Abstract
Background
The aim of this study was to investigate whether the anisotropy parameters are helpful in the detection and discrimination of breast cancers, and to determine its value in predicting the risk of cancers.
Material/Methods
There were 56 patients with 56 lesions (34 malignant, 22 benign) included in the study. DTI was performed in every patient and apparent diffusion coefficient (ADC), fractional anisotropy (FA), and eigenvalues E1, E2, and E3 were measured in every lesion and the normal breast tissue.
Results
ADC, FA, and eigenvalues of E1, E2, E3, and E1–E3 in breast cancers were all significantly lower than in normal tissue (P<0.001 for all) with mean reduction of (32±17)%, (24±13)%, (33±19)%, (32±17)%, (31±18)%, and (37±20)% for ADC, FA, E1, E2, E3, and E1–E3, respectively. These parameters were also statistically lower in cancers than in benign lesions (P<0.01 for all), except FA (P>0.05). ADC, E1, E2, and E3 were very similar in discriminating breast cancers and benign lesions, with area under the curve (AUC) 0.885–0.898, sensitivity 73.5–85.3%, and specificity 90.9–100%.
Conclusions
ADC, E1, E2, E3, and E1–E3 are much lower in breast cancers than in normal tissue and benign lesions. The reduction of ADC, E1, E2, E3, and E1–E3 of a mass in the breast is highly associated with the risk of breast cancer, but the FA has no utility in breast cancer risk prediction.
MeSH Keywords: Anisotropy, Breast Neoplasms, Diffusion
Background
Diffusion-weighted imaging (DWI) is increasingly regarded as a promising modality in breast lesion detection and characterization [1–4]. ADC values were demonstrated to be reduced in breast cancers compared to normal tissue and benign lesions [2,4] due to the increased cellularity. DWI is now regarded as a very useful adjunct method to dynamic contrast-enhanced (DCE) MR imaging, which can improve the accuracy of diagnosis compared to DCE MR imaging alone [5–7].
The histological difference between carcinomas and normal breast fibroglandular tissue lies not only in the increasing abnormal cell density, but also in the disordered organization and arrangement of fibroglandular tissues. The diffusion and ADC changes detected by DWI are non-directional and cannot demonstrate the 3-dimensional diffusion process resulting from the disordered structure. In normal breast tissue, the ducts, vessels, and other parenchyma around them are organized in approximately parallel patterns and have their main directions; therefore, the water molecules within the extracellular space diffuse mainly along the axis of this space and they show anisotropy. DWI imaging can only detect the mean diffusion mobility without directional dependency, due to the limited number of gradients used in these sequences.
On the other hand, the diffusion tensor imaging (DTI) advanced from DWI can vividly exhibit the 3-dimensional diffusion process in healthy tissue and in lesions of the breast and other organs. Because the number of diffusion gradients used in DTI is much more than that used in DWI, a specific 3-dimensional ellipsoid tensor unit can be uniquely determined in each pixel, within which the diffusion ability in any direction can be accurately calculated. By measuring the anisotropic diffusion in different tissues, DTI can provide more information about microstructure and pathophysiology than DWI, and could be helpful to describe and identify the characteristics of different kinds of tissue and even to discriminate different lesions. Prior studies in anisotropy organs such as the brain, prostate, and kidney have shown its great potential [8–14]. Similar to the brain, prostate, and kidney, the breast is also an anisotropy tissue, with parenchyma composed of ducts, vessels, and fibrous glandular tissue spreading radially from the nipple. Initial studies have shown its can detect breast cancers.
The purpose of this study was to investigate whether the anisotropy parameters are helpful in the detection and discrimination of breast cancers, and to determine its value in predicting the risk of cancers.
Material and Methods
Subjects
This study was approved by the local ethics committee, and all patients provided written informed consent before examination. Between June 2012 and July 2014, 106 female patients with breast focal lesions underwent dynamic contrast-enhanced MRI and DTI. Inclusion criteria were: (1) focal lesions found in contrast-enhanced MRI were more than 10 mm in diameter; (2) biopsy or surgery were performed after MRI with a less than 7-day interval; (3) benign or malignant histological result. We excluded anyone with previous surgery, radiation or chemotherapy of breast cancer, lesion in bilateral breast, or incomplete examination due to artifacts. A total of 56 patients were included, with age ranging from 37 to 68 years (median, 47 years).
MR protocol
All patients were examined in prone position on the same 1.5T MRI system device (AVANTO, Siemens Healthcare, Erlangen, Germany) using a dedicated receiving bilateral breast matrix coil (Breast matrix, Siemens Healthcare, Erlangen, Germany). A routine axial turbo-spin echo inversion recovery sequence of fat-suppressed T2WI (TR 5800 ms, TE 56 ms, FOV 275×275 mm, matrix 314×320, slice thickness 6 mm with no intersection gap, NEX 2) was first performed after tomography, followed by a DTI sequence. DTI was performed using an axial 2-dimensional diffusion-weighted echo planer imaging sequence (TR 6900 ms, TE 90 ms, slice thickness 5 mm with zero gap, NEX 4, FOV 380 mm×285 mm, matrix 144×192), and the diffusion gradients were applied in 6 directions with b=0 and 1 000 s/mm2.
Finally, a dynamic contrast-enhanced sequence containing an axial T1-weighted 3D fast-spoiled gradient-recalled echo sequence (TR 20 ms, TE 1.19 ms, FOV 300×300 mm, matrix 128×128, slice thickness 2 mm) was performed. One precontrast acquisition and 5 postcontrast acquisitions were performed before and after the contrast of Gd-DTPA (Omniscan, GE) with a dose of 0.1 mmol/kg.
