Abstract
Background
Acute exacerbations are major drivers of COPD deterioration. However, limited data are available for the prevalence of severe exacerbations and impact of asthma on severe exacerbations, especially in patients with mild-to-moderate COPD.
Methods
Patients with mild-to-moderate COPD (≥40 years) were extracted from Korean National Health and Nutrition Examination Survey data (2007–2012) and were linked to the national health insurance reimbursement database to obtain medical service utilization records.
Results
Of the 2,397 patients with mild-to-moderate COPD, 111 (4.6%) had severe exacerbations over the 6 years (0.012/person-year). Severe exacerbations were more frequent in the COPD patients with concomitant self-reported physician-diagnosed asthma compared with only COPD patients (P<0.001). A multiple logistic regression presented that asthma was an independent risk factor of severe exacerbations in patients with mild-to-moderate COPD regardless of adjustment for all possible confounding factors (adjusted odds ratio, 1.67; 95% confidence interval, 1.002–2.77, P=0.049). In addition, age, female, poor lung function, use of inhalers, and low EuroQoL five dimensions questionnaire index values were independently associated with severe exacerbation in patients with mild-to-moderate COPD.
Conclusion
In this population-based study, the prevalence of severe exacerbations in patients with mild-to-moderate COPD was relatively low, compared with previous clinical interventional studies. Coexisting asthma significantly impacted the frequency of severe exacerbations in patients with mild-to-moderate COPD, suggesting application of an exacerbation preventive strategy in these patients.
Keywords: bronchial asthma, chronic obstructive pulmonary disease, acute exacerbation
Introduction
Acute exacerbations of COPD are associated with a decline in lung function,1,2 reduced health-related quality of life (QoL),3,4 and high morbidity and mortality.5–9 Moreover, ~50%–75% of health care service costs for COPD are attributed to acute exacerbations, particularly severe episodes requiring hospitalization.10 To date, vigorous efforts have been made to evaluate the risk factors for exacerbation of COPD, and the important determinants of frequent exacerbations, such as history of exacerbation,11,12 severe airflow limitation,11,12 or chronic bronchitis,13 were found in previous studies.
Bronchial asthma has received attention as a risk factor for frequent exacerbation of COPD. A primary care population study showed that comorbidities including bronchial asthma are independent factors for moderate-to-severe exacerbations over 1 year in patients with COPD.14 Furthermore, asthma-COPD overlap syndrome (ACOS) is related to increased frequency and severity of exacerbations and hospitalizations.15,16 Given that decline of lung function is faster in mild-to-moderate COPD patients than in patients with severe or very severe COPD17 and exacerbations accelerate the deterioration in lung function,1,2 it is necessary to explore whether bronchial asthma is associated with severe exacerbations in COPD patients with mild-to-moderate airflow limitations. However, as patients with mild-to-moderate COPD have lower rates of hospital use and relatively infrequent severe exacerbations compared with those with severe COPD, data from clinical interventional studies are limited.
A moderate exacerbation is defined when a patient with an exacerbation is treated with specific antibiotics or oral corticosteroids.18 COPD patients often receive prescription of both antibiotics and prednisone for COPD exacerbation action plan in the clinics and are educated to increase the frequency of inhaler use or to initiate these medications on worsening symptoms including dyspnea, sputum color change, or sputum volume increase. Thus, the number of moderate exacerbations based on prescription records and event coding of National Health Insurance (NHI) data is probably inaccurate due to the possibility of steroid or antibiotic prescriptions for COPD exacerbation action plans or the possibility of an unrecorded exacerbation event. A severe exacerbation is defined as an exacerbation requiring hospital admission or a visit to the emergency department.18 As all diagnostic and medication records during a hospital admission or emergency department visit are sent to NHI for the reimbursement, the number of severe exacerbation based on NHI data is more reliable. Thus, our study concerns severe exacerbations as an outcome.
The purposes of this study were to investigate the frequency of severe exacerbations in COPD patients having mild-to-moderate airflow limitations and to evaluate whether asthma is associated with severe exacerbations in patients with mild-to-moderate COPD using both the Korean National Health and Nutrition Examination Survey (KNHANES) and the NHI reimbursement databases during 2007–2012 in Korea.
Methods
Study design
The KNHANES (2007–2012) is a cross-sectional observational study conducted using complex and stratified multistage cluster sampling to select a representative nationwide sample of the noninstitutionalized Korean population. In addition, South Korea has a compulsory universal health insurance system that includes medical reimbursement records for the entire Korean population. The NHI reimbursement database provides a unique and advantageous mechanism for assessing the nationwide magnitude of an illness and consequent health care use. Two databases were linked, thus complementary information can be obtained through both databases.
