genotypes and sub-phenotypes. Prog Neuropsychopharmacol Biol Psychiatry. 2012;38(2):328–335. [DOI] [PubMed] [Google Scholar]
- 36. Webb JB, Applegate KL, Grant JP. A comparative analysis of type 2 diabetes and binge eating disorder in a bariatric sample. Eat Behav. 2011;12(3):175–181. [DOI] [PubMed] [Google Scholar]
- 37. Cukierman T, Gerstein H, Williamson J. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460–2469. [DOI] [PubMed] [Google Scholar]
- 38. Han M, Huang X-F, Xiu M, et al. Diabetes and cognitive deficits in chronic schizophrenia: a case-control study. PLoS One. 2013;8(6):e66299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sommerfield AJ, Deary IJ, Frier BM. Acute hyperglycemia alters mood state and impairs cognitive performance in people with type 2 diabetes. Diabetes Care. 2004;27(10):2335–2340. [DOI] [PubMed] [Google Scholar]
- 40. Akisaki T, Sakurai T, Takata T, et al. Cognitive dysfunction associates with white matter hyperintensities and subcortical atrophy on magnetic resonance imaging of the elderly diabetes mellitus Japanese elderly diabetes intervention trial (J-EDIT). Diabetes Metab Res Rev. 2006;22(5):376–384. [DOI] [PubMed] [Google Scholar]
- 41. Xu H, Kellendonk CB, Simpson EH, et al. DRD2 C957 T polymorphism interacts with the COMT Val158Met polymorphism in human working memory ability. Schizophr Res. 2007;90(1):104–107. [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez-Jimenez R, Hoenicka J, Jimenez-Arriero M, et al. Performance in the Wisconsin Card Sorting Test and the C957 T polymorphism of the DRD2 gene in healthy volunteers. Neuropsychobiology. 2005;54(3):166–170. [DOI] [PubMed] [Google Scholar]
- 43. Sagud M, Mück-Seler D, Mihaljević-Peles A, et al. Catechol-O-methyl transferase and schizophrenia. Psychiatr Danub. 2010; 22(2):270–274. [PubMed] [Google Scholar]
- 44. Huang SY, Chen HK, Ma KH, et al. Association of promoter variants of human dopamine transporter gene with schizophrenia in Han Chinese. Schizophr Res. 2010;116(1):68–74. [DOI] [PubMed] [Google Scholar]
- 45. Alkelai A, Greenbaum L, Lupoli S, et al. Association of the type 2 diabetes mellitus susceptibility gene, TCF7L2, with schizophrenia in an Arab-Israeli family sample. PLoS One. 2012;7(1):e29228. [DOI] [PMC free article] [PubMed] [Google Scholar]