Abstract
Obesity is an unconstrained worldwide epidemic. Unraveling molecular controls in adipose tissue development holds promise to treat obesity or diabetes. Although numerous immortalized adipogenic cell lines have been established, adipose-derived stem cells from the stromal vascular fraction of subcutaneous white adipose tissues provide a reliable cellular system ex vivo much closer to adipose development in vivo. Pig adipose-derived stem cells (pADSC) are isolated from 7- to 9-day old piglets. The dorsal white fat depot of porcine subcutaneous adipose tissues is sliced, minced and collagenase digested. These pADSC exhibit strong potential to differentiate into adipocytes. Moreover, the pADSC also possess multipotency, assessed by selective stem cell markers, to differentiate into various mesenchymal cell types including adipocytes, osteocytes, and chondrocytes. These pADSC can be used for clarification of molecular switches in regulating classical adipocyte differentiation or in direction to other mesenchymal cell types of mesodermal origin. Furthermore, extended lineages into cells of ectodermal and endodermal origin have recently been achieved. Therefore, pADSC derived in this protocol provide an abundant and assessable source of adult mesenchymal stem cells with full multipotency for studying adipose development and application to tissue engineering of regenerative medicine.
Keywords: Developmental Biology, Issue 109, Adipocytes, adipocyte differentiation, adipose-derived stem cells, mesenchymal stem cells, pigs, regenerative medicine, stromal-vascular fraction, tissue engineering
Introduction
Obesity, present in about 30% of the population in the USA, with a body mass index over 30, has emerged as a prevalent worldwide phenomenon1. Obesity tends to lead to related complications including cardiovascular diseases, type-2 diabetes, and cancer2-4. Therefore, dealing with obesity is an important priority. Obesity is manifested by massive expansion of adipose tissues, and is attributed to excessive food consumption and a sedentary life style in modern society. Hence, deciphering the transcriptional regulation of adipogenesis and lipogenesis could hold promise to treat obesity or diabetes5.
The 3T3-L1, 3T3-F442A and other mouse adipogenic cell lines have been applied to study adipogenesis or lipogenesis during adipose tissue development. However, there are some discrepancies in regulatory mechanisms between cell lines in vitro and animals in vivo6. Primary adipose-derived stem cells (ADSC) in the stromal-vascular cell fraction can be isolated directly from white adipose tissues and induced to differentiate. Differentiation of ADSC into adipocytes most likely recapitulates the process of adipogenesis and lipogenesis in adipose tissue development in vivo7.
Pigs are a suitable animal model for studying adipogenesis and lipogenesis in adipose tissue development. Our previous porcine studies8-10 demonstrate that expression of sterol regulatory element-binding transcription factor 1c (SREBP1c), an important transcription factor known to modulate transcription of lipogenic fatty acid synthase, is inhibited by polyunsaturated fatty acids (PUFA) in the porcine liver and adipose tissues. The expression of porcine SREBP1c decreased by PUFA in vivo and in vitro is similar to other species such as humans and mice11-13. These pig studies in vitro are primarily in differentiated adipocytes derived from porcine ADSC (pADSC). Therefore, this primary cell culture of pADSC can be used to serve as a reliable cellular system to study adipose tissue development or other stem cell applications.
Protocol
Note: This method has been established and used in research reported previously14-17 from this laboratory; over time the methodology was modified. The current procedure was performed using an average of 60 g of porcine subcutaneous adipose tissues from one piglet (7 to 9 days old) with seeding on 6-well tissue culture plates. All procedures were performed at RT unless otherwise designated. All the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at National Taiwan University.
1. Prepare Digestion Medium
Obtain subcutaneous adipose tissues from the neck and back; 40 to 80 g per piglet (7 to 9 days old), depending on the size of the pigs. Here, use 60 g of subcutaneous adipose tissues obtained from one pig.
Prepare digestion medium: weigh collagenase II powder with a total of 54,000 units and dissolve it in 90 ml Dulbecco's Modified Eagle Medium (DMEM, for 60 g fat) in a 100 ml serological bottle (900 units of collagenase/1.5 ml DMEM/g fat).
Gently agitate the digestion medium on a shaker table (100 rpm) for at least 15 min to dissolve and then pass the digestion medium through a 0.22 µm filter for sterilization. Store at 4 °C before use.
2. Dissect Subcutaneous Adipose Tissues from Pigs
Sterilize all instruments, glass and plastic ware and warm all the media to 37 °C before use.
Sacrifice the piglet with electrical stunning and exsanguination or by using a method in accordance with the local IACUC regulations. Perform dissection carefully and immediately after piglets are sacrificed.
