Abstract
High thoracic or cervical spinal cord injury (SCI) can lead to cardiovascular dysfunction. To monitor cardiovascular parameters, we implanted a catheter connected to a radio transmitter into the femoral artery of rats that underwent a T4 spinal cord transection with or without grafting of embryonic brainstem-derived neural stem cells expressing green fluorescent protein. Compared to other methods such as cannula insertion or tail-cuff, telemetry is advantageous to continuously monitor blood pressure and heart rate in freely moving animals. It is also capable of long term multiple data acquisitions. In spinal cord injured rats, basal cardiovascular data under unrestrained condition and autonomic dysreflexia in response to colorectal distension were successfully recorded. In addition, cardiovascular parameters before and after SCI can be compared in the same rat if a transmitter is implanted before a spinal cord transection. One limitation of the described telemetry procedure is that implantation in the femoral artery may influence the blood supply to the ipsilateral hindlimb.
Keywords: Medicine, Issue 92, spinal cord injury, telemetric recording, blood pressure, heart rate, autonomic dysreflexia, embryonic neural stem cell
Introduction
Cardiovascular dysfunction occurs after spinal cord injury (SCI) at high levels. It is manifested in disordered blood pressure and heart rate at rest, orthostatic hypotension, exercise-induced hypotension, and autonomic dysreflexia characterized by episodes of hypertension and baroreflex-mediated bradycardia in response to sensory stimuli below the injury level1,2. These symptoms interfere with the daily living of spinal cord injured patients. Thus, it is important to establish effective tools for the investigation of cardiovascular changes in animals with SCI and experimental treatments.
To investigate cardiovascular function in animals, several techniques have been used to monitor blood pressure and heart rate. Central cardiovascular parameters can be recorded by cannula insertion and telemetry, whereas noninvasive tail-cuffs can be employed to measure peripheral blood pressure3. Compared to other methods, telemetry has the main advantage that it allows for continuous recording in freely moving animals and long-term monitoring of cardiovascular function4. In SCI animal models, the changes in peripheral blood pressure after experimental stimulation may not be large enough to be detected. Accordingly, suitable cardiac monitoring technique should be selected for animals with SCI.
In the present study, a radio-telemetric system was introduced to monitor cardiovascular function in adult rats after complete spinal cord transection. Rats received grafts of syngeneic rat embryonic day 14 (E14) brainstem-derived neural stem cells (BS-NSCs) in the lesion site. Rats with injury and no transplant and naïve, uninjured rats served as controls. The procedure of telemetry includes transmitter sterilization and implantation (Figure 1), recording of basal cardiovascular parameter, colorectal distension-induced responses, and transmitter cleaning and storage.
Protocol
All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC). NIH guidelines for laboratory animal care and safety are strictly followed. Animals with surgical procedures were adequately treated for minimizing pain and discomfort.
1. Spinal Cord Curgery and Cell Grafting
Autoclave surgical instruments prior to all surgeries. Use Hot Bead Sterilizer (Fine Science Tools) to remove pathogens and microbial contaminants from instruments between procedures on different animals. Use sterile surgical gloves, gown, and drapes during the surgery. Employ aseptic surgical technique for each surgical procedure.
Anesthetize female Fischer 344 rats with a combination (2 ml/kg) of ketamine (25 mg/ml), xylazine (1.3 mg/ml), and acepromazine (0.25 mg/ml) administered via intraperitoneal injection (i.p.).
Shave the back area and clean the skin repeatedly with Betadine and ethanol.
Cut the skin using a #10 blade and bluntly dissect muscles layers. Use a scalpel to isolate the vertebrae, expose the T3 vertebra, and perform a dorsal laminectomy using a fine-tipped rongeurs.
Incise the dura longitudinally and transect the spinal cord at T4 level using a combination of iridectomy scissors and microaspiration to create an approximately 1 mm rostrocaudal gap between the spinal cord stumps.
Wait about 1 - 2 min until the bleeding fully stops, then suture muscles with 3-0 Vycril and close the skin with wound clips.
