Abstract
The cytoskeleton is a complex of detergent-insoluble components of the cytoplasm playing critical roles in cell motility, shape generation, and mechanical properties of a cell. Fibrillar polymers—actin filaments, microtubules, and intermediate filaments—are major constituents of the cytoskeleton, which constantly change their organization during cellular activities. The actin cytoskeleton is especially polymorphic, as actin filaments can form multiple higher order assemblies performing different functions. Structural information about cytoskeleton organization is critical for understanding its functions and mechanisms underlying various forms of cellular activity. Because of the nanometer-scale thickness of cytoskeletal fibers, electron microscopy (EM) is a key tool to determine the structure of the cytoskeleton.
This article describes application of rotary shadowing (or metal replica) EM for visualization of the cytoskeleton. The procedure is applicable to thin cultured cells growing on glass coverslips and consists of detergent extraction of cells to expose their cytoskeleton, chemical fixation to provide stability, ethanol dehydration and critical point drying to preserve three-dimensionality, rotary shadowing with platinum to create contrast, and carbon coating to stabilize replicas. This technique provides easily interpretable three-dimensional images, in which individual cytoskeletal fibers are clearly resolved, and individual proteins can be identified by immunogold labeling. More importantly, replica EM is easily compatible with live cell imaging, so that one can correlate the dynamics of a cell or its components, e.g., expressed fluorescent proteins, with high resolution structural organization of the cytoskeleton in the same cell.
Keywords: Electron microscopy, Cytoskeleton, Critical point drying, Rotary shadowing, Actin, Microtubules, Immunogold, Correlative microscopy
1 Introduction
Electron microscopy (EM) has been instrumental in discovering the cytoskeleton in the first place, and also in investigating its structural organization in different cells and conditions. The initial progress in the cytoskeletal studies closely paralleled the development of EM techniques. Thus, the introduction of heavy metal fixation led to the discovery of actin filaments in non-muscle cells [1], while the discovery of microtubules [2] was made possible after the introduction of aldehyde fixation [3].
A great value of EM is its ability to obtain structural information at a high resolution level, which for biological samples is limited by a sample preparation procedure rather than by the power of a transmission electron microscope (TEM). Vacuum in the TEM column and electron beam irradiation impose strict restrictions on how samples should be prepared, which in turn greatly affect the quality of images and the rate of success. A large number of different EM protocols have been developed over the years to improve the quality of samples and the amount of collected information, and to avoid artifacts. Each technique has its pluses and minuses, making it more suitable for some applications than for others.
The thin sectioning technique was initially a dominant way to visualize the cytoskeleton [4, 5]. It involves the embedding of chemically fixed specimens into a resin followed by thin sectioning to allow for beam penetration. Contrast is generated by positive staining of the sections with heavy metal salts, and the limited ability of stains to bind bioorganic material reduces the resolution of this technique. Thin sections provide a 2D view of the sample at a single plane, and a series of sections is required to retrieve the 3D information. Such reconstruction works well with relatively large and simple objects, but is not efficient in revealing the details of complex and delicately organized cytoskeletal structures, such as, for example, the actin filament networks in lamellipodia of locomoting cells.
Different versions of whole mount EM have been used to investigate the structural organization of the cytoskeleton in its entirety. Thus, the structural arrangement of actin filaments in lamellipodia was first visualized by the negative staining EM of cultured cells [6]. In this technique, partially permeabilized cells growing on EM grids are immersed into a heavy metal stain solution, which is blotted off shortly after, and the samples are dried in open air. The dried stain generates a dark amorphous background on which the structures appear as translucent shapes. Negative staining EM provides high resolution and allows one to see the thin regions of a cell all the way through. Weaknesses of the procedure include sample flattening during air drying, relatively low contrast, and low stability of the samples.
In cryo EM technique, the samples are quickly frozen (to prevent ice crystal formation) and viewed while still embedded in amorphous ice, either as whole mounts or after cryosectioning, so that they remain hydrated and the proteins retain their natural conformation [7, 8]. To view frozen samples, a TEM should be equipped with a chilled sample holder and electron beam power and the observation time should be minimized to keep the specimen frozen. No contrasting procedures are used in this technique except for the specimen’s own contrast, which is quite low. Therefore, significant image processing is required for the presentation and analysis of images and many structures still remain undetected. The major limitation of the technique is the significant difficulty in obtaining successful samples.
In metal replica EM, heavy metals are evaporated onto a 3D sample at an angle, which reveals its surface topography [9]. The quality of the samples is greatly enhanced if rotary, and not unilateral, coating is used, as it helps to avoid deep featureless shadows. As metal coating is not cohesive, it is subsequently stabilized by a layer of carbon, which keeps metal grains together and is fairly transparent for the electron beam. The coated sample, or just a metal–carbon replica, is subsequently removed from its original support and placed onto EM grids. The resolution of replica EM is quite high, but it depends on the metal grain size, the thickness of the coating, and the angle of shadowing. Platinum is the most popular metal, as it provides a good compromise between the grain size and an ease of evaporation. The replica technique was initially introduced to study freeze-fractured samples [10], but it is applicable for a large range of samples, such as single molecules [11, 12], cells [13–15], and tissues [16, 17]. This approach can reveal the 3D structure in great detail, but it is limited by the depth of shadowing penetration.
For our studies of the cytoskeleton organization in cultured cells, we chose platinum replica EM, in which detergent extraction is used to expose the cytoskeleton; chemical fixation helps to preserve the sample structure; ethanol dehydration followed by critical point drying (CPD) preserves the cell’s 3D organization; and rotary shadowing with platinum creates contrast. Over the years, we have found a good combination of individual steps to develop a reliable and relatively simple protocol that consistently produces highly informative images with excellent yield that can be combined with immunochemistry [18–20]. However, this approach is not universal, but is limited to relatively thin samples attached to glass surfaces. Also, because of extensive fixation and dehydration, it can achieve the molecular level of resolution only for very large molecules [21, 22], but is optimal for analyses of the fine cytoskeletal architecture with a single filament resolution at the scale of a whole cell.
