Abstract
The natural fatty acids (5Z)-5-pentacosenoic and (9Z)-9-pentacosenoic acids were synthesized for the first time in eight steps starting from either 4-bromo-1-butanol or 8-bromo-1-butanol and in 20-58% overall yields, while the novel fatty acids 5-pentacosynoic and 9-pentacosynoic acids were also synthesized in six steps and in 34-43% overall yields. The Δ5 acids displayed the best IC50’s (24-38 µM) against the HIV-1 reverse transcriptase (RT) enzyme, comparable to nervonic acid (IC50 = 12 µM). The Δ9 acids were not as effective towards HIV-RT with the (9Z)-9-pentacosenoic acid displaying an IC50 = 54 µM. Fatty acid chain length and position of the unsaturation was critical for the observed inhibition. Molecular modeling studies indicated the structural determinants underlying the biological activity of the most potent compounds. These results provide new insights into the structural requirements that must be present in fatty acids so as to enhance their inhibitory potential towards HIV-RT.
Keywords: Fatty Acids, Human immunodeficiency virus, Pentacosenoic acid, Reverse Transcriptase, Synthesis
Introduction
The potential of fatty acids to combat infectious diseases such as malaria, tuberculosis, and fungal infections continues to be a focus of recent research activity [1]. Fatty acids are important in a diversity of pathological conditions and have been postulated as possible drug candidates against viruses, bacteria, fungi, and cancerous cells by inducing death responses [1-6]. For example, it has been postulated that fatty acids, such as palmitic acid, play a key role in regulating the characteristic loss of homeostasis in cancer [4]. Numerous fatty acids, mainly of bacterial origin, also inhibit DNA polymerase and Topoisomerase I, and display anti-malarial and anti-viral activity [1, 7-11].
The World Health Organization (WHO) considers the Human Immunodeficiency Virus (HIV) infection pandemic [12]. In 2012 alone, around 2.3 million adults and children were infected with HIV, bringing the total population living with HIV to 35.3 million, mostly on the African continent [12]. There are two sub-types of HIV, namely HIV-1 and HIV-2, but HIV-1 is more virulent and far more common than HIV-2, which is confined to West Africa [13].
Even after more than twenty years of research, a HIV vaccine has yet to be discovered and current research focuses on the search for novel anti-HIV agents [14]. There are more than twenty antiretroviral drugs approved for clinical use today [15], but due to their long-term use in drug therapies these drugs must be relatively nontoxic [16]. This implies that our present arsenal to combat the disease is limited due to adverse effects and toxicities that normally arise from long-term use coupled to the emergence of drug resistance [14]. Since HIV-1 can acquire drug resistance to any single inhibitor quite easily, multiple drugs are typically used simultaneously [14].
The retrotranscriptase (RT) is a multifunctional enzyme that plays a key role in HIV-1 replication [16, 17-22]. It possesses two distinct enzymatic activities, DNA polymerase and RNase H, which are used to convert the viral RNA genome into double-stranded linear proviral DNA that are subsequently integrated into the host genome [16-24]. Since RT inhibitors prevent the RNA-DNA conversion, a vital role of the viral life cycle, it is no wonder that more than half the currently approved HIV-1 antiretrovirals are RT inhibitors [21]. There are two classes of RT inhibitors, namely nucleoside/nucleotide retrotranscriptase inhibitors (NRTIs) and non-nucleoside retrotranscriptase inhibitors (NNRTIs) [15, 25-27]. Together they are the backbone of the highly active-antiretroviral therapy (HAART) regime [24], the standard treatment that usually combines two NRTIs with one NNRTI or with one protease inhibitor (PI) [14, 16, 24]. However, over the years, NNRTIs have become more popular than the PIs [21].
Even though the NNRTIs are a chemically heterogeneous group of compounds, they possess a common mode of binding and they all bind to the same site on the RT enzyme [28-29]. This binding site is located in the palm sub-domain between two beta-sheets of the p66 subunit about 10 Å away from the DNA polymerase catalytic site and approximately 60 Å from the RNase H active site of the p66 subunit [24, 28, 30].
The HAART regime currently used is not potent enough to completely suppress virus replication to the point of eradication due to the development of drug-resistance [13, 25]. It is still necessary to develop new less cytotoxic antiretrovirals for the treatment of this disease. Natural products from various marine sources have been known to possess diverse biological activities including antiviral [11, 31].
Nervonic acid (NA) is a cis-15-tetracosenoic acid found in the sphingolipids of white matter in human brain where it plays an important role in the biosynthesis of nerve cell myelin [32]. NA is also abundantly found in the spotted seal, King salmon and beluga whale. NA is used to treat adrenoleukodystrophy and multiple sclerosis where the decreased level of nervonic acid in sphingolipids causes demyelination [32]. This FA possesses great inhibitory activity against several enzymes involved in replication [33-36]. Mizushina and co-workers discovered that NA was a potent non-competitive inhibitor of HIV-1 RT with an IC50 of 4.8 μM and almost complete inhibition (more than 80%) at 8 μM [36]. Modification of the carboxyl group of NA to a carboxyl ester causes the loss of inhibitory activity thus indicating that the carboxyl group in NA is important for RT inhibition [33]. Therefore, the carboxyl group and length of the alkyl chain in NA play crucial roles in HIV-RT inhibition.
Since nervonic acid was a potent HIV-1 RT inhibitor, other monounsaturated long chain fatty acids could be potential HIV-1 RT inhibitors as well. The pentacosenoic acids are a minor class of lipids found in a wide range of organisms including bacteria and marine sponges [37]. The (5Z)-5-pentacosenoic acid is naturally found in the marine sponge Pseudaxinella cf. lunaecharta and in the bacterium Mycobacterium tuberculosis [38-39]. On the other hand, the (9Z)-9-pentacosenoic acid, which is more ubiquitous, was identified in various sponges including the marine sponges Dysidea fragilis, Desmapsamma anchorata, Geodinella robusta, Spheciospongia cuspidifera and Hymeniacidon sanguinea as well as in the bacterium Mycobacterium tuberculosis [38, 40-44]. These long-chain monounsaturated fatty acids could be potential RT inhibitors.
In this work we synthesized, for the first time, the naturally occurring fatty acids (5Z)-5-pentacosenoic and (9Z)-9-pentacosenoic acids as well as the alkynoic analogs 5-pentacosynoic, 9-pentacosynoic, and 2- pentacosynoic acids. Their HIV-RT inhibitory activity was determined and compared to other shorter chain analogs. Molecular modeling studies indicated the structural basis underlying the inhibitory activities of the most potent compounds and this was compared to nervonic acid.
Materials and Methods
Instrumentation
All compounds were analyzed by 1H NMR (300 or 500 MHz) and 13C NMR (75 or 125 MHz) by using a Bruker Avance DPX-300 or a Bruker Avance DRX-500 spectrometer. The samples were diluted in chloroform-d (CDCl3) and the solvent signals at 7.26 and 77.0 ppm were used as internal standard for proton and carbon, respectively. Mass spectral data was acquired on a GC-MS (Hewlett-Packard 5972A MS ChemStation or Agilent 5975C MS Chemstation) instrument at 70 eV, equipped with a 30 x 0.25 mm special performance capillary column (HP-5MS) of polymethylsiloxane cross-linked with 5% phenyl methylpolysiloxane. Infrared spectra were recorded on a Nicolet Magna 750 FT-IR spectrophotometer (Thermo-Nicolet, Madison, WI, USA).
2-(4-Bromobutoxy)-tetrahydro-2H-pyran (4a)
Into a round-bottomed flask equipped with a magnetic stirrer was added a solution of 4-bromo-1-butanol (1.29 g, 8.4 mmol) in 20 mL of CHCl3. To this solution, the 3,4-dihydro-2H-pyran (1.77 g, 21.1 mmol) was added drop wise followed by the addition of catalytic amounts of p-toluenesulfonic acid (p-TSA). The reaction mixture was stirred for 3h at rt. The chloroform was evaporated in vacuo and the organic layer was washed with an aqueous NaHCO3 saturated solution (3 x 30 mL), dried over MgSO4, filtered and concentrated in vacuo. The crude product was initially purified under Kugel Rohr distillation (95-120 °C/3 mm Hg) for 3 h and then further purified using silica gel column chromatography with hexane/ether (9:1 v/v) as the mobile phase affording 4a as a colorless oil in 99% yield with spectral data comparable to those previously reported in the literature [45].