DTI data postprocessing and analysis
Diffusion tensor data were post-processed and analyzed by an experienced MRI physician blinded to histopathological findings, on the MR Syngo station (Siemens Healthcare) using the Neuro3D toolbox (Siemens Healthcare, Erlangen, Germany). By browsing the contrast-enhanced subtraction images, the slice showing the lesion’s maximum diameter was determined, and then the same slice was found in the axial ADC map. By referring to the DCE images, an ROI in ADC maps corresponding to the hyperintensity in DCE images was drawn along the lesion margin, omitting hemorrhagic, cystic, and calcific areas. In the same patient, another ROI was drawn in the contralateral healthy breast containing normal breast tissue only. Then the anisotropy parameters, such as ADC, FA, and eigenvalues (E1, E2, E3) were automatically calculated, where E1, E2, and E3 are the maximum, intermediate, and minimum diffusion tensor eigenvalues, respectively. Every lesion and normal breast tissue was measured 3 times to produce the final averaged measurements.
Statistical analysis
Statistical analysis was performed using SPSS 19.0. Differences of ADC, FA, eigenvalues E1, E2, E3, and the maximum anisotropy index E1–E3 between breast cancers and normal breast tissue in the same patient were compared by 2-tailed paired t-test. DTI-derived metrics between breast cancer and benign lesions were compared by non-parametric test. Multi-variant logistic analysis was performed to determine if ADC, FA, eigenvalues E1, E2, E3, and the maximum anisotropy index E1–E3 are independent predictors in characterization of different breast lesions, and their predictive values were calculated. Then the best-fitting regression model combining multiple predictors was created. The full range of ADC, FA, and E1 measurements were divided into equal quartiles and their values in predicting risk of breast cancer in every quartile were calculated. Then, to compare diagnostic performance of ADC, FA, E1, E2, E3, E1–E3, and the multivariate model, receiver operating characteristic (ROC) curve analysis was performed to calculate the area under the curve (AUC) and 95% confidence interval (CI). Z-tests were used to compare the difference of AUC in detecting and charactering different breast lesions using MedCalc 11.0 software (MedCalc Software, Mariakerke, Belgium). We calculated the optimal critical value defined as the point with the maximum sum of sensitivity and specificity as well as the corresponding sensitivity and specificity. P-values less than 0.05 were considered to be statistically significant.
Results
In this study, 56 lesions were identified on DCE-MRI in 56 women and categorized as BI-RADS 3, 4, or 5. All lesions were histologically confirmed by operation or puncture sampling. The lesions and their histological results are listed in Table 1.
Table 1.
Characteristics of lesions in breast.
| N | % | |
|---|---|---|
| BI-RADS score | 56 | |
| 3 | 12 | 21 |
| 4 | 19 | 34 |
| 5 | 25 | 45 |
| Histologic type | ||
| Invasive carcinoma | 23 | 41 |
| Ductal carcinoma in situ | 11 | 20 |
| Benign | 22 | 39 |
| Fibroadenomas | 10 | |
| Fibrocystic changes | 5 | |
| Adenosis | 6 | |
| Sclerosing Adenosis | 1 | |
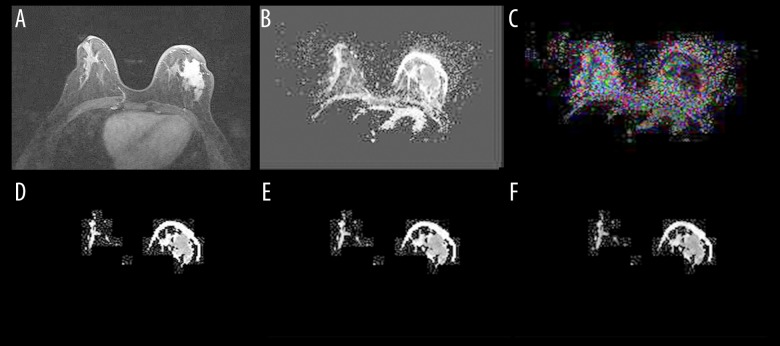
ADC, FA, E1, E2, and E3 were successfully measured in every lesion and the contralateral healthy breast tissue in carcinoma patients. The DTI maps for invasive ductal carcinoma are shown in Figure 1.
Figure 1.
Contrast-enhanced T1WI and DTI images of a 43-year-old woman with irregular invasive ductal carcinoma in the left breast. The figures are the axial images of contrast-enhanced T1-weighted image (A), ADC map (B), FA map (C), E1 map (D), E2 map (E), and E3 map (F). The ADC, FA, and E1 of tumor tissue were compared to the corresponding contralateral breast normal tissue.
