Abstract
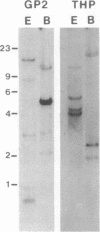
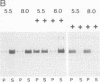
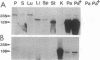
A family of homologous genes is shown to encode GP-2, the major glycosylphosphatidylinositol (GPI)-linked glycoprotein of pancreatic zymogen granule membranes, and Tamm-Horsfall protein (THP), a GPI-linked glycoprotein associated with apical vesicles in kidney thick ascending limb of Henle (TALH) cells. The C-terminal regions of GP-2 (Asp54-Phe530) and THP (Asp175-His644) from rat show 53% identity, 86% similarity, and 26 conserved cysteine residues including one epidermal growth factor motif. The unique N-terminal domain of rat THP (unique-THP, Pro29-Gln174) shows four conserved epidermal growth factor motifs, three in tandem and one in reverse orientation. GP-2 homologues are observed in a wide variety of epithelial cells, several of which contain highly regulated secretory processes. GP-2 released from zymogen granule membranes with phosphatidylinositol phospholipase C reacts with anti-cross-reactive determinant antibody (anti-CRD), confirming the GPI nature of the pancreatic homologue. In contrast, GP-2 and THP, released endogenously from pancreas and kidney, respectively, do not react with anti-cross-reactive determinant antibody, suggesting alternative enzymatic mechanisms for their physiological release. Globular domains of GP-2 and THP, but not albumin, show pH- and ion-dependent self-association in vitro. The GP-2/THP family appears to represent a newly discovered class of GPI-anchored proteins, which may utilize pH- and ion-dependent self-association mechanisms for establishing membrane (micro)domains targeted to intracellular secretory compartments.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argraves W. S., Suzuki S., Arai H., Thompson K., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1183–1190. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann S., Koeppen-Hagemann I., Kriz W. Ultrastructural localization of Tamm-Horsfall glycoprotein (THP) in rat kidney as revealed by protein A-gold immunocytochemistry. Histochemistry. 1985;83(6):531–538. doi: 10.1007/BF00492456. [DOI] [PubMed] [Google Scholar]
- Baron M., Norman D. G., Campbell I. D. Protein modules. Trends Biochem Sci. 1991 Jan;16(1):13–17. doi: 10.1016/0968-0004(91)90009-k. [DOI] [PubMed] [Google Scholar]
- Beaudoin A. R., St-Jean P., Grondin G. Ultrastructural localization of GP2 in acinar cells of pancreas: presence of GP2 in endocytic and exocytic compartments. J Histochem Cytochem. 1991 May;39(5):575–588. doi: 10.1177/39.5.2016510. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Stengelin S., Gimbrone M. A., Jr, Seed B. Endothelial leukocyte adhesion molecule 1: an inducible receptor for neutrophils related to complement regulatory proteins and lectins. Science. 1989 Mar 3;243(4895):1160–1165. doi: 10.1126/science.2466335. [DOI] [PubMed] [Google Scholar]
- Bjugn R., Flood P. R. Scanning electron microscopy of human urine and purified Tamm-Horsfall's glycoprotein. Scand J Urol Nephrol. 1988;22(4):313–315. doi: 10.3109/00365598809180806. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clemente F., Meldolesi J. Calcium and pancreatic secretion. I. Subcellular distribution of calcium and magnesium in the exocrine pancreas of the guinea pig. J Cell Biol. 1975 Apr;65(1):88–102. doi: 10.1083/jcb.65.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., MacGillivray R. T. Characterization of the human blood coagulation factor XII gene. Intron/exon gene organization and analysis of the 5'-flanking region. J Biol Chem. 1987 Oct 5;262(28):13662–13673. [PubMed] [Google Scholar]
- Cross G. A. Glycolipid anchoring of plasma membrane proteins. Annu Rev Cell Biol. 1990;6:1–39. doi: 10.1146/annurev.cb.06.110190.000245. [DOI] [PubMed] [Google Scholar]
- Dulawa J., Jann K., Thomsen M., Rambausek M., Ritz E. Tamm Horsfall glycoprotein interferes with bacterial adherence to human kidney cells. Eur J Clin Invest. 1988 Feb;18(1):87–91. doi: 10.1111/j.1365-2362.1988.tb01171.x. [DOI] [PubMed] [Google Scholar]