Inject Lactated Ringer’s (5 ml), buprenorphine (0.035 mg/kg), and Ampicillin (33 mg/kg) subcutaneously immediately after surgery and maintain rats in a warm incubator until awake.
Inject the Ringer’s and Ampicillin solution once daily up to 10 days, and buprenorphine twice daily for 3 days or until signs of pain and distress disappear. Do not return an animal to the company of other animals until fully recovered.
Manually empty the bladder twice a day for about two weeks until the establishment of reflex bladder emptying, and then empty the bladder once a day throughout the survival if necessary.
Two weeks later, re-anesthetize SCI rats as described above and re-expose the spinal cord lesion site. Keep the dura closed to retain implanted cells in the lesion site.
Inject 10 µl of cell solution (3.5 × 105 µl), collected from E14 ubiquitous GFP transgenic rat embryos and embedded in fibrinogen and thrombin5,6, into the epicenter of the lesion cavity and the rostral and caudal interface of lesion with multiple injection sites, using a pulled glass micropipette with an inner diameter of 40 µm, connected to a Hamilton syringe.
Suture the muscle layers and close the skin with wound clips.
Perform post surgery injection and bladder care as described in steps 1.6 – 1.8.
One week before perfusion, inject 0.5% Fluorogold (FG, 0.4 ml in distilled water) intraperitoneally to retrogradely label sympathetic preganglionic neurons in the spinal cord7.
2. Transmitter Implantation
Soak transmittersin 2% glutaraldehyde solution (4 ml of 50% glutaraldhyde in 96 ml distilled water) for at least 1 - 2 hr (up to 10 hr) at room temperature for sterilization.
Wash transmitters thoroughly with sterile 0.9% saline 3 times and store them in saline until use (no more than 1 hr).
Eight weeks after E14 cell transplantation (10 weeks post injury), reanesthetize the rats that underwent a SCI with or without cell grafting and naïve control rats.
Shave the abdominal area and hindlimbs. Clean the skin with Betadine. Place the rat on the surgical table in supine position.
Incise the skin on the ventral abdomen and inner thigh on the right side using a #15 blade.
Cut through the subcutaneous connective tissues to expose the bundle of femoral vessels and nerve using a small scissors.
Separate the femoral artery from the vein and the nerve using a fine forceps with curved tips.
Put three silk sutures underneath the artery and make a loose knot in each suture.
Apply 0.1 ml Lidocaine (2%) to the surface of the artery to elicit vasodilation for subsequent catheterization.
Secure the vessel distally with a permanent silk knot and temporary block proximally by stretching a loose silk suture.
Puncture the artery using a 20 gauge curved needle and insert the tip of the telemeter catheter (8 cm long) using a catheter insertion tool.
Insert the catheter rostrally up to 4 cm thereby placing the tip in the thoracic aorta.
Anchor the catheter within the vessel by tying three silk sutures around the femoral artery.
Make a subcutaneous pocket along the flank between the caudal edge of ribcage and the most cranial extension of knee's range with a blunt scissors.
Insert the transmitter body into the pocket and suture to the connective tissue surrounding the transmitter to avoid excessive movement.
Suture the skin with No. 6 silk thread.
Inject Lactated Ringer’s (5 ml), buprenorphine (0.035 mg/kg), and Ampicillin (33 mg/kg) subcutaneously immediately after surgery and maintain rats in a warm incubator until awake.
3. Basal Mean Arterial Pressure (MAP) and Heart Rate (HR) Recording
As early as one day following transmitter implantation, put a single animal on the receiver pad and turn the transmitter on. Wait for around 10 - 15 min to habituate the animal and stabilize the cardiovascular parameters.
Record resting MAP and HR, which are derived from the pulse arterial pressure with a computerized data acquisition system for at least 1 hr. Collect data every 5 sec.
Monitor the animals continuously and remove data points during the occurrence of visible spasms. For each animal, average data points to obtain mean values.
4. Colorectal Distension-induced Autonomic Dysreflexia
Restrain NSC-grafted or SCI control rats in a towel supplied with food pellets inside on the transmitter receiver. Usually rats can stay cooperatively during the procedure.