Comparison of DTI measurements between carcinoma and normal tissue
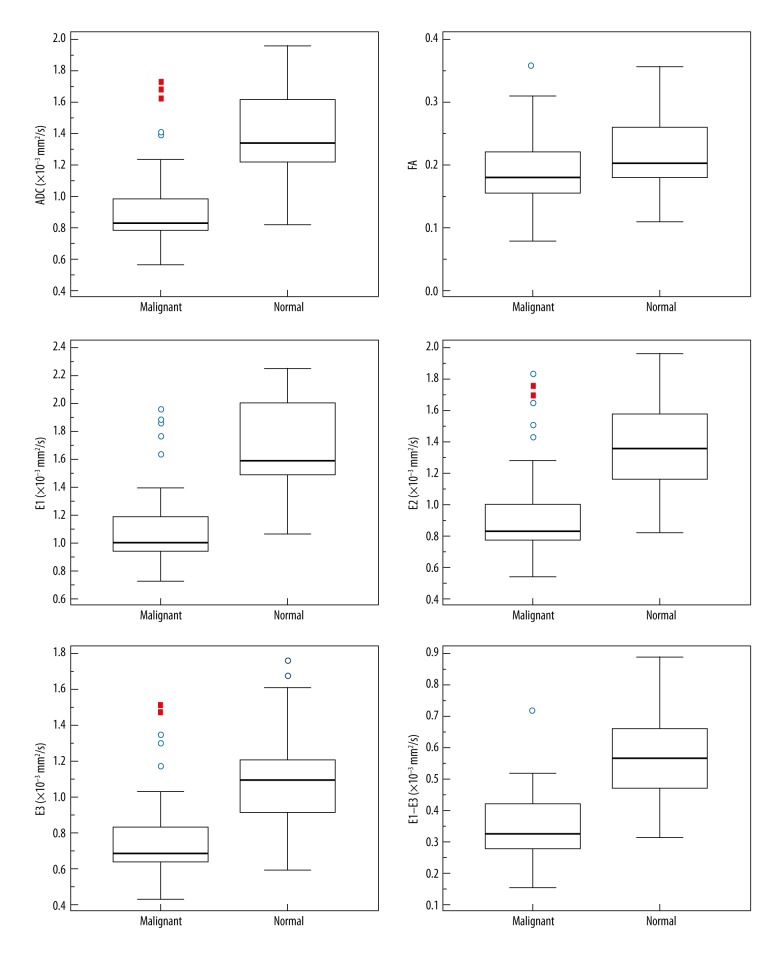
All 34 breast cancer patients were included in the paired DTI measures analysis. ADC, FA, E1, E2, E3, and E1–E3 were all significantly lower in breast cancers than those in normal breast tissue (Figure 2 and Table 2). The averaged differences between cancers and normal tissue in the same patients were calculated. The averaged differences of ADC, FA, E1, E2, E3, and E1–E3 between breast cancer and normal tissue were (0.46±0.31)×10−3 mm2/s, 0.05±0.04, (0.57±0.38)×10−3 mm2/s, (0.45±0.31)×10−3 mm2/s, (0.36±0.26)×10−3 mm2/s, and (0.23±0.16)×10−3 mm2/s, respectively, and the averaged reducing ratios were (32±17)%, (24±13)%, (33±19)%, (32±17)%, (31±18)%, and (37±20)%, respectively. The subgroups of invasive ductal cancers (IDC) and ductal carcinoma in situ (DCIS) were also compared to the normal tissue, and all of these 6 parameters were obviously lower in IDC and DCIS than in normal tissue, with a statistically significant difference (P<0.01).
Figure 2.
Diffusion tensor imaging parameters comparison between breast cancers and normal tissue. All of the parameters in breast cancer tissues were significantly lower than in normal tissue.
Table 2.
DTI derived parameters comparison between malignant lesions and normal tissue (mean ±sd).
| Tissue classification | N | ADC (×10−3 mm2/s) | FA | E1 (×10−3 mm2/s) | E2 (×10−3 mm2/s) | E3 (×10−3 mm2/s) | E1–E3 (×10−3 mm2/s) |
|---|---|---|---|---|---|---|---|
| Breast cancer | 34 | 0.95±0.29 | 0.19±0.06 | 1.13±0.32 | 0.95±0.31 | 0.78±0.28 | 0.35±0.11 |
| Normal tissue | 34 | 1.38±0.27 | 0.22±0.06 | 1.68±0.30 | 1.37±0.27 | 1.10±0.26 | 0.58±0.15 |
| Z | −4.778 | −2.804 | −4.898 | −4.710 | −4.471 | −4.984 | |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
ADC – apparent diffusion coefficient; FA – fractional anisotropy; E1 – maximum eigenvalue; E2 – medium eigenvalue; E3 – minimum eigenvalue.
Comparison of DTI measurements between carcinoma and benign lesions
All of the 34 breast cancer patients and 22 benign lesions were included in this analysis. DTI-derived parameters of ADC, E1, E2, E3, and E1–E3 were all significantly lower in breast cancers than those in benign lesions (P<0.01; Figure 3 and Table 3). However, there was no difference in FA between malignant and benign lesions (P>0.05).
Figure 3.
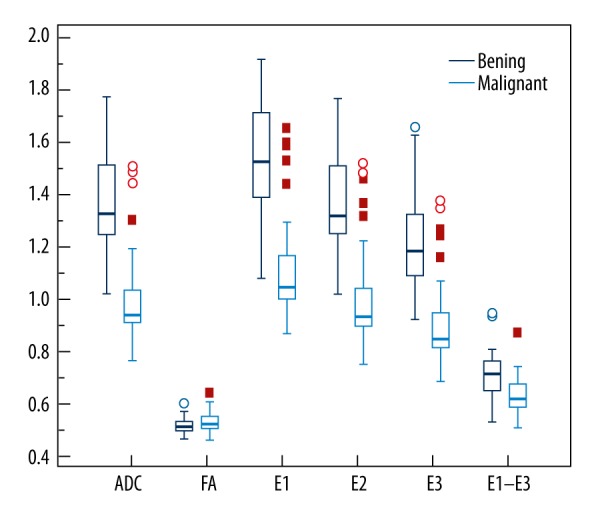
Comparison of DTI measures between breast cancers (IDC N=23 and DCIS N=11) and benign lesions (N=22). ADC, E1, E2, E3, and E1–E3 were significantly lower in breast cancers than in benign lesions, while there was no difference in FA between malignant and benign lesions.
Table 3.
DTI derived parameters comparison between Malignant and benign breast lesions (mean ±sd).
| Tissue | N | ADC (×10−3 mm2/s) | FA | E1 (×10−3 mm2/s) | E2 (×10−3 mm2/s) | E3 (×10−3 mm2/s) | E1–E3 (×10−3 mm2/s) |
|---|---|---|---|---|---|---|---|
| Invasive cancer | 23 | 0.94±0.33 | 0.20±0.06 | 1.12±0.35 | 0.93±0.35 | 0.76±0.30 | 0.36±0.12 |
| DCIS | 11 | 0.96±0.21 | 0.17±0.06 | 1.13±0.25 | 0.97±0.23 | 0.81±0.22 | 0.32±0.09 |
| Benign lesion | 22 | 1.52±0.33 | 0.17±0.05 | 1.77±0.35 | 1.5±0.34 | 1.28±0.32 | 0.49±0.15 |
| χ2 | 25.213 | 3.189 | 25.216 | 24.024 | 24.983 | 17.028 | |
| P | <0.01 | 0.203 | <0.01 | <0.01 | <0.01 | <0.01 |
A further detailed analysis was performed for IDC, DICS, and benign lesions (Table 3). There were no differences in ADC, FA, E1, E2, E3, and E1–E3 between IDC and DCIS (P>0.05). The ADC, E1, E2, E3, and E1–E3 were all significantly lower in invasive cancer (P<0.01) and DCIS (P<0.01) than in benign lesions, except for FA (P>0.05).