- Fukuoka S., Freedman S. D., Scheele G. A. A single gene encodes membrane-bound and free forms of GP-2, the major glycoprotein in pancreatic secretory (zymogen) granule membranes. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2898–2902. doi: 10.1073/pnas.88.7.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka S., Scheele G. Nucleotide sequence encoding the major glycoprotein (GP2) of rat pancreatic secretory (zymogen) granule membranes. Nucleic Acids Res. 1990 Oct 11;18(19):5900–5900. doi: 10.1093/nar/18.19.5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes H. H., Rosa P., Phillips E., Baeuerle P. A., Frank R., Argos P., Huttner W. B. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989 Jul 15;264(20):12009–12015. [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., van der Ley P. A., Scheffer R. C. Use of colloidal gold particles in double-labeling immunoelectron microscopy of ultrathin frozen tissue sections. J Cell Biol. 1981 Jun;89(3):653–665. doi: 10.1083/jcb.89.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. Properties and diversity of (Na-K-Cl) cotransporters. Annu Rev Physiol. 1989;51:443–457. doi: 10.1146/annurev.ph.51.030189.002303. [DOI] [PubMed] [Google Scholar]
- Handford P. A., Mayhew M., Baron M., Winship P. R., Campbell I. D., Brownlee G. G. Key residues involved in calcium-binding motifs in EGF-like domains. Nature. 1991 May 9;351(6322):164–167. doi: 10.1038/351164a0. [DOI] [PubMed] [Google Scholar]
- Havinga J. R., Strous G. J., Poort C. Intracellular transport of the major glycoprotein of zymogen granule membranes in the rat pancreas. Demonstration of high turnover at the plasma membrane. Eur J Biochem. 1984 Oct 1;144(1):177–183. doi: 10.1111/j.1432-1033.1984.tb08446.x. [DOI] [PubMed] [Google Scholar]
- Hession C., Decker J. M., Sherblom A. P., Kumar S., Yue C. C., Mattaliano R. J., Tizard R., Kawashima E., Schmeissner U., Heletky S. Uromodulin (Tamm-Horsfall glycoprotein): a renal ligand for lymphokines. Science. 1987 Sep 18;237(4821):1479–1484. doi: 10.1126/science.3498215. [DOI] [PubMed] [Google Scholar]
- Hoops T. C., Rindler M. J. Isolation of the cDNA encoding glycoprotein-2 (GP-2), the major zymogen granule membrane protein. Homology to uromodulin/Tamm-Horsfall protein. J Biol Chem. 1991 Mar 5;266(7):4257–4263. [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- LeBel D., Beattie M. The major protein of pancreatic zymogen granule membranes (GP-2) is anchored via covalent bonds to phosphatidylinositol. Biochem Biophys Res Commun. 1988 Jul 29;154(2):818–823. doi: 10.1016/0006-291x(88)90213-6. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Schwartz R. H., Schenk E. A. Tamm-Horsfall mucoprotein. II. Ontogenetic development. Lab Invest. 1972 Jun;26(6):728–730. [PubMed] [Google Scholar]
- Lisanti M. P., Caras I. W., Davitz M. A., Rodriguez-Boulan E. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989 Nov;109(5):2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Darnell J. C., Chan B. L., Rodriguez-Boulan E., Saltiel A. R. The distribution of glycosyl-phosphatidylinositol anchored proteins is differentially regulated by serum and insulin. Biochem Biophys Res Commun. 1989 Oct 31;164(2):824–832. doi: 10.1016/0006-291x(89)91533-7. [DOI] [PubMed] [Google Scholar]
- Low M. G. Glycosyl-phosphatidylinositol: a versatile anchor for cell surface proteins. FASEB J. 1989 Mar;3(5):1600–1608. doi: 10.1096/fasebj.3.5.2522071. [DOI] [PubMed] [Google Scholar]
- Micanovic R., Gerber L. D., Berger J., Kodukula K., Udenfriend S. Selectivity of the cleavage/attachment site of phosphatidylinositol-glycan-anchored membrane proteins determined by site-specific mutagenesis at Asp-484 of placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1990 Jan;87(1):157–161. doi: 10.1073/pnas.87.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Yoon K., Rodan G. A., Koppel D. E. High lateral mobility of endogenous and transfected alkaline phosphatase: a phosphatidylinositol-anchored membrane protein. J Cell Biol. 1987 Oct;105(4):1671–1677. doi: 10.1083/jcb.105.4.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Anderson R. G. The condensing vacuole of exocrine cells is more acidic than the mature secretory vesicle. Nature. 1987 Mar 5;326(6108):77–79. doi: 10.1038/326077a0. [DOI] [PubMed] [Google Scholar]