Lay the piglet on a clean surgical table (back facing up and belly down). Shave off the hair from the piglet back removing all hair from the neck to the tail and on both sides down to the midline.
Scrub the pig's dorsal skin with 7.5% povidone-iodine three times (with three new independent-scrubs) and then allow the iodine to sit on the surface of the skin for about 10 min.
Remove the povidone-iodine with multiple sprays of 70% ethanol. Use gauze pads or tissue papers containing 70% ethanol to wipe the skin in one direction, repeating until no obvious color of povidone-iodine is observed.
Use a scalpel to separate the porcine dorsal fat layer of subcutaneous adipose tissues along with the attached skin layer from the muscles while holding up the fat and skin using forceps.
Immediately immerse the fat layer of subcutaneous adipose tissues with attached skin in a sterilized beaker (200 ml) containing serum-free DMEM.
Spray the outside of the beaker containing the fat layer with 70% ethanol and place in a laminar flow cell culture hood. Place a large (40 cm x 30 cm) sterilized triple-layer cover foil in the hood. [A triple layer is used to ensure continual integrity.]
Place the tissue with the skin facing down onto the cover foil.
Trim the remaining muscle tissue off of the adipose tissue using forceps and scissors to avoid contamination with muscle tissue.
Cut the fat into square pieces (~7 cm X 7 cm) with a scalpel or scissors. Put these pieces of the fat layer into a new sterilized beaker (200 ml) containing serum-free DMEM.
Set a customized slice holder on the cover foil assembled with a carbon steel slicer blade (Figure 1). Take one piece of fat out of the beaker and place it on the slicer (skin layer on top and fat layer below).
Slice the fat layer of subcutaneous adipose tissues into approximately 1 mm thick pieces. Slice the fat layer from the skin as close as possible but avoid slicing the skin.
Mince sliced adipose tissues with scissors as fine as possible.
Add filtered digestion medium containing collagenase to a 250 ml Erlenmeyer flask or a serological bottle with minced adipose tissues (54,000 units of collagenase/90 ml DMEM/60 g fat).
Incubate and swirl the Erlenmeyer flask at 45 rpm in an orbital shaker for 90 min at 37 °C to allow the collagenase to digest the tissues. Note: Check every 15 to 30 min to avoid over-digestion. The digestion process is completed if the digestion medium is a slurry without significant tissue clumps.
Add an equal volume (equal to the digestion medium) of culture medium containing DMEM/F12 with 10% fetal bovine serum (FBS) to stop the collagenase digestion.
 Figure 1. A customized slicer used for isolation of pADSC. Dissected adipose tissues from porcine dorsal subcutaneous adipose tissues are composed of the fat layer with attached skin layers. A slicer is required to slice the fat layer approximately 1 mm thick with avoidance of over-slicing into the skin layers. From left to right: slice holder, slicer pad, carbon steel slicer blade, and screws. Slicer blade is inserted between slice holder and slicer pad when assembled. Please click here to view a larger version of this figure.
Figure 1. A customized slicer used for isolation of pADSC. Dissected adipose tissues from porcine dorsal subcutaneous adipose tissues are composed of the fat layer with attached skin layers. A slicer is required to slice the fat layer approximately 1 mm thick with avoidance of over-slicing into the skin layers. From left to right: slice holder, slicer pad, carbon steel slicer blade, and screws. Slicer blade is inserted between slice holder and slicer pad when assembled. Please click here to view a larger version of this figure.
3. Collection of pADSC from the Stromal-vascular Fraction
Pass the digestion medium containing the digested adipose tissues through a single layer of chiffon into a clean sterilized 250 ml Erlenmeyer flask or a 250 ml serological bottle. Use a forceps to depress the middle of the chiffon to guide and assist passage of the digest.
Drain and twist the chiffon with a forceps to complete passage.
Distribute the digestion medium into four 50 ml conical centrifuge tubes (~40 ml medium per tube).
Centrifuge at 700 x g for 10 min to collect the pellet of stromal-vascular cells.
Decant the supernatant without disturbing the pellets to remove most of the top fat layer containing mature adipocytes. Wash the pellet by adding 10 ml DMEM into each tube to resuspend the pellet with pipetting and gentle shaking of the tube.
Centrifuge at 700 x g for 6 min. Decant the supernatant.
Add 10 ml ACK lysis buffer and then resuspend the pellet by pipetting. Let it stand for 7 min (5 to 10 min) at RT to lyse red blood cells in the stromal-vascular fraction.
Add equal amounts of DMEM (10 ml) to stop the reaction with gentle shaking of the tube and then centrifuge at 700 x g for 10 min.
Decant the supernatant, add 10 ml DMEM into each tube, resuspend the pellet with repeated pipetting, and centrifuge at 700 x g for 6 min. Repeat twice.