Because of the collinearity between ADC, E1, E2, E3, and E1–E3, these 5 parameters cannot be combined in a single multivariate model, but 2-parameter models of ADC, E1, E2, and E3 combined with FA all showed that ADC (P<0.01), E1 (P<0.01), E2 (P<0.01), and E3 (P<0.01) were the significant discriminating factors between breast cancers and benign lesions, except for FA (P>0.05). Multiple logistic regression showed E1–E3 (P<0.01) and FA (P<0.01) can be combined to produce a predicting model of y=1.9+29.9*FA-16.5*(E1–E3), which means that when FA increases by 0.1 unit or E1–E3 decreases by 0.1×10−3 mm2/s, the risk of a mass being malignant would increase by 20 or 5 times, respectively.
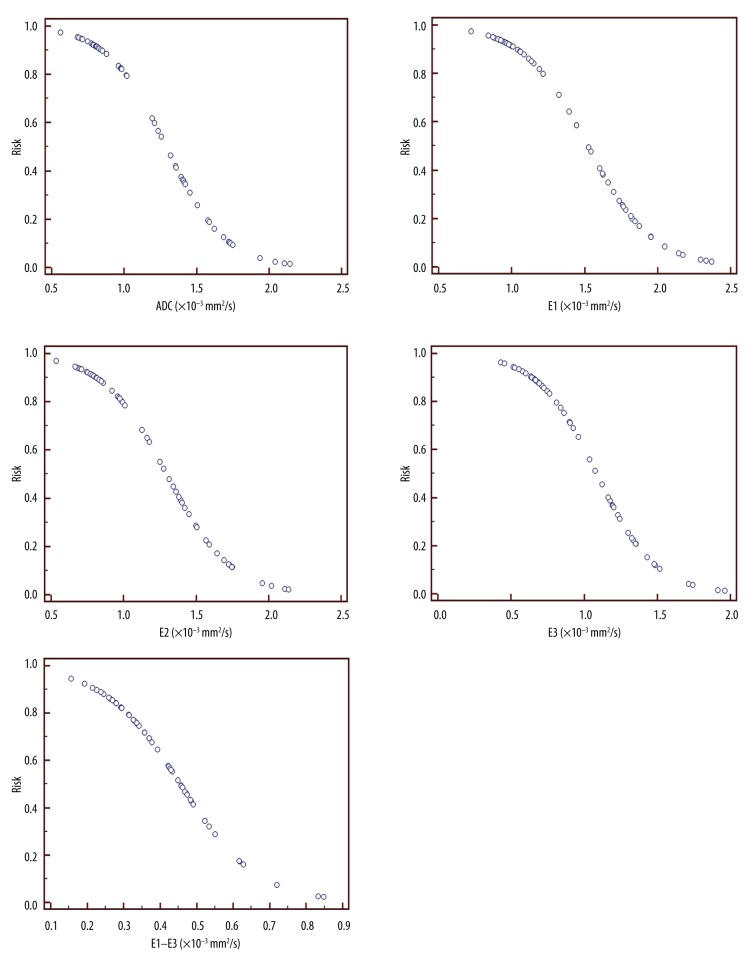
To confirm the value of every parameter in predicting the risk of breast cancer, the values of ADC, FA, E1, E2, E3, and E1–E3 of breast cancer and benign lesions were divided into equal quartiles, as shown in Table 4. ADC, E1, E2, E3, and E1–E3 were strongly associated with breast cancers. A mass with ADC, E1, E2, E3, or E1–E3 in the lowest quartiles was over 50–90 times more likely to be a malignancy, and the odds ratios were 95.0, 95.0, 67.67, 67.67, and 47.7, respectively. No benign lesions were found, but we did find breast cancers with ADC <0.811×10−3 mm2/s, E1 <0.977×10−3 mm2/s, E2 <0.800×10−3 mm2/s, or E3 <0.667×10−3 mm2/s. The discriminating values of FA were very low, with odds ratios around 1–2 (P>0.05). The coefficients, odds ratios, and the corresponding P valves of each DTI parameter and the combining model are shown in Table 5. The predicted risk of malignancy based on each DTI parameters is shown in Figure 4.
Table 4.
Risk assess of a mass in breast to be malignant according to DTI metrics.