2-(8-Bromooctoxy)-tetrahydro-2H-pyran(4b)
The tetrahydropyran 4b was obtained as colorless oil in 93% yield from the reaction of 8-bromo-1-octanol (1.98 g, 6.8 mmol) with 3,4-dihydro-2H-pyran (0.7 g, 8.2 mmol) and catalytic amounts of p-TSA in CHCl3 (20 mL) following the procedure described above and with spectral data similar to the one reported in the literature [45].
Trimethyl [6-(tetrahydro-2H-pyran-2-yloxy)-1-hexynyl] silane (5a)
To a stirred solution of (trimethylsilyl)acetylene (1.94 g, 19.7 mmol) in dry THF (10.0 mL) and under a nitrogen atmosphere, n-BuLi (2.5 M, 10.0-26.0 mmol) in hexane was added drop wise at −78.0 °C. After 5 min HMPA (3.0 mL) was added followed by the drop wise addition of 4a (1.0 g, 6.6 mmol) in THF (10.0 mL) while maintaining the temperature at −78 °C. After 24 h, the reaction mixture was quenched with water, extracted with diethyl ether (2 x 15 mL) and washed with brine (2 x 20 mL). The organic phase was dried with MgSO4, filtered, and the solvent rotoevaporated. Final purification of the product by Kugel-Rohr distillation (110-120 °C/3 mm Hg) afforded 5a as a colorless oil in a 92 % yield with spectral data comparable to those previously reported in the literature [46].
Trimethyl [10-(tetrahydro-2H-pyran-2-yloxy)-1-decynyl] silane (5b)
Silane 5b was obtained as a colorless oil [47] in 94 % yield from the reaction of 1.70 mL of (trimethylsilyl)acetylene (1.21 g, 12.3 mmol) and 4b (1.20 g, 4.1 mmol) following the procedure described for 5a.
2-[(5-Hexynyl)oxy]-tetrahydro-2H-pyran (6a)
A mixture of 5a (0.99 g, 3.9 mmol) in 20.0 mL of dry THF was stirred at 0 °C and then tetrabutylammonium fluoride (2.3 mL, 7.8 mmol) was added drop-wise to the stirred solution. After 2 h, the reaction mixture was quenched with a 2M HCl solution and extracted with diethyl ether (2 x 20 mL). The organic extracts were dried over MgSO4 and concentrated in vacuo. The product was purified by Kugel-Rohr distillation (110-120 °C/3 m Hg), affording 6a in a 99 % yield as a colorless oil and with spectral data similar to the one reported in the literature [48].
2-[(9-Decynyl)oxy]-tetrahydro-2H-pyran (6b)
The tetrahydropyran 6b was obtained as a colorless oil [47] and in a 98% yield from the reaction of 1.66 g (5.3 mmol) of 5b and 3.1 mL (10.7 mmol) of TBAF following the procedure described for 6a.
2-[(5-Pentacosynyl)oxy]-tetrahydro-2H-pyran (7a)
To a solution of 6a (0.19 g, 1.0 mmol) and dry THF (15 mL) at −78°C, n-BuLi (2.5 M, 2.1-3.2 mmol) in hexane was added drop-wise while stirring under an argon atmosphere. After 45 min, HMPA (3.0 mL) was added followed by the addition of 1-bromononadecane (0.32 g, 0.9 mmol). The reaction was left stirring for 24 h at room temperature. The reaction mixture was then quenched with water and the organic products extracted with diethyl ether (1 x 50 mL), dried over MgSO4, filtered and evaporated in vacuo. The crude products were initially purified under Kugel Rohr distillation (160-195 °C/3 mm Hg) for 4-5 h and then further purified using alumina column chromatography with hexane first and then diethyl ether as the mobile phases affording 0.34 g of 7a in an overall 81% yield. The product was used as such for the next step without further purification, mp 61-63°C, IR (neat) νmax 2919, 2851, 1726, 1464, 1119, 1023, 811, 721, 626 cm−1; 1H NMR (CDCl3, 300 MHz) δ 4.54-4.53 (1H, t, J = 2.7 Hz), 3.85-3.68 (2H, m), 3.48-3.33 (2H, m), 2.19-2.07 (4H, m, H-4, H-7), 1.84-1.61 (6H, m), 1.59-1.48 (6H, m), 1.45-1.22 (32H, m, −CH2−), 0.86-0.82 (3H, t, J = 7.2 Hz, H-25); 13C NMR (CDCl3, 75 MHz) δ 98.7 (d), 80.4 (s), 79.7 (s), 67.0 (t, C-1), 62.1 (t), 31.9 (t, C-23), 30.7 (t), 29.7 (t), 29.6 (t), 29.5 (t), 29.4 (t), 29.3 (t), 29.2 (t), 29.1 (t), 28.9 (t), 28.7 (t), 28.1 (t), 25.9 (t), 25.5 (t, C-3), 22.7 (t, C-24), 19.7 (t), 19.6 (t), 18.7 (t, C-7), 18.6 (t, C-4), 14.1 (q, C-25).
2-[(9-Pentacosynyl)oxy]-tetrahydro-2H-pyran (7b)
The tetrahydropyran 7b was obtained in a 60% yield as a white solid from the reaction of 0.20 g (0.8 mmol) of 6b and 1-bromopentadecane (0.22 g, 0.8 mmol) following the procedure described for 7a. The product was used as such for the next step without further purification, mp 57-59°C, IR (neat) νmax 2915, 2848, 1462, 1122, 1027, 719 cm−1; 1H NMR (CDCl3, 300 MHz) δ 4.58-4.56 (1H, t, J = 2.7 Hz), 3.90-3.83 (1H, m), 3.76-3.69 (1H, m), 3.53-3.46 (1H, m), 3.43-3.33 (1H, m), 2.20-2.10 (4H, m, H-8, H-11), 1.94-1.65 (6H, m), 1.62-1.42 (6H, m), 1.31-1.25 (32H, m, −CH2−), 0.89-0.85 (3H, t, J = 6.9 Hz, H-25); 13C NMR (CDCl3, 75 MHz) δ 98.8 (d), 84.8 (s), 80.3 (s), 67.7 (t, C-1), 62.3 (t), 31.9 (t, C-23), 30.8 (t, C-2), 29.69 (t), 29.65 (t), 29.6 (t), 29.4 (t), 29.35 (t), 29.2 (t), 29.1 (t), 28.9 (t), 28.8 (t), 28.76 (t), 28.7 (t), 28.4 (t), 28.2 (t), 26.2 (t), 25.5 (t), 22.7 (t, C-24), 19.7 (t), 18.7 (t, C-11), 18.4 (t, C-8), 14.1 (q, C-25).
5-Pentacosyn-1-ol (8a)
Pyran 7a (0.34 g, 0.8 mmol) and catalytic amounts of p-TSA in methanol (15.0 mL) were stirred at 45 °C for 3 h. The reaction mixture was quenched with a 5% NaHCO3 solution and extracted with diethyl ether (2 x 20 mL). The organic extracts were dried over MgSO4 and concentrated in vacuo. The product was purified by Kugel Rohr distillation (160-190°C/ 3 mm Hg), affording 8a in a 99% yield as a white solid, mp 57-59 °C, IR (neat) νmax 3455 (br, −OH), 2921, 2852, 2179, 1460, 1377, 1258, 1121, 1076, 1023, 798, 721 cm−1; 1H NMR (CDCl3, 300 MHz) δ 3.69-3.65 (2H, t, J = 6.3 Hz, H-1), 2.22-2.10 (4H, m, H-4, H-7), 1.72-1.63 (2H, m, H-2), 1.61-1.51 (4H, m, H-3, H-8), 1.49-1.42 (2H, br. quintet, J = 7.1 Hz, H-24), 1.34-1.25 (31H, m, −CH2−, −OH), 0.89-0.85 (3H, t, J = 6.9 Hz, H-25); 13C NMR (CDCl3, 75 MHz) δ 80.8 (s), 79.7 (s), 62.5 (t, C-1), 31.9 (t, C-2), 31.85 (t, C-23), 29.7 (t), 29.65 (t), 29.55 (t), 29.4 (t), 29.2 (t), 29.1 (t), 28.9 (t), 25.3 (t, C-3), 22.7 (t, C-24), 18.7 (t), 18.5 (t), 14.1 (q, C-25). HRMS (APCI) Calcd for C25H49O1 [M + H]+ 365.3778, found 365.3778.