Collect and pass the DMEM (total of 40 ml DMEM from 4 tubes representing 60 g fat) with suspended cells through a 100 µm strainer into a new 50 ml conical tube.
Gently pipette medium several times to mix well and aliquot 20 µl of cell-containing medium mixed with 180 µl of 0.4% trypan blue solution (1:10 dilution) in a new 1.5 ml Eppendorf tube.
Count the cells with a hemocytometer and then seed pADSC at a density of 60,000 cells/cm2 onto a desired size of culture dish or plate with culture medium containing DMEM/F12, 10% fetal bovine serum (FBS) and 1% antibiotics of penicillin-streptomycin-amphotericin B solution (P/S/A). Generally, seed the pADSC on the 6-well tissue culture plates for adipocyte or osteocyte differentiation. Seed the pADSC on 10-cm dishes for surface marker staining of stem cells or chondrocyte differentiation.
Incubate the plates or dishes in the 37 °C incubator in air with 5% CO2 to allow cell attachment to the plates.
4. Identification Stem-cell Surface Markers of pADSC by Flow Cytometry
After 24 hr, remove the medium completely, wash the 10-cm dish twice with phosphate-buffered saline (PBS), and harvest cells with 1 ml of 0.25% trypsin-EDTA for 5 min at 37 °C.
Neutralize trypsin-EDTA with equal amounts of culture medium (1 ml), collect cells in a new 15-ml conical tube, and then centrifuge at 400 × g for 7 min.
Decant the supernatant. Wash the pellet twice in 3 ml ice-cold FCS-wash buffer (PBS containing 10% FBS) using re-suspension by pipetting coupled with centrifugation at 400 x g for 7 min.
Resuspend, count, and adjust pADSC to 106 cells/ml in ice-cold FCS-wash buffer. Place cells (100 µl/each tube) into multiple new 15-ml conical tubes and incubate tubes containing pADSC at 4 °C for 30 min with antibodies against either phycoerythrin-conjugated CD4a (CD4a-PE), CD29-PE, CD31-PE, CD44-PE, CD45-PE, CD90-PE, MHC I-PE or MHC II-PE for direct staining. Stop the reaction by washing the cells twice in 10 ml FCS-wash buffer coupled with 400 × g centrifugation for 7 min.
Fix and resuspend cells in fixation buffer (PBS with 0.01% FBS and 1% formaldehyde) for flow cytometry according to the manufacturer's instructions and our previous publication18.
5. Differentiation of pADSC into Adipocytes, Osteocytes and Chondrocytes
- Differentiation of pADSC into adipocytes
- Prepare adipocyte induction medium and adipocyte maintenance medium
- For adipocyte induction medium, prepare 1 L of serum-free DMEM/F12 (with antibiotics of 1% P/S/A) containing the following: 1 ml insulin stock (10 mg/ml HEPES buffer, pH 8), final conc = 10 µg/ml; 1 µl T3 stock (3,3',5-Triiodo-L-thyronine, 1 mM in DMSO), final conc = 1 nM; 200 µl transferrin stock (50 mg/ml double distilled H2O), final conc = 10 µg/ml; 100 µl dexamethasone stock (10 mM in ethanol), final conc = 1 µM; 100 µl rosiglitazone stock (10 mM in DMSO), final conc = 1 µM.
- Prepare adipocyte maintenance medium with the same additions as the induction medium, but with omission of dexamethasone.
- Differentiation process for adipocytes Note: After seeding pADSC on 6-well plates, pADSC will be confluent within 72 h.
- After 3 days, remove the medium completely and then add 3 ml of adipocyte induction medium into each well. Return the plates to the incubator. This is day zero of differentiation.
- After 3 days, remove the adipocyte induction medium completely and replace with 3 ml of adipocyte maintenance medium every three days. Mature adipocytes will be terminally differentiated by about 9 days. Over 90% of adipocytes are well differentiated using this protocol. These adipocytes are ready for Oil Red O staining.
- Differentiation of pADSC into osteocytes
- Prepare osteocyte induction medium: complete culture medium (DMEM/F12 with 10% FBS and 1% P/S/A) containing 1 µM dexamethasone, 10 mM β-glycerophosphate and 50 µg/ml ascorbate-2-phosphate.
- Differentiation process for osteocytes Note: After seeding pADSC on 6-well plates, pADSC will be confluent within 72 h.
- After 3 days, remove the medium completely and add 3 ml of osteocyte induction medium into each well. Return the plates to the 37 °C incubator. This is day zero of differentiation.
- Replace with osteocyte induction medium every three days. Mature osteocytes will form by 14 days of differentiation. These osteocytes are ready for Alizarin Red S staining.
