Abstract
Patient: Male, 41
Final Diagnosis: Olfactory neuroblastoma
Symptoms: Left nasal obstruction • occasional left epistaxis • headache
Medication: None
Clinical Procedure: Nasal endoscopic examination • neck palpation • CT • bilateral endoscopic resection • MRI • PET-CT • postoperative radiotherapy
Specialty: Otolaryngology
Objective:
Unusual clinical course
Background:
Olfactory neuroblastoma (ONB), also known as esthesioneuroblastoma, is a rare malignant head and neck cancer thought to originate from the olfactory epithelium. It typically invades contiguous structures at presentation. We report a very rare case of multifocal and ectopic ONB.
Case Report:
A 41-year-old man presented with left nasal obstruction and occasional left epistaxis associated with headache. Endoscopic examination of the nasal cavities and computed tomography suggested bilateral polypoid masses. Histopathological diagnosis after endoscopic resection established bilateral olfactory neuroblastoma of the ethmoid sinuses. The patient received postoperative radiotherapy. He remains free of disease 4 years after treatment.
Conclusions:
To the best of our knowledge this is the second documented case of multifocal ectopic olfactory neuroblastoma. Clinicians should consider ONB in the differential diagnosis of bilateral synchronous nasal and paranasal masses to avoid delayed diagnosis. Endoscopic resection of ONB could be an option in selected cases.
MeSH Keywords: Endoscopy; Esthesioneuroblastoma, Olfactory; Nasal Surgical Procedures; Nose Neoplasms; Paranasal Sinus Neoplasms
Background
Olfactory neuroblastoma (ONB), also known as esthesioneuroblastoma, is thought to arise from the specialized olfactory epithelium at the nasal roof and rarely originates in a different location. It represents about 5% of the cases of malignant nasal and paranasal sinus (NPS) tumors [1,2].
At presentation, ONB typically extends by direct infiltration to adjacent structures, including the orbit, the skull base, and the contralateral nasal cavity. This feature, combined with its unspecific clinical presentation, often leads to a bad prognosis. To date, there are neither well-defined treatment guidelines nor a universally accepted staging system. The main prognostic factors are clinical stage, lymph node metastasis, and Hyams’ histopathological grade.
We report a very unusual clinical case of multifocal noncontiguous ectopic ONB. To the best of our knowledge, this is the second published case report of multifocal ONB.
Case Report
A 41-year-old white man presented with left nasal obstruction and occasional left epistaxis associated with headache beginning about 1.5 years before admission. Endoscopic examination revealed a polypoid mass completely obstructing the left nasal cavity and on the right side a polypoid mass originated from the middle meatus filling the nasal cavity. The rest of the examination results were unremarkable and no associated cervical lymph nodes were found.
Computed tomography (CT) showed a left nasal polypoid mass extending from the ethmoid sinus with partial opacification of the left maxillary sinus, and on the right side complete opacification of the ethmoid, maxillary, and sphenoid sinuses. There appeared to be no invasion of the cribriform plate, orbit, or skull base. Radiological findings suggested bilateral polyps (Figure 1).
Figure 1.

Coronal computed tomography (CT) image revealing bilateral nasal masses with partial extension into the left maxillary sinus and partial opacification of the ethmoidal cells of the same side, as well as complete opacification of the right ethmoid and maxillary sinuses. The cribriform plate, the orbit walls, and the nasal septum seem to be uninvolved.
The patient underwent endoscopic resection. Complete bilateral ethmoidectomy with wide middle meatal antrostomy as well as right sphenoidectomy and tailored left lamina papyracea resection were performed because of suspicion of left sinonasal inverted papilloma. No postoperative complications occurred.
Hematoxylin-eosin staining of the tumor showed a lobular growth pattern and round cells surrounded by neurofibrillary material (Figures 2, 3). Immunohistochemical staining for synaptophysin, chromogranin, neuron specific enolase, and S-100 protein proved to be positive, revealing the histological diagnosis of bilateral Hyams’ grade II ONB of the ethmoid sinuses.
Figure 2.

Microscopic image of the typical lobular growth pattern of ONB. Hematoxylin – eosin staining, original magnification ×4.