9-Pentacosyn-1-ol (8b)
The 9-pentacosyn-1-ol (8b) was obtained in an 83% yield as a white solid from the reaction of 0.21 g (0.5 mmol) of 7b in methanol with catalytic amounts of p-TSA according to the procedure described for 8a, mp 54-55°C, IR (neat) νmax 3265 (br, −OH), 2915, 2848, 2116, 1485, 1471, 1462, 1405, 1353, 1122, 1061, 1031, 1014, 729, 719, 682 cm−1; 1H NMR (CDCl3, 300 MHz) δ 3.65-3.61 (2H, t, J = 6.7 Hz, H-1), 2.15-2.10 (4H, t, J = 6.7 Hz, H-8, H-11), 1.58-1.51 (2H, m, H-2), 1.46-1.44 (4H, quintet, J = 6.9 Hz, H-7, H-12), 1.38-1.24 (33H, m, –CH2−, −OH), 0.89-0.85 (3H, t, J = 6.9 Hz, H-25); 13C NMR (CDCl3, 75 MHz) δ 80.3 (s), 80.1 (s), 63.0 (t, C-1), 32.7 (C-2), 31.9 (t, C-23), 29.7 (t), 29.6 (t), 29.55 (t), 29.34 (t), 29.30 (t), 29.2 (t), 29.1 (t), 28.9 (t), 28.8 (t), 25.7 (t, C-3), 22.7 (t, C-24), 18.7 (t), 14.1 (q, C-25). HRMS (APCI) Calcd for C25H49O1 [M + H]+ 365.3778, found 365.3778.
5-Pentacosynoic acid (1a)
To a stirred solution of 8a (0.10 g, 0.3 mmol) in 10.0 mL of DMF was slowly added a solution of pyridinium dichromate (0.42 g, 1.1 mmol) at rt. After 24 h, the reaction mixture was washed with water (3 x 25 mL), diethyl ether (1 x 25 mL), dried over MgSO4, filtered, and concentrated in vacuo. The crude product was purified utilizing fluorisil column chromatography eluting with diethyl ether affording 1a as a white solid in a 59% yield, mp 64-66°C, IR (neat) νmax 3151-3091, 2916, 2849, 1705 (C=O), 1463, 1378, 1295, 1186, 1099, 937, 721 cm−1; 1H NMR (CDCl3, 500 MHz) δ 2.51-2.48 (2H, t, J = 7.4 Hz, H-2), 2.26-2.23 (2H, t, J = 6.8 Hz, H-7), 2.14-2.13 (2H, t, J = 7.2 Hz, H-4), 1.84-1.78 (2H, br. quintet, J = 6.9 Hz, H-3), 1.50-1.44 (2H, br. quintet, J = 7.3 Hz, H-8), 1.35-1.25 (32H, m, −CH2−), 0.89-0.87 (3H, t, J = 6.9 Hz, H-25); 13C NMR (CDCl3, 75 MHz) δ 179.5 (s, C-1), 81.5 (s, C-6), 78.5 (s, C-5), 32.8 (t, C-2), 31.9 (t, C-23), 29.7 (t), 29.5 (t), 29.4 (t), 29.2 (t), 29.0 (t), 28.9 (t), 23.9 (t, C-3), 22.7 (t, C-24), 18.7 (t, C-7), 18.1 (t, C-4), 14.1 (q, C-25). HRMS (APCI) Calcd for C25H47O2 [M + H]+ 379.3570, found 379.3571.
9-Pentacosynoic acid (1b)
The 9-pentacosynoic acid (1b) was obtained in an 80% yield as a white solid from the reaction of 0.019 g (0.05 mmol) of 8b and 0.12 g (0.3 mmol) of PDC following the procedure described for 1a, mp 61-63°C, IR (neat) νmax 3091 (br, −OH), 2916, 2849, 1705 (C=O), 1463, 1432, 1412, 1378, 1295, 1099, 937, 721 cm−1; 1H NMR (CDCl3, 500 MHz) δ 2.36-2.32 (2H, t, J = 7.5 Hz, H-2), 2.15-2.09 (4H, m, H-8, H-11), 1.63-1.50 (2H, quintet, J = 6.6 Hz, H-3), 1.48-1.37 (4H, quintet, J = 6.9 Hz, H-7, H-12), 1.31-1.25 (30H, m, −CH2−) 0.89-0.86 (3H, t, J = 6.9 Hz, H-25); 13C NMR (CDCl3, 125 MHz) δ 179.6 (s, C-1), 80.3 (s, C-10), 80.0 (s, C-9), 34.0 (t, C-2), 31.9 (t), 29.71 (t), 29.67 (t), 29.6 (t), 29.44 (t), 29.36 (t), 28.9 (t), 28.8 (t), 28.5 (t), 24.7 (t), 22.7 (t), 18.75 (t), 18.71 (t), 14.1 (q, C-25).
(5Z)-5-Pentacosen-1-ol (9a)
Into a 25-mL two-necked round-bottomed flask were placed dry hexane, 8a (72 mg, 0.2 mmol), quinoline (0.3 mL), and palladium in activated carbon (Lindlar’s catalyst). One of the necks was capped with a rubber septum and the other was connected via tygon tubing to a 25-mL graduated pipet ending in a 150-mL beaker with distilled water. While stirring at rt a 20-mL syringe with needle was used to withdraw air from the system and to draw water up the graduated pipet to the 0.0 mL mark. Hydrogen was then introduced into the system using a balloon filled with hydrogen attached to a hose barb-to-luer lock adapter with a stopcock and a needle. The reaction mixture consumed 4.8-9.3 mL of hydrogen during 1 h. The mixture was filtered and the solvent removed in vacuo. The pentacosenol 9a was purified under Kugel Rohr distillation (160-190 °C/ 3 mm Hg), affording a white solid, in a 97% yield. The alkenol was used as such for the next step without further purification, mp 50-52 °C, IR (neat) νmax 3346 (OH, broad), 2916, 2849, 1621, 1468, 1352, 1259, 1200, 1121, 1064, 1023, 978, 905, 868, 810, 718 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.40-5.31 (2H, m, H-5, H-6), 3.66-3.63 (2H, t, J = 6.5 Hz, H-1), 2.08-1.97 (5H, m, H-4, H-7, −OH), 1.61-1.56 (2H, br. quintet, J = 7.1 Hz, H-2), 1.45-1.39 (2H, br. quintet, J = 7.5 Hz, H-3), 1.32-1.25 (34H, m, −CH2−), 0.89-0.86 (3H, t, J = 6.4 Hz, H-25); 13C NMR (CDCl3, 125 MHz) δ 130.4 (d), 129.3 (d), 62.9 (t, C-1), 32.4 (t, C-2), 32.3 (t, C-23), 31.9 (t), 31.74 (t), 29.69 (t), 29.65 (t), 29.6 (t), 29.4 (t), 29.3 (t), 29.2 (t), 27.3 (t), 26.9 (t), 25.8 (t, C-3), 22.7 (t, C-24), 14.1 (q, C-25).
(9Z)-Pentacosen-1-ol (9b)
The pentacosenol 9b was obtained in a 70 % yield as a white solid from the catalytic hydrogenation, using Lindlar’s catalyst, of 8b (0.14 g, 0.4 mmol) following the procedure described for 9a, mp 47-49°C, IR (neat) νmax 3311-2900 (br, −OH), 2920, 2851, 1641, 1463, 1259, 1083, 1013, 867, 793 cm−1; 1H NMR (CDCl3, 300 MHz) δ 5.35-5.32 (2H, m, H-9, H-10), 3.66-3.61 (2H, t, J = 6.7 Hz, H-1), 2.13-2.11(4H, m, H-8, H-11), 1.56-1.18 (38H, m, −CH2−), 0.89-0.85 (3H, t, J = 6.7 Hz, H-25); 13C NMR (CDCl3, 75 MHz) δ 130.0 (d, C-10), 129.8 (d, C-9), 63.1 (t, C-1), 32.8 (t, C-2), 31.9 (t, C-23), 29.7 (t), 29.6 (t), 29.5 (t), 29.4 (t), 29.2 (t), 28.9 (t), 28.8 (t), 28.1 (t), 27.2 (t), 25.7 (t, C-3), 22.7 (t, C-24), 14.1 (q, C-25).