- Differentiation of pADSC into chondrocytes
- Prepare chondrocyte induction medium: αMEM containing 1% FBS, 6.25 µg/ml insulin, 50 µg/ml ascorbate-2-phosphate, and 10 ng/ml transforming growth factor-β1.
- Differentiation process for chondrocytes
- After seeding pADSC on 10-cm culture dishes for 24 h, remove culture medium completely and wash dishes twice with PBS.
- Trypsinize pADSC with 1 ml of 0.25% trypsin-EDTA for 5 min, and then neutralize with 1 ml culture medium. Collect, count and adjust pADSC in 15 ml conical tubes with a density of 2.5 × 105 cells per tube. Use a hemocytometer to count the cells.
- After centrifugation at 400 × g for 7 min, discard the supernatant without disturbing the pellet and add 1 ml of chondrocyte induction medium in a 15 ml tube. The tube is returned to the 37 °C incubator. This is day zero of differentiation.
- Replace the chondrocyte induction medium every three days without removing cells on the bottom. Mature chondrocytes will form in approximately 14 days. These chondrocytes are ready for Toluidine Blue O staining.
6. Staining of Differentiated Adipocytes, Osteocytes and Chondrocytes
- Oil Red O staining for differentiated adipocytes
- At day 9, remove adipocyte maintenance medium in 6-well culture plates with differentiated adipocytes and then wash the plate twice with PBS. [In the following steps, add enough designated reagents to cover each well of the 6-well plate.]
- Fix adipocytes with 10% formalin solution for at least 10 min.
- Remove the 10% formalin solution and wash the plate twice with double-distilled water.
- After two washes, add 100% propylene glycol to the culture plate and let stand for 1 min.
- Remove the 100% propylene glycol and then add Oil Red O solution (0.5% in propylene glycol) to the culture plate. Let stand for at least 10 min on a rocker shaker with gentle agitation (100 rpm).
- Remove the Oil Red O solution and then replace with 60% propylene glycol. Let stand for 1 min.
- Remove the 60% propylene glycol and then wash the plate twice with double-distilled water.
- Replace with 10% formalin solution. The stained lipid droplets inside of adipocytes are ready for observation by light microscopy.
- Quantification of intracellular Oil Red O (optional steps below): after microscopic observation, remove 10% formalin solution and wash the plate twice with double-distilled water.
- Drain the plate completely and add 500 µl of isopropanol to the 6-well plate.
- Let isopropanol stand in the 6-well plate on a gentle rocker shaker (100 rpm) for at least 10 min to dissolve the dye of Oil Red O.
- Aspirate the isopropanol containing Oil Red O and distribute on a 96-well plate. Quantify the extracted Oil Red O using a spectrophotometric reading at 510 nm.
- Alizarin Red S staining for differentiated osteocytes
- At day 14, remove osteocyte induction medium from 6-well plates with differentiated osteocytes and wash the plates twice with PBS. [In the following steps, add enough designated reagents to cover each well of the 6-well plate.]
- Fix osteocytes in 10% formalin solution for at least 10 min.
- Remove the 10% formalin solution from each well and wash the plate twice with double-distilled water.
- After two washes, add 2% Alizarin Red S solution (pH = 4.1-4.3) to the 6-well plate and let stand for at least 15 min on a gentle rocker shaker (100 rpm).
- Remove the Alizarin Red S solution and then wash the plate twice with double-distilled water.
- Replace with 10% formalin solution. Osteocytes are ready for observation by light microscopy.
- Toluidine Blue O staining for differentiated chondrocytes
- At day 14, aspirate chondrocyte induction medium from the 15 ml conical tube without removing sediments of differentiated chondrocytes in the bottom of the tube and wash the tube twice with PBS. [In the following steps, add enough designated reagents to cover chondrocytes in the bottom of the tube or on the section slide.]
- Fix chondrocytes in 10% formalin solution for at least 10 min.
- Remove the 10% formalin solution from each tube and wash the tube twice with double-distilled water.
- After two washes, embed pellets in OCT Compound and section with a cryostat at a thickness of 5 µm.
- Stain the slide of the cryostat sections with Toluidine Blue O solution (0.1% with pH 4.1).
- Remove Toluidine Blue O solution and then wash the section twice with double-distilled water. Note: Chondrocytes on slides are ready for observation by light microscopy.