Insert a latex balloon-tipped catheter into the rectum for approximately 2 cm and secure it to the tail with tape8.
Turn the transmitter on and wait for 10 - 15 min allowing the blood pressure to return to preinsertion baseline.
Induce colorectal distension by inflation of the balloon slowly over 10 sec with 1.4 ml of air for 1 min, to generate a pressure of approximately 30 mmHg.
Record MAP and HR 1 min before, 1 min during, and 1 min after colorectal distension; sample data every 3 sec during the 3 min procedure.
Perform 2 - 3 trials per animal with at least 15 min recovery interval between two trials.
Overdose animals (i. p.) with double dose anesthesia combination described above if no further evaluation. Perfuse animals with saline followed by 4% paraformaldehyde.
For each animal, average the values before and during colorectal distension respectively; calculate the difference between baseline and distension-induced MAP and HR changes for each trial; average over the 2 - 3 trials to obtain mean values.
5. Transmitter Cleaning
Remove the transmitter from the animal body after anesthesia but before perfusion. Soak immediately in a beaker filled with distilled water until cleaning; avoid drying of the telemeter device.
Transfer the telemeter to 1% Terg-A-Zyme enzymatic cleaning solution (10 g/L water) for 24 hr at room temperature.
Regel the tip of transmitter catheter with a blunt 30 gauge needle connected to the regel syringe.
Carefully dry the transmitter using a folded soft tissue and store it in the original plastic tray.
Representative Results
Using the above described telemetry technique, we successfully recorded cardiovascular parameters in spinal cord injured animals. In animals with SCI alone, MAP was significantly reduced whereas HR increased compared to naïve animals, consistent with previous reports9. In animals with BS-NSC grafting, MAP and HR approached levels measured in naïve animals (Figure 2). During colorectal distension, a rat was regarded as dysreflexic if the noxious stimulation produced a rise in MAP and a decrease in HR3,8. Episodic hypertension and baroreflex-mediated bradycardia were inevitably triggered in all animals but the extent differed between groups. MAP increase in animals grafted with BS-NSCs was much lower (16.9 ± 3.1 mmHg, n = 3) than animals with injury alone (64.1 ± 1.6 mmHg, n = 3) (Figure 3). However, the decrease in HR during colorectal distension did not significantly differ between groups. As bradycardia is mediated by baroreflex mechanisms, this discrepancy may be due to reduced sensitivity of baroreceptors after SCI10. Spasm occurred during colorectal distension in most rats regardless of the treatment.
Upon histological analysis, immunolabeling indicated excellent graft survival in spinal cords implanted with BS-NSCs but a gap in the spinal cord of injured control animals without graft. Fetal NSCs completely filled the lesion site and only minor cavities were observed. Implants integrated into the host gray and white matter both rostrally and caudally. A large number of TH+ catecholaminergic and 5-HT+ serotonergic neurons were found within BS-NSC implants, and numerous axons topographically innervated caudal sympathetic preganglionic neurons in the intermediolateral cell column over long distances (Figure 4). In contrast, in injured animals without graft, TH+/5-HT+ fibers were not detected in thoracolumbar spinal cord below the lesion (not shown).
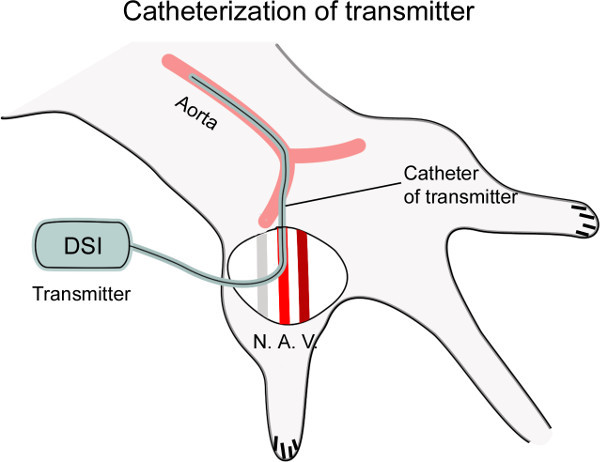 Figure 1: Diagram showing catheterization of the femoral artery. The femoral artery (A.) is dissected from the nerve (N.) and vein (V.), and then punctured with a curved needle. The catheter connected to the transmitter is inserted into the artery, placing the tip of catheter at the level of the thoracic aorta for cardiovascular recording (~4 cm).