Figure 3.

Microscopic image showing the uniform-appearing round cells surrounded by neurofibrillary material. Hematoxylin – eosin staining, original magnification ×40.
A magnetic resonance imaging (MRI) scan was carried out 1 month after surgery, confirming postoperative changes with no evidence of intracranial or orbital extension. Positron emission tomography-CT (PET-CT) showed no distant metastases or local uptake (Figure 4). The patient was staged Kadish stage B and Dulgerov stage T1N0M0 bilaterally. He subsequently received postoperative 3-D conformal radiotherapy on the primary site. The total dose was 60 Gy, which were divided into 2 Gy/fraction. During the 48-month follow-up the patient has remained clinically and radiologically free of disease recurrence.
Figure 4.
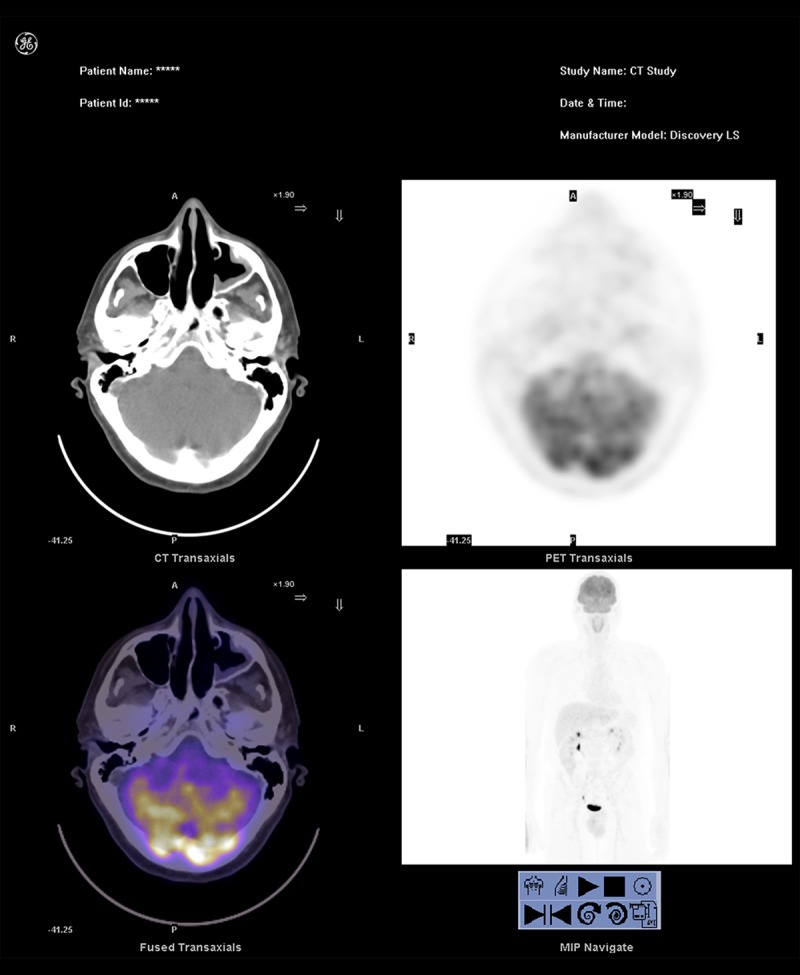
Axial PET-CT images taken 1.5 months after endoscopic resection, showing no local or distant pathological uptake. The images are cut through the maxillary antrostomies.
Discussion
Olfactory neuroblastoma, also known as esthesioneuroblastoma, is the most prevalent of the malignant neuroectodermal NPS tumors. It occurs in about 5% of the cases of malignant NPS neoplasms [1–3].