| N | Benign | Malignant | Odd | Odds ratio | P-value | |
|---|---|---|---|---|---|---|
| ADC (×10−3 mm2/s) | ||||||
| 0.562–0.809 | 14 | 0 | 14 | 95 | <0.01 | |
| 0.811–0.982 | 14 | 2 | 12 | 6.0 | 22.0 | <0.01 |
| 1.016–1.421 | 14 | 9 | 5 | 0.6 | 2.0 | 0.41 |
| 1.453–2.144 | 14 | 11 | 3 | 0.3 | 1.0 | |
| FA | ||||||
| 0.079–0.148 | 14 | 7 | 7 | 1.0 | 1 | |
| 0.149–0.174 | 14 | 6 | 8 | 1.3 | 1.3 | 0.71 |
| 0.177–0.209 | 14 | 5 | 9 | 1.8 | 1.8 | 0.45 |
| 0.217–0.358 | 14 | 4 | 10 | 2.5 | 2.5 | 0.25 |
| E1 (×10−3 mm2/s) | ||||||
| 0.721–0.968 | 14 | 0 | 14 | 95 | <0.01 | |
| 0.977–1.189 | 14 | 2 | 12 | 6 | 22.0 | <0.01 |
| 1.217–1.758 | 14 | 9 | 5 | 0.6 | 2.0 | 0.41 |
| 1.765–2.369 | 14 | 11 | 3 | 0.3 | 1.0 | |
| E2(×10−3 mm2/s) | ||||||
| 0.535–0.789 | 14 | 0 | 14 | 67.67 | <0.01 | |
| 0.800–0.992 | 14 | 2 | 12 | 6 | 15 | <0.01 |
| 1.010–1.450 | 14 | 10 | 4 | 0.4 | 1.0 | 1.00 |
| 1.499–2.138 | 14 | 10 | 4 | 0.4 | 1.0 | |
| E3(×10−3 mm2/s) | ||||||
| 0.429–0.663 | 14 | 0 | 14 | 67.67 | <0.01 | |
| 0.667–0.862 | 14 | 2 | 12 | 6 | 15 | <0.01 |
| 0.900–1.230 | 14 | 10 | 4 | 0.4 | 1.0 | 1.00 |
| 1.243–1.962 | 14 | 10 | 4 | 0.4 | 1.0 | |
| E1–E3 (×10−3 mm2/s) | ||||||
| 0.156–0.294 | 14 | 1 | 13 | 13 | 47.7 | <0.01 |
| 0.295–0.369 | 14 | 4 | 10 | 2.5 | 9.2 | <0.05 |
| 0.377–0.471 | 14 | 6 | 8 | 1.3 | 4.9 | 0.06 |
| 0.474–0.848 | 14 | 11 | 3 | 0.3 | 1 | |
Table 5.
The discriminating values of DTI parameters in differentiating breast cancers and benign lesions.
| DTI parameters | Threshold | Sensitivity (%) | Specificity (%) | AUC | 95%CI (for AUC) | OR per 0.1×10−3 mm2/s decrease | P for OR |
|---|---|---|---|---|---|---|---|
| ADC (×10−3 mm2/s) | ≤1.017 | 82.4 | 90.9 | 0.897 | 0.786–0.962 | 1.64 | <0.001 |
| E1 (×10−3 mm2/s) | ≤1.393 | 85.3 | 90.9 | 0.898 | 0.788–0.963 | 1.57 | <0.001 |
| E2 (×10−3 mm2/s) | ≤1.127 | 82.4 | 90.9 | 0.885 | 0.772–0.955 | 1.57 | <0.001 |
| E3 (×10−3 mm2/s) | ≤0.762 | 73.5 | 100 | 0.892 | 0.780–0.959 | 1.65 | <0.001 |
| E1–E3 (×10−3 mm2/s) | ≤0.357 | 67.7 | 86.4 | 0.882 | 0.697–0.911 | 2.59 | <0.001 |
| FA | ≥0.189 | 44.1 | 77.3 | 0.607 | 0.467–0.735 | ||
| Regression model | >0.576 | 85.3 | 90.9 | 0.897 | 0.786–0.962 |
Figure 4.
The predicted probability of malignancy based on each DTI parameter. Because FA could not differentiate breast cancer from benign lesions, FA was not used in breast cancer risk prediction.
The ROC analysis results are shown in Table 5. ADC, E1, E2, E3, and E1–E3 produced AUCs of 0.897, 0.898, 0.885, 0.892, and 0.882, respectively, and FA only produced 0.607. The multivariate model combining (E1–E3) and FA produced an AUC of 0.897, similar to ADC (Figure 5). No statistically significant difference was found in AUC between ADC, E1, E2, E3, and the combined model (P>0.05), and the difference between FA and other parameters was statistically significant (P<0.01).
Figure 5.
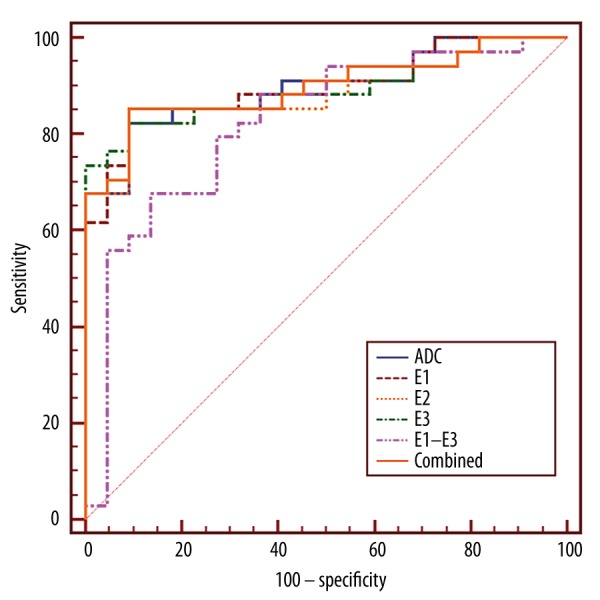
ROC curves of DTI parameters and the regression model showed that ADC, E1, E2, E3, and the multivariate model combining (E1–E3) and FA had almost the same AUCs (P>0.05), which were much larger than FA alone (P<0.01).
Discussion
DWI is now widely used in breast cancer detection and characterization. Developed from DWI, DTI not only provides traced weighted images and ADC maps, but also other parameters maps such as FA and eigenvalues, which can show the microstructures and arrangements of tissues. Studies have shown that ADC and FA values are useful tools in breast carcinoma detection from normal breast tissue, and that ADC is very useful in characterization and differentiation of malignant breast lesions from benign ones. ADC values calculated from DTI were shown to be highly consistent with those from DWI [15]. In our study, the eigenvalues E1, E2, and E3, the maximum anisotropy index E1–E3, and ADC and FA were included, and their potential values in breast cancers detection and differentiation were investigated and evaluated with a 1.5T MR scanner. Our results showed that ADC, E1, E2, E3, and E1–E3 were significantly lower in cancer lesions compared to normal breast tissue. FA in breast cancers was also decreased by a lesser degree than ADC, E1, and E1–E3. In breast cancer differentiation, ADC, E1, E2, and E3, as malignancy predictors, were highly associated with breast cancers risk, which had much more high-differentiation efficacy than other DTI parameters used in our study. The FA alone did not provide any useful information in distinguish malignant from benign breast lesions.