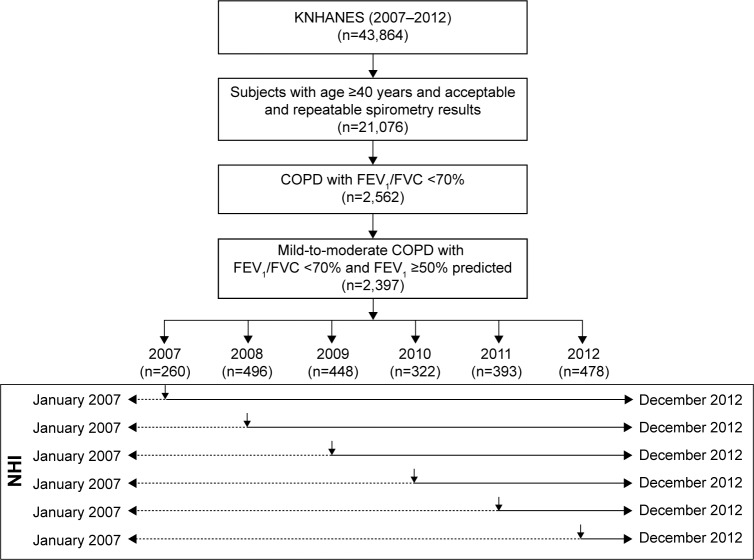
We constructed a patient cohort from KNHANES using data obtained from January 2007 to December 2012 who were aged ≥40 years and were diagnosed with mild-to-moderate COPD by spirometry (prebronchodilator ratio of forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) of less than 0.70 and FEV1 of more than 50% of the predicted value). Absolute values of FEV1 and FVC were obtained and percentage of the predicted values (% predicted) for FEV1 and FVC was calculated using the reference equation obtained based on analysis of a representative Korean sample.19 This cohort was linked to the NHI data to analyze their health care service utilization from January 2007 to December 2012 (Figure 1). Since the annual rate of decline in FEV1 is reduced especially in Asian patients,20 we assumed that the change of lung function would be minimal for 6 years. Thus, for the COPD patients enrolled to the KNHANES in 2007, the outcome of severe exacerbation was prospectively assessed until 2012 for 6 years, and for those from the KNHANES in 2012, the outcome of severe exacerbation was retrospectively assessed to 2007 for 6 years.
Figure 1.
Study flow chart and outcome assessment flow using KNHANES and NHI reimbursement database.
Note: Vertical arrows in the rectangle box indicate the year of enrollment from KNHANES. Dashed arrows in the rectangle box represent the retrospective assessment of NHI data and solid arrows in the rectangle box represent the prospective assessment of NHI data.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; KNHANES, Korean National Health and Nutrition Examination Survey; NHI, National Health Insurance.
Emergency department visits or hospitalizations requiring treatment with systemic corticosteroids and/or antibiotics were considered a severe exacerbation when the principal or secondary diagnosis was COPD (J42.x–J44.x [unspecified chronic bronchitis: J42.x, emphysema: J43.x, other COPD: J44.x], except unilateral pulmonary emphysema: J430) or a COPD-related disease (pneumonia: J12.x–J17.x, pulmonary thromboembolism: I26, I26.0, and I26.9, dyspnea: R06.0, or acute respiratory distress syndrome: J80) as from our previous studies.21–24 Medical utilization were excluded for analysis if they were not considered to be COPD-related.
We obtained information such as age, sex, body mass index (BMI), smoking history, physical activity, the EuroQoL five dimensions questionnaire (EQ-5D) index values, which range between 0 (worst imaginable health state) and 1 (best imaginable health state), and pulmonary function test results from the KNHANES database. The prevalence of self-reported physician-diagnosed diseases – ischemic heart disease, depressive disorder, diabetes mellitus (DM), hypertension, atopic dermatitis, stroke, bronchial asthma, and previous pulmonary tuberculosis – were also obtained from questionnaire conducted by the KNHANES. Hospitalizations, emergency department visits, intensive care unit admissions, and medication service use were obtained from the NIH database. We also contained the use of inhalers (long-acting beta2-agonist, long-acting muscarinic antagonist, or inhaled corticosteroid/long-acting beta2-agonist), which had been prescribed before the patients experienced severe exacerbations.
This study was approved by The National Evidence-based Healthcare Collaborating Agency Ethics Committee of Korea. The requirement for informed consent from the patients was waived due to the retrospective nature of this study by the ethics review board.
Statistical analysis
Data are presented as mean and standard deviation for continuous variables and as numbers (percentages) for categorical variables. The data were compared using Student’s t-test for continuous and Pearson’s χ2 test for categorical variables. To analyze an impact of FEV1 and EQ-5D index values on the severe exacerbations in mild-to-moderate COPD patients, we classified the patients into three groups based on FEV1 (those with FEV1% predicted ≥80%, those with 65%≤ FEV1% predicted <80%, and those with 50%≤ FEV1% predicted <65%) and two groups based on EQ-5D index values (those with EQ-5D index values <0.9 and those with EQ-5D index values ≥0.9) and analyzed these factors as categorical variables.