Figure 1: Diagram showing catheterization of the femoral artery. The femoral artery (A.) is dissected from the nerve (N.) and vein (V.), and then punctured with a curved needle. The catheter connected to the transmitter is inserted into the artery, placing the tip of catheter at the level of the thoracic aorta for cardiovascular recording (~4 cm).
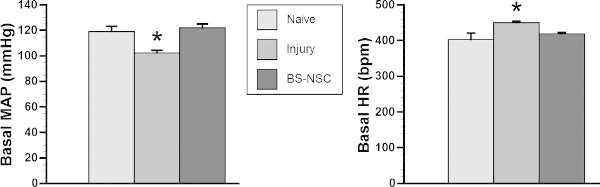 Figure 2: Basal MAP and HR recover in spinal cord injured rats grafted with BS-NSCs. In a representative cohort (n = 3/group), telemetry recording indicates significantly lower basal MAP and higher HR in rats with SCI and no treatment 10 weeks after injury (*p < 0.05). In contrast, both cardiovascular parameters recover to the level of naïve rats 8 weeks after BS-NSC grafting (10 weeks post-injury) (both p > 0.05; ANOVA followed by Fisher’s post hoc).
Figure 2: Basal MAP and HR recover in spinal cord injured rats grafted with BS-NSCs. In a representative cohort (n = 3/group), telemetry recording indicates significantly lower basal MAP and higher HR in rats with SCI and no treatment 10 weeks after injury (*p < 0.05). In contrast, both cardiovascular parameters recover to the level of naïve rats 8 weeks after BS-NSC grafting (10 weeks post-injury) (both p > 0.05; ANOVA followed by Fisher’s post hoc).
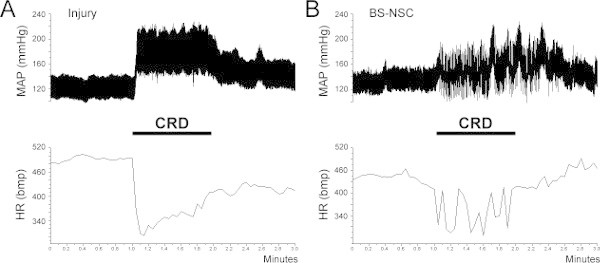 Figure 3: Colorectal distension (CRD)-induced autonomic dysreflexic. Autonomic dysreflexia is triggered during a 1 min CRD in both SCI and BS-NSC grafted animals, manifested as episodic hypertension accompanied by bradycardia. (A) In an injured control rat, MAP increases by approximately 60 mmHg during colon distension. (B) However, the increase of MAP is strongly reduced to 10 - 20 mmHg in a rat grafted with BS-NSCs.
Figure 3: Colorectal distension (CRD)-induced autonomic dysreflexic. Autonomic dysreflexia is triggered during a 1 min CRD in both SCI and BS-NSC grafted animals, manifested as episodic hypertension accompanied by bradycardia. (A) In an injured control rat, MAP increases by approximately 60 mmHg during colon distension. (B) However, the increase of MAP is strongly reduced to 10 - 20 mmHg in a rat grafted with BS-NSCs.