(5Z)-5-Pentacosenoic acid (2a)
Was obtained in 83% yield as a white solid [38-39] from the reaction of 0.070 g (0.2 mmol) of 9a and PDC (0.43 g, 1.1 mmol) using the procedure described for 1a, mp 56-58°C, IR (neat) νmax 3091(br, −OH), 2915, 2848, 1693 (C=O), 1464, 1412, 1294, 1225, 1206, 1187, 1099, 1039, 1026, 932, 720, 688 cm−1; 1H NMR (CDCl3, 500 MHz) δ 5.46-5.29 (2H, m, H-5, H-6), 2.43-2.33 (2H, m, H-2), 2.12-1.96 (4H, m, H-4, H-7), 1.73-1.63 (2H, m, H-3), 1.32-1.25 (36H, m, −CH2−) 0.89-0.86 (3H, t, J = 6.8 Hz, H-25); 13C NMR (CDCl3, 125 MHz) δ 179.8 (s, C-1), 131.4 (d, C-6), 128.1 (d, C-5), 33.3 (t, C-2), 31.9 (t, C-23), 29.7 (t), 29.6 (t), 29.4 (t), 27.2 (t, C-7), 26.4 (t, C-4), 24.6 (t, C-3), 22.7 (t, C-24), 14.1 (q, C-25).
(9Z)-9-Pentacosenoic acid (2b)
Acid 2b was obtained in 66% yield as a white solid [38, 40-44] from the reaction of 0.098 g (0.3 mmol) of 9b and 0.60 g (1.6 mmol) of PDC using the procedure described for 1a, mp 54-56°C, IR (neat) νmax 3084 (br, −OH), 2915, 2848, 1702 (C=O), 1463, 1411, 1296, 1099, 1026, 937, 721 cm−1; 1H NMR (CDCl3, 300 MHz) δ 5.34-5.33 (2H, m, H-9, H-10), 2.36-2.30 (2H, t, J = 7.4 Hz, H-2), 2.01-1.99 (4H, m, H-8, H-11), 1.62-1.60 (2H, m, H-3), 1.29-1.24 (37H, m, −CH2−) 0.88-0.85 (3H, t, J = 6.9 Hz, H-25); 13C NMR (CDCl3, 75 MHz) δ 180.0 (s, C-1), 130.0 (d, C-10), 129.7 (d, C-9), 34.0 (t, C-2), 31.9 (t, C-23), 29.7 (t), 29.64 (t), 29.59 (t), 29.53 (t), 29.4 (t), 29.35 (t), 29.3 (t), 29.23 (t), 29.19 (t), 29.12 (t), 29.07 (t), 28.97 (t), 27.2 (t), 27.1 (t), 24.7 (t, C-3), 22.7 (t, C-24), 14.1 (q, C-25).
2-Pentacosynoic acid
The 2-pentacosynoic acid was obtained in a 13% yield as a white solid from the reaction of 1-tetracosyne (0.035 g, 1.0 mmol) and excess dry CO2 following the standard procedure, mp 69-70°C, IR (neat) νmax 3456-3045 (br), 2956, 2915, 2850, 2251, 1723, 1467, 1379, 1269, 1072, 742, 714 cm−1; 1H NMR (CDCl3, 300 MHz) δ 2.37-2.32 (2H, t, J = 7.2 Hz, H-4), 1.63-1.54 (2H, quintet, J = 7.4 Hz, H-5), 1.41-1.26 (38H, m, −CH2−), 0.89-0.85 (3H, t, J = 7.2 Hz, H-25); 13C NMR (CDCl3, 125 MHz) δ 158.2 (s, C-1), 92.7 (s, C-3), 72.6 (s, C-2), 31.9 (t, C-23), 29.64 (t), 29.62 (t), 29.55 (t), 29.4 (t), 29.3 (t), 29.0 (t), 28.8 (t), 27.4 (t), 22.7 (t, C-24), 18.8 (t, C-4), 14.1 (q, C-25).
Reverse Transcriptase Colorimetric Assay
The Reverse Transcriptase Colorimetric Assay Kit was purchased from Roche Diagnostics Corporation, Indianapolis, Indiana. The assay was carried out according to the procedure described in Roche’s Reverse Transcriptase Colorimetric Assay Kit manual with some minor modifications [49-50]. First a 1000 μM stock solution of each of the fatty acids in dimethyl sulfoxide (DMSO) was prepared (final concentration of DMSO = 1%). The stock solutions were sonicated for 1 h, and then each stock solution was further diluted to final concentrations of 100, 10, 1, 0.1 and 0.01 μM with Lysis buffer. The recombinant HIV-1-RT was briefly dissolved in Tris-buffer solution [50 mM Tris, 80 mM KCl, 2.5 mM DTT, 0.75 mM EDTA and 0.5 % Triton X-100 , pH 7.8] to reach a final concentration of 2 ng/ μL. The HIV-1-RT enzyme solutions with the experimental agents were incubated for 60 min at 37 °C. A 20 µL aliquot of a reaction mixture [incubation buffer: 50 mM Tris-buffer, containing 319 mM KCl, 33 mM magnesium chloride, and 11 mM DTT; nucleotides: 50 mM Tris-HCl at pH 7.8 with DIG-dUTP, biotin-dUTP and dTTP; and template: template/primer hybrid poly (A) x oligo (dT)15] was added and incubated for 60 min at 37°C. The reactions were transferred to MP modules coated with streptavidin and incubated for 60 min at 37°C. After the incubation the plate was washed 5 times with the kit’s washing buffer followed by the addition of 200μL of peroxidase-labeled antibody against digoxigenin (anti-DIG-POD) and incubated for 60 min at 37 °C. After that, the wells were washed five times with the kit’s 250 µL washing buffer and incubated with 200 µL of ABTS substrate solution for about 10 min at rt (25 °C) at 250 rpm. The absorbance was taken at 405 nm (with reference wavelength at 490 nm) on a microplate reader (MRX II; Dynex Technologies, Chantilly, VA). The IC50 values were calculated using the Prism Software (Graphpad, San Diego, CA) from titration curves generated from the experimental values.
Molecular Modeling
The 3D structures of the inhibitors 1a and 2a were constructed using standard geometric parameters provided by the molecular modeling software package SYBYL 8.0. Single optimized conformation of each molecule was energetically minimized employing the Tripos force field [51] and the Powell conjugate gradient algorithm [52] with a convergence criterion of 0.05 kcal/mol•Å and Gasteiger–Hückel charges [53]. Molecular docking and scoring protocols were carried out with GOLD 5.1 (Cambridge Crystallographic Data Centre, Cambridge, UK) [54]. To investigate the binding mode of 1a and 2a GOLD 5.1 default parameters were used. The coordinates for HIV-1 RT solved at 2.8 Å (PDB ID 2HMI) [55] were used during the molecular modeling investigation. Hydrogen atoms were added in standard geometry using the GOLD 5.1 wizard. Histidine, glutamine, and asparagine residues within the binding site were manually checked for possible flipped orientation, protonation, and tautomeric states GOLD side chain wizard. The binding cavity of HIV-1 RT was defined as all the amino acid residues encompassed within a 20 Å radius sphere centered on the three dimensional coordinates (–4.029; 122.631; 11.880) of the side chain OH of Tyr188 in the p51 subunit. For each ligand the docking protocols were repeated twenty five times. The ASP (Astex Statistical Potential) scoring function and visual inspection were employed to select the representative conformation for each inhibitor.
Cytotoxicity
The cytotoxicity of the unsaturated fatty acids against peripheral blood mononuclear cells (PBMC) was tested as described by Sanabria-Ríos et al [56]. Briefly, PBMCs were cultured in a culture medium supplemented with interleukin-2 (IL-2). These cells were seeded into a 96-well microplate (20,000 cells/200 µL/well) and fatty acids were added to the cell cultures. The final concentrations of fatty acids ranged from 5 to 500 µM. The cells were incubated at 37 °C for 3 days in a humidified 5% CO2 incubator. The cytotoxicity of the cells was evaluated by the MTT assay.