Representative Results
The pADSC derived from pig dorsal subcutaneous fat were seeded on the culture plates or dishes and shown in Figure 2. The morphology of the pADSC derived from the stromal-vascular fraction is similar to mouse or human ADSC. Twenty-four h after seeding, subconfluent pADSC are adhered and have an expanded fibroblast-like morphology (Figure 2A). The pADSC will become confluent within 72 h and are ready for adipocyte or other mesenchymal-type differentiation (Figure 2B). pADSC exhibit strong adipogenic potential after chemical induction and mature adipocytes can be observed after 9 days of differentiation with over 90 % of the pADSCs showing adipogenic differentiation (Figure 2C).
To address the characteristics of pADSC derived in this protocol, surface markers of pADSC were evaluated by flow cytometry analysis. As shown in Figure 3, surface markers for mesenchymal stem cells, including CD29, CD44, CD90 and MHC I (or HLA I), were highly expressed. Negative surface markers, such as CD4a, CD31, CD45 and MHC II (or HLA II) were barely detectable in pADSC derived in the protocol (Figure 3). These results demonstrate that these pADSC exhibit mesenchymal-type stem cell characteristics without significant endothelial or haematopoietic stem cell contamination, including myeloid or lymphoid progenitors.
To further confirm that pADSC represent mesenchymal stem cells, the multipotency of pADSC was examined by differentiation into adipocytes, osteocytes and chondrocytes. These adipocytes, osteocytes and chondrocytes were stained by specific dyes, Oil Red O, Alizarin Red S, and Toluidine Blue O, respectively (Figure 4). These data indicate that this protocol generated pADSC that retained multipotency with full characteristics resembling mesenchymal-type progenitors.
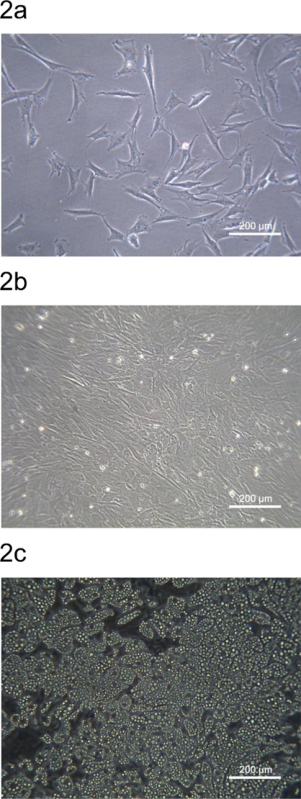 Figure 2. Morphology of pADSC from porcine back fat region. (A) Subconfluent pADSC adhered and expanded after 24-h seeding on a 6-well culture plate. (B) pADSC became confluent after 72-h seeding on a 6-well culture plate. (C) Mature adipocytes were observed after 9 days of adipogenic differentiation from pADSC. Images were taken at 100 x magnification using phase contrast microscopy. Please click here to view a larger version of this figure.
Figure 2. Morphology of pADSC from porcine back fat region. (A) Subconfluent pADSC adhered and expanded after 24-h seeding on a 6-well culture plate. (B) pADSC became confluent after 72-h seeding on a 6-well culture plate. (C) Mature adipocytes were observed after 9 days of adipogenic differentiation from pADSC. Images were taken at 100 x magnification using phase contrast microscopy. Please click here to view a larger version of this figure.
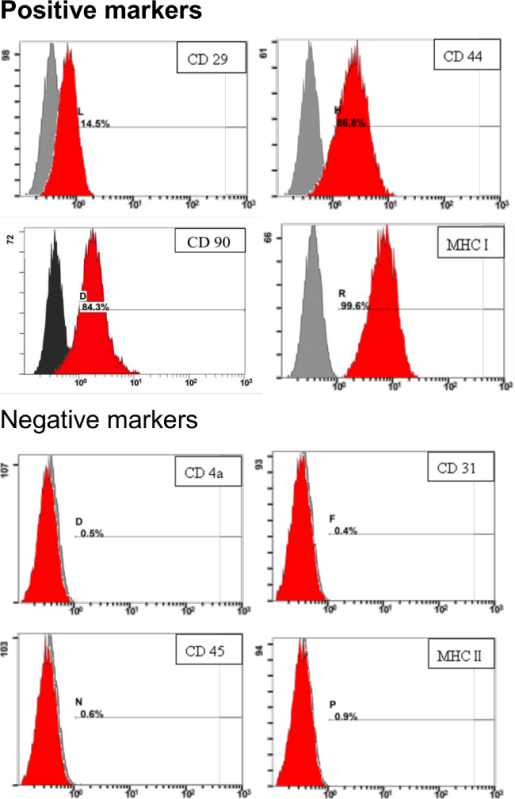 Figure 3. Identification of stem-cell surface markers for pADSC. 1 x 105 of pADSC were reacted with specific antibodies and analyzed for stem cell markers by flow cytometry analysis. Numbers indicate the percentage of stained cells in the population (red) compared with the unstained control. The x-axis represents the relative fluorescence intensity. The y-axis represents the population of cells. Please click here to view a larger version of this figure.