ONB occurs at any age, from 3 to 90 years, with a bimodal distribution in the second and sixth decades of life without any sex predilection [1,3–5]. It was first described by Berger et al. in 1924 and has also been termed as the “great impostor” by Ogura and Schenck [6] due to its histopathological features that often require further histochemical, immunochemical, and ultrastructural investigations, and due to the biological features, which range from indolent to highly aggressive growth [4,6,7]. It is thought to arise from specialized sensory neuroectodermal olfactory basal cells from the upper nasal cavity, but its origin is still controversial [1]. ONB arises from the olfactory mucosa and when found in other locations other than the roof of the nasal cavity it is considered to be ectopic. Some of the alternative origin hypotheses, like Jacobson’s vomero-nasal organ or autonomic ganglia, support the concept of ectopic origin of ONB. Ectopic ONBs are particularly rare and have been previously reported in 21 cases, such as atypical nasal cavity locations, paranasal sinuses, nasopharynx, petrous apex, pituitary gland, sphenoclival region, and pterygopalatine fossa [8–11]. Only 1 multifocal synchronous ONB case besides ours has been documented in the literature [12]. The present case should be regarded both as multifocal and ectopic, given the tumor’s origin in both ethmoid sinuses.
Histological features include the characteristic nests of small, round, blue cells with rosette or pseudorosette formation and neurofibrillary matrix. Immunohistochemical staining is positive for neuron-specific enolase, S-100 protein, synaptophysin, chromogranin, NFP, and CD 56. S-100 staining is typically limited to the sustentacular cells situated along the periphery of the lobules. Our case was tested for the first 4 markers, which were all positive. These immunohistochemical studies as well as electron microscopy may be necessary for differential diagnosis, especially for high-grade tumors, which may be undistinguishable from other small round cell cancers [13]. Hyams’ grading system proposes 4 levels of differentiation with different histological features (Table 1). Most ONBs are included in Hyams’ lower grades I and II (62%) [2,13,14] ], as was our case.
Table 1.
Hyams’ grading system for olfactory neuroblastoma [14].
| Microscopic features | Grade I | Grade II | Grade III | Grade IV |
|---|---|---|---|---|
| Lobular architecture | Lobular | Lobular | ±Lobular | ±Lobular |
| Nuclear polymorphism | Absent to slight | Present | Prominent | Marked |
| Neurofibrillary matrix | Prominent | Present | May be present | Absent |
| Rosettes | HW* | HW | FW** | FW |
| Mitosis | Absent | Present | Prominent | Marked |
| Necrosis | Absent | Absent | Present | Prominent |
| Glands | May be present | May be present | May be present | May be present |
| Calcification | Variable | Variable | Absent | Absent |
Homer Wright;
Flexner-Wintersteiner.
Clinical manifestations may be similar to the ones observed in benign pathology, especially at a low-stage of disease or if bilateral pathology is present. Therefore, delay between the onset of symptoms, diagnosis, and treatment is common, which accounts for the bad prognosis and outcome of these patients [4]. ONB typically invades the orbit, the skull base, and the paranasal sinuses, often being locally advanced (61%) and showing cervical lymph node metastases in 5% of cases at presentation [2,15]. Neck masses eventually appear in 25–35% of patients. Distant metastases at onset have been reported in 6.6% of the cases, with bone and lung metastases being the commonest [2,5,7,16].
Tumor staging is best established by the combination of CT and MRI. CT shows a homogenous or heterogeneous contrast-enhanced mass with areas of necrosis, T1-weighted MRI a hypointense mass, and T2-weighted MRI intermediate to high intensity [1,17]. Cysts along the margins of intracranial lesions are typically found in Kadish stage C ONB. Intralesional calcification may be considered pathognomonic [18]. Hyperostosis of adjacent bones is also typically seen. Distant metastases have been successfully diagnosed with PET.
Kadish et al. proposed 3 clinical staging categories – A, B, and C – depending on whether the tumor is localized in the nasal cavity (A), nasal cavity and paranasal sinuses (B), or extends beyond these structures (C) [19]. Morita et al. modified this by adding stage D, which includes those cases with regional or distant metastases [3]. The staging system of Dulguerov et al. is TNM-based (Table 2) after radiological findings, but neither of them is universally accepted [4]. None of the staging systems is adequate for ectopic ONB, as shown by our patient, who presented with 2 ectopic tumors arising directly from the ethmoid sinuses without a nasal cavity origin or further extension, both being staged as T1 with the Dulguerov classification. Using the Kadish system, our case could be staged as B or even as A due to its lack of nasal cavity involvement, based on its ectopic localization.