As EM, in general, cannot work with live samples, investigators can only guess the kind of activity the cell was involved in at the moment of fixation, and what it would do next. A partial solution for this problem is provided by correlative light and EM, in which the dynamics of a living cell is followed by time-lapse optical imaging, and the same sample is subsequently analyzed by EM. Our EM protocol made it possible to perform correlative light and EM routinely, as it allowed us to obtain high quality structural information for a cell of interest with high probability [15, 18–20]. Several other EM techniques have also been used in a correlative approach, including thin sections of resin-embedded samples [23–25], cryoEM [26–29], and negatively contrasted cells [30].
2 Materials
2.1 Cell Culture and Extraction
Small (6–12 mm) coverslips that can be made by cutting regular coverslips with a diamond pencil. Trapezoidal (square with one oblique side) coverslips lacking mirror symmetry are helpful to easily determine the cell-containing side. Commercially available 12 mm round coverslips are also acceptable (see Note 1).
Phosphate-buffered saline (PBS) with Ca2+ and Mg2+.
PEM buffer: 100 mM PIPES (free acid), pH 6.9 (adjust with KOH), 1 mM MgCl2, and 1 mM EGTA. Working buffer is prepared from 2× stock solution, which can be stored up to 1 month at 4°C (see Note 2).
Extraction solution: 1 % Triton X-100 in PEM buffer supplemented (optionally) with 1–4% polyethyleneglycol (PEG) (MW 20,000–40,000), 2 μM phalloidin, and/or 2 μM taxol (paclitaxel) (see Note 3). Use a stirrer and allow 15–20 min to dissolve PEG. Extraction solutions can be stored for up to 3 days at 4°C, but phalloidin and taxol should be added before use. Stock solutions (1000×) of phalloidin and taxol are made in dimethylsulfoxide (DMSO) and stored at −20°C in aliquots.
2.2 Fixation
Glutaraldehyde solution: 2 % EM grade glutaraldehyde in 0.1 M sodium cacodylate, pH 7.3. The working solution can be stored at 4 °C for up to 1 week, 2× stock solution of sodium cacodylate is stable at 4 °C. Caution: Glutaraldehyde is toxic and volatile, so a fume hood should be used when working; sodium cacodylate is toxic.
Tannic acid solution: 0.1 % tannic acid in distilled water. Use within a day.
Aqueous uranyl acetate solution: 0.2 % uranyl acetate in distilled water. Use a stirrer to dissolve. Store at room temperature. Caution: Uranyl acetate is toxic.
2.3 Dehydration and Critical Point Drying
Graded ethanol solutions: 10, 20, 40, 60, 80 and 100 % ethanol in distilled water.
Alcohol uranyl acetate solution: 0.2 % uranyl acetate in 100 % ethanol. Use a stirrer to dissolve. Use within a day. Caution: Uranyl acetate is toxic.
Dehydrated ethanol: Wash molecular sieves (4 Å, 8–12 mesh) with several changes of water to remove dust. Dry in the air, bake at 160 °C overnight, cool down, and add to a bottle of 100 % ethanol (~50 g per 500 mL). Seal with Parafilm and store at room temperature. Do not shake, as the beads are fragile and easily generate dust.
CPD sample holder and scaffolds: A holder with a lid and two scaffolds are homemade with a stainless-steel wire mesh. The holder should fit into the chamber of the CPD apparatus. A scaffold is required to maintain the holder above the stirring bar during stirring. It should fit a beaker in which dehydration will be processed, e.g., a 50 mL glass beaker.
CPD device: We use Samdri PVT-3D (Tousimis) CPD with manual operation, but other devices are also appropriate.
Carbon dioxide: Use liquid dehydrated CO2 (bone dry grade) in a tank with a siphon (deep tube) and a water and oil-absorbing filter (Tousimis). Siphonized tanks make it possible to take the liquid phase of CO2 from the bottom of the tank.
2.4 Platinum and Carbon Coating
Vacuum evaporator: We use Auto 306 coater (Boc Edwards) equipped with a water-cooled diffusion pumping system, carbon and metal evaporation sources, a rotary stage, and a thickness monitor.
Metals for evaporation (Ted Pella): Tungsten wire (0.76 mm), platinum wire (0.2 mm), carbon rods (3 mm).
2.5 Preparation of Replicas
Hydrofluoric acid (HF): 5–10 % HF in distilled water is prepared from the concentrated acid (49 %). Do not use glassware to handle acid containing solutions. Caution: Extremely volatile and toxic, it causes severe skin burns. Use a fume hood and gloves.
Platinum loop: The optimal loop diameters are 3–5 mm.
EM Grids: Formvar-coated EM grids with low mesh size (e.g., 50) to provide a large viewing area. Other options are acceptable.
2.6 Immunogold EM
Immunogold buffer: 20 mM Tris–HCl, pH 8.0, 0.5 M NaCl, and 0.05 % Tween 20. For dilution of antibodies, the buffer is supplemented with 1 % bovine serum albumin (BSA); for washing, 0.1 % BSA is added. Stock solutions (5×) are stable at 4 °C for several months. Sodium azide can be added to the stock solution to prevent microbial contamination. Caution: Sodium azide is toxic.
Quenching solution: 2 mg/mL sodium borohydrate (NaBH4) in PBS. Use immediately.
Blocking solution: 1 mg/mL glycine (or lysine) in PBS. Stable at 4 °C.
2.7 Correlative EM
Marked coverslips: Homemade coverslips with reference marks are prepared by evaporating gold through a finder grid placed in the middle of a 22 × 22 square or 25 mm round coverslip. A variety of finder grids are available commercially. Baking (160 °C overnight) of gold-coated coverslips is necessary for the firm adhesion of gold to glass (see Note 4). For light microscopy, choose cells on the clear footprint of the finder grid.
Light microscopy: The light microscopic system should be equipped with an environmental chamber to maintain normal cell behavior; it should allow for fast exchange of culture medium to extraction solution to quickly stop cellular activity during imaging. A simple option is to use open dishes on a heated stage with the cells growing in a bicarbonate-free medium.
3 Methods
In a basic form, replica EM can be used to study the cytoskeleton architecture in a cell population. In an advanced form, it can be combined with immunogold staining to detect specific proteins in the cytoskeleton (Fig. 1), and with light microscopy to correlate the cytoskeleton organization with cell behavior or with the distribution and dynamics of fluorescent probes (Figs. 1 and 2). The basic procedure consisting of extraction, fixation, dehydration, CPD, metal shadowing, and preparation of replicas is described first, and it is followed by the description of the advanced applications.