A multiple logistic regression model was used to adjust for potential confounding factors in severe exacerbations in COPD patients. Four models were constructed: Model 1 was adjusted for age, sex, and BMI; Model 2 was additionally adjusted for pulmonary-related variables generally considered to be important in severe exacerbations in COPD (smoking history and severity of airflow limitation); Model 3 additionally included extrapulmonary-related variables generally considered to be important in severe exacerbations in COPD or extrapulmonary-related variables with P<0.05 in the univariate analyses with considering multicollinearity (DM, cardiovascular disease [stroke, myocardial infarction, and angina pectoris], and QoL); finally, Model 4 was additionally adjusted for use of inhalers with all of the above-mentioned variables. All tests were two-sided, and P<0.05 was considered to indicate significance. All statistical analyses were performed using SAS ver. 9.2 (SAS Institute, Cary, NC, USA).
Results
Clinical characteristics of patients
A total of 2,397 patients with mild-to-moderate COPD were identified from the KNHANES databases between 2007 and 2012 (Figure 1). As shown in Table 1, there were 1,692 males (70.6%) and 705 females (29.4%) with a mean age of 63.5 years. Mean BMI was 23.5±2.9 kg/m2 and 1,621 patients (67.6%) were current or ex-smokers. The common comorbidities were hypertension (n=847, 35.3%), followed by DM (n=331, 13.8%), depressive disorder (n=296, 12.3%), and bronchial asthma (n=261, 10.9%). The mean FEV1/FVC and FEV1 were 60% and 2.3 L (78.6% predicted), respectively. Whereas 44.8% (1,075/2,397) of the patients had mild airflow limitations (FEV1 ≥80% predicted), 55.2% (1,322/2,397) had moderate airflow limitations (50%≤ FEV1% predicted <80%). The mean EQ-5D index values were 0.9. Only 5.9% of mild-to-moderate COPD patients used inhalers such as long-acting muscarinic antagonist (3.4%, n=82) and inhaled corticosteroid/long-acting beta2-agonist (4.1%, n=98) before severe exacerbations.
Table 1.
Baseline characteristics (n=2,397)
| N (%) or mean ± SD | |
|---|---|
| Age, years | 63.5±11.9 |
| Sex, male | 1,692 (70.6) |
| Body mass index, kg/m2 | 23.5±2.9 |
| Body mass index ≥25 kg/m2 | 689 (28.7) |
| Smoking historya | |
| Nonsmoker | 747 (31.2) |
| Current or ex-smoker | 1,621 (67.6) |
| Pack-years | 23.9±23.3 |
| Previous pulmonary tuberculosis | 323 (13.5) |
| Comorbidities | |
| Hypertension | 847 (35.3) |
| Diabetes mellitus | 331 (13.8) |
| Depression | 296 (12.3) |
| Bronchial asthma | 261 (10.9) |
| Coronary heart diseaseb | 96 (4.0) |
| Atopic dermatitis | 69 (2.9) |
| Stroke | 66 (2.8) |
| Physical activity | |
| Days of moderate active physical activity per week | 2.3±2.1 |
| Days of high active physical activity per week | 1.8±1.7 |
| Baseline pulmonary function test | |
| FVC, L | 3.6±0.9 |
| FVC, % predicted | 90.9±13.6 |
| FEV1, L | 2.3±0.6 |
| FEV1, % predicted | 78.6±13.7 |
| FEV1/FVC | 0.6±0.07 |
| COPD severity | |
| FEV1% predicted ≥80% | 1,075 (44.8) |
| 65%≤ FEV1% predicted <80% | 924 (38.6) |
| 50%≤ FEV1% predicted <65% | 398 (16.6) |
| EQ-5D index values | 0.9±0.1 |
| EQ-5D index values ≥0.9 | 1,611 (67.2) |
| Use of inhalers | 141 (5.9) |
| LAMA | 82 (3.4) |
| LABA | 0 (0) |
| ICS/LABA | 98 (4.1) |
Notes: The data are presented as number (%) or mean and SD.
Data of 29 patients are missed.
Coronary heart disease includes myocardial infarction and angina pectoris.
Abbreviations: EQ-5D, The EuroQoL five dimensions questionnaire; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; SD, standard deviation.
Regarding COPD-related medical utilization, overall 18.6% had history of medical service use over the duration of the study period.
Comparison of clinical characteristics between patients with and without severe exacerbations
As shown in Figure 2, 95.4% of patients (n=2,286) had no history of a severe COPD exacerbation, whereas 4.6% of patients (n=111) experienced one or more severe exacerbations during the 6-year study period. The rate of severe exacerbations during the 6-year follow-up period was 0.012/person-year.
