Abstract
Background
Advanced stage/recurrent clear cell ovarian cancers (CCOCs) are characterized by a low response to chemotherapy and a poor prognosis. There is growing interest in investigating novel/molecular targeted therapies in patients with CCOC in histotype-specific trials. However, CCOCs are not a uniform entity and comprise a number of molecular subtypes and it is unlikely that a single approach to treatment will be appropriate for all patients. The aim of this study was to analyze the results of a multiplatform profiling panel in CCOCs to identify potential therapeutic targets.
Patients and Methods
Tumor profiling was performed on 521 CCOCs. They were grouped into pure (n = 422) and mixed (n = 99) CCOC for analysis. Testing included a combination of DNA sequencing (including next-generation sequencing) using a 46-gene panel, immunohistochemistry, fluorescent or chromogenic in situ hybridization, and RNA fragment analysis.
Results
The most common findings were in the PIK3CA/Akt/mTOR pathway, with 61% of all CCOCs showing a molecular alteration in one of these pathway components. Next-generation sequencing revealed PIK3CA mutations in 50% of pure CCOCs. Significant differences were observed between pure and mixed CCOCs with respect to hormone receptor expression (9% vs 34.7% for ER, 13.45 vs 26.4% for PR), cMET (24.1% vs 11.6%), PD-1 tumor infiltrating lymphocytes (48.1% vs 100%), expression of PD-L1 (7.4% vs 25%), and TOPO1 (41% vs 27.1%) on immunohistochemistry, whereas next-generation sequencing revealed significant differences in mutation frequency in PIK3CA (50% vs 18.5%), TP53 (18.1% vs 57.7%), KRAS (12.4% vs 3.7%), and cMET (1.9% vs 11.1%).
Conclusions
This large study confirms that the PIK3CA/Akt/mTOR pathway is commonly altered in CCOCs, and highlights the significant differences between pure and mixed CCOCs. Clear cell ovarian cancers are molecularly heterogeneous and there are a number of potential therapeutic targets which could be tested in clinical trials.
Key Words: Clear cell ovarian cancer, Next-generation sequencing, Molecular profiling, Biomarkers, Targeted therapies
Clear cell ovarian cancer (CCOC) represents 5% to 13% of epithelial ovarian cancers (EOCs) in Europe and North America, but has a much higher prevalence of up to 25% of EOC in Japan and other parts of Asia.1–3 They are a distinct histologic subtype of EOC with a very different biology to the more common high-grade serous ovarian cancer (HGSOC). They are characterized by a low response to platinum-based chemotherapy and a worse prognosis and are a challenge to treat.4,5 Attempts to develop more effective first-line treatment, for example, by substituting irinotecan for paclitaxel in patients with CCOC, have been unsuccessful.6 There is increasing recognition that patients with recurrent/advanced CCOC should be recruited into histotype-specific clinical trials rather than be included together with more chemotherapy responsive subtypes such as HGSOC in the so-called generic “EOC” trials.3 However, CCOCs are molecularly heterogeneous and not necessarily a single entity, which underscores the importance of identifying specific patient subsets that may be more likely to benefit from targeted therapies rather than treating all patients with the same treatment. Molecular profiling of CCOCs could help identify potential targets and select patients for clinical trials with molecular targeted therapies.7
High-resolution microarray analysis of 50 primary pure CCOCs identified distinct subgroups of CCOC with very different clinical outcomes.8 Although the tumors shared similar histological features, comparative genomic hybridization yielded 2 clusters with distinct gene expression patterns and with significant differences in median progression-free survival (11 vs 65 months; P = 0.065). Molecular heterogeneity has also been observed with respect to gene copy number, point mutations, and other alterations, particularly in the PI3K/AKT/mTOR pathway, all of which could impact on likelihood of response to targeted therapies.7,8
There are an increasing number of examples of how effective molecularly targeted therapies can be in tumors that are chemotherapy resistant such as metastatic melanoma and renal cancer.9,10 A better understanding of the genomic heterogeneity and specific molecular aberrations in CCOC should pave the way toward more effective and personalized therapy.