Results and Discussion
Synthesis
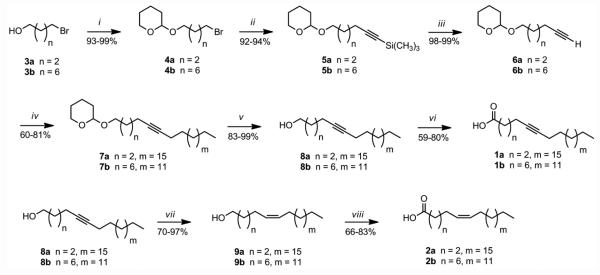
The 5- and 9-pentacosynoic acids 1a and 1b were prepared according to Scheme 1. The synthesis of 1a and 1b started with the protection of either commercially available 4-bromo-1-butanol (3a) or 8-bromo-1-butanol (3b) utilizing 3,4-dihydro-2H-pyran (DHP) in chloroform with catalytic amounts of p-toluenesulfonic acid (p-TSA) at rt for 3h, affording 4a or 4b in 93-99% yields. The alkyne coupling of 4a or 4b with (trimethylsilyl)acetylene using n-BuLi in THF-HMPA at –78 °C, afforded silanes 5a or 5b in 92-94% yields. The removal of the trimethylsilyl protecting group in 5a or 5b was achieved with tetrabutylammonium fluoride (TBAF) in THF, which resulted in the terminal alkynes 6a or 6b in 98-99% yields. A second acetylenic coupling of 6a with 1-bromononadecane or 6b with 1-bromopentadecane using n-BuLi in THF-HMPA at −78 °C to 0 °C (the temperature was increased in order to increase the solubility of the bromo alkane which was added slowly for a period of 1h) resulted in the alkynes 7a or 7b in 60-81% yields. The deprotection of either 7a or 7b with p-TSA in methanol at 45 °C yielded the desired 5-pentacosyn-1-ol (8a) or 9-pentacosyn-1-ol (8b) in 83-99% yields. Subsequent oxidation of 8a or 8b with pyridinium dichromate (PDC) in dimethylformamide (DMF) afforded the 5-pentacosynoic acid (1a) or the 9-pentacosynoic acid (1b) in 59-80% yields. The overall yields for these six-step syntheses were 43% for 1a and 34% for 1b.
Scheme 1.
Synthesis of the pentacosynoic acids 1a and 1b, and the pentacosenoic acids 2a and 2b. (i) DHP, p-TSA, CHCl3, 3 h; (ii) n-BuLi, (trimethylsilyl)acetylene, HMPA, THF, − 78 °C; (iii) TBAF, THF, 0 °C; (iv) 1-bromononadecane or 1-bromopentadecane, n-BuLi, HMPA, THF, −78 °C to 0 °C, 24 h; (v) p-TSA, MeOH, 45 °C, 3 h; (vi) PDC, DMF, rt, 24 h; (vii) H2, Pd/C (10%), quinoline, hexane; (viii) PDC, DMF, rt , 24 h.
The natural products (5Z)-5-pentacosenoic acid (2a) and (9Z)-9-pentacosenoic acid (2b) were also synthesized as described in Scheme 1. This divergent synthetic procedure used the corresponding alkynols 8a and 8b obtained in the previous synthesis as starting points. Therefore, the synthesis of 2a or 2b started from the previously synthesized 8a or 8b, which were hydrogenated utilizing Lindlar’s conditions (hydrogen gas, 10% Pd/C and quinoline in hexane) in order to obtain the (5Z)-5-pentacosen-1-ol (9a) or the (9Z)-9-pentacosen-1-ol (9b) in 70-97% yields. The pentacosenols 9a or 9b were oxidized with PDC in DMF at rt resulting in either the (5Z)-5-pentacosenoic acid (2a) in an 83% yield or in the (9Z)-9-pentacosenoic acid (2b) in a 66% yield. The synthesis of 2a was completed with an overall yield of 58% for the 8 steps (last two steps having a combined yield of 81%), while the synthesis of 2b was completed with an overall yield of 20%. This is the first total synthesis for either 2a or 2b, which permits the full characterization of these fatty acids that were previously identified in nature by only gas chromatographic means [38-44].
When comparing the values of the 13C chemical shifts (ppm) for the sp carbons of the monoalkynoic fatty acids 1a and 1b, it can be seen that the further away the alkyne group is from the carboxylic acid the smaller the difference between the chemical shifts of the two alkyne signals. For example, 1a displays a difference of 3.0 ppm (81.5 ppm, 78.5 ppm) for these carbons while 1b only displays a difference of 0.3 ppm (80.3 ppm, 80.0 ppm). The same principle applies to the monoenoic fatty acids, where the difference between the 13C NMR sp2 carbons of 2a was 3.3 ppm (131.4 ppm, 128.1 ppm), while the difference in the chemical shifts values for 2b was 0.3 ppm (130.0 ppm, 129.7 ppm).
According to Gunstone these defined differences in chemical shifts can be utilized to characterize unsaturated fatty acids and determine the position of the unsaturation [57]. In a monoacetylenic fatty acid where the triple bond is far away from the carboxyl group it has a characteristic 13C NMR chemical shift of 80.19 ppm, however, due to the differing effect of the carboxyl group on the two sp carbon atoms this signal frequently appears as two signals [57]. In his work, Gunstone discovered that the differences for the sp carbons of Δ5 and Δ9 C18 monoacetylenic fatty acids were 2.91 and 0.30 ppm, respectively [57]. These values concur with the experimental values of our C25 monoacetylenic fatty acids 1a and 1b.
In the case of a monoenoic cis fatty acid, if the double bond is sufficiently far from the carboxyl group as well as from the end methyl group, the two olefinic carbon atoms display the same chemical shift at ~129.9 ppm. However, the closer the two olefinic carbons are to the carboxyl group, the greater the difference between their chemical shifts having the greatest differential value the olefinic carbons in a Δ2 fatty acid and decreasing until it is not apparent for the olefinic carbons in a Δ12 fatty acid. In the case of Δ5 and Δ9 C18 monoenoic fatty acids the 13C chemical shifts differences for the twosp2 carbons are 3.23 and 0.26 ppm, respectively [57]. These values also coincide with the experimental values of our C25 monoenoic fatty acids; for 2a and 2b the 13C NMR differences for the sp2 carbons chemical shifts were 3.3 and 0.30 ppm, respectively. This data confirms how the carboxyl group influences the olefinic 13C NMR chemical shifts in very long chain fatty acids as well as it provides additional corroboration of their characterization.
HIV-RT Inhibitory Studies
Long-chain unsaturated fatty acids, such as nervonic acid, are non-competitive HIV-1 RT inhibitors [36]. Based on this, our goal was to assess the natural fatty acids 2a and 2b, as well as other analogs as potential inhibitors of the DNA polymerase, reverse transcriptase (RT) and compare their inhibitory activities to nervonic acid. In this regard, we modified an existing protocol for screening fatty acid candidates using a non-radioactive colorimetric assay method, which assesses the activity of HIV-1 RT [49-50]. The colorimetric enzyme immunoassay quantitatively determines the retroviral reverse transcriptase activity by measuring the incorporation of digoxigenin- and biotin-labeled dUTP into DNA. The DNA molecule labeled with biotin nucleotides binds to the streptavidin coated MP module, followed by the binding of the peroxidase-labeled digoxigenin antibody molecule to the DNA molecule. Then, the 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS) is added. The peroxidase catalyzes the cleavage of ABTS resulting in a colored product. The absorbance is directly correlated to the amount of DNA synthesized, thus it is proportional to the level of RT activity.
In this study, RT inhibitory assays were performed in duplicate in a range of 0.01-1000 μM for each acid along with a positive control (no inhibitor of HIV-1 RT), negative control (no enzyme HIV-1-RT), inhibitor control (nervonic acid instead of experimental agent) and solvent control (DMSO instead of experimental agent). Our results first confirmed that DMSO can be used as solvent for the experimental agents at maximal and final concentration of 2% with no effect on the activity of the HIV-1 RT. The results of the experimental agents were compared with a positive control, nervonic acid (NA), which according to our results displayed an IC50 of 12 ± 1 mM. These results support previous studies indicating that NA inhibits HIV-1 RT in the micromolar range [36].