Table 2.
Dulgerov et al. TNM-based staging system [4].
| Stage | Extent of tumor |
|---|---|
| T1 | Tumor involving the nasal cavity and/or paranasal sinuses (excluding the sphenoid sinus) sparing the most superior ethmoidal cells |
| T2 | Tumor involving the nasal cavity and/or paranasal sinuses (including the sphenoid sinus) with extension to or erosion of the cribriform plate |
| T3 | Tumor extending into the orbit or protruding into the anterior cranial fossa without dural invasion |
| T4 | Tumor involving the brain |
| N0 | No cervical lymph node metastasis |
| N1 | Any form of lymph node metastasis |
| M0 | No distant metastasis |
| M1 | Any distant metastasis |
Treatment of ONB includes combinations of surgery, radiation, and chemotherapy, with no well-defined guidelines. Surgery with postoperative radiotherapy is the most accepted treatment modality in all different stages, with the best survival rates (65%), followed by radiotherapy as the only treatment [2]. Craniofacial en bloc resection is the most frequently used technique and has long been considered to be the standard treatment. However, Devaiah and Andreoli stated that endonasal endoscopic surgery is a valid treatment option with comparable outcomes to open surgery in selected cases with no local invasion, showing even better survival rates [20]. Folbe et al. presented one of the largest studies of ONB treated with endoscopic resection, including 23 patients. Only 1 patient, who already had recurrent disease at presentation, had recurrence after treatment [21].
The low 5-year overall survival rate of 45% is due to recurrence of ONB, but improves in low-grade tumors. Wormald’s review of the ectopic cases of ONB does not show any differences in treatment between ectopic and localized ONB [11]. Hence, the main prognostic factors are clinical stage, Hyams’ grade of differentiation, and lymph node metastasis status [2,5].
Clinical and radiological follow-up has been suggested to be warranted for the remainder of the patient’s life.
Conclusions
To the best of our knowledge, this is the second reported case of multifocal ectopic non-invasive olfactory neuroblastoma [12]. Clinicians should consider ONB in the differential diagnosis of bilateral nasal and paranasal masses to avoid delayed diagnosis. Endoscopic endonasal surgery of selected NPS tumors does not compromise oncologic results and provides lower complication rates with possible complete resection at the same time.
Acknowledgments
The authors thank Sonia Rodado Marina, Department of Nuclear Medicine, for her assistance.
Footnotes
Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors declare no potential conflicts of interest.
References:
- 1.Broich G, Pagliari A, Ottaviani F. Esthesioneuroblastoma: A general review of the cases published since the discovery of the tumour in 1924. Anticancer Res. 1997;17(4A):2683–706. [PubMed] [Google Scholar]
- 2.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: A meta-analysis and review. Lancet Oncol. 2001;2(11):683–90. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 3.Morita A, Ebersold MJ, Olsen KD, et al. Esthesioneuroblastoma: Prognosis and management. Neurosurgery. 1993;32(5):706–14. doi: 10.1227/00006123-199305000-00002. discussion 714–15. [DOI] [PubMed] [Google Scholar]
- 4.Dulguerov P, Calcaterra T. Esthesioneuroblastoma: the UCLA experience 1970–1990. Laryngoscope. 1992;102(8):843–49. doi: 10.1288/00005537-199208000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Koka VN, Julieron M, Bourhis J, et al. Aesthesioneuroblastoma. J Laryngol Otol. 1998;112(7):628–33. doi: 10.1017/s0022215100141295. [DOI] [PubMed] [Google Scholar]