At a more fundamental level, it is imperative that patients with CCOC are correctly diagnosed. Although gynecologic pathologists can consistently diagnose “pure” CCOC, this expertise may not exist in community practice.11 For example, high-grade serous cancers with clear cell features can be confused with CCOC.11 The variations in response rates and outcomes for patients with the so-called “CCOC” may be due to misdiagnosis in some cases as well as inclusion of mixed CCOC tumors, with a serous component which would be expected to respond more favorably to platinum therapy.11 In view of this, we divided CCOC into pure and mixed subtypes in our analyses based on the original pathology reports rather than analyze them as a single entity.
We report results of an analysis of a large number of CCOCs that underwent comprehensive tumor profiling to identify potential druggable targets. The findings of this study could help inform the design of clinical trials and selection of patients for treatment with targeted therapies.
PATIENTS AND METHODS
Five hundred twenty-one patients with CCOC tumors were referred to Caris Life Sciences between 2009 and 2014. The clinical details and histological diagnosis of CCOC was based on the information including reported pathology provided by referring physicians. Four hundred twenty-two tumors were reported to be pure CCOC and 99 were mixed CCOC based on the original pathology report. No data on disease stage, recurrence or prior treatments were provided. Paraffin-embedded tumor samples included those obtained from the primary tumor or metastases either at initial diagnosis or at recurrence (Table 1, Supplemental Digital Content 1, http://links.lww.com/IGC/A363). The Western Institutional Review Board, the IRB for Caris Life Sciences deemed the study exempt from additional patient consent as it used previously collected de-identified data.
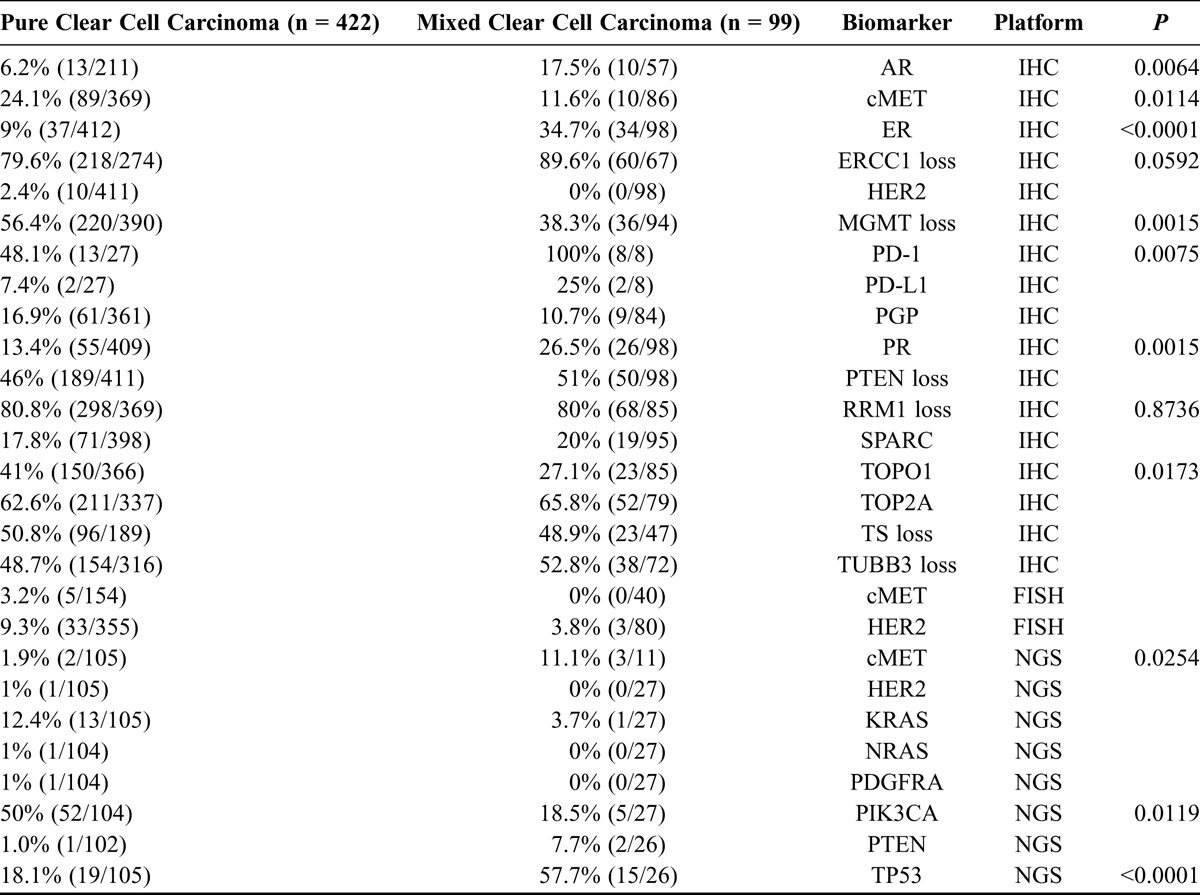
TABLE 1.