Figure 2.
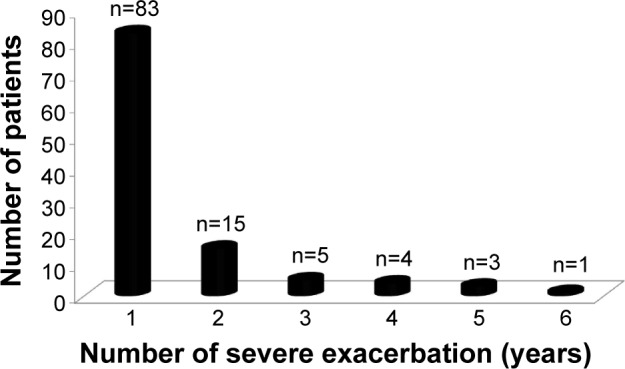
Number of severe exacerbations in overall population over 6-year follow-up.
Compared with patients without severe exacerbations during the study period, patients who suffered a severe exacerbation were more likely to be older (69.5 vs 63.2 years, P<0.001), female (46.8% vs 28.6%, P<0.001), nonsmokers (43.2% vs 30.6%, P=0.005), and more likely to have fewer days of high active physical activity per week (1.5 vs 1.8 days, P=0.008) and fewer proportions of patients with EQ-5D index values ≥0.9 (29.7% vs 69.0%, P<0.001). In addition, patients with severe exacerbations showed poorer lung function (mean FEV1, % predicted: 74.2% predicted vs 78.8% predicted, P<0.001) and used inhalers more (36.9% vs 4.4%, P<0.001) than those without severe exacerbations. Comorbidities including DM (20.7% vs 13.5%, P=0.031) and bronchial asthma (25.2% vs 10.2%, P<0.001) were more frequently observed in patients with severe exacerbations than in those without severe exacerbations during the 6-year study period. No differences were observed in BMI, smoking history (pack-years), comorbidities including hypertension, depression, coronary heart disease, atopic dermatitis, stroke, and previous pulmonary tuberculosis, and days of moderate physical activity per week between patients with and without severe exacerbations during the study period (Table 2).
Table 2.
Comparison of characteristics between patients with and without severe exacerbations
| Patients without severe exacerbations (n=2,286, 95.4%) |
Patients with severe exacerbations (n=111, 4.6%) |
P-value | |
|---|---|---|---|
| Age, yearsa | 63.2±11.9 | 69.5±8.2 | <0.001 |
| Sex, male | 1,633 (71.4) | 59 (53.2) | <0.001 |
| Body mass index, kg/m2a | 23.5±2.8 | 23.5±2.9 | 0.821 |
| Body mass index ≥25 kg/m2 | 659 (28.8) | 30 (27.0) | 0.682 |
| Smoking historyb | |||
| Nonsmoker | 699 (30.6) | 48 (43.2) | 0.005 |
| Current or ex-smoker | 1,559 (68.2) | 62 (55.9) | |
| Pack-yearsa | 24.0±23.0 | 20.9±28.9 | 0.310 |
| Previous pulmonary tuberculosis | 304 (13.3) | 19 (17.1) | 0.250 |
| Comorbidities | |||
| Diabetes mellitus | 308 (13.5) | 23 (20.7) | 0.031 |
| Hypertension | 801 (35.0) | 46 (41.4) | 0.168 |
| Depression | 276 (12.1) | 20 (18.0) | 0.063 |
| Bronchial asthma | 233 (10.2) | 28 (25.2) | <0.001 |
| Coronary heart diseasec | 92 (4.0) | 4 (3.6) | 0.825 |
| Atopic dermatitis | 65 (2.8) | 4 (3.6) | 0.640 |
| Stroke | 61 (2.7) | 5 (4.5) | 0.248 |
| Physical activity | |||
| Days of moderate active physical activity per weeka | 2.3±2.1 | 2.3±2.1 | 0.862 |
| Days of high active physical activity per weeka | 1.8±1.7 | 1.5±1.4 | 0.008 |
| Baseline pulmonary function test | |||
| FVC, La | 3.6±0.9 | 3.1±0.9 | <0.001 |
| FVC, % predicteda | 91.0±13.6 | 87.8±14.0 | 0.016 |
| FEV1, La | 2.3±0.6 | 1.8±0.6 | <0.001 |
| FEV1, % predicteda | 78.8±13.6 | 74.2±14.4 | <0.001 |
| FEV1/FVC, %a | 0.6±0.1 | 0.6±0.1 | <0.001 |
| COPD severity | <0.001 | ||
| FEV1, % predicted ≥80% | 1,034 (45.2) | 41 (36.9) | |
| 65%≤ FEV1, % predicted <80% | 888 (38.9) | 36 (32.5) | |
| 50%≤ FEV1, % predicted <65% | 364 (15.9) | 34 (30.6) | |
| EQ-5D index values ≥0.9 | 1,578 (69.0) | 33 (29.7) | <0.001 |
| Use of inhalers | 100 (4.4) | 41 (36.9) | <0.001 |
Notes: The data are presented as number (%) or mean and standard deviation.