- 6.Schenck NL, Ogura JH. Esthesioneuroblastoma. An enigma in diagnosis, a dilemma in treatment. Arch Otolaryngol. 1972;96(4):322–24. doi: 10.1001/archotol.1972.00770090498005. [DOI] [PubMed] [Google Scholar]
- 7.Berger L, Luc R, Richard D. L’esthesioneuroepitheliome olfactif. Bull Assoc Fr Etude Cancer. 1924;13:410–21. [in French] [Google Scholar]
- 8.Purohit B, Winder T, Maggio EM, Kollias SS. Aggressive primary olfactory neuroblastoma of the sphenoclival region: A case report and literature review. Laryngoscope. 2015;125(4):822–25. doi: 10.1002/lary.24925. [DOI] [PubMed] [Google Scholar]
- 9.Kodama S, Kawano T, Suzuki M. Endoscopic transnasal resection of ectopic esthesioneuroblastoma in the pterygopalatine fossa: technical case report. Neurosurgery. 2009;65(6 Suppl.):E112–13. doi: 10.1227/01.NEU.0000346268.69786.88. discussion E3. [DOI] [PubMed] [Google Scholar]
- 10.Seccia V, Lenzi R, Casani AP, Muscatello L. Ectopic olfactory neuroblastoma arising in the pterygopalatine fossa. Otolaryngol Head Neck Surg. 2010;142(3):460–61. doi: 10.1016/j.otohns.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 11.Wormald R, Lennon P, O’Dwyer TP. Ectopic olfactory neuroblastoma: Report of four cases and a review of the literature. Eur Arch Otorhinolaryngol. 2011;268(4):555–60. doi: 10.1007/s00405-010-1423-8. [DOI] [PubMed] [Google Scholar]
- 12.Lee A, Goldstein DP, Irish J, Gentili F, Perez-Ordonez B. Noncontiguous bilateral esthesioneuroblastoma: A case report. Skull Base. 2007;17(6):405–7. doi: 10.1055/s-2007-986459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirose T, Scheithauer BW, Lopes MB, et al. Olfactory neuroblastoma. An immunohistochemical, ultrastructural, and flow cytometric study. Cancer. 1995;76(1):4–19. doi: 10.1002/1097-0142(19950701)76:1<4::aid-cncr2820760103>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Hyams V, Batsakis J, Michaels L. Olfactory neuroblastoma Tumors of the upper respiratory tract and ear. Washington DC: Armed Forces Institute of Pathology; 1988. pp. 240–48. [Google Scholar]
- 15.Bradley PJ, Jones NS, Robertson I. Diagnosis and management of esthesioneuroblastoma. Curr Opin Otolaryngol Head Neck Surg. 2003;11(2):112–18. doi: 10.1097/00020840-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Rinaldo A, Ferlito A, Shaha AR, et al. Esthesioneuroblastoma and cervical lymph node metastases: Clinical and therapeutic implications. Acta Otolaryngol. 2002;122(2):215–21. doi: 10.1080/00016480252814261. [DOI] [PubMed] [Google Scholar]
- 17.Pickuth D, Heywang-Kobrunner SH, Spielmann RP. Computed tomography and magnetic resonance imaging features of olfactory neuroblastoma: An analysis of 22 cases. Clin Otolaryngol Allied Sci. 1999;24(5):457–61. doi: 10.1046/j.1365-2273.1999.00295.x. [DOI] [PubMed] [Google Scholar]
- 18.Manelfe C, Bonafe A, Fabre P, Pessey JJ. Computed tomography in olfactory neuroblastoma: One case of esthesioneuroepithelioma and four cases of esthesioneuroblastoma. J Comput Assist Tomogr. 1978;2(4):412–20. [PubMed] [Google Scholar]
- 19.Kadish S, Goodman M, Wang CC. Olfactory neuroblastoma. A clinical analysis of 17 cases. Cancer. 1976;37(3):1571–76. doi: 10.1002/1097-0142(197603)37:3<1571::aid-cncr2820370347>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Devaiah AK, Andreoli MT. Treatment of esthesioneuroblastoma: A 16-year meta-analysis of 361 patients. Laryngoscope. 2009;119(7):1412–16. doi: 10.1002/lary.20280. [DOI] [PubMed] [Google Scholar]
- 21.Folbe A, Herzallah I, Duvvuri U, et al. Endoscopic endonasal resection of esthesioneuroblastoma: a multicenter study. Am J Rhinol Allergy. 2009;23(1):91–94. doi: 10.2500/ajra.2009.23.3269. [DOI] [PubMed] [Google Scholar]