Fig. 1.
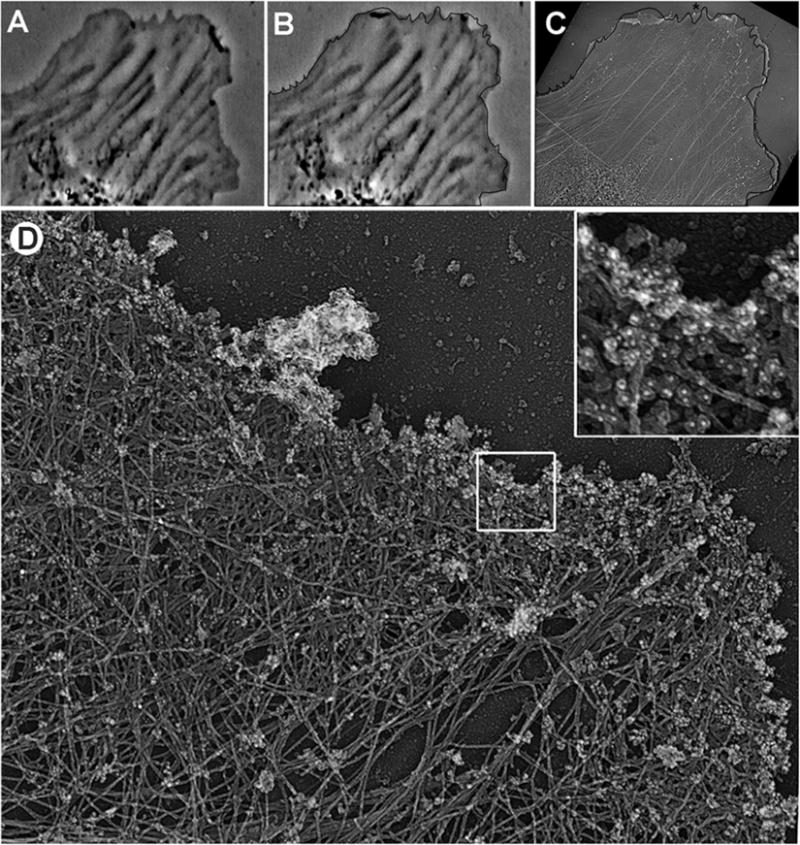
Correlative phase-contrast and EM of cultured Rat-2 fibroblast combined with immunogold staining of ADF/cofilin. (a and b) Frames from time-lapse sequence showing the last live cell image (b) and an image 12 s earlier (a). Black line in (b) shows the cell edge outline from (a). (c) Low magnification EM image of the cell overlaid with the cell outline, as in (b). (d) EM of the protruding edge (asterisk in (c)) comprising a lamellipodium filled with dense actin network. Before the EM processing, the sample was immunogold labeled by cofilin antibody; inset in (d) shows gold particles as white dots
Fig. 2.
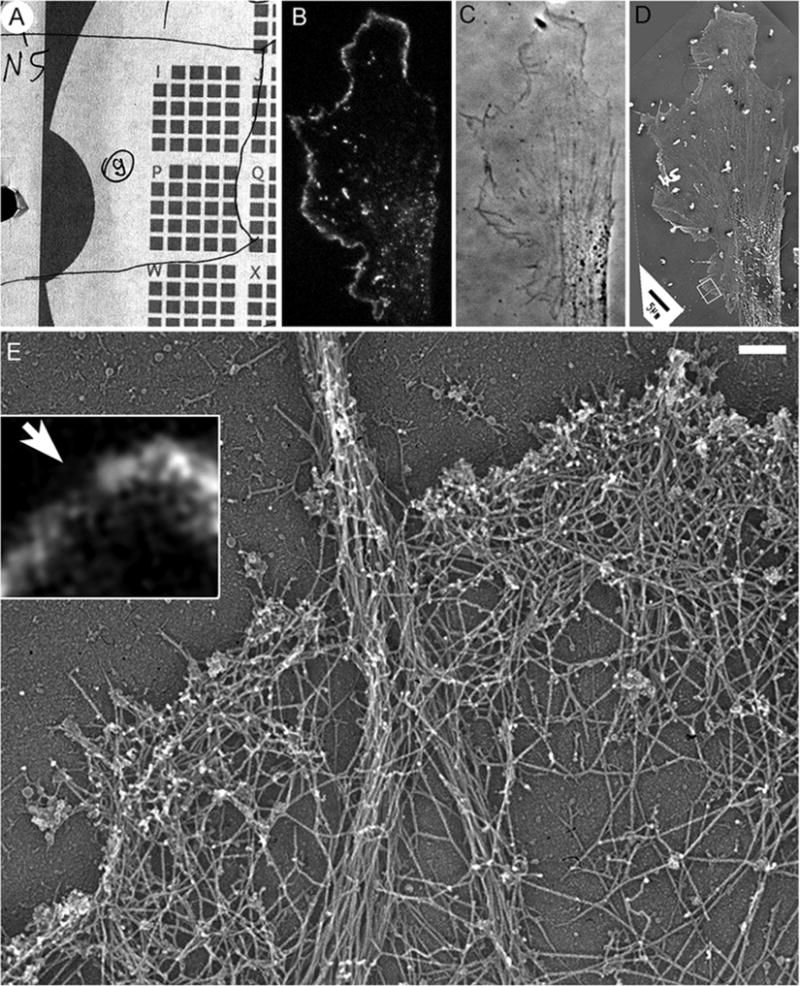
Correlative fluorescence and EM of cultured B16F1 mouse melanoma cell expressing EGFP-capping protein. (a) Map showing position of the cell (number 9 in a circle) relative to the reference marks on the coverslip. (b) Fluorescence image the cell showing localization of EGFP-capping protein to the edge of lamellipodia and puncta in lamella. (c) Phase contrast image of the same cell. (d) Low magnification EM image of the same cell. Box indicates a region enlarged in (e). (e) High magnification EM of the boxed region from (d) showing actin filament bundle in a filopodium in the center and dense branched network of actin filaments in lamellipodia. Inset shows the same region by fluorescence microscopy. Bright fluorescence corresponds to lamellipodia, while the dim region (arrow) corresponds to the filopodium
A major source of artifacts in this technique is the failure to perform a genuine CPD, which may occur if wet samples are transiently exposed to air, or water is not fully exchanged to ethanol or ethanol to CO2, or if the dried samples absorb ambient humidity. In order to get a high quality preparation, it is critical not to allow a liquid–gas interface to touch the samples at any point during the procedure. Practically, it means keeping the cells away from air while they are wet, and away from water, while they are dry. Changes of solutions need to be done quickly, with a layer of liquid always being retained above the cells. After drying, the cells should be kept at low humidity until they are coated with carbon.