These data are analyzed by using Student’s t-test.
Data of 29 patients are missed including 28 patients without severe exacerbations and one patient with severe exacerbation.
Coronary heart disease includes myocardial infarction and angina pectoris.
Abbreviations: EQ-5D, The EuroQoL five dimensions questionnaire; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Factors associated with severe exacerbations in patients with mild-to-moderate COPD
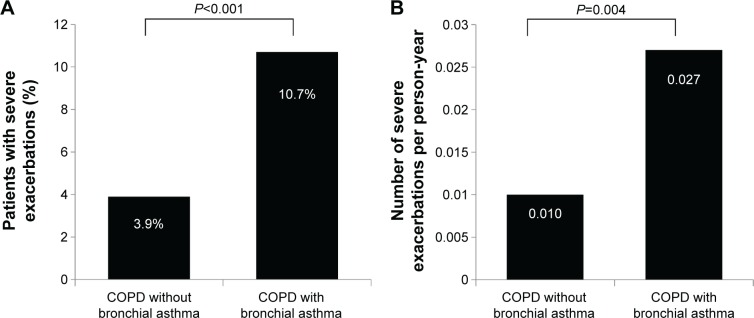
Severe exacerbations were more frequent in COPD patients with concomitant bronchial asthma compared with COPD patients without concomitant bronchial asthma (10.7% vs 3.9%, P<0.001 and 0.027/person-year vs 0.010/person-year, P=0.004, Figure 3).
Figure 3.
The comparison of severe exacerbation between COPD patients with coexisting self-reported physician-diagnosed bronchial asthma and those with COPD only.
Notes: (A) Percentage of patients with severe exacerbations and (B) number of severe exacerbations/person-year.
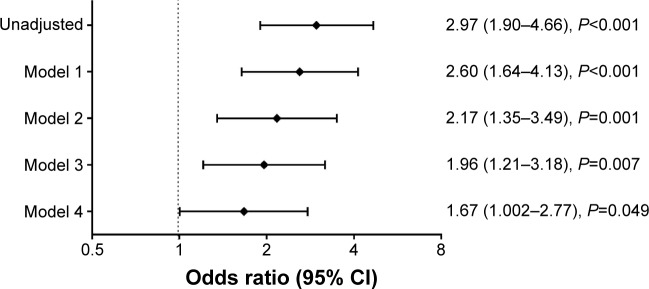
In the overall population, bronchial asthma (adjusted odds ratio [aOR], 1.96; 95% confidence interval [CI], 1.21–3.18, P=0.007) was independently associated with the development of severe exacerbations after adjustment for all possible confounding factors except for use of inhalers (Model 3). With the addition of use of inhalers as a confounding factor (Model 4), the significance of bronchial asthma associated with the development of severe exacerbations persisted (aOR, 1.67; 95% CI, 1.002–2.77, P=0.049) (Figure 4). In addition, Model 4 showed that age (aOR, 1.04; 95% CI, 1.02–1.06, P<0.001), female (aOR, 1.82; 95% CI, 1.01–3.33, P=0.045), the severity of airflow limitations (50%≤ FEV1, % predicted <65% vs FEV1, % predicted ≥80%, aOR, 1.93; 95% CI, 1.15–3.24, P=0.013), the use of inhalers (aOR, 2.34; 95% CI, 1.25–4.38, P=0.008) and poor QoL (EQ-5D index values <0.9 vs EQ-5D index values ≥0.9, aOR, 3.47; 95% CI, 2.22–5.43, P<0.001) were correlated with the development of severe exacerbations in mild-to-moderate COPD patients (Table 3).
Figure 4.
Association between asthma and severe exacerbations in mild-to-moderate COPD patients. Diamonds and whiskers show odds ratio and 95% CI values, respectively. Model 1 was adjusted for age, sex, and BMI; Model 2 was additionally adjusted for pulmonary-related variables generally considered to be important in severe exacerbations in COPD (smoking history and severity of airflow limitation); Model 3 additionally included extrapulmonary-related variables generally considered to be important in severe exacerbations in COPD or extrapulmonary-related variables with P<0.05 in the univariate analyses with considering multicollinearity (diabetes mellitus, cardiovascular disease [stroke, myocardial infarction, and angina pectoris], and quality of life); finally, Model 4 was additionally adjusted for use of inhalers with all of the above mentioned variables.
Abbreviations: BMI, body mass index; CI, confidence interval.
Table 3.