Several studies have demonstrated that ADC and FA are lower in breast cancers compared with normal breast tissue and benign lesions [16–18], and this was supported by our results. We also found that eigenvalue E1, E2, E3, and the maximum anisotropy index E1–E3 all were significantly lower in malignant lesions. To the best of our knowledge, no investigations have ever measured the decrease in those DTI metrics. In our study, we first quantitatively investigated the decreasing degree of these parameters, and found they decreased by about 24–37%.
Studies have examined the histologic changes of breast fibroglandular tissue in different phases of the menstrual cycle and found morphologic changes in epithelial and stromal components [19,20]. As a result, breast morphology and density influenced by hormonal level have demonstrated regular fluctuation during the menstrual cycle [21,22]. However, previous studies that examined these changes by using DTI showed that ADC and other anisotropy parameters, such as FA and E1, remained almost unchanged during the menstrual cycle and showed low mean within-subject variations, with coefficient of variance around 1–2% for ADC and E1, and around 5% for FA and E1–E3 [23]. Besides the influence of hormonal level, the heterogeneous fibroglandular (especially the collagen) fibers may also result in the reduction of signal and mimic breast carcinomas, which may in turn produce the variations in the breast. Reports have shown within-subject coefficient of variance is about 4.5–22% for ADC, 11.4% for FA, and 17.6% for E1 [17,24,25]. However, our results suggest that the decrease in these DTI parameters in breast cancer are far greater than the normal fluctuations in normal breast tissue, which means that the lower magnitude of these DTI parameters in breast cancer result from the histologic difference between cancer lesions and normal tissue rather than from normal fluctuations. Compared to normal breast glands, the cellularity density and microvessel density in breast cancer lesions increased significantly [26]. Although the relationship between DTI anisotropy indices and breast cellularity density and microvessel density has not been reported, studies of other organs, such as the brain, lung, and prostate, have displayed a negative correlation between cellularity, microvessel density, and anisotropy parameters such as ADC and FA values [27–30], which may to some extent explain the reduction of ADC and FA values in breast cancers. In the cancerous tissue, more disordered structure and increased necrotic material of gland cells may also play a role. The reduction in eigenvalues may also be due to do this, and further research on this topic is needed.
Our study found that the DTI parameters ADC, E1, E2, E3, and E1–E3 were reduced much more than FA in breast cancer compared to normal tissue. Changes in tumor cellular and microvessel density and the disorder of fibroglandular tissue may limit diffusion activity of water molecules, which, in turn, reduces E1, E2, and E3. Especially interesting in our results is that the degrees of reduction of E1, E2, and E3 in breast cancer were very close, with a mean of around 30–35%, which suggests that the 3 orthogonal eigenvectors of diffusion tensor ellipsoid unit was similarly scaled down. ADC, as the average of the 3 eigenvectors, was significantly reduced, while FA was less reduced. This can also be confirmed by the different situation in breast cancers versus benign lesions, in which E1, E2, and E3 were significantly different between caners and benign lesions, while FA values had no significant difference, and the ratios of E1, E2, and E3 between both were also very close, at around 0.61–0.64. This suggests that the proliferation of cells in fibroglandular tissue, as well as the structural arrangement in tumors and normal tissue, have a relatively slighter influence on FA, perhaps because the diffusion distance of water molecular and the disordered microstructure is much smaller than a voxel in scale and the impact is significantly reduced in FA due to the arithmetic averages.
While values of DTI indices in breast cancers varied widely in previous studies (0.7–1.6 mm2/s for ADC, 2.26–0.24 for FA), the mean ADC of breast cancers in our results (0.95±0.29 mm2/s) are comparable with previous studies, and the indices of E1, E2, E3, and E1–E3 in our study also agree with results in the literature [17]. The FA in our study was 0.19±0.06, which is lower than in previous reports [16,31]. No significant differences were found in anisotropy indices between DCIS and invasive breast cancers in our study, which were all significantly lower than in normal tissue and benign lesions. Partridge et al. [16] reported that while the differences in FA values between DICS and invasive breast cancers have no statistical significance, the ADC value of invasive breast cancers were significantly lower than DCIS, which is quite different from our results. The reasons for the difference in ADC values between the 2 studies may lie in the variations among individual lesions. The manifestation and diagnosis of DCIS remains controversial. DCIS has great variation in grade and cellularity, and some high-grade DCIS has relatively higher cellularity, which may affect the measured DTI parameters. The results of ADC and FA in our study for DICS and invasive cancer were all lower than the results of Partridge [16], which, from another perspective, may confirm the reason mentioned early.
Comparisons between benign and malignant tumors showed no significant difference in FA values between the 2 different type of lesions, while ADC, E1, E2, E3, and E1–E3, as predictors for breast cancer, were significantly lower in breast cancer than in benign lesions, which is consistent with the findings of Partridge [16] and Cakir [18], but opposite to the results of Tsougos [32]. Our results suggest that ADC, E1, E2, E3, and E1–E3 all help to distinguish breast cancers from benign lesions. The ROC curves show that ADC and E1 have the highest discriminative values, with a slightly larger AUC than E1–E3, but no significant difference was found among the AUC of those 5 parameters. Although the regression model combining FA and E1–E3 has similar diagnostic efficiency as ADC, the computational complexity of the model restricts its use in clinical settings. For the discrimination between malignant and benign lesions, the sensitivity and specificity of ADC was 82.4% and 90.9%, respectively, which are comparable to the results of Baltzer [31]. In addition, in our study the performance of E1 and E2 was very close to ADC in sensitivity and specificity.