3.1 Cell Culture and Extraction
Detergent extraction is used to expose the internal cytoskeletal structures, while the carrier buffer is designed to maximally preserve them until fixation. Additional preservation may be achieved using specific and nonspecific stabilizers.
Put small glass coverslips into a culture dish and plate the cells. Cell culture conditions are specific for each system and are not discussed here. If several coverslips are placed into the same dish, make sure that they do not overlap and that there are no bubbles underneath.
When the cells are ready, remove the culture medium from the dish using a pipette or by pouring out; quickly rinse with the pre-warmed to 37 °C PBS (see Note 5).
Immediately, but gently, add extraction solution equilibrated to room temperature; gently stir the dish to ensure that extraction solution instantly reaches all the cells (see Note 6). Incubate for 3–5 min at room temperature.
Rinse cells with PEM buffer 2–3 times, for a few seconds each time, at room temperature (see Note 7).
3.2 Fixation
Chemical fixation provides cytoskeletal structures with physical resistance against subsequent procedures, especially dehydration and CPD. It is a three-step procedure using different fixatives: glutaraldehyde, tannic acid and uranyl acetate.
After the last PEM rinse, add glutaraldehyde solution and incubate for at least 20 min at room temperature. If necessary, the samples can be stored at this stage at 4 °C for up to 3 weeks. Take care to prevent evaporation during storage.
Transfer coverslips to another container with tannic acid solution, or change solutions in the same dish (see Note 8). Make sure that the cells remain covered with liquid during transfer. No washing is necessary before this step. Incubate for 20 min at room temperature.
Take the coverslips out of the tannic acid solution one by one, rinse by dipping sequentially into two water-filled beakers, and place in a new plate with distilled water. Do not keep the coverslips out of the solution longer than necessary. Incubate for 5 min (see Note 9).
Take the coverslips out of the water one by one, rinse twice again by dipping into water, and place in a new plate with aqueous uranyl acetate solution. Avoid drying during transfer. Incubate for 20 min at room temperature.
Wash off uranyl acetate solution with distilled water by transferring the samples or exchanging the solutions.
3.3 Dehydration and CPD
Drying of the samples is necessary to expose the surfaces for metal coating in a vacuum. However, plain drying in the open air generates major structural distortions. When the liquid–gas interface passes through the samples, the forces of the surface tension that are enormous at the cellular scale flatten the samples. During CPD, the temperature and pressure of a liquid are raised above its critical point, at which the phase boundary and surface tension do not exist. In this state, the liquid can well be considered as compressed gas. When the pressure is released, the samples remain dry with the 3D organization intact, because they never experienced the surface tension. Carbon dioxide has reasonably low values of critical point pressure and temperature that can be tolerated by biological samples. However, a direct transfer of the samples from water to CO2 is not possible and ethanol, which is freely miscible with both water and CO2, is used as an intermediate. For dehydration and CPD, the coverslips are stacked in the sample holder with pieces of lens tissue as spacers, and processed simultaneously.
Cut lens tissue with loosely arranged fibers into pieces fitting the size of the CPD sample holder or a little larger (see Note 10).
Place the CPD sample holder into a wide container with distilled water. Put a piece of lens tissue at the bottom of the holder, place a coverslip with the cell side up on the lens tissue, and cover with another piece of lens tissue. Continue loading the coverslips into the holder, alternating them with pieces of lens tissue. All loading should be done under water. Make sure that the coverslips are minimally exposed to air during loading. Do not overload the holder, as it will interfere with the exchange of solutions (see Note 11). Keep track of sample identity based on its position in the holder.
Put a stirrer bar into a 50 mL glass beaker, place a scaffold over it, and add 10 % ethanol in the amount sufficient to cover the CPD holder. Quickly transfer the CPD holder with samples from the water-filled container into the beaker. Place the beaker on a magnetic stirrer, and stir for 5 min.
Prepare another beaker with a stirrer bar and a scaffold and add 20 % ethanol. Transfer the CPD holder from the first beaker into the second, and stir for 5 min.
Continue dehydration by transferring the CPD holder sequentially through the remaining graded ethanols, 5 min in each: 40, 60, 80, and 100 % (twice). Alternate the two beakers with their scaffolds and stirrer bars (see Note 12).
Place the CPD holder into a beaker with 0.2 % uranyl acetate in ethanol to fully cover it. Incubate for 20 min. Stirring is not necessary.
Transfer the CPD holder through beakers with 100 % ethanol (twice) and dehydrated 100 % ethanol dried over a molecular sieve (twice), as in steps 3–5. Stir for 5 min in each.
Fill the chamber of the CPD apparatus with dehydrated ethanol, just sufficient to cover the CPD holder. Place the holder in the ethanol and close the lid. Operate the CPD according to the manufacturer’s instructions or following the procedure below (see Note 13).
Open the CO2 tank. Cool down the CPD chamber to 10–15 °C to keep the CO2 in a liquid state. Maintain this temperature until the heating step (see Note 14).
Open the inlet valve on the CPD to allow the CO2 to fill the chamber. If the device is equipped with a magnetic stirrer, turn it on and keep it running till the heating step. Wait for 5 min. This is a mixing step, during which the ethanol in the chamber, the sample holder, and the samples, are equilibrated with the liquid CO2 from the tank.