Multivariate analysis for factors associated with severe exacerbations in mild-to-moderate COPD patients
| All patients (n=2,397)
|
||
|---|---|---|
| aOR (95% CI) | P-value | |
| Age, years | 1.04 (1.02–1.06) | <0.001 |
| Sex, female | 1.82 (1.01–3.33) | 0.045 |
| Body mass index, kg/m2 | 1.0 (0.93–1.07) | 0.954 |
| Smoking historya | ||
| Nonsmokerc | 1.0 | |
| Current or ex-smoker | 1.02 (0.57–1.82) | 0.961 |
| Comorbidities | ||
| Diabetes mellitus | 1.44 (0.87–2.38) | 0.157 |
| Cardiovascular diseaseb | 0.89 (0.42–1.86) | 0.752 |
| Bronchial asthma | 1.67 (1.002–2.77) | 0.049 |
| Severity of airflow limitation | ||
| FEV1, % predicted ≥80%c | 1.0 | |
| 65%≤ FEV1, % predicted <80% | 1.14 (0.70–1.85) | 0.599 |
| 50%≤ FEV1, % predicted <65% | 1.93 (1.15–3.24) | 0.013 |
| Quality of life | ||
| EQ-5D index values ≥0.9c | 1.0 | |
| EQ-5D index values <0.9 | 3.47 (2.22–5.43) | <0.001 |
| Use of inhalers | 2.34 (1.25–4.38) | 0.008 |
Notes:
Data of 29 patients are missed.
Cardiovascular disease includes stroke, myocardial infarction, and angina pectoris.
Subjects in this category served as the reference group.
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; EQ-5D, The EuroQoL five dimensions questionnaire; FEV1, forced expiratory volume in 1 second.
Discussion
This is the first population-based observational study using a 6-year nationwide database to explore the prevalence of severe exacerbations in mild-to-moderate COPD. We found that 4.6% of patients with mild-to-moderate COPD experienced more than one severe exacerbation requiring a hospital admission or visit to the emergency department with administration of systemic corticosteroids and/or antibiotics over the 6-year study period. In addition, we provided robust data showing that coexisting bronchial asthma was associated with severe exacerbation in mild-to-moderate COPD patients.
This study evaluated the prevalence of severe exacerbations in a representative nationwide sample with mild-to-moderate COPD, which might reflect the real prevalence of the general COPD population including both symptomatic and nonsymptomatic patients. The prevalence rates of severe exacerbations were 0.009/person-year in patients with FEV1 ≥80% predicted and 0.013/person-year in patients with 50% predicted ≤FEV1 <80% predicted, which is approximately one-tenth of that reported previously for patients with moderate COPD, which was estimated at 0.11–0.2/person-year.11,25 This substantial difference can be explained by the composition of symptomatic patients. About half of the patients in the ECLIPSE study had symptoms (modified Medical Research Council score ≥2) and more than half of the patients in the TORCH study had a history of inhaler treatment for COPD,11,26 whereas 81.4% of our subjects had no history of COPD-related medical service use. When we restricted subjects who ever used COPD-related medical service, the prevalence of severe exacerbation was 0.062/person-year, which is comparable to the rates in the ECLIPSE and TORCH studies considering that our study included patients with mild COPD.
We showed that coexisting bronchial asthma was independently associated with a higher risk for severe exacerbation. Our data agree with a previous study regarding ACOS, which showed that patients with ACOS experience up to 1.9-fold more severe COPD exacerbations than those with COPD alone.16 Subjects with ACOS in the PLATINO study had a higher risk for hospitalization due to exacerbations at a prevalence ratio of 4.11, compared to those with only COPD.15 In addition, this observation replicates earlier findings from a large retrospective cohort study showing that bronchial asthma is one of the independent predictors of moderate-to-severe exacerbation over a 1-year follow-up in a primary care population.14 We extended the findings of this study with a prolonged follow-up of 6 years, as 1 year is insufficient to obtain the frequency of exacerbations in patients with mild-to-moderate COPD. Furthermore, we investigated utilization of the health care system in all patients with mild-to-moderate COPD, as confirmed by spirometry in the KNHANES, which overcomes a limitation of a previous study that the true population of patients with a milder airflow obstruction (mild-to-moderate COPD) may be underestimated, as these patients would not require health care or undergo spirometry.14
We observed that severe exacerbations in mild-to-moderate COPD patients are more frequent as FEV1 decreases and the EQ-5D index representing health-related QoL is low. It is well established that exacerbations deteriorate lung function and health-related QoL and, in turn, poor lung function and low QoL are risk factors for subsequent severe exacerbations in patients with moderate-to-severe COPD.8,11,12,25,27–30 In particular, EQ-5D index is related to poor clinical outcome of exacerbations.31 We extended these independent associations to patients with mild COPD, suggesting that mild COPD patients with relatively preserved lung function showed characteristics similar to those with moderate-to-severe COPD. In addition, we found that the use of inhalers was associated with severe exacerbations in mild-to-moderate COPD patients. A recent retrospective observational study showed that COPD patients with more symptoms increased the likelihood of receiving inhalers in the Optimum Patient Care Research Database, which is a quality-controlled, longitudinal, primary care respiratory database.32 Thus, the use of inhalers in the present study might indicate more symptomatic COPD patients with mild-to-moderate airflow limitation in the real practice.