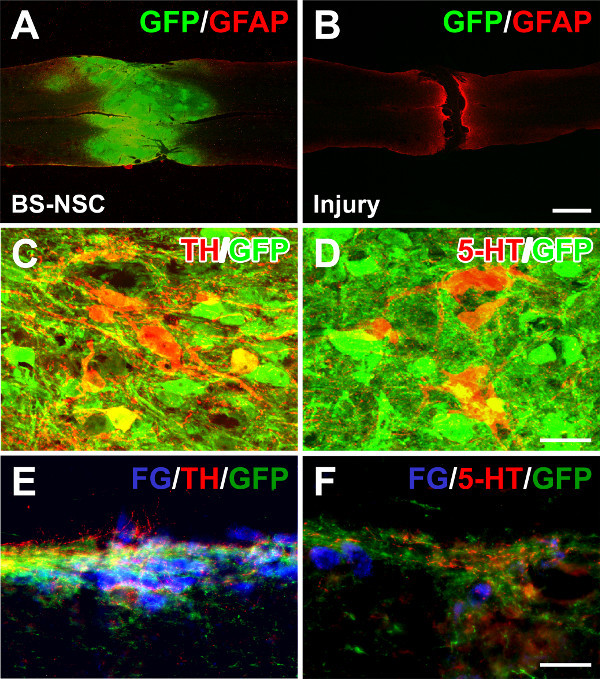 Figure 4: Grafted BS-NSCs integrate into the host adult spinal cord and differentiate into catecholaminergic and serotonergic neurons. (A) Eight weeks after BS-NSC grafting, GFP and GFAP double immunolabeling demonstrates filling of the lesion site with implanted GFP labeled cells in a longitudinal spinal cord section. (B) In contrast, a gap exists in a transected spinal cord without graft. (C, D) Immunolabeling for tyrosine hydroxylase (TH) and serotonin (5-HT) reveals many TH and 5-HT positive neurons within graft. (E, F) Graft-derived TH+ and 5-HT+ axons extend and innervate caudal sympathetic preganglionic neurons labeled with fluorogold in the intermediolateral cell column. Scale bars: 1 mm (B), 25 µm (D), and 50 µm (F).
Figure 4: Grafted BS-NSCs integrate into the host adult spinal cord and differentiate into catecholaminergic and serotonergic neurons. (A) Eight weeks after BS-NSC grafting, GFP and GFAP double immunolabeling demonstrates filling of the lesion site with implanted GFP labeled cells in a longitudinal spinal cord section. (B) In contrast, a gap exists in a transected spinal cord without graft. (C, D) Immunolabeling for tyrosine hydroxylase (TH) and serotonin (5-HT) reveals many TH and 5-HT positive neurons within graft. (E, F) Graft-derived TH+ and 5-HT+ axons extend and innervate caudal sympathetic preganglionic neurons labeled with fluorogold in the intermediolateral cell column. Scale bars: 1 mm (B), 25 µm (D), and 50 µm (F).
Discussion
Traditionally, a fluid filled cannula is inserted into the artery and connected to a pressure transducer to record cardiovascular parameters as a terminal snapshot in each animal11. To continuously monitor cardiovascular performance for a long time, radio-telemetric systems are employed in many laboratories. This more refined tool can record blood pressure in conscious, freely moving animals. Compared to fluid filled catheters, telemetry is considered a more advanced technique to accurately evaluate cardiovascular function in SCI animals. Although in the present study a transmitter was implanted only 1 day before recording, it can be implanted before or after SCI for multiple data acquisition depending on the experimental requirements12. For basal blood pressure and HR, either central or peripheral recording can be achieved. However, cardiovascular response to colorectal distension cannot be detected by noninvasive tail-cuff-based method in our experience. Recent telemetric analysis verified the long-term persistence of hypotension and tachycardia after complete high thoracic spinal cord transection in rats9,13. The lower basal MAP and higher HR in our SCI control rats are consistent with previous report using same recording technique. Colorectal-induced autonomic dysreflexia was alleviated in rats that received cell graft. Based on our histological evidence, catecholaminergic and serotonergic inputs to SPNs across multiple spinal segments may account for the physiological improvements.
Transmitter catheterization is critical for successful implantation and recording. In spinal cord transected rats, the diameter of femoral artery is smaller than usual due to the lack of motor and sensory function of the hindlimbs. Complete paralysis usually results in muscular atrophy. Surgeons may find the tip of the catheter to be bigger than the artery. In this case, applying 2% Lidocaine on the surface of the artery eliciting vasodilation allows for the tip of catheter to be easily inserted into the punctured vessels. Notably, lidocaine induced-vasodilation persists approximately 4 - 5 min. Thus, vessel puncture and catheterization should be performed as quickly as possible. In the period of data acquisition of basal parameters, we collected data points every 5 sec for 1 hr. During the 3 min colorectal distension-induced recording, however, data were sampled every 3 sec in order to obtain more data points for a more accurate averaging throughout this short period.