With the inlet valve still open, open the outlet valve slightly until you clearly see that the liquid exchange is happening in the chamber, but do not allow the level of the liquid to go below the top of the sample holder. Wait for 30 s. This is a washing step, when the ethanol–CO2 mixture is released from the chamber and replaced with pure CO2 from the tank. If the CPD is not equipped with a magnetic stirrer, shake the CPD manually during the washing to mix the chamber contents better (see Note 15).
Close the outlet valve. Wait for 5 min.
Repeat steps 11 and 12 nine more times (total ten washes). After the last wash, wait only until the chamber is completely filled with CO2; that may take less than 5 min.
Close both the inlet and outlet valves and turn on the heat. As the chamber is isolated, heating will raise both the temperature and the pressure. Wait until both parameters exceed the critical values for CO2 (critical pressure = 1072 psi or 73 atm, critical temperature = 31 °C) and reach values of ~1250 psi (85 atm) and ~40 °C. If one value is reached sooner than the other, maintain the former at a steady-state level by turning the heater or the outlet valve on and off until both the values are reached.
Open the outlet valve slightly to slowly release the pressure until it reaches the atmospheric pressure. It should take about 10 min (see Note 16).
Open the CPD chamber, remove the sample holder and immediately put it into a desiccator.
3.4 Platinum and Carbon Coating
Platinum shadowing generates the contrast of the samples. The angle and the thickness of the coating are critical parameters influencing the quality of the image. Lower angles provide higher contrast, but do not penetrate deep into the sample. Thinner coats provide higher resolution, but lower contrast. For cellular studies, we shadow platinum at a ~45° angle with rotation to achieve a ~2 nm thickness of the coat, which is controlled by the thickness monitor. Carbon is applied from the top of the samples with a thickness of 3.5–5 nm. The basic steps of coating are listed below. Use the equipment manual for detailed operation.
Open the coating chamber of the vacuum evaporator. Load the evaporation materials, platinum and carbon. Adjust the angles of coating.
Mount the samples onto the rotary stage of the vacuum evaporator using a double-sided tape. This will prevent the dislodging of the samples during rotation (see Note 17). To prevent damage of the samples by ambient humidity, perform mounting as quickly as possible (see Note 18).
Pump down the coating chamber till ~5 × 10−6 atm.
Turn on the stage rotation, shadow with platinum (2 nm) and then with carbon (3.5–5 nm) (see Note 19).
Vent the coating chamber. Remove the samples together with the mounting tape and place in a Petri dish. The samples can be safely stored in a room at this stage.
3.5 Preparation of Replicas
The release of the replicas from the coverslips is achieved by floating the coverslips onto the surface of hydrofluoric acid solution, which dissolves glass. After that, the replicas are washed and mounted on EM grids.
While the coverslips are still attached to the sticky tape, scratch the coated surface of each coverslip with a needle or a razor blade to make regions fitting the size of an EM grid.
Fill the wells of a 12-well plate with ~10 % HF almost to the top, which makes replica handling easier.
Detach a coverslip from the sticky tape and place it on the surface of the HF solution, so that the coverslip remains floating. Wait until the glass sinks, leaving the platinum replica floating. The replica will fall apart along the scratches made in step 1 (see Note 20).
Fill the wells of another 12-well plate with distilled water, and add a trace amount of a detergent to decrease the surface tension of water. Stock solution of the detergent is prepared by dissolving ~1 drop of detergent in ~20 mL of water. To make a working solution, dip a platinum loop into the stock solution, take it out (this will create a film on the loop), and dip the loop into a water-filled well. The final concentration of detergent is ~10−6 % (see Note 21).
Using a platinum loop, transfer the replica pieces onto the surface of the detergent-containing water. Wait for 1 min or more.
Fill the wells of another 12-well plate with distilled water without detergent. Transfer the replica pieces onto the surface of the water. Wait for 1 min or more.
Pick up the replica pieces onto Formvar-coated EM grids with the lower side of the replica attached to the Formar film. The technique of replica mounting is similar to the way thin sections are picked up onto grids. Fasten a grid in a pair of forceps, partially submerge the grid into water at a ~45° angle, bring the grid close to a piece of replica and allow them to make contact; then, gently pull the grid out of the water making sure that the replica piece remains attached to, and spreads over the grid (see Note 22).
Examine the samples in TEM. Present the images in inverse contrast (as negatives) because it gives a more natural view of the structure, as if illuminated with scattered light.
3.6 Immunogold EM
Structural information has much greater value if the identity of the structures is known. Immunostaining is a conventional way to identify cellular components. For EM purposes, the antibodies are labeled with electron-dense markers. A popular marker, colloidal gold, has a higher electron density than platinum and thus is appropriate for platinum replica EM. For successful immunogold replica EM, a primary antibody should work after glutaraldehyde fixation, which optimally preserves the structure (see Note 23).
After glutaraldehyde fixation (Subheading 3.2, step 1), wash the samples with three changes of PBS. Incubate for at least 5 min in the last change.
Quench samples with NaBH4 for 10 min at room temperature. Shake off the bubbles occasionally. Rinse with PBS as in step 1.
Incubate with blocking solution for 20 min at room temperature.
Apply primary antibody at concentration giving bright staining by light microscopy. Incubate for 30–45 min at room temperature. Wash with PBS as in step 1.
Rinse once in immunogold buffer with 0.1 % BSA. Apply gold-conjugated secondary antibody diluted ~1:10 in immunogold buffer with 1 % BSA. Incubate overnight at room temperature in a sealed container in moist conditions (see Note 24).
Rinse in immunogold buffer with 0.1 % BSA as in step 1, and perform the remaining steps starting from Subheading 3.2, step 1.
3.7 Correlative EM
The correlative light and EM combines the advantages of both the microscopic techniques, namely, the high spatial resolution of EM and the high temporal resolution of live imaging. In this procedure, the cell dynamics is recorded by light microscopy, and then the same cell is analyzed by EM. The correlative analysis is extremely important from at least two points of view: to control for potential artifacts and to establish functional connections between the cytoskeletal organization and the cell’s motile behavior or the dynamics of cytoskeletal components [31, 32]. Modifications of the basic procedure as required for correlative EM are described below.