Potentially actionable targets in pure (n = 422) versus mixed (n = 99) CCOC
Tumor Testing
Specific testing was performed per physician request and included a combination of sequencing [Sanger or next-generation sequencing (NGS)], protein expression by immunohistochemistry (IHC), gene amplification by fluorescent in situ hybridization (FISH) or chromogenic in situ hybridization, and/or RNA fragment analysis. The available tests examine a wide range of markers that are of interest in a wide range of tumor types. The type of analyses performed and the specific biomarkers tested depended on the amount of tissue sample available. A technical description of the technologies used is presented in Table 2, Supplemental Digital Content 2, http://links.lww.com/IGC/A362. The panel of tests evolved over time as new biomarker information was published. Next-generation sequencing was introduced in January 2013 and therefore only 105 pure CCOC and 27 mixed CCOC tumor samples were analyzed using NGS. PD1 and PDL1 testing was introduced in 2014.
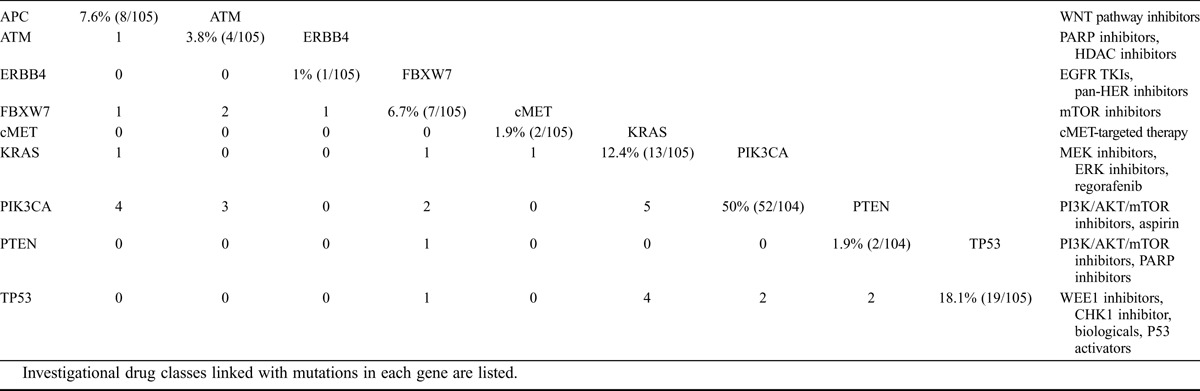
TABLE 2.
The prevalence and overlap of mutations observed by NGS in a cohort of 105 pure CCOC patients
Statistical Analysis
Biomarker expression was compared across histologic subtypes via unpaired t tests using GraphPad software (GraphPad Software Inc, La Jolla, CA).
RESULTS
IHC Results
On the basis of IHC, the protein expression observed with the highest frequency in pure CCOC in at least 50% of samples included RRM1 loss (80.8%; 298 of 369 cases tested), ERCC1 loss (79.6%; 218/274), TOP2A (62.6%; 211/337) overexpression, MGMT loss (56.4%; 220/390), and TS loss (50.8%; 96/189; Table 1). In mixed CCOC, the most common findings on IHC were infiltration of PD-1+ lymphocytes (100%; 8/8), ERCC1 loss (89.6%; 60/67), RRM1 loss (80.0%, 68/85), TOP2A (65.8%; 52/79), TUBB3 loss (52.8%; 38/72), and PTEN loss (51.0%; 50/85; Table 1). There were also differences between pure and mixed CCOC with respect to cMET, AR, ER, PR, PD-L1, MGMT, and TOPO1 (Table 1).