Implantation of a catheter into the femoral artery may interfere with the blood supply of the hindlimb and induce tissue necrosis, especially if the distal artery is sutured. Sealing the catheter insertion point into the artery with a small drop of tissue glue instead of a silk suture can at least partially restore blood flow. Thereby, animals would be healthier for long-term monitoring. Alternatively, the catheter of the transmitter can be implanted directly into the abdominal aorta placing the transmitter body into the abdominal cavity. This method avoids tissue decay in the ipsilateral hindlimb but has a risk of influencing gastrointestinal function.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The work was supported by grants from NIH/NINDS (NS054883), Craig H. Neilsen Foundation (280072), and the Veterans Administration and Canadian Spinal Research Organization. We thank the Rat Resource and Research Center, University of Missouri, Columbia, Missouri, for providing GFP rats.
References
- Krassioukov AV, Furlan JC, Fehlings MG. Autonomic dysreflexia in acute spinal cord injury: an under-recognized clinical entity. J Neurotrauma. 2003;20:707–716. doi: 10.1089/089771503767869944. [DOI] [PubMed] [Google Scholar]
- Lindan R, Joiner E, Freehafer AA, Hazel C. Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Paraplegia. 1980;18:285–292. doi: 10.1038/sc.1980.51. [DOI] [PubMed] [Google Scholar]
- Inskip JA, Ramer LM, Ramer MS, Krassioukov AV. Autonomic assessment of animals with spinal cord injury: tools, techniques and translation. Spinal Cord. 2009;47:2–35. doi: 10.1038/sc.2008.61. [DOI] [PubMed] [Google Scholar]
- Mayorov DN, Adams MA, Krassioukov AV. Telemetric blood pressure monitoring in conscious rats before and after compression injury of spinal cord. J Neurotrauma. 2001;18:727–736. doi: 10.1089/089771501750357663. [DOI] [PubMed] [Google Scholar]
- Hou S, Tom VJ, Graham L, Lu P, Blesch A. Partial restoration of cardiovascular function by embryonic neural stem cell grafts after complete spinal cord transection. J Neurosci. 2013;33:17138–17149. doi: 10.1523/JNEUROSCI.2851-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhavan M, Hoang TX, Havton LA. Improved detection of fluorogold-labeled neurons in long-term studies. J Neurosci Methods. 2006;152:156–162. doi: 10.1016/j.jneumeth.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Maiorov DN, Fehlings MG, Krassioukov AV. Relationship between severity of spinal cord injury and abnormalities in neurogenic cardiovascular control in conscious rats. J Neurotrauma. 1998;15:365–374. doi: 10.1089/neu.1998.15.365. [DOI] [PubMed] [Google Scholar]
- Laird AS, Carrive P, Waite PM. Cardiovascular and temperature changes in spinal cord injured rats at rest and during autonomic dysreflexia. J Physiol. 2006;577:539–548. doi: 10.1113/jphysiol.2006.116301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AA, Krassioukov AV, Ainslie PN, Warburton DE. Baroreflex function after spinal cord injury. J Neurotrauma. 2012;29:2431–2445. doi: 10.1089/neu.2012.2507. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Taylor RF, Schramm LP. Determinants of arterial pressure after chronic spinal transection in rats. Am J Physiol. 1989;256:666–673. doi: 10.1152/ajpregu.1989.256.3.R666. [DOI] [PubMed] [Google Scholar]
- Rabchevsky AG, et al. Effects of gabapentin on muscle spasticity and both induced as well as spontaneous autonomic dysreflexia after complete spinal cord injury. Front Physiol. 2012;3:329. doi: 10.3389/fphys.2012.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Lu P, Blesch A. Characterization of supraspinal vasomotor pathways and autonomic dysreflexia after spinal cord injury in F344 rats. Auton Neurosci. 2013;176:54–63. doi: 10.1016/j.autneu.2013.02.001. [DOI] [PubMed] [Google Scholar]