As starting point for our structural studies of fatty acids, we used commercially available palmitic acid (16:0) as well as pentacosanoic acid (25:0). Our results revealed that the saturated as well as other shorter chain acetylenic fatty acids (C17 or C20) were the least potent of the acids tested (IC50 > 1000 mM). Among the acetylenic fatty acids evaluated (Table 1), acid 1a was the best inhibitor of HIV-1 RT with an IC50 of 24 ± 1 μM. We were not able to effectively determine the inhibitory activity of acid 1b towards the HIV-RT enzyme. When the position of the triple bond in the C25 acyl chain was changed from Δ5 to Δ2, a decrease in the inhibitory activity towards HIV-1 RT was observed, as exemplified by an IC50 of 566 ± 1 μM displayed by the 2-pentacosynoic acid. Moreover, when a shorter chain (C17 or C20) acetylenic fatty acid was tested, as exemplified by the natural fatty acids 6-heptadecynoic and 6-icosynoic acids, no inhibition of the HIV-1 RT enzyme was observed [58]. Therefore, we can say that both fatty acid chain length and the triple bond position are directly related to the effectiveness of inhibition against the HIV-1 RT.
Table 1.
The reverse transcriptase (RT) inhibitory activity of the tested fatty acids
| Experimental agents | IC50 (μM)a |
|---|---|
| Palmitic acid (16:0) | >1000 |
| 6-Heptadecynoic acid (17:1)b | >1000 |
| 6-Icosynoic acid (20:1)b | >1000 |
| 2-Pentacosynoic acidc | 566 ± 1 |
| 5-Pentacosynoic acid (1a) | 24 ± 1 |
| (5Z)-5-Pentacosenoic acid (2a) | 38 ± 1 |
| (9Z)-9-Pentacosenoic acid (2b) | 54 ± 1 |
| Nervonic acid (NA) | 12 ± 1 |
| Pentacosanoic acid (25:0) | >1000 |
| 2% DMSO | >1000 |
RT inhibitory assays were performed in duplicate. The IC50 values were calculated using Prism Software (Graphpad, San Diego, CA) from titration curves generated from the experimental values.
The synthesis of the shorter-chain acetylenic fatty acids was previously described [58].
Obtained from the reaction of 1-tetracosyne with CO2 using n-BuLi in THF.
With respect to the monoenoic fatty acids, changing the position of the double bonds from Δ5 to Δ9 in the C25 acyl chain had some minor effect on the inhibitory activity towards HIV-RT (Table 1). For example, acid 2a displayed an IC50 of 38 ± 1 μM against HIV-1 RT, while acid 2b displayed an IC50 of 54 ± 1 µM. This result also confirms that the unsaturation sites in C25 monoenoic fatty acids are critical for the inhibitory activity towards HIV-RT to take place.
The degree of unsaturation in fatty acids affects its inhibitory activity towards DNA polymerase enzymes such the DNA polymerase β [33-35]. Since HIV-1 RT shares structural similarities with the DNA pol β enzyme [36], this would imply that the unsaturation should also affect the inhibitory activity of fatty acids towards the RT enzyme as we have shown. Our results do reveal a dependency between the degree of unsaturation and the inhibitory activity of these fatty acids, e.g., 25:1 vs. 25:0. We were also able to establish that the longer the fatty acid chain (C25 vs. C20 or C17) the greater its inhibitory activity. This coincides with previous studies with the HIV-1 RT as well as with DNA pol β, where the long-chain fatty acids had greater inhibitory activity against these enzymes [33].
Molecular Modeling Studies
To better understand the relationships between fatty acid chain length, degree of unsaturation and inhibitory activity, we modeled the binding modes of acids 1a and 2a to the p51 subunit of HIV-1 RT. Both fatty acids tested herein have similar molecular interactions in the HIV-RT binding pocket as those exhibited by nervonic acid [36] (Figures 1A and 1B). The carboxylate group of these fatty acids interacts with key amino acids such as Lys65, Lys66, Lys220 and Tyr232, while their long alkyl chain establishes attractive hydrophobic interactions with the side chain of Lys104, Ile195, and His235. The modeling studies also indicated that shorter carbon chains would not be capable of stabilizing the molecule within the binding site. An ideal length of 25 carbons is required to fulfill the binding site, thereby inhibiting the enzymatic activity. Furthermore, the small difference in the inhibitory activities between 1a and 2a might be due to the cis-configuration of acid 2a preventing it from fully occupying the HIV-RT hydrophobic cavity formed by Lys104, Ile195 and His235 (Figures 1C and 1D). It is also important to note that the unsaturations constrain the alkyl chain degrees of freedom, thereby orienting the carbon chain toward the hydrophobic pocket close to Ile195, Lys104 and His235. The latter can explain the fact that pentacosanoic acid does not inhibit HIV-1 RT at all.
Fig. 1.
Modeled binding modes of the fatty acids 1a (green) and 2a (orange) in the p51 subunit of HIV-1 RT. (A and B) Inhibitors and residues involved in the ligand-receptor binding are indicated as stick models, protein structure is indicated as cartoon and electrostatic interactions as yellow dashed lines. (C and D) View of the fatty acids binding site indicating the solvent accessible surface of p51 subunit and the spatial complementarity of the inhibitors (sphere model).
Cytotoxicity
The cytotoxicity of the fatty acids 1a, 1b, 2a, 2b as well as nervonic acid were tested against peripheral blood mononuclear cells (PBMCs) isolated from healthy volunteers following a methodology previously described by us [56]. In this assay none of the synthetic fatty acids tested, including nervonic acid, displayed significant toxicity at concentrations higher than 500 µM. Comparing these results with the effective dose necessary for HIV-1 RT inhibition, both acids 1a and 2a would not be cytotoxic at their optimum inhibitory dose concentrations of 24 μM and 38 μM, respectively.
Conclusions
In summary, we have shown that 2a, as well as its acetylenic analogue 1a, are inhibitors of the HIV-1 RT enzyme with similar molecular interactions as those previously described for nervonic acid on the basis of molecular modeling and enzyme inhibitory studies. These results open the door to the synthesis of other structurally related analogs, which can combine in a single molecule, favorable structural features of both NA and 1a for a more efficient HIV-RT inhibition. On the other hand, we accomplished the first total synthesis for the naturally occurring fatty acids 1b and 2b, which also allowed us to validate the use of 13C NMR spectroscopy as a tool for the identification of the position of the unsaturation in fatty acids with C25 chain lengths.
Table 2.
Cytotoxicity of the unsaturated fatty acids against peripheral blood mononuclear cells (PBMCs) isolated from healthy volunteers.a
| Compound | IC50 (μM) |
|---|---|
| Nervonic Acid | > 500 |
| 1a | > 500 |
| 2a | > 500 |
| 1b | > 500 |
| 2b | > 500 |
Experiments were performed in six replicates (n = 6).
Acknowledgments
Part of the work described herein was initially supported by Award Number SC1GM084708 from the National Institutes of General Medical Sciences of the NIH. K. Rosado acknowledges the support of the UPR RISE program for an undergraduate fellowship. We thank Dr. Fred Strobel (Emory University) for the high resolution mass spectral data.