Cell Culture: Grow cells in dishes with marked coverslips mounted over a hole in the bottom of the dish. To make a dish, drill a 18-mm hole in the bottom of a 35-mm dish; polish the edges to remove any burrs; apply a thin layer of vacuum grease just outside the edges of the hole; place a marked coverslip symmetrically over the hole with the coated side facing up; press firmly to spread the grease until it forms a clear circle and all the air bubbles are gone (see Note 25).
Light microscopy: While imaging a region of interest, mark its position on a map with a pattern of reference marks. Because of the large difference in the resolution of light and EM, perform light microscopy at the highest possible resolution. EM is able to reveal even minor photodamage, not recognizable at the light microscopic level; therefore, keep the illumination of the samples to a minimum.
Extraction: Change the culture medium to extraction solution as soon as possible after the acquisition of the last live images, and take another image after extraction. It will serve as a reference to correlate the light and EM images.
Fixation: Perform all the fixation and washing steps with the marked coverslips still attached to the dishes, by exchanging solutions.
Dehydration: Before loading the coverslips into the CPD sample holder, excise the central marked area of the coverslip as described in steps 6–10.
Wipe away the immersion oil from the bottom of the dish using dry cotton swabs first, followed by ethanol-soaked swabs.
Detach the marked coverslip from the bottom of the dish and immediately place it into a water-filled 100 mm Petri dish.
Lightly press down the coverslip to allow the vacuum grease on the underside to secure the coverslip in the dish. Make sure that grease does not contaminate the region of interest.
Use a diamond pencil as a cutter and a razor blade as a ruler to cut off the greasy margins of the coverslip. Do not press the pencil hard, but instead make several light cuts along the same line, guided by the razor blade, until the margin detaches. Move it out of the way and cut off another margin. This procedure should be done with the coverslip completely submerged in the water (see Note 26). When done, transfer the excised central region to another water-filled container. Use a new dish for the next coverslip, as the remaining glass crumbs may cause shattering of the coverslip.
All subsequent processing, including coating, is performed as in the basic procedure.
Preparation of replicas: After the samples are coated, the region of interest needs to be specifically recovered for EM analysis as described in steps 12–16.
Immobilize a coated coverslip in the middle of a 100 mm Petri dish, with two pieces double-sided tape positioned under opposite corners of the coverslip, so that the region of interest is not obstructed and the coverslip is not attached too strongly.
Under a dissection microscope, localize the cells of interest using locator marks. Dried and shadowed cells have sufficient contrast for their shape to be seen even at low magnification. If necessary, use a regular light microscope with a low power lens.
Using a razor blade (or a needle), make cuts in the platinum–carbon layer around the region of interest (see Note 27). To facilitate the separation of this region from the rest of the replica after HF treatment, make additional cuts connecting the region of interest with the edges of the coverslip.
Make a drawing on the map to depict the exact shape of the outlined region of interest, which will help to identify it from among the other replica pieces during replica preparation.
Perform the washing and mounting on grids, as described in the basic protocol, handling only the replica piece with the region of interest. While mounting on a grid, use a dissection microscope to make sure that the cell of interest does not go to a grid bar; or use single-hole grids.
Acknowledgments
The author acknowledges the current support from NIH grant R01 GM 095977.
Footnotes
Small coverslips allow for better exchange of solutions during dehydration and CPD, and thus for better quality of samples at the end.
Stock solutions with a concentration of more than 2× change pH significantly after dilution to the working concentration. Free acid PIPES is not soluble in water and forms a milky suspension, but becomes soluble upon neutralization. KOH granules can be used for neutralization initially, until the solution almost clears. However, remember to allow enough time for the granules to dissolve before adding more. Finish the pH adjustment with 1 N KOH. KOH is preferable over NaOH, because K+ -containing buffer, more faithfully imitates the cytoplasm composition.
PEG is a nonspecific stabilizer of the cytoskeleton; phalloidin and taxol are specific stabilizers of actin filaments and microtubules, respectively.
Commercially available etched coverslips are not suitable for replica EM, as the marks are not visible in TEM.
Rinsing with PBS is optional, but if omitted, the extraction solution at the next step should be added in sufficient quantity to overcome the potentially harmful effects from the remaining medium and serum.
The choice of the extraction solution depends on a cell type and a goal. For a new experimental system, try different options in the preliminary experiments. Basic extraction solution (Triton X-100 in PEM) gives a better clarity of the cytoskeleton, but it is easier to damage the cells during extraction. If using this protocol, handle the samples extremely gently, and use phalloidin and taxol to better preserve the actin filaments and microtubules, respectively. The addition of PEG to the extraction solution provides for better preservation of the cells, but it also retains many cytoskeleton-associated components, which may partially obscure the filament arrangement. Such an effect is increased with PEG concentration and molecular weight, but PEGs in the range of 20,000–40,000 act similarly. We typically use 2 % PEG (35,000). Phalloidin and taxol are not as necessary in this case. For extremely fragile and poorly attached cells, low concentrations of glutaraldehyde can be used as stabilizing supplements for the extraction solution. In this case, the detergent and fixative compete with each other, and the results depend on their ratio. The extraction solution containing 0.5 % Triton X-100 and 0.25 % glutaraldehyde in PEM buffer worked well in our experiments.
For PEG-containing extraction solutions, use a longer washing time, at least 1 min in each change. If drugs are used during extraction, add them also to the rinsing buffer in a fourfold to fivefold lower concentration.
It is convenient to use a multiwell plate with numbered wells (24-well for 6–8 mm coverslips or 12-well for 9–12 mm coverslips) to transfer the samples. This makes it possible to combine samples from different experiments for EM processing while keeping parallel samples in the original container as a backup.
Uranyl acetate and tannic acid react with each other and form a precipitate. Extensive washing is important to avoid the formation of debris on the samples.
Pieces of lens tissue slightly larger, than the holder’s bottom area, will make minor wrinkles which promote looser packing of the coverslips in the holder and facilitate the liquid exchange.
The acceptable number of samples for a load depends on the sizes of the holder and the coverslips. For an 18 × 12 mm holder and ~7 × 7 mm coverslips, the maximum load is 12. For larger coverslips, the load should be decreased. Larger holders may accept more samples, especially if the coverslips are staggered.