PD-1/PD-L1
Thirteen of 27 pure CCOC tumors showed infiltration by PD-1+ lymphocytes, and 2 of these had aberrant PD-L1 overexpression on carcinoma cells. Eight of 13 that were positive for PD-1 or PD-L1 did not have mutations in any RTK, or in any components of the ERK or mTOR pathways. Aberrant PD-L1 overexpression was observed in 3 of 8 mixed CCOC, whereas PD-1+ tumor infiltrating lymphocytes were present in all 8 mixed CCOC patients tested.
NGS Results
The most common mutations identified by NGS in pure CCOCs included mutations in PIK3CA (50.0%; 52 of 104 cases tested), TP53 (18.1%; 19/105), and KRAS (12.4%; 13/105; Table 2 and Fig. 1). Most PIK3CA mutations occurred in exon 9 (21 mutations) or exon 20 (22 mutations). Of the exon 20 mutations, the most common was H1047R, observed in 13 (22.8%) of 57 patients with any PIK3CA mutation. TP53 mutations were observed in exons 4 to 8. TP53 mutations were present in 19 (18.1%) of 105 of pure CCOC tumors tested and in 15 (57.7%) of 26 mixed CCOCs. Most TP53 mutations were only observed once, with R175H being the most prevalent in 3 tumors. There were significant differences in the frequency of mutations in pure versus mixed CCOCS for the PIK3CA, TP53, KRAS, and cMET genes.
FIGURE 1.
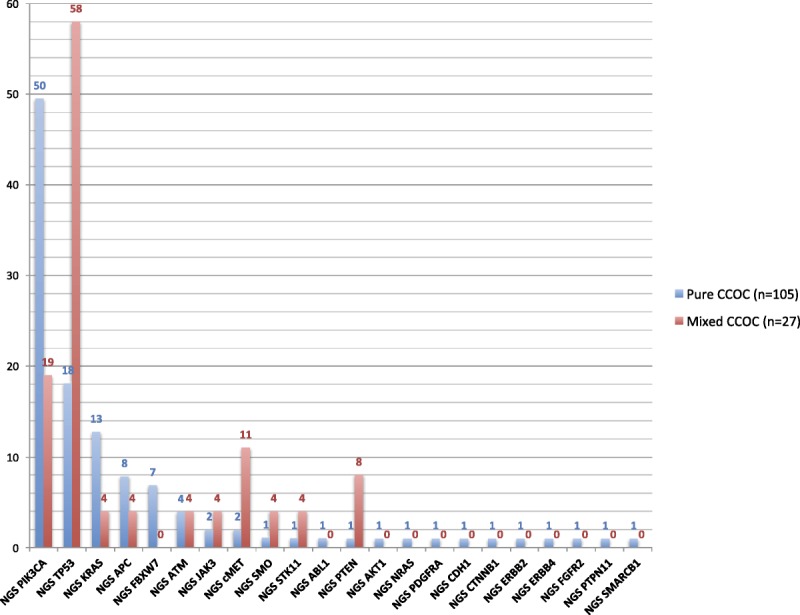
Prevalence of all mutations in pure (n = 105) versus mixed (n = 27) CCOCs by NGS. P values for the comparison of pure versus mixed CCOC samples are as follows: PIK3CA, P = 0.0119; TP53, P < 0.0001; KRAS, P = 0.1944; cMET, P = 0.0254. For all of the other comparisons shown in the graph: P ≥ 0.05. Tested genes that showed no alterations included the following: ALK, BRAF, CSF1R, EGFR, FGFR1, FLT3, GNA11, GNAQ, GNAS, HNF1A, IDH1, JAK2, KDR, MPL, NOTCH1, NPM1, RB1, RET, SMAD4, and VHL.