In our study, the reduction of ADC, as well as E1, E2, E3, and E1–E3, was found to be very closely related to breast cancer risk. Our results show that the ADC values of benign lesions, as well as E1, E2, E3 and E1–E3, are mostly in the higher range, while the corresponding parameters for malignant lesions are mostly in the lower values. In fact, we did not observe a single malignant lesion when ADC <0.56 mm2/s or E1 <0.97 mm2/s or E2 <0.8 mm2/s or E3 <0.6 mm2/s, and only 1 lesion with E1–E3 less than 0.29 mm2/s. With the reduction of ADC, E1, E2, E3, and E1–E3 from the values that most benign lesions are in, the risk of breast cancer increases rapidly. If the ranges in which most benign lesions are concentrated are regarded as reference groups, when the ADC, E1, E2, or E3 is located in the lowest group (which strongly suggests breast cancer), the odds ratio reached about 97 or 67, which suggests that the risk of breast cancer was more than 90 or 70 times higher than in the reference group. Due to the impact of cyclical changes in hormone levels, the DTI indices of tissues may be affected, especially in the 2nd week, which means the detection and differentiation of breast cancers by using ADC, E1, E2, E3, and E1–E3 may be influenced. Fortunately, research shows that normal breast tissue has very slight fluctuations in ADC, E1, E2, E3, and E1–E3 values during the menstrual cycle, and no statistical significance was observed [23]. Therefore, the relatively stable mammary glands in DTI parameters provide a reliable indication that these DTI parameters have utility in breast lesions characterization.
There are some limitations in this study. First, to ensure the accuracy of measurement, the smaller lesions were excluded because the margin of small lesions cannot be accurately defined, which may have influenced the values of DTI indices. Therefore, the DTI measurements in this study are not representative of all tumor lesions. Fibroadenomas are relatively common in benign lesions; therefore, the DTI parameters in the benign group may reflect the characteristics of fibroadenomas to a relative larger degree, so further studies with expanded sample sizes are needed.
Conclusions
We investigated the diffusion tensor indices of breast cancers, and found that ADC, E1, E2, E3, and E1–E3 were significantly lower in cancer lesions compared to normal breast tissue and benign lesions. ADC, E1, E2, E3, and E1–E3 of a breast mass were highly associated with the risk of breast cancer in a reverse relationship, while the FA is useless in breast cancer risk prediction. Our results show the potential value of DTI indices in breast cancer. In previous studies, DWI and ADC were combined with contrast-enhanced MRI to detect and differentiate breast cancers, so further studies are also required to assess the added value of DTI indices in breast cancers as complementary tools to contrast-enhanced MRI.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest in this study.
Source of support: This study was supported by a grant from the Taishan Scholars Project no. 81371547 from the National Natural Science Foundation of China
References
- 1.Partridge SC, Demartini WB, Kurland BF, et al. Differential diagnosis of mammographically and clinically occult breast lesions on diffusion-weighted MRI. J Magn Reson Imaging. 2010;31(3):562–70. doi: 10.1002/jmri.22078. [DOI] [PubMed] [Google Scholar]
- 2.Ochi M, Kuroiwa T, Sunami S, et al. Diffusion-weighted imaging (b value = 1500 s/mm(2)) is useful to decrease false-positive breast cancer cases due to fibrocystic changes. Breast Cancer-Tokyo. 2013;20(2):137–44. doi: 10.1007/s12282-011-0319-9. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa MI, Ohsumi S, Sugata S, et al. Relation between cancer cellularity and apparent diffusion coefficient values using diffusion-weighted magnetic resonance imaging in breast cancer. Radiat Med. 2008;26(4):222–26. doi: 10.1007/s11604-007-0218-3. [DOI] [PubMed] [Google Scholar]
- 4.Park MJ, Cha ES, Kang BJ, et al. The role of diffusion-weighted imaging and the apparent diffusion coefficient (ADC) values for breast tumors. Korean J Radiol. 2007;8(5):390–96. doi: 10.3348/kjr.2007.8.5.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinker K, Bogner W, Baltzer P, et al. Improved diagnostic accuracy with multiparametric magnetic resonance imaging of the breast using dynamic contrast-enhanced magnetic resonance imaging, diffusion-weighted imaging, and 3-dimensional proton magnetic resonance spectroscopic imaging. Invest Radiol. 2014;49(6):421–30. doi: 10.1097/RLI.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 6.Parsian S, Rahbar H, Allison KH, et al. Nonmalignant breast lesions: ADCs of benign and high-risk subtypes assessed as false-positive at dynamic enhanced MR imaging. Radiology. 2012;265(3):696–706. doi: 10.1148/radiol.12112672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn SY, Ko EY, Han BK, et al. Role of diffusion-weighted imaging as an adjunct to contrast-enhanced breast MRI in evaluating residual breast cancer following neoadjuvant chemotherapy. Eur J Radiol. 2014;83(2):283–88. doi: 10.1016/j.ejrad.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Zhu W, Tao L, Qian Z, et al. [Study of the degree in white matter structural networks in the glioma based on diffusion tensor tractography]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2013;30(6):1309–14. [in Chinese] [PubMed] [Google Scholar]