It is not necessary to dry the beakers before the next incubation, as the ethanol concentration may not be exact, except for 100 % ethanol, when it is better to dry the beakers and scaffolds with tissue. Incubation for 5 min is minimal. For larger coverslips or greater loads, increase the incubation time.
The process of CPD is most commonly used for scanning EM and production of microelectronics. Consequently, the protocols suggested by the manufacturers or incorporated into automated procedures of CPDs are designed for those applications. Replica TEM, however, is more demanding in terms of sample quality. We adjusted the CPD processing to fully remove all traces of ethanol from the samples before bringing the CO2 to the critical point; this helps to eliminate minor artifacts that appear as a fusion of closely positioned filaments in the cytoskeleton. The CPD operation described here is applicable to manual CPDs, such as Samdri PVD-3D (Tousimis), which we use in the lab, or to semi-automatic CPDs switched to a manual mode of operation, e.g., Samdri-795 (Tousimis).
Lower temperatures are acceptable, but the diffusion of ethanol from the samples will be slower, so that longer washing time is needed. Warming up the chamber till the ambient temperature is allowed if the outlet valve of the CPD is closed, and the CO2 remains pressurized and in liquid form. However, it is important to cool down the chamber back to 15 °C before opening the outlet valve for purging out the ethanol–CO2 mixture.
Letting the liquid level go below the samples will irreversibly damage them. On the other hand, too low a rate of liquid exchange is also a mistake. Adjust the outlet valve to get a steady-state liquid level, about halfway from the top of the holder to the top of the chamber. This will also make the liquid mixing more efficient. Although the shaking step sounds a bit amusing, it does make a difference by helping to remove the ethanol from the samples.
Fast release of pressure may cause condensation of CO2 back to liquid state and ruin the dried samples.
Conventional Scotch double-sided tape becomes too sticky in a vacuum, preventing the safe detachment of the samples after coating. To avoid this problem, sandwich the double-sided tape between the glued parts of two Post-It notes, with the sticky sides exposed.
Humidity in the room should be below 35 %; the 35–50 % humidity level may be acceptable, but much caution and the speedy mounting of the samples is required; humidity >50 % is not acceptable. Try to run a powerful dehumidifier in the latter case.
If the evaporator is not equipped with a thickness monitor, the thickness of the coating may be adjusted in the preliminary experiments based on the amount of coating material loaded (for platinum) or used (carbon) for evaporation.
To safely float a coverslip, grab it with the forceps from the top for parallel edges, lift the coverslip, and carefully place it onto the liquid surface, keeping it in a horizontal position. Practice first by placing a coverslip onto a clean solid surface. Alternatively, a coverslip can be placed first on a platinum loop bent at 90° angle and then loaded horizontally onto the liquid surface. If the replica does not fall apart along the scratches, use the platinum loop to reach the replica from below, lightly touch it and pull or shake it to detach it from other pieces. Extreme care should be used not to ruin the replicas with these manipulations.
Water has much greater surface tension than HF, which may cause severe replica breakage, if detergent is not added. Test the detergent concentration before applying it to the samples. An overdose of detergent causes shrinkage and drowning of the replicas. Stock solutions should be changed at least every 2 weeks. Old detergents leave contamination on the samples, looking like semi-transparent films between filaments. Household non-colored detergent, such as Ivory, works fine. Triton X-100 can also be used, but it should be prepared fresh every time.
Sometimes, replicas appear to be repelled by the grid, making it difficult to establish the initial contact between a replica piece and a grid. Try to gently guide a piece of replica to the wall of the well to restrict its motility, and then pick it up. However, there is a danger of smashing the replica against the wall with this approach. In severe cases, use glow discharge to treat grids.
The efficiency of staining may be improved if the cells are fixed with a lower (e.g., 0.2 %) glutaraldehyde concentration before staining. For some antibodies that do not work after glutaraldehyde fixation, it may be possible to stain unfixed samples by incubating them with primary antibodies diluted in PEM for 10–15 min, then fixing with glutaraldehyde, and quenching and staining with a secondary antibody.
Gold size of 10–20 nm is optimal for this technique, as smaller particles are poorly visible, and larger particles are too disruptive for an image.
The coverslips can be mounted either inside or outside the dish, but inside mounting is more convenient at later stages, when the centerpiece of the coverslip needs to be cut out. For mounting, use a minimal amount of grease, just sufficient to seal the dish; excessive grease causes complications at later stages. Commercially available glass-bottom dishes have cover-slips permanently glued to the bottom, which makes it difficult to remove them for EM processing.
Cutting under water is more difficult than in the air; therefore, use a sharp diamond pencil and avoid glass crumbs.
To reduce the effect of shaky hands, hold a razor blade with one hand with the sharp blade corner pointing down; stabilize the blade by putting the index finger of the other hand onto the blunt blade corner pointing up; rest the forearms on the table and the other fingers of both hands on the microscope stage and/or dish edges; keep the blade above the sample and find its unfocused image in the microscope; slowly bring down the sharp corner of the blade until it almost comes to focus; bring the blade corner to a region where a cut is to be made; under microscope control, bring it down to the sample and make a scratch.