FISH Results
On the basis of FISH, cMET was amplified in 3.2% (5/154) of pure versus 0% (0/40) of mixed CCOC samples (P = 0.1955), and HER2 was amplified in 9.3% (33/355) of pure versus 3.8% (3/80) of mixed CCOC samples (P = 0.0218).
Other Potentially Actionable Mutations
Mutations were also observed in the ATM (3.7%; 4/102) and APC (7.8%; 8/102) genes in pure CCOC tumors.
Comparison of Biomarker Expression in Biopsies From Primary Site With Metastatic Sites
The expression of all biomarkers was performed within the CCOC patients with NGS performed cohort to see if differences between biopsies taken from primary and metastatic sites could be observed. The frequencies of specific mutations seemed similar in biopsies from primary sites (n = 62) and metastatic sites (n = 40). Only TOPO2A protein expression was significantly different (P = 0.042) with less TOP2A expressed on primary tumors (61.8%; 34/55) than metastatic lesions (69%; 31/38).
DISCUSSION
To the best of our knowledge, this study is the largest and most comprehensive analysis of potential predictive biomarkers and molecular profiling in CCOCs reported to date. We subdivided patients into those with pure (n = 422) and mixed (n = 99) CCOC based on the pathology reports due to the likely differences between them with respect to biological behavior and response to chemotherapy. Molecular profiling and biomarker analysis using IHC, FISH, and NGS suggests that most pure and mixed CCOC tumors have potentially actionable targets that could be tested in clinical trials. The most common alterations were observed in the PIK3CA/AKT/mTOR pathway, with 61% of tumors tested having a mutation one of these pathway components. These mutations overlapped infrequently with mutations in the Ras–Raf–Mek–Erk pathway or with mutations in the receptor tyrosine kinases (Fig. 2). Next-generation sequencing analysis of pure CCOCs demonstrated a high frequency of mutations in PIK3CA (50.0%).
FIGURE 2.
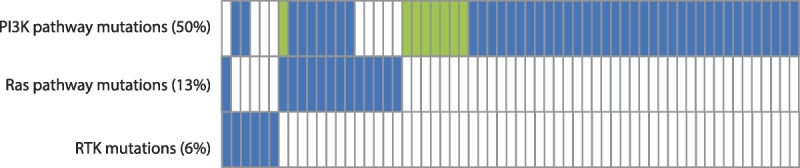
Overlapping mutations in pure CCOCs with a mutation in a component of the PI3K pathway. A total of 105 pure CCOCs were analyzed, but results are shown only for the 61 samples that had a mutation in any of the 3 categories. Blue represents 1 mutation found, green represents 2 mutations found, and blank represents no mutation found.
Immunohistochemistry of pure CCOC demonstrated RRM1 loss (80.8%), ERCC1 loss (79.6%), TOP2A overexpression (62.6%), and TS loss (50.8%). These have been reported to predict response to chemotherapy and suggest that a significant percentage of CCOC may respond to chemotherapy. However, the response rates to platinum- and taxane-based chemotherapy in the first-line setting are relatively low in patients with measureable disease (11%–27%) and less than 10% response rates have been reported with a wide range of agents in patients with recurrent disease.3 Unfortunately, we do not have clinical information available and do not know what percentage of patients in this series responded initially to chemotherapy or how they were treated after receiving the results of the CARIS testing which makes it difficult interpret the IHC results with respect to predicting chemosensitivity. Although CCOCs are often chemoresistant, some patients do respond to platinum-based chemotherapy and it would be important to identify this subset of patients. It is tempting to speculate that the patients most likely to respond to chemotherapy have “mixed” CCOCs. A number of mechanisms of chemoresistance in CCOC have been reported including decreased drug accumulation, increased drug detoxification, increased DNA repair, abnormal growth factor signaling, and cell cycle control which could explain the low response rates, particularly in the second-line setting.12 Loss of ARID1A expression is common in CCOC and has also been correlated to shorter progression-free survival and chemoresistance.13 The high frequency of mutations or alterations in the PIK3CA/Akt/mTOR pathway may also explain the chemotherapy resistance in CCOC as they are associated with intrinsic and acquired resistance to chemotherapy in a number of cancer types.14
We found significant differences in selected biomarkers’ protein expression, gene amplification, and mutation prevalence in pure compared to mixed CCOC, suggesting that a significant proportion of mixed CCOC may either have been misdiagnosed as clear cell cancers and were serous cancers with clear cell features. The results of TP53 mutation analysis in mixed compared to pure CCOC are indicative of this. TP53 mutations are considered a hallmark of HGSOC.15 In contrast, pure CCOC have a much lower rate of TP53 mutation, with different studies reporting TP53 mutation rates ranging from 0% to 52%.16–18 Our analysis by NGS found a mutation rate of 18.1% for TP53 (19 of 105 samples). Mixed CCOC had a significantly higher rate of TP53 mutation (57.7% vs 18.1%; P < 0.0001) supporting the premise that they constitute a distinct subtype of CCOC and they should be either excluded or stratified in clinical trials of CCOC.