Abbreviations
- DMSO
Dimethyl sulfoxide
- FA
Fatty acids
- GC-MS
Gas chromatography-mass spectrometry
- HAART
Highly Active-antiretroviral therapy
- HIV
Human immunodeficiency virus
- IC50
Inhibitory concentration for half-life maximal inhibition
- NA
Nervonic acid
- NNRTI
Non-nucleoside retrotranscriptase inhibitors
- NRTI
Nucleoside retrotranscriptase inhibitors
- PBMC
Peripheral blood mononuclear cells
- PDC
Pyridinium dichromate
- p-TSA
p-Toluenesulfonic acid
- RT
Reverse transcriptase
- THF
Tetrahydrofuran
References
- 1.Carballeira NM. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog Lipid Res. 2008;47:50–61. doi: 10.1016/j.plipres.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parang K, Wiebe LI, Knaus EE, Huang JS, Tyrrell DL, Csizmadia F. In vitro antiviral activities of myristic acid analogs against human immunodeficiency and hepatitis B viruses. Antiviral Research. 1997;34:75–90. doi: 10.1016/s0166-3542(96)01022-4. [DOI] [PubMed] [Google Scholar]
- 3.Carballeira NM, Miranda C, Orellano EA, Gonzalez FA. Synthesis of a novel series of 2-methylsulfanyl fatty acids and their toxicity on the human K-562 and U-937 leukemia cell lines. Lipids. 2005;40:1063–1068. doi: 10.1007/s11745-005-1470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy S, Langelier Y, Prentki M. Oleate activates phosphatidylinositol 3-kinase and promotes proliferation and reduces apoptosis of MDA-MB-231 breast cancer cells, whereas palmitate has opposite effects. Cancer Res. 2000;60:6353–6358. [PubMed] [Google Scholar]
- 5.Schwarz S, Hufnagel B, Dworak M, Klumpp S, Krieglstein J. Protein phosphatase type 2Calpha and 2Cbeta are involved in fatty acid-induced apoptosis of neuronal and endothelial cells. Apoptosis. 2006;11:1111–1119. doi: 10.1007/s10495-006-6982-1. [DOI] [PubMed] [Google Scholar]
- 6.Lin T, Yin XB, Cai Q, Fan X, Xu K, Huang L, Luo J, Zheng J, Huang J. 13-Methyltetradecanoic acid induces mitochondrial-mediated apoptosis in human bladder cancer cells. Urologic Oncology. 2012;30:339–345. doi: 10.1016/j.urolonc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Heath RJ, Rubin JR, Holland DR, Zhang E, Snow ME, Rock CO. Mechanism of triclosan inhibition of bacterial fatty acid synthesis. J Biol Chem. 1999;274:11110–1114. doi: 10.1074/jbc.274.16.11110. [DOI] [PubMed] [Google Scholar]
- 8.Perozzo R, Kuo M, Sidhu AbS, Valiyaveettil JT, Bittman R, Jacobs WR, Jr, Fidock DA, Sacchettini JC. Structural elucidation of the specificity of the antibacterial agent triclosan for malarial enoyl acyl carrier protein reductase. J Biol Chem. 2002;277:13106–13114. doi: 10.1074/jbc.M112000200. [DOI] [PubMed] [Google Scholar]
- 9.Pillai S, Rajagopal C, Kapoor M, Kumar G, Gupta A, Surolia N. Functional characterization of beta-ketoacyl-ACP reductase (FabG) from Plasmodium falciparum. Biochem Biophys Res Comm. 2003;303:387–392. doi: 10.1016/s0006-291x(03)00321-8. [DOI] [PubMed] [Google Scholar]
- 10.Tasdemir D, Topaloglu B, Perozzo R, Brun R, O’neill R, Carballeira NM, Zhang X, Tonge PJ, Lindeng A, Rüedi P. Marine natural products from the Turkish sponge Agelas oroides that inhibit the enoyl reductases from Plasmodium falciparum, Mycobacterium tuberculosis and Escherichia coli. Bioorg Med Chem. 2007;15:6834–6845. doi: 10.1016/j.bmc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 11.Pungitore CR. Natural products as inhibitors of DNA related enzymes. Current Enzyme Inhibition. 2008;4:194–215. [Google Scholar]
- 12.Richardson ET, Collins SE, Kung T, Jones JH, Hoan Tram K, Boggiano VL, Bekker LG, Zolopa AR. Gender inequality and HIV transmission: a global analysis. J Int AIDS Soc. 2014;17:19035. doi: 10.7448/IAS.17.1.19035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ntemgwa ML, d’Aquin Toni T, Brenner BG, Camacho RJ, Wainberg MA. Antiretroviral drug resistance in human immunodeficiency virus type 2. Antimicrob Agents Chemother. 2009;53:3611–3619. doi: 10.1128/AAC.00154-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan JJ, Cong XJ, Hu LM, Wang CX, Jia L, Liang X-J. Therapeutic strategies underpinning the development of novel techniques for the treatment of HIV infection. Drug Discovery Today. 2010;15:186–197. doi: 10.1016/j.drudis.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adamson CS, Freed EO. Novel approaches to inhibiting HIV-1 replication. Antiviral Res. 2010;85:119–141. doi: 10.1016/j.antiviral.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarafianos SG, Marchand B, Das K, Himmel DM, Parnaik MA, Hughes SH, Arnold E. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J Mol Biol. 2009;385:693–713. doi: 10.1016/j.jmb.2008.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goff SP. Retroviral reverse transcriptase: synthesis, structure, and function. J Acquir Immune Defic Syndr. 1990;3:817–831. [PubMed] [Google Scholar]
- 18.Ivetac A, McCammon JA. Elucidating the inhibition mechanism of HIV-1 non-nucleoside reverse transcriptase inhibitors through multicopy molecular dynamics simulations. J Mol Biol. 2009;388:644–658. doi: 10.1016/j.jmb.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Béthune M-P. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years (1989-2009) Antiviral Res. 2010;85:75–90. doi: 10.1016/j.antiviral.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Götte M, Rausch JW, Marchand B, Sarafianos S, Le Grice SF. Reverse transcriptase in motion: conformational dynamics of enzyme-substrate interactions. J Biochim Biophys Acta. 2010;1804:1202–1212. doi: 10.1016/j.bbapap.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alcaro S, Alteri C, Artese A, Ceccherini-Silberstein F, Costa G, Ortuso F, Parrotta L, Perno CF, Svicher V. Molecular and structural aspects of clinically relevant mutations related to the approved non-nucleoside inhibitors of HIV-1 reverse transcriptase. Drug Resist Updat. 2011;14:141–149. doi: 10.1016/j.drup.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Ravich VL, Masso M, Vaisman II. A combined sequence-structure approach for predicting resistance to the non-nucleoside HIV-1 reverse transcriptase inhibitor. Nevirapine Biophys Chem. 2011;153:168–172. doi: 10.1016/j.bpc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Jacobo-Molina A, Ding J, Nanni RG, Clark AD, Jr, Lu X, Tantillo C, Williams RL, Kamer G, Ferris AL, Clark P, Hizi A, Hughes SH, Arnold E. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 Å resolution shows bent DNA. Proc Natl Acad Sci USA. 1993;90:6320–6324. doi: 10.1073/pnas.90.13.6320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menéndez-Arias L, Betancor G, Matamoros T. HIV-1 reverse transcriptase connection subdomain mutations involved in resistance to approved non-nucleoside inhibitors. Antiviral Res. 2011;92:139–149. doi: 10.1016/j.antiviral.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Loya S, Rudi A, Kashman Y, Hizi A. Mode of inhibition of HIV-1 reverse transcriptase by polyacetylenetriol, a novel inhibitor of RNA- and DNA-directed DNA polymerases. Biochem J. 2002;362:685–692. doi: 10.1042/0264-6021:3620685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cihlar T, Ray AS. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antiviral Res. 2010;85:39–58. doi: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Vadivelan S, Deeksha TN, Arun S, Machiraju PK, Gundla R, Sinha BN, Jagarlapudi SA. Virtual screening studies on HIV-1 reverse transcriptase inhibitors to design potent leads. Eur J Med Chem. 2011;46:851–859. doi: 10.1016/j.ejmech.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Ren J, Esnouf R, Garman E, Somers D, Ross C, Kirby I, Keeling J, Darby G, Jones Y, Stuart D, Stammers D. High resolution structures of HIV-1 RT from four RT-inhibitor complexes. Nat Struct Biol. 1995;2:293–302. doi: 10.1038/nsb0495-293. [DOI] [PubMed] [Google Scholar]