There are several limitations in this study. First, as lung function test were performed only during the time of enrollment to KNHANES, some patients who were classified as moderate COPD from data of 2012 KNHANES would have mild COPD at 2007. In addition, the events of severe exacerbation were not assessed prospectively for all patients. In other words, some events of severe exacerbation would be assessed retrospectively from the point of the risk assessment obtained from KNHANES, as such the directionality of the relationship between severe exacerbation and lung function or EQ-5D could be uncertain. It is possible that severe exacerbation caused poor lung function and low EQ-5D index but reverse causation was also possible. However, as severe exacerbation cannot cause bronchial asthma to develop and there was self-reported past history of physician-diagnosed bronchial asthma, the effect of our study model for the association between severe exacerbation and bronchial asthma would be minimal. Third, the other prognosis associated with severe exacerbation, such as lung function decline or mortality, were not assessed in the present study. Further studies to evaluate impact of concomitant bronchial asthma with COPD on natural course of disease are needed. Finally, this study was performed in a single country in Asia and to generalize our findings, additional studies in other countries are necessary.
In conclusion, the prevalence of severe exacerbations in patients with mild-to-moderate COPD was 0.012/person-year in this population-based observational study using a nationwide database. Bronchial asthma was an independent factor associated with severe exacerbations in patients with mild-to-moderate COPD. Given the impact of coexisting bronchial asthma on the frequency of severe exacerbations in patients with mild-to-moderate COPD, exacerbation preventive strategies are necessary in patients with a bronchial asthma history across COPD disease severities.
Acknowledgments
The authors would like to thank Kyungjoo Kim for the confident statistical analyses in this work. This study was supported by a grant (2014P3300300) from the Korea Centers for Disease Control and Prevention. The abstract of this paper was presented at the Asian Pacific Society of Respirology 20th Congress as an oral presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Respirology.
Footnotes
Disclosure
David Price has Board Membership with Aerocrine, Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva. Consultancy: A Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, and Teva; Grants and unrestricted funding for investigator-initiated studies from UK National Health Service, British Lung Foundation, Aerocrine, AKL Ltd, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Eli Lilly, GlaxoSmithKline, Meda, Merck, Mundipharma, Napp, Novartis, Orion, Pfizer, Respiratory Effectiveness Group, Takeda, Teva, and Zentiva; Payments for lectures/speaking: Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, SkyePharma, Takeda, and Teva; Payment for manuscript preparation: Mundipharma and Teva; Patents (planned, pending or issued): AKL Ltd.; Payment for the development of educational materials: GlaxoSmithKline, Novartis; Stock/Stock options: Shares in AKL Ltd which produces phytopharmaceuticals and owns 80% of Research in Real Life Ltd and its subsidiary social enterprise Optimum Patient Care; received Payment for travel/accommodations/meeting expenses from Aerocrine, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva; Funding for patient enrolment or completion of research: Almirral, Chiesi, Teva, and Zentiva; and Peer reviewer for grant committees: Medical Research Council (2014), Efficacy and Mechanism Evaluation programme (2012), HTA (2014). The authors report no other conflicts of interest.