The PI3K/AKT/mTOR pathway has been established as a key mediator of oncogenic signaling.19 In the current study, PIK3CA mutations were observed in 50% (52 of 104 samples) of pure CCOCs and in 18.1% of mixed CCOCs. The mutation rates are similar to results from a previous study that found that PIK3CA was mutated in 33% of CCOCs and in 46% of affinity-purified CCOC cell lines, with the higher mutation frequency observed in the cell lines being consistent with the higher mutation frequency observed in pure CCOCs.20 In addition the PIK3CA/AKT/MTOR pathway may be overactive due to PTEN loss, which was observed in almost 50% of CCOC, underscoring the potential importance of targeting this pathway. There are a number of targeted therapies available including mTOR, PIK3CA, and AKT inhibitors, some of which are currently being tested in clinical trials in CCOC.21 However, the PI3K pathway is a complex signaling network coordinating signals from a number other membrane receptors such as Met, as well as cross talk with the Ras–Raf–Mek–Erk pathway.22–24 Parallel molecular networks and feedback loops suggest that it may be better to investigate a combinatorial treatment strategy rather than using single agents. There are many potential combinations that could and should be investigated and there are a number of possible options for clinical trials (Fig. 3). For example, we found that 70% of CCOC with a PIK3CA mutation also overexpressed MET. The signaling pathway of the receptor tyrosine kinase MET and its ligand hepatocyte growth factor is important for cell growth, survival, and motility and is functionally linked to the signaling pathway of VEGF, which is a key effector in angiogenesis. Cabozantinib is a small-molecule kinase inhibitor, which targets MET and VEGF receptor 2, as well as a number of other receptor tyrosine kinases and has significant activity in clear cell renal cancer and is one of many possible agents that should be tested in CCOC.25 Analysis of PD-1 and PD-L1 by IHC was performed for only a small number of samples and larger studies are necessary to provide more robust numbers given the potential treatment implications of immune checkpoint inhibitors in selected patient subsets.
FIGURE 3.
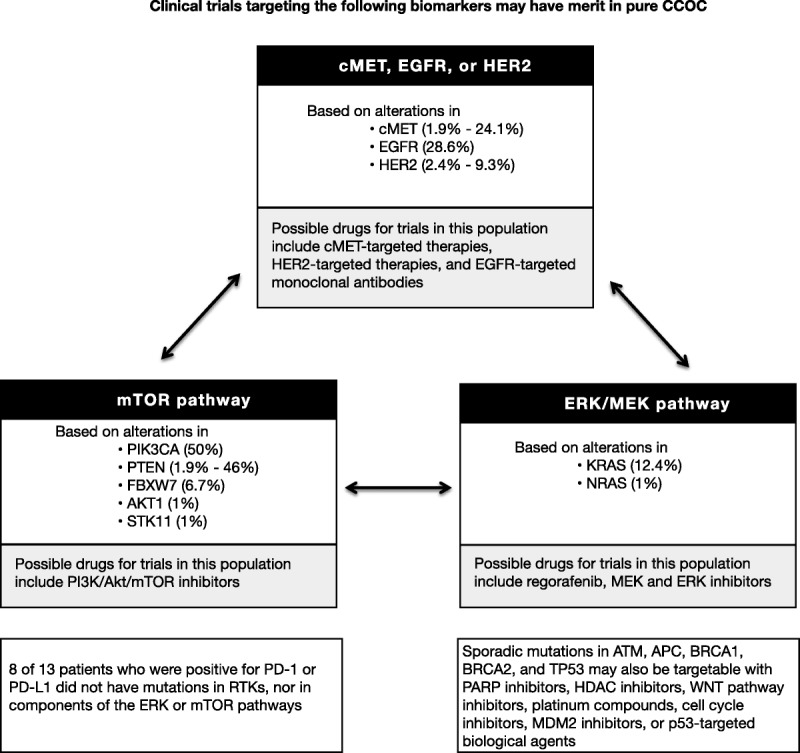
Schema showing potential targeted therapy combinations which may be tested in future clinical trials in pure CCOC.