Figure 3. Identification of stem-cell surface markers for pADSC. 1 x 105 of pADSC were reacted with specific antibodies and analyzed for stem cell markers by flow cytometry analysis. Numbers indicate the percentage of stained cells in the population (red) compared with the unstained control. The x-axis represents the relative fluorescence intensity. The y-axis represents the population of cells. Please click here to view a larger version of this figure.
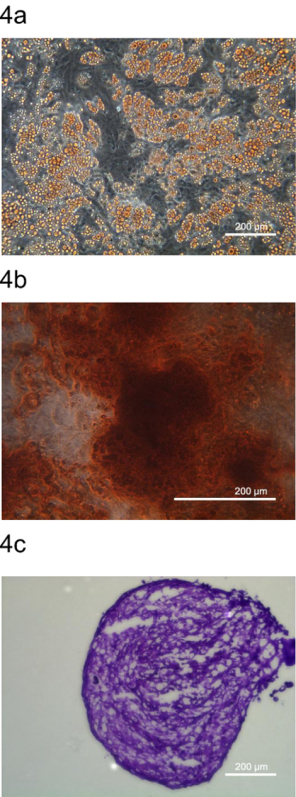 Figure 4. Multipotent differentiation of pADSC. Multipotency of pADSC was determined by differentiating pADSC into (A) adipocytes, (B) osteocytes (C) chondrocytes and stained by representative dyes, Oil Red O, Alizarin Red S, and Toluidine Blue O, respectively. Images were taken at (A) 100 x, (B) 200 x, and (C) 100 x magnification using phase contrast microscopy, respectively. Please click here to view a larger version of this figure.
Figure 4. Multipotent differentiation of pADSC. Multipotency of pADSC was determined by differentiating pADSC into (A) adipocytes, (B) osteocytes (C) chondrocytes and stained by representative dyes, Oil Red O, Alizarin Red S, and Toluidine Blue O, respectively. Images were taken at (A) 100 x, (B) 200 x, and (C) 100 x magnification using phase contrast microscopy, respectively. Please click here to view a larger version of this figure.
Discussion
Here we present a reliable cellular system to study adipose tissue development in primary cell culture of pADSC. Compared to other immortalized cell lines, this method provides a convenient way to isolate large quantities of high quality adult mesenchymal stem cells that can be applied to study differentiation processes of adipocytes or other mesenchymal lineages related to animal development in vivo. The critical modified step in this protocol is that we derive pADSC using a 7- to 9-day old piglet because it is easy to handle the small piglet compared to older pigs and similar to other species19,20, the yield and multipotency of pADSC decreases as the pig ages21.
Potential stem cell sources include embryonic stem cells (ESC), induced pluripotent stem cells (iPSC), and postnatal adult stem cells. The constraint of ADSC, classified as adult multipotent stem cells, is that multipotency of adult stem cells in differentiating divergent lineages is relatively limited compared with ESC or iPSC. However, ethical issues regarding derivation of ESC and oncogenic properties of iPSC restrain the application of ESC and iPSC22,23. Therefore, numerous investigators have focused on adult stem cells with efforts to enhance pluripotency. The most common source of adult mesenchymal stem cells (MSC), which has long been studied, is bone marrow-derived mesenchymal stem cells24. However, harvesting bone marrow is considered a relatively painful procedure. Another concern is that the yield of stem cells from the bone marrow is finite. Bone marrow aspirates yield an average of 6 × 106 nucleated cells per ml, and MSC only represent 0.001 to 0.01% of all the nucleated cells. After considering these drawbacks, ADSC is suggested as a less obtrusive source to obtain multipotent stem cells25,26.
Limitations on the use of ADSC in regenerative medicine are dependent, to a large extent on cell yield and quality. Therefore, the significance of employing pigs to isolate ADSC in this protocol is to yield a large quantity of high quality adult stem cells. The pig is a useful animal model representing humans because of the comparable organ size and many physiological and biochemical similarities between the species27-30. Acquiring hADSC from commercial companies is expensive and in many cases the cells have been manipulated, passaged or cryopreserved. Acquiring human clinical samples is relatively difficult because of ethical issues and production of ADSC is limited. We derive approximately 6 x 105 hADSC per g fat after collagenase digestion. With 100 g of female breast adipose tissue (an average sampling), a total of 6 x 107 cells can be harvested. Using an individual mouse, the yield is even more limited. A total of 1 x 106 cells can be harvested from 0.4 g of subcutaneous mouse inguinal adipose tissue from both legs of an adult FVB mouse (6-8 weeks old). However in one individual pig (7 to 9 days old), a total of 2 x 108 cells can be easily harvested from 60 g of subcutaneous adipose tissue obtained from the dorsal fat depot. The pADSC derived in this protocol have full mesenchymal-type multipotency and appropriate mesenchymal stem cell markers. Therefore, pADSC are a favorable source to obtain large quantities of adult stem cells without compromising stem cell quality.