References
- 1.Wohlfarth-Bottermann KE. Weitreichende fibrillare Protoplasmadifferenzierungen und ihre Bedeutung fur die Protoplasmastromung. I. Elektronenmikroskopischer Nachweis und Feinstruktur. Protoplasma. 1962;54:514–539. [Google Scholar]
- 2.Ledbetter MC, Porter KR. A “microtubule” in plant cell fine structure. J Cell Biol. 1963;19(1):239–250. doi: 10.1083/jcb.19.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabatini DD, Bensch K, Barrnett RJ. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67(2):359–367. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- 5.Wohlfarth-Bottermann KE. Differentiations of the ground cytoplasm and their significance for the generation of the motive force of amoeboid movement. In: Allen RD, Kamiya N, editors. Primitive motile systems in cell biology. Academic; New York: 1964. pp. 79–109. [Google Scholar]
- 6.Small JV, Isenberg G, Celis JE. Polarity of actin at the leading edge of cultured cells. Nature. 1978;272(5654):638–639. doi: 10.1038/272638a0. [DOI] [PubMed] [Google Scholar]
- 7.Lucic V, Rigort A, Baumeister W. Cryo-electron tomography: the challenge of doing structural biology in situ. J Cell Biol. 2013;202(3):407–419. doi: 10.1083/jcb.201304193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Harush K, Maimon T, Patla I, Villa E, Medalia O. Visualizing cellular processes at the molecular level by cryo-electron tomography. J Cell Sci. 2010;123(Pt 1):7–12. doi: 10.1242/jcs.060111. [DOI] [PubMed] [Google Scholar]
- 9.Heuser J. Preparing biological samples for stereomicroscopy by the quick-freeze, deep-etch, rotary-replication technique. Methods Cell Biol. 1981;22:97–122. doi: 10.1016/s0091-679x(08)61872-5. [DOI] [PubMed] [Google Scholar]
- 10.Steere RL. Electron microscopy of structural detail in frozen biological specimens. J Cell Biol. 1957;3(1):45–60. doi: 10.1083/jcb.3.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heuser J. Protocol for 3-D visualization of molecules on mica via the quick-freeze, deep-etch technique. J Electron Microsc Tech. 1989;13(3):244–263. doi: 10.1002/jemt.1060130310. [DOI] [PubMed] [Google Scholar]
- 12.Loesser KE, Franzini-Armstrong C. A simple method for freeze-drying of macromolecules and macromolecular complexes. J Struct Biol. 1990;103(1):48–56. doi: 10.1016/1047-8477(90)90085-q. [DOI] [PubMed] [Google Scholar]
- 13.Heuser JE, Kirschner MW. Filament organization revealed in platinum replicas of freeze-dried cytoskeletons. J Cell Biol. 1980;86(1):212–234. doi: 10.1083/jcb.86.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svitkina TM, Shevelev AA, Bershadsky AD, Gelfand VI. Cytoskeleton of mouse embryo fibroblasts. Electron microscopy of platinum replicas. Eur J Cell Biol. 1984;34(1):64–74. [PubMed] [Google Scholar]
- 15.Svitkina TM, Verkhovsky AB, Borisy GG. Improved procedures for electron microscopic visualization of the cytoskeleton of cultured cells. J Struct Biol. 1995;115(3):290–303. doi: 10.1006/jsbi.1995.1054. [DOI] [PubMed] [Google Scholar]
- 16.Hirokawa N. Quick-freeze, deep-etch electron microscopy. J Electron Microsc (Tokyo) 1989;38(Suppl):S123–S128. [PubMed] [Google Scholar]
- 17.Meyer HW, Richter W. Freeze-fracture studies on lipids and membranes. Micron. 2001;32(6):615–644. doi: 10.1016/s0968-4328(00)00050-0. [DOI] [PubMed] [Google Scholar]
- 18.Svitkina T. Electron microscopic analysis of the leading edge in migrating cells. Methods Cell Biol. 2007;79:295–319. doi: 10.1016/S0091-679X(06)79012-4. [DOI] [PubMed] [Google Scholar]
- 19.Svitkina TM, Borisy GG. Correlative light and electron microscopy studies of cytoskeletal dynamics. In: Celis J, editor. Cell biology: a laboratory handbook. 3rd. Vol. 3. Elsevier; Amsterdam: 2006. pp. 277–285. [Google Scholar]
- 20.Svitkina TM, Borisy GG. Correlative light and electron microscopy of the cytoskeleton of cultured cells. Methods Enzymol. 1998;298:570–592. doi: 10.1016/s0076-6879(98)98045-4. [DOI] [PubMed] [Google Scholar]
- 21.Shutova MS, Spessott WA, Giraudo CG, Svitkina T. Endogenous species of mammalian nonmuscle myosin IIA and IIB include activated monomers and heteropolymers. Curr Biol. 2014;24(17):1958–1968. doi: 10.1016/j.cub.2014.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Svitkina TM, Verkhovsky AB, Borisy GG. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J Cell Biol. 1996;135(4):991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kukulski W, Schorb M, Welsch S, Picco A, Kaksonen M, Briggs JA. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J Cell Biol. 2011;192(1):111–119. doi: 10.1083/jcb.201009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perkovic M, Kunz M, Endesfelder U, Bunse S, Wigge C, Yu Z, Hodirnau VV, Scheffer MP, Seybert A, Malkusch S, Schuman EM, Heilemann M, Frangakis AS. Correlative light-and electron microscopy with chemical tags. J Struct Biol. 2014;186(2):205–213. doi: 10.1016/j.jsb.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe S, Punge A, Hollopeter G, Willig KI, Hobson RJ, Davis MW, Hell SW, Jorgensen EM. Protein localization in electron micrographs using fluorescence nanoscopy. Nat Methods. 2011;8(1):80–84. doi: 10.1038/nmeth.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Busnadiego R, Schrod N, Kochovski Z, Asano S, Vanhecke D, Baumeister W, Lucic V. Insights into the molecular organization of the neuron by cryo-electron tomography. J Electron Microsc (Tokyo) 2011;60(Suppl 1):S137–S148. doi: 10.1093/jmi-cro/dfr018. [DOI] [PubMed] [Google Scholar]
- 27.Kandela IK, Bleher R, Albrecht RM. Immunolabeling for correlative light and electron microscopy on ultrathin cryosections. Microsc Microanal. 2008;14(2):159–165. doi: 10.1017/S1431927608080239. [DOI] [PubMed] [Google Scholar]
- 28.Sartori A, Gatz R, Beck F, Rigort A, Baumeister W, Plitzko JM. Correlative microscopy: bridging the gap between fluorescence light microscopy and cryo-electron tomography. J Struct Biol. 2007;160(2):135–145. doi: 10.1016/j.jsb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Verkade P. Moving EM: the rapid transfer system as a new tool for correlative light and electron microscopy and high throughput for high-pressure freezing. J Microsc. 2008;230(Pt 2):317–328. doi: 10.1111/j.1365-2818.2008.01989.x. [DOI] [PubMed] [Google Scholar]
- 30.Nemethova M, Auinger S, Small JV. Building the actin cytoskeleton: filopodia contribute to the construction of contractile bundles in the lamella. J Cell Biol. 2008;180(6):1233–1244. doi: 10.1083/jcb.200709134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol. 2003;160(3):409–421. doi: 10.1083/jcb.200210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5(11):e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]