References
- 1.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 3.Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. Eur Respir J. 2004;23(5):698–702. doi: 10.1183/09031936.04.00121404. [DOI] [PubMed] [Google Scholar]
- 4.Kessler R, Stahl E, Vogelmeier C, et al. Patient understanding, detection, and experience of COPD exacerbations: an observational, interview-based study. Chest. 2006;130(1):133–142. doi: 10.1378/chest.130.1.133. [DOI] [PubMed] [Google Scholar]
- 5.Piquet J, Chavaillon JM, David P, Martin F, Blanchon F, Roche N. High-risk patients following hospitalisation for an acute exacerbation of COPD. Eur Respir J. 2013;42(4):946–955. doi: 10.1183/09031936.00180312. [DOI] [PubMed] [Google Scholar]
- 6.Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2013;10(2):81–89. doi: 10.1513/AnnalsATS.201208-043OC. [DOI] [PubMed] [Google Scholar]
- 7.Gunen H, Hacievliyagil SS, Kosar F, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J. 2005;26(2):234–241. doi: 10.1183/09031936.05.00024804. [DOI] [PubMed] [Google Scholar]
- 8.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoogendoorn M, Hoogenveen RT, Rutten-van Molken MP, Vestbo J, Feenstra TL. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508–515. doi: 10.1183/09031936.00043710. [DOI] [PubMed] [Google Scholar]
- 10.Toy EL, Gallagher KF, Stanley EL, Swensen AR, Duh MS. The economic impact of exacerbations of chronic obstructive pulmonary disease and exacerbation definition: a review. COPD. 2010;7(3):214–228. doi: 10.3109/15412555.2010.481697. [DOI] [PubMed] [Google Scholar]
- 11.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 12.Beeh KM, Glaab T, Stowasser S, et al. Characterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trial. Respir Res. 2013;14:116. doi: 10.1186/1465-9921-14-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foreman MG, DeMeo DL, Hersh CP, Reilly JJ, Silverman EK. Clinical determinants of exacerbations in severe, early-onset COPD. Eur Respir J. 2007;30(6):1124–1130. doi: 10.1183/09031936.00009307. [DOI] [PubMed] [Google Scholar]
- 14.Mullerova H, Shukla A, Hawkins A, Quint J. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4(12):e006171. doi: 10.1136/bmjopen-2014-006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menezes AM, Montes de Oca M, Perez-Padilla R, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest. 2014;145(2):297–304. doi: 10.1378/chest.13-0622. [DOI] [PubMed] [Google Scholar]
- 16.Hardin M, Silverman EK, Barr RG, et al. The clinical features of the overlap between COPD and asthma. Respir Res. 2011;12:127. doi: 10.1186/1465-9921-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welte T, Vogelmeier C, Papi A. COPD: early diagnosis and treatment to slow disease progression. Int J Clin Pract. 2015;69(3):336–349. doi: 10.1111/ijcp.12522. [DOI] [PubMed] [Google Scholar]
- 18.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi JK, Paek D, Lee JO. Normal predictive values of spirometry in Korean population. Tuberc Respir Dis. 2005;58(3):230–242. [Google Scholar]
- 20.Fukuchi Y, Fernandez L, Kuo HP, et al. Efficacy of tiotropium in COPD patients from Asia: a subgroup analysis from the UPLIFT trial. Respirology. 2011;16(5):825–835. doi: 10.1111/j.1440-1843.2011.01982.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Lee JH, Kim Y, et al. Association between chronic obstructive pulmonary disease and gastroesophageal reflux disease: a national cross-sectional cohort study. BMC Pulm Med. 2013;13:51. doi: 10.1186/1471-2466-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Rhee CK, Yoo KH, et al. The health care burden of high grade chronic obstructive pulmonary disease in Korea: analysis of the Korean Health Insurance Review and Assessment Service data. Int J Chron Obstruct Pulmon Dis. 2013;8:561–568. doi: 10.2147/COPD.S48577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim C, Yoo KH, Rhee CK, et al. Health care use and economic burden of patients with diagnosed chronic obstructive pulmonary disease in Korea. Int J Tuberc Lung Dis. 2014;18(6):737–743. doi: 10.5588/ijtld.13.0634. [DOI] [PubMed] [Google Scholar]
- 24.Rhee CK, Yoon HK, Yoo KH, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. COPD. 2014;11(2):163–170. doi: 10.3109/15412555.2013.831061. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 27.Miravitlles M, Guerrero T, Mayordomo C, Sanchez-Agudo L, Nicolau F, Segu JL. Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000;67(5):495–501. doi: 10.1159/000067462. [DOI] [PubMed] [Google Scholar]
- 28.Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation – systematic review. Int J Chron Obstruct Pulmon Dis. 2007;2(3):241–251. [PMC free article] [PubMed] [Google Scholar]
- 29.Miravitlles M, Calle M, Alvarez-Gutierrez F, Gobartt E, Lopez F, Martin A. Exacerbations, hospital admissions and impaired health status in chronic obstructive pulmonary disease. Qual Life Res. 2006;15(3):471–480. doi: 10.1007/s11136-005-3215-y. [DOI] [PubMed] [Google Scholar]
- 30.Gadoury MA, Schwartzman K, Rouleau M, et al. Self-management reduces both short- and long-term hospitalisation in COPD. Eur Respir J. 2005;26(5):853–857. doi: 10.1183/09031936.05.00093204. [DOI] [PubMed] [Google Scholar]
- 31.Miravitlles M, Izquierdo I, Herrejon A, et al. COPD severity score as a predictor of failure in exacerbations of COPD. The ESFERA study. Respir Med. 2011;105(5):740–747. doi: 10.1016/j.rmed.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Gruffydd-Jones K, Brusselle G, Jones R, et al. Changes in initial COPD treatment choice over time and factors influencing prescribing decisions in UK primary care: a real-world study. NPJ Prim Care Respir Med. 2016 doi: 10.1038/npjpcrm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]