The application of pADSC is not restricted to deciphering adipocyte differentiation including adipogenesis and lipogenesis. Recently, ADSC have become a popular source of stem cells in the field of regenerative medicine22,31,32. Compared to other stem cell sources, ADSC retain a unique advantage of being easily accessible and abundant, and their robust multipotency has been demonstrated to be a promising source for stem cell therapy and tissue engineering22,33,34. The easy accessibility of adipose tissue makes ADSC one of the least intrusive ways to get multipotent progenitors. Recently, we differentiated pADSC into glucose-responsive insulin-secreting clusters, indicating that pADSC are not limited to mesenchymal differentiation (unpublished data). Others have also been demonstrated that ADSC can be differentiated into many cell types derived from other germ layers such as endodermal hepatocytes (from hADSC35 or pADSC36) or ectodermal neurons (from hADSC37 or pADSC38). Thus, pADSC could be used for high-throughput drug or biomaterial screening by directing cells to divergent differentiation processes to yield desired lineages. Therefore, pADSC derived in this protocol have potential application in stem cell therapy and tissue transplantation for regenerative medicine research.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors would like to express gratitude to all the lab members for the extensive discussion and technique supports in this protocol. Research performed in the lab was supported by grants from Ministry of Science and Technology (MOST 103-2314-B-002-126 and MOST 102-2313-B-002-026-MY3) and by grants from Aim for the Top University Plan (104R350144) of the National University, Taiwan.
References
- Farese RV, Zechner R, Newgard CB, Walther TC. The Problem of Establishing Relationships between Hepatic Steatosis and Hepatic Insulin Resistance. Cell Metab. 2012;15:570–573. doi: 10.1016/j.cmet.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubes G. Cancer research. Unraveling the obesity-cancer connection. Science. 2012;335(28):30–32. doi: 10.1126/science.335.6064.28. [DOI] [PubMed] [Google Scholar]
- Apovian CM, Gokce N. Obesity and cardiovascular disease. Circulation. 2012;125:1178–1182. doi: 10.1161/CIRCULATIONAHA.111.022541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer SR. Transcriptional control of adipocyte formation. Cell Metabolism. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune UL, Ruiz L, Kajimura S. Isolation and Differentiation of Stromal Vascular Cells to Beige/Brite Cells. J Vis Exp. 2013. [DOI] [PMC free article] [PubMed]
- Hsu JM, Ding ST. Effect of polyunsaturated fatty acids on the expression of transcription factor adipocyte determination and differentiation-dependent factor 1 and of lipogenic and fatty acid oxidation enzymes in porcine differentiating adipocytes. Brit J Nutr. 2003;90:507–513. doi: 10.1079/bjn2003918. [DOI] [PubMed] [Google Scholar]
- Hsu JM, Wang PH, Liu BH, Ding ST. The effect of dietary docosahexaenoic acid on the expression of porcine lipid metabolism-related genes. J Anim Sci. 2004;82:683–689. doi: 10.2527/2004.823683x. [DOI] [PubMed] [Google Scholar]
- Liu BH, Kuo CF, Wang YC, Ding ST. Effect of docosahexaenoic acid and arachidonic acid on the expression of adipocyte determination and differentiation-dependent factor 1 in differentiating porcine adipocytes. J Anim Sci. 2005;83:1516–1525. doi: 10.2527/2005.8371516x. [DOI] [PubMed] [Google Scholar]
- Ou JF, et al. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. P Natl Acad Sci USA. 2001;98:6027–6032. doi: 10.1073/pnas.111138698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya M, et al. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology. 2003;38:1529–1539. doi: 10.1016/j.hep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Xu J, Nakamura MT, Cho HP, Clarke SD. Sterol regulatory element binding protein-1 expression is suppressed by dietary polyunsaturated fatty acids - A mechanism for the coordinate suppression of lipogenic genes by polyunsaturated fats. J Biol Chem. 1999;274:23577–23583. doi: 10.1074/jbc.274.33.23577. [DOI] [PubMed] [Google Scholar]
- Ding ST, McNeel RL, Mersmann HJ. Conjugated linoleic acid increases the differentiation of porcine adipocytes in vitro. Nutr Res. 2000;20:1569–1580. [Google Scholar]