- 9.Ma L, Song ZJ. Differentiation between low-grade and high-grade glioma using combined diffusion tensor imaging metrics. Clin Neurol Neurosurg. 2013;115(12):2489–95. doi: 10.1016/j.clineuro.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Inano R, Oishi N, Kunieda T, et al. Voxel-based clustered imaging by multiparameter diffusion tensor images for glioma grading. Neuroimage Clin. 2014;5:396–407. doi: 10.1016/j.nicl.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Xu Y, Zhang J, et al. Chronic kidney disease: pathological and functional assessment with diffusion tensor imaging at 3T MR. Eur Radiol. 2015;25(3):652–60. doi: 10.1007/s00330-014-3461-x. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Margolis DJ, Deng M, et al. Correlation of gleason scores with magnetic resonance diffusion tensor imaging in peripheral zone prostate cancer. J Magn Reson Imaging. 2015;42(2):460–67. doi: 10.1002/jmri.24813. [DOI] [PubMed] [Google Scholar]
- 13.Ries M, Jones RA, Basseau F, et al. Diffusion tensor MRI of the human kidney. J Magn Reson Imaging. 2001;14(1):42–49. doi: 10.1002/jmri.1149. [DOI] [PubMed] [Google Scholar]
- 14.Park SY, Kim CK, Park BK, et al. Diffusion-tensor MRI at 3 T: differentiation of central gland prostate cancer from benign prostatic hyperplasia. Am J Roentgenol. 2014;202(3):W254–62. doi: 10.2214/AJR.13.11015. [DOI] [PubMed] [Google Scholar]
- 15.Tagliafico A, Rescinito G, Monetti F, et al. Diffusion tensor magnetic resonance imaging of the normal breast: reproducibility of DTI-derived fractional anisotropy and apparent diffusion coefficient at 3.0 T. Radiol Med. 2012;117(6):992–1003. doi: 10.1007/s11547-012-0831-9. [DOI] [PubMed] [Google Scholar]
- 16.Partridge SC, Ziadloo A, Murthy R, et al. Diffusion tensor MRI: preliminary anisotropy measures and mapping of breast tumors. J Magn Reson Imaging. 2010;31(2):339–47. doi: 10.1002/jmri.22045. [DOI] [PubMed] [Google Scholar]
- 17.Eyal E, Shapiro-Feinberg M, Furman-Haran E, et al. Parametric diffusion tensor imaging of the breast. Invest Radiol. 2012;47(5):284–91. doi: 10.1097/RLI.0b013e3182438e5d. [DOI] [PubMed] [Google Scholar]
- 18.Cakir O, Arslan A, Inan N, et al. Comparison of the diagnostic performances of diffusion parameters in diffusion weighted imaging and diffusion tensor imaging of breast lesions. Eur J Radiol. 2013;82(12):e801–6. doi: 10.1016/j.ejrad.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Longacre TA, Bartow SA. A correlative morphologic study of human breast and endometrium in the menstrual cycle. Am J Surg Pathol. 1986;10(6):382–93. doi: 10.1097/00000478-198606000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Vogel PM, Georgiade NG, Fetter BF, et al. The correlation of histologic changes in the human breast with the menstrual cycle. Am J Pathol. 1981;104(1):23–34. [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow M, Chatterton RJ, Rademaker AW, et al. A prospective study of variability in mammographic density during the menstrual cycle. Breast Cancer Res Treat. 2010;121(3):565–74. doi: 10.1007/s10549-009-0496-9. [DOI] [PubMed] [Google Scholar]
- 22.Hovhannisyan G, Chow L, Schlosser A, et al. Differences in measured mammographic density in the menstrual cycle. Cancer Epidemiol Biomarkers Prev. 2009;18(7):1993–99. doi: 10.1158/1055-9965.EPI-09-0074. [DOI] [PubMed] [Google Scholar]
- 23.Nissan N, Furman-Haran E, Shapiro-Feinberg M, et al. Diffusion-tensor MR imaging of the breast: hormonal regulation. Radiology. 2014;271(3):672–80. doi: 10.1148/radiol.14132084. [DOI] [PubMed] [Google Scholar]
- 24.Partridge SC, Murthy RS, Ziadloo A, et al. Diffusion tensor magnetic resonance imaging of the normal breast. Magn Reson Imaging. 2010;28(3):320–28. doi: 10.1016/j.mri.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 25.O’Flynn EA, Morgan VA, Giles SL, et al. Diffusion weighted imaging of the normal breast: reproducibility of apparent diffusion coefficient measurements and variation with menstrual cycle and menopausal status. Eur Radiol. 2012;22(7):1512–18. doi: 10.1007/s00330-012-2399-0. [DOI] [PubMed] [Google Scholar]
- 26.Li JY, Zhang Y, Zhang WH, et al. Effects of differential distribution of microvessel density, possibly regulated by miR-374a, on breast cancer prognosis. Asian Pac J Cancer Prev. 2013;14(3):1715–20. doi: 10.7314/apjcp.2013.14.3.1715. [DOI] [PubMed] [Google Scholar]
- 27.Chen L, Zhang J, Chen Y, et al. Relationship between apparent diffusion coefficient and tumour cellularity in lung cancer. PLoS One. 2014;9(6):e99865. doi: 10.1371/journal.pone.0099865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Liu M, Bao J, et al. The correlation between apparent diffusion coefficient and tumor cellularity in patients: A meta-analysis. PLoS One. 2013;8(11):e79008. doi: 10.1371/journal.pone.0079008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doskaliyev A, Yamasaki F, Ohtaki M, et al. Lymphomas and glioblastomas: differences in the apparent diffusion coefficient evaluated with high b-value diffusion-weighted magnetic resonance imaging at 3T. Eur J Radiol. 2012;81(2):339–44. doi: 10.1016/j.ejrad.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Gibbs P, Liney GP, Pickles MD, et al. Correlation of ADC and T2 measurements with cell density in prostate cancer at 3.0 Tesla. Invest Radiol. 2009;44(9):572–76. doi: 10.1097/RLI.0b013e3181b4c10e. [DOI] [PubMed] [Google Scholar]
- 31.Baltzer PA, Schafer A, Dietzel M, et al. Diffusion tensor magnetic resonance imaging of the breast: a pilot study. Eur Radiol. 2011;21(1):1–10. doi: 10.1007/s00330-010-1901-9. [DOI] [PubMed] [Google Scholar]
- 32.Tsougos I, Svolos P, Kousi E, et al. The contribution of diffusion tensor imaging and magnetic resonance spectroscopy for the differentiation of breast lesions at 3T. Acta Radiol. 2014;55(1):14–23. doi: 10.1177/0284185113492152. [DOI] [PubMed] [Google Scholar]