Limitations of this study include lack of a comprehensive central pathology review (only one representative block was submitted), no clinical information detailing prior therapies or response, and no details on ethnicity. It is encouraging that similar mutation frequencies were observed in the results of NGS testing on biopsies from primary sites (n = 62) and metastatic sites (n = 40), but more work is required to look at tumor heterogeneity to confirm the value of a single biopsy to assign treatment. Although a comprehensive central pathology review would be ideal, the patients with CCOC included in this study do reflect “real world” diagnosis and practice. Central review by expert gynecologic pathologists is however essential for patients entered onto clinical trials and should be encouraged in clinical practice as well.
Patients with recurrent CCOC have a very poor prognosis and very limited treatment options. The results of this extensive biomarker and mutation analysis are consistent with other studies confirming the molecular heterogeneity of CCOC and highlight the potential targets and opportunities for clinical trials to investigate the potential activity of targeted therapies.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Maggie Merchant, PhD, of JFK Communications for the editorial assistance.
Footnotes
Kenneth Russell, Sherri Millis, Zoran Gatalica, Ryan Bender, and Andreas Voss are employees of Caris Life Sciences. Professor Friedlander has participated in advisory boards for Pfizer, Roche, Astra Zeneca, and Clovis, and has received honoraria from Roche, Astra Zeneca and Pfizer.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.ijgc.net).
REFERENCES
- 1. Aoki D. Annual report of Gynecologic Oncology Committee, Japan Society of Obstetrics and Gynecology, 2013. J Obstet Gynaecol Res. 2014; 40: 338– 348. [DOI] [PubMed] [Google Scholar]
- 2. Quirk JT, Natarajan N. Ovarian cancer incidence in the United States, 1992–1999. Gynecol Oncol. 2005; 97: 519– 523. [DOI] [PubMed] [Google Scholar]
- 3. Takano M, Tsuda H, Sugiyama T. Clear cell carcinoma of the ovary: is there a role of histology-specific treatment? J Exp Clin Cancer Res. 2012; 31: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crozier MA, Copeland LJ, Silva EG, et al. Clear cell carcinoma of the ovary: a study of 59 cases. Gynecol Oncol. 1989; 35: 199– 203. [DOI] [PubMed] [Google Scholar]
- 5. Chan JK, Teoh D, Hu JM, et al. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008; 109: 370– 376. [DOI] [PubMed] [Google Scholar]
- 6. Okamoto A, Sugiyama T, Hamano T, et al. Randomized phase III trial of paclitaxel/carboplatin (PC) versus cisplatin/irinotecan (CPT-P) as first-line chemotherapy in patients with clear cell carcinoma (CCC) of the ovary: a Japanese Gynecologic Oncology Group (JGOG)/GCIG study. J Clin Oncol. 2014; 32(suppl 15): 5507. [Google Scholar]
- 7. Tan DS, Miller RE, Kaye SB. New perspectives on molecular targeted therapy in ovarian clear cell carcinoma. Br J Cancer. 2013; 108: 1553– 1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tan DS, Iravani M, McCluggage WG, et al. Genomic analysis reveals the molecular heterogeneity of ovarian clear cell carcinomas. Clin Cancer Res. 2011; 17: 1521– 1534. [DOI] [PubMed] [Google Scholar]