- Ding S, Mersmann HJ. Fatty acids modulate porcine adipocyte differentiation and transcripts for transcription factors and adipocyte-characteristic proteins. J Nutr Biochem. 2001;12:101–108. doi: 10.1016/s0955-2863(00)00136-4. [DOI] [PubMed] [Google Scholar]
- Liu LR, et al. Serum amyloid A induces lipolysis by downregulating perilipin through ERK1/2 and PKA signaling pathways. Obesity (Silver Spring. 2011;19:2301–2309. doi: 10.1038/oby.2011.176. [DOI] [PubMed] [Google Scholar]
- Chen YJ, et al. Docosahexaenoic acid suppresses the expression of FoxO and its target genes. J Nutr Biochem. 2012;23:1609–1616. doi: 10.1016/j.jnutbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Lin YY, et al. Modulation of glucose and lipid metabolism by porcine adiponectin receptor 1-transgenic mesenchymal stromal cells in diet-induced obese mice. Cytotherapy. 2013;15:971–978. doi: 10.1016/j.jcyt.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Schipper BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimenko A, et al. Adipose-Derived Mesenchymal Stromal Cells From Aged Patients With Coronary Artery Disease Keep Mesenchymal Stromal Cell Properties but Exhibit Characteristics of Aging and Have Impaired Angiogenic Potential. Stem Cell Transl Med. 2014;3:32–41. doi: 10.5966/sctm.2013-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akanbi KA, Brodie AE, Suryawan A, Hu CY. Effect of age on the differentiation of porcine adipose stromal-vascular cells in culture. J Anim Sci. 1994;72:2828–2835. doi: 10.2527/1994.72112828x. [DOI] [PubMed] [Google Scholar]
- Mizuno H, Tobita M, Uysal AC. Concise Review: Adipose-Derived Stem Cells as a Novel Tool for Future Regenerative Medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Cir Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobita M, Orbay H, Mizuno H. Adipose-derived Stem Cells: Current Findings and Future Perspectives. Discov Med. 2011;57:160–170. [PubMed] [Google Scholar]
- Baer PC. Adipose-Derived Stem Cells and Their Potential to Differentiate into the Epithelial Lineage. Stem Cell Dev. 2011;20:1805–1816. doi: 10.1089/scd.2011.0086. [DOI] [PubMed] [Google Scholar]
- Kakudo N, et al. Adipose-derived regenerative cell (ADRC)enriched fat grafting: optimal cell concentration and effects on grafted fat characteristics. J Transl Med. 2013;11 doi: 10.1186/1479-5876-11-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney JK. Advances in swine biomedical model genomics. Int J Biol Sci. 2007;3:179–184. doi: 10.7150/ijbs.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather RS, Lorson M, Ross JW, Whyte JJ, Walters E. Genetically Engineered Pig Models for Human Diseases. Annu Rev Anim Biosci. 2013;1:203–219. doi: 10.1146/annurev-animal-031412-103715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodicka P, et al. The miniature pig as an animal model in biomedical research. Ann Ny Acad Sci. 2005;1049:161–171. doi: 10.1196/annals.1334.015. [DOI] [PubMed] [Google Scholar]
- Wolf E, et al. Transgenic pigs as models for translational biomedical research. Transgenic Res. 2011;20:1150–1150. [Google Scholar]
- Lindroos B, Suuronen R, Miettinen S. The Potential of Adipose Stem Cells in Regenerative Medicine. Stem Cell Rev Rep. 2011;7:269–291. doi: 10.1007/s12015-010-9193-7. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cignarelli A, et al. Human adipose tissue stem cells: relevance in the pathophysiology of obesity and metabolic diseases and therapeutic applications. Expert Rev Mol Med. 2012;14 doi: 10.1017/erm.2012.13. [DOI] [PubMed] [Google Scholar]
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells: the great WAT hope. Trends Endocrinol Metab. 2012;23:270–277. doi: 10.1016/j.tem.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas A, et al. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- Bruckner S, et al. A fat option for the pig: hepatocytic differentiated mesenchymal stem cells for translational research. Exp Cell Res. 2014;321:267–275. doi: 10.1016/j.yexcr.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Anghileri E, et al. Neuronal Differentiation Potential of Human Adipose-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2008;17:909–916. doi: 10.1089/scd.2007.0197. [DOI] [PubMed] [Google Scholar]
- Huang TT, He DS, Kleiner G, Kuluz J. Neuron-like differentiation of adipose-derived stem cells from infant piglets in vitro. J Spinal Cord Med. 2007;30:S35–S40. doi: 10.1080/10790268.2007.11753967. [DOI] [PMC free article] [PubMed] [Google Scholar]