- Pennica D., Kohr W. J., Kuang W. J., Glaister D., Aggarwal B. B., Chen E. Y., Goeddel D. V. Identification of human uromodulin as the Tamm-Horsfall urinary glycoprotein. Science. 1987 Apr 3;236(4797):83–88. doi: 10.1126/science.3453112. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Hoops T. C. The pancreatic membrane protein GP-2 localizes specifically to secretory granules and is shed into the pancreatic juice as a protein aggregate. Eur J Cell Biol. 1990 Oct;53(1):154–163. [PubMed] [Google Scholar]
- Rindler M. J., Naik S. S., Li N., Hoops T. C., Peraldi M. N. Uromodulin (Tamm-Horsfall glycoprotein/uromucoid) is a phosphatidylinositol-linked membrane protein. J Biol Chem. 1990 Dec 5;265(34):20784–20789. [PubMed] [Google Scholar]
- Santoso A. W., Scott D. M., Kinne R. Localization of Tamm-Horsfall protein in chloride transporting epithelia: lack of correlation with the Na, K, Cl cotransporter. Eur J Cell Biol. 1987 Feb;43(1):104–109. [PubMed] [Google Scholar]
- Sarkar P., Sengupta D., Basu S., Maitra U. Nucleotide sequence of a major class-III phage-T3 RNA-polymerase promoter located at 98.0% of phage-T3 genetic map. Gene. 1985;33(3):351–355. doi: 10.1016/0378-1119(85)90243-4. [DOI] [PubMed] [Google Scholar]
- Scheffer R. C., Poort C., Slot J. W. Fate of the major zymogen granule membrane-associated glycoproteins from rat pancreas. A biochemical and immunocytochemical study. Eur J Cell Biol. 1980 Dec;23(1):122–128. [PubMed] [Google Scholar]
- Sikri K. L., Foster C. L., Bloomfield F. J., Marshall R. D. Localization by immunofluorescence and by light- and electron-microscopic immunoperoxidase techniques of Tamm-Horsfall glycoprotein in adult hamster kidney. Biochem J. 1979 Sep 1;181(3):525–532. doi: 10.1042/bj1810525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikri K. L., Foster C. L., Marshall R. D. Localization of Tamm-Horsfall-glycoprotein-like immunoreactivity in cultured baby-hamster kidney cells, shown by immunofluorescence and by light- and electron-microscopic immunoperoxidase techniques. Biochem J. 1985 Jan 15;225(2):481–486. doi: 10.1042/bj2250481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue R. J., Bahadur G., Zambon M. C., Hall-Smith M., Douglas A. R., Hay A. J. Specific structural alteration of the influenza haemagglutinin by amantadine. EMBO J. 1990 Nov;9(11):3469–3476. doi: 10.1002/j.1460-2075.1990.tb07555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr Characterization and separation of an inhibitor of viral hemagglutination present in urine. Proc Soc Exp Biol Med. 1950 May;74(1):106–108. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartakoff A. M. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983 Apr;32(4):1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Worcester E. M., Nakagawa Y., Wabner C. L., Kumar S., Coe F. L. Crystal adsorption and growth slowing by nephrocalcin, albumin, and Tamm-Horsfall protein. Am J Physiol. 1988 Dec;255(6 Pt 2):F1197–F1205. doi: 10.1152/ajprenal.1988.255.6.F1197. [DOI] [PubMed] [Google Scholar]
- Yochem J., Greenwald I. glp-1 and lin-12, genes implicated in distinct cell-cell interactions in C. elegans, encode similar transmembrane proteins. Cell. 1989 Aug 11;58(3):553–563. doi: 10.1016/0092-8674(89)90436-4. [DOI] [PubMed] [Google Scholar]
- Yoo S. H., Albanesi J. P. Ca2(+)-induced conformational change and aggregation of chromogranin A. J Biol Chem. 1990 Aug 25;265(24):14414–14421. [PubMed] [Google Scholar]
- Zimmermann D. R., Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989 Oct;8(10):2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G., Simons K. Lipid polarity and sorting in epithelial cells. J Cell Biochem. 1988 Jan;36(1):51–58. doi: 10.1002/jcb.240360106. [DOI] [PubMed] [Google Scholar]