- 29.Rios A, Quesada J, Anderson D, Goldstein A, Fossum T, Colby-Germinario S, Wainberg MA. Complete inactivation of HIV-1 using photo-labeled non-nucleoside reverse transcriptase inhibitors. Viral Res. 2011;155:189–194. doi: 10.1016/j.virusres.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal structure at 3.5 Å resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 31.Goud TV, Reddy NS, Swamy NR, Ram TS, Venkateswarlu Y. Two new bromotyrosine-derived metabolites from the sponge Psammaplysilla purpurea. Biol Pharm Bull. 2003;51:990–993. doi: 10.1248/cpb.51.990. [DOI] [PubMed] [Google Scholar]
- 32.Sargent JR, Coupland K, Wilson R. Nervonic acid and demyelinating disease. Med Hypothesese. 1994;42:237–242. doi: 10.1016/0306-9877(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 33.Mizushina Y, Yoshida S, Matsukage A, Sakaguchi K. The inhibitory action of fatty acids on DNA polymerase beta. Biochim Biophys Acta. 1997;1336:509–521. doi: 10.1016/s0304-4165(97)00067-6. [DOI] [PubMed] [Google Scholar]
- 34.Mizushina Y, Ohkubo T, Date T, Yamaguchi T, Saneyoshi M, Sugawara F, Sakaguchi K. Mode analysis of a fatty acid molecule binding to the N-terminal 8-kDa domain of DNA polymerase beta. A 1:1 complex and binding surface. J Biol Chem. 1999;274:25599–25607. doi: 10.1074/jbc.274.36.25599. [DOI] [PubMed] [Google Scholar]
- 35.Mizushina Y, Sugawara F, Lida A, Sakaguchi K. Structural homology between DNA binding sites of DNA polymerase beta and DNA topoisomerase II. J Mol Biol. 2000;304:385–395. doi: 10.1006/jmbi.2000.4223. [DOI] [PubMed] [Google Scholar]
- 36.Kasai N, Mizushina Y, Sugawara F, Sakaguchi K. Three-dimensional structural model analysis of the binding site of an inhibitor, nervonic acid, of both DNA polymerase beta and HIV-1 reverse transcriptase. J Biochem. 2002;132:819–828. doi: 10.1093/oxfordjournals.jbchem.a003292. [DOI] [PubMed] [Google Scholar]
- 37.Rezanka T, Sigler K. Odd-numbered very-long-chain fatty acids from the microbial, animal and plant kingdoms. Prog Lipid Res. 2009;48:206–238. doi: 10.1016/j.plipres.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Takayama K, Qureshi N. Isolation and characterization of the monounsaturated long chain fatty acids of Mycobacterium tuberculosis. Lipids. 1978;13:575–579. doi: 10.1007/BF02535818. [DOI] [PubMed] [Google Scholar]
- 39.Barnathan G, Kornprobst JM, Doumenq P, Miralles J. New unsaturated long-chain fatty acids in the phospholipids from the Axinellida sponges Trikentrion loeve and Pseudaxinella cf. lunaecharta. Lipids. 1996;31:193–200. doi: 10.1007/BF02522620. [DOI] [PubMed] [Google Scholar]
- 40.Christie WW, Brechany EY, Stefanov K, Popov S. The fatty acids of the sponge Dysidea fragilis from the Black Sea. Lipids. 1992;27:640–644. [Google Scholar]
- 41.Carballeira NM, Shalabi F. Unusual lipids in the Caribbean sponges Amphimedon viridis and Desmapsamma anchorata. J Nat Prod. 1994;57:1152–1159. doi: 10.1021/np50110a004. [DOI] [PubMed] [Google Scholar]
- 42.Makarieva TN, Santalova EA, Gorshkova IA, Dmitrenok AS, Guzii AG, Gorbach VI, Svetashev VI, Stonik VA. A new cytotoxic fatty acid (5Z,9Z)-22-methyl-5,9-tetracosadienoic acid and the sterols from the far Eastern sponge Geodinella robusta. Lipids. 2002;37:75–80. doi: 10.1007/s11745-002-0866-6. [DOI] [PubMed] [Google Scholar]
- 43.Carballeira NM, Alicea J. Novel methoxylated FA from the Caribbean sponge Spheciospongia cuspidifera. Lipids. 2002;37:305–308. doi: 10.1007/s11745-002-0895-1. [DOI] [PubMed] [Google Scholar]
- 44.Nechev J, Christie WW, Robaina R, de Diego F, Popov S, Stefanov K. Chemical composition of the sponge Hymeniacidon sanguinea from the Canary Islands. Comp Biochem Physiol. 2004;137A:365–374. doi: 10.1016/j.cbpb.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 45.Kumar KR, Satyanarayana PVV, Reddy BS. Simple and efficient method for tetrahydropyranylation of alcohols and phenols by using silica supported sodium hydrogen sulphate as a catalyst. Asian J Chem. 2012;24:3876–3878. [Google Scholar]
- 46.Shipman M, Thorpe HR, Clemens IR. Generation and trapping of allene oxides: an approach to chiral, nonracemic α-alkoxyketones. Tetrahedron. 1998;54:14265–14282. [Google Scholar]
- 47.Bjoerkling F, Norin T, Unelius CR, Miller RB. A stereospecific synthesis of all four isomers of 9,11-tetradecadienyl acetate using a general method applicable to 1,3-dienes. J Org Chem. 1987;52:292–294. [Google Scholar]
- 48.Albu S, Sverko E, Arts MT, Capretta A. Synthesis of deuterated 5(Z),11(Z)-eicosadienoic acid as a biomarker for trophic transfer. Tetrahedron Lett. 2011;52:787–788. [Google Scholar]
- 49.Suzuki K, Craddock BP, Kano T, Steigbigel RT. Colorimetric reverse transcriptase assay for HIV-1. J Virol Methods. 1993;41:21–28. doi: 10.1016/0166-0934(93)90159-o. [DOI] [PubMed] [Google Scholar]
- 50.Bustanji Y, Al-Masri IM, Qasem A, Al-Bakri AG, Taha MO. In silico screening for non-nucleoside HIV-1 reverse transcriptase inhibitors using physicochemical filters and high-throughput docking followed by in vitro evaluation. Chem Biol Drug Des. 2009;74:258–265. doi: 10.1111/j.1747-0285.2009.00852.x. [DOI] [PubMed] [Google Scholar]
- 51.Clark M, Cramer RD, van Opdenbosch N. Validation of the general purpose tripos 5.2 force field. J Comput Chem. 1989;10:982–1012. [Google Scholar]
- 52.Powell MJD. Restart procedures for the conjugate gradient method. Math Program. 1977;12:241–254. [Google Scholar]
- 53.Gasteiger J, Marsili M. Iterative partial equalization of orbital electronegativity—a rapid access to atomic charges. Tetrahedron. 1980;36:3219–3228. [Google Scholar]
- 54.Jones G, Willett P, Glen RC, Leach AR, Taylor RD. Development and validation of a genetic algorithm for flexible docking. J Mol Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 55.Ding J, Das K, Hsiou Y, Sarafianos SG, Clark AD Jr, Jacobo-Molina A, Tantillo C, Hughes SH, Arnold E. Structure and functional implications of the polymerase active site region in a complex of HIV-1 RT with a double-stranded DNA template-primer and an antibody Fab fragment at 2.8 Å resolution. J Mol Biol. 1998;284:1095–1111. doi: 10.1006/jmbi.1998.2208. [DOI] [PubMed] [Google Scholar]
- 56.Sanabria-Ríos DJ, Rivera-Torres Y, Maldonado-Domínguez G, Domínguez I, Ríos C, Díaz D, Rodríguez JW, Altieri-Rivera JS, Rios-Olivares E, Cintrón G, Montano N, Carballeira NM. Antibacterial activity of 2-alkynoic fatty acids against multidrug-resistant bacteria. Chem Phys Lipids. 2014;178:84–91. doi: 10.1016/j.chemphyslip.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gunstone FD, Pollard MR, Scrimgeour CM, Gilman, NW, Hollard, BC. Fatty acids. Part 48. 13C nuclear magnetic resonance studies of acetylenic fatty acids. Chem Phys Lipids. 1976;17:1–13. doi: 10.1016/0009-3084(76)90031-1. [DOI] [PubMed] [Google Scholar]
- 58.Carballeira NM, Cartagena MM, Fernández Prada C, Fernández Rubio C, Balaña-Fouce R. Total synthesis and antileishmanial activity of the naturally occurring acetylenic fatty acids 6-heptadecynoic acid and 6-icosynoic acid. Lipids. 2009;44:953–961. doi: 10.1007/s11745-009-3345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]