- 9. Girotti MR, Saturno G, Lorigan P, et al. No longer an untreatable disease: how targeted and immunotherapies have changed the management of melanoma patients. Mol Oncol. 2014; 8: 1140– 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Su D, Stamatakis L, Singer EA, et al. Renal cell carcinoma: molecular biology and targeted therapy. Curr Opin Oncol. 2014; 26: 321– 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han G, Gilks CB, Leung S, et al. Mixed ovarian epithelial carcinomas with clear cell and serous components are variants of high-grade serous carcinoma: an interobserver correlative and immunohistochemical study of 32 cases. Am J Surg Pathol. 2008; 32: 955– 964. [DOI] [PubMed] [Google Scholar]
- 12. Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008; 99: 653– 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katagiri A, Nakayama K, Rahman MT, et al. Loss of ARID1A expression is related to shorter progression-free survival and chemoresistance in ovarian clear cell carcinoma. Mod Pathol. 2012; 25: 282– 288. [DOI] [PubMed] [Google Scholar]
- 14. Burris HA., 3rd Overcoming acquired resistance to anticancer therapy: focus on the PI3K/AKT/mTOR pathway. Cancer Chemother Pharmacol. 2013; 71: 829– 842. [DOI] [PubMed] [Google Scholar]
- 15. Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011; 474: 609– 615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho CM, Huang YJ, Chen TC, et al. Pure-type clear cell carcinoma of the ovary as a distinct histological type and improved survival in patients treated with paclitaxel-platinum-based chemotherapy in pure-type advanced disease. Gynecol Oncol. 2004; 94: 197– 203. [DOI] [PubMed] [Google Scholar]
- 17. Okuda T, Otsuka J, Sekizawa A, et al. p53 mutations and overexpression affect prognosis of ovarian endometrioid cancer but not clear cell cancer. Gynecol Oncol. 2003; 88: 318– 325. [DOI] [PubMed] [Google Scholar]
- 18. Rechsteiner M, Zimmermann AK, Wild PJ, et al. TP53 mutations are common in all subtypes of epithelial ovarian cancer and occur concomitantly with KRAS mutations in the mucinous type. Exp Mol Pathol. 2013; 95: 235– 241. [DOI] [PubMed] [Google Scholar]
- 19. Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014; 13: 140– 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuo KT, Mao TL, Jones S, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009; 174: 1597– 1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ClinicalTrials.gov. Sunitinib® in Patients With Recurrent Ovarian Clear Cell Carcinoma. Identifier NCT01824615. Available at: http://clinicaltrials.gov/show/NCT01824615 Accessed September 24, 2014.
- 22. Takano M, Kikuchi Y, Kudoh K, et al. Weekly administration of temsirolimus for heavily pretreated patients with clear cell carcinoma of the ovary: a report of six cases. Int J Clin Oncol. 2011; 16: 605– 609. [DOI] [PubMed] [Google Scholar]
- 23. Takano M, Kouta H, Ikeda Y, et al. Combination chemotherapy with temsirolimus and trabectedin for recurrent clear cell carcinoma of the ovary: a phase II single arm clinical trial. J Clin Oncol. 2014; 32(suppl 15): 5517. [Google Scholar]
- 24. Leary A, Auclin E, Pautier P, et al. The PI3K/Akt/mTOR pathway in ovarian cancer: biological rationale and therapeutic opportunities. In: Diaz-Padilla I, ed. Ovarian Cancer—A Clinical and Translational Update. 2013. Available at: http://www.intechopen.com/books/ovarian-cancer-a-clinical-and-translational-update/the-pi3k-akt-mtor-pathway-in-ovarian-cancer-biological-rationale-and-therapeutic-opportunities. [Google Scholar]
- 25. Smyth EC, Sclafani F, Cunningham D. Emerging molecular targets in oncology: clinical potential of MET/hepatocyte growth-factor inhibitors. Onco Targets Ther. 2014; 7: 1001– 1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


