Abstract
Sympathetic vasomotor tone is elevated in obesity-related hypertension. Orexin importantly regulates energy metabolism and autonomic function. We hypothesized that alteration of orexin receptor in the paraventricular nucleus (PVN) of the hypothalamus leads to elevated sympathetic vasomotor tone in obesity. We used in vivo measurement of sympathetic vasomotor tone and microinjection into brain nucleus, whole-cell patch clamp recording in brain slices, and immunocytochemical staining in obese Zucker rats (OZRs) and lean Zucker rats (LZRs). Microinjection of orexin 1 receptor (OX1R) antagonist SB334867 into the PVN reduced basal arterial blood pressure (ABP) and renal sympathetic nerve activity (RSNA) in anesthetized OZRs but not in LZRs. Microinjection of orexin A into the PVN produced greater increases in ABP and RSNA in OZRs than in LZRs. Western blot analysis revealed that OX1R expression levels in the PVN were significantly increased in OZRs compared with LZRs. OX1R immunoreactivity was positive in retrogradely labeled PVN-spinal neurons. The basal firing rate of labeled PVN-spinal neurons was higher in OZRs than in LZRs. SB334867 decreased the basal firing activity of PVN-spinal neurons in OZRs but had no effect in LZRs. Orexin A induced a greater increase in the firing rate of PVN-spinal neurons in OZRs than in LZRs. In addition, orexin A induced larger currents in PVN-spinal neurons in OZRs than in LZRs. These data suggest that upregulation of OX1R in the PVN promotes hyperactivity of PVN presympathetic neurons and elevated sympathetic outflow in obesity.
Keywords: Hypothalamus, Sympathetic nerve activity, Obesity, Orexin receptors
1. Introduction
Obesity is associated with increased sympathetic nerve activity, which contributes to high blood pressure in both obese humans and animal models of obesity (Carlson et al., 2000). Obese Zucker rats (OZRs), which have nonfunctional leptin receptors owing to gene mutation, possess metabolic abnormalities such as hyperphagia, insulin resistance, hyperinsulinemia, and hyperlipidemia (Kasiske et al., 1992). This rat model of obesity provides additional insight into the etiology of obesity-related hypertension. Although the common form of obesity-related hypertension in humans is not caused by deficiency in leptin receptors, leptin receptors mutation has been found in obese humans (Clement et al., 1998). Furthermore, diet-induced obesity is associated with leptin resistance in both the central nervous system (CNS) and peripheral tissue (Sainz et al., 2015). Thus, OZR is a widely used animal model to study obesity-related cardiovascular complications (Alonso-Galicia et al., 1996; Guimaraes et al., 2014; Schreihofer et al., 2007), in which elevated sympathetic outflow contributes to hypertension (Carlson et al., 2000; Morgan et al., 1995). However, the mechanisms underlying the elevated sympathetic outflow in obesity-related hypertension remain unclear.
The paraventricular nucleus (PVN) of the hypothalamus is an important brain region that regulates neuroendocrine and autonomic functions (Swanson and Sawchenko, 1983). The pre-sympathetic PVN neurons with projection to the rostral ventrolateral medulla and the intermediolateral cell column in the spinal cord importantly regulate sympathetic outflow (Li and Pan, 2007b; Pyner and Coote, 2000; Ranson et al., 1998). Previous studies have shown that the PVN is an important source of excitatory drive for sympathetic vasomotor tone in physiological and pathophysiological conditions (Allen, 2002; Li and Pan, 2007a). Orexins (orexin A and orexin B, also called hypocretin-1 and hypocretin-2) are synthesized in the lateral hypothalamus and play important roles in the regulation of neuroendocrine function, sleep-wakefulness, feeding behavior, and energy homeostasis (de Lecea et al., 1998; Sakurai et al., 1998). The two known orexin receptors (OX1R and OX2R) belong to the G protein-coupled receptor superfamily, which has 7 transmembrane domains (Sakurai et al., 1998). Because orexin A binds with higher affinity (30–100 times greater) than orexin B to OX1R and both orexin A and B bind with equal affinity to OX2R (Sakurai et al., 1998), many studies have focused on the pharmacologic effects of orexin A. In this regard, orexin A increases the neuronal activity of neurons in various brain regions such as the lateral hypothalamus (Liu et al., 2002), arcuate nucleus (Rauch et al., 2000), and PVN (Shirasaka et al., 2001) through diverse mechanisms. Whereas orexin-producing neurons are exclusively localized in the hypothalamus, orexinergic nerve fibers and orexin receptors are distributed widely throughout the CNS (Date et al., 1999; de Lecea et al., 1998). For example, OX1R and OX2R mRNA (Marcus et al., 2001; Trivedi et al., 1998) and protein (Cluderay et al., 2002; Hervieu et al., 2001) are expressed, but not homogenously, throughout brain nucleus.
Orexins are involved in autonomic regulation, including hormone secretion and cardiovascular function control (Date et al., 1999; Shirasaka et al., 1999). In this regard, intracerebroventricular administration of orexin A significantly increases concentrations of plasma norepinephrine, renal sympathetic activity, and mean ABP in rats (Date et al., 1999; Shirasaka et al., 1999). Furthermore, an orally administered antagonist for OX1R and OX2R markedly reduces blood pressure in spontaneous hypertensive rats (Li et al., 2013). Because orexin receptor mRNA (Sakurai et al., 1998; Trivedi et al., 1998) and orexin-containing nerve fibers (Date et al., 1999) are abundant in the PVN, and OX1R mRNA in the hypothalamus was increased in OZRs (Beck et al., 2001), we hypothesized that the alteration of orexin receptors results in hyperactivity of the PVN presympathetic neurons and elevated sympathetic outflow in obesity.
2. Materials and methods
2.1. Animals
Male lean Zucker rats (LZRs) and obese Zucker rats (OZRs) (6 weeks old and 15 weeks old, Harlan Laboratory, Indianapolis, IN) were used in this study. The rats were housed in the animal facility in the Department of Veterinary Medicine & Surgery at The University of Texas M.D. Anderson Cancer Center. The surgical procedures and experimental protocols were approved by the Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center (approved animal protocol number: 020602143) and conformed to the National Institutes of Health guidelines on the ethical use of animals. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques.
2.2. In vivo recordings of hemodynamics and sympathetic nerve activity
Renal sympathetic nerve activity (RSNA), arterial blood pressure (ABP), and heart rate (HR) were recorded as described previously (Li and Pan, 2007a,b). Briefly, the rats were anesthetized with a mixture of α-chloralose (60–75 mg/kg) and urethane (800 mg/kg, intraperitoneal injection). The trachea was cannulated for mechanical ventilation through a rodent ventilator (CWE, Ardmore, PA) with 100% O2. The CO2 concentration in the expiration was monitored by using a CO2 analyzer (Capstar 100; CWE) and maintained at 4–5% by adjusting the ventilation rate (about 60 breaths/min) or tidal volume (about 2.5 ml) throughout the experiment. ABP was measured through a cannula surgically inserted into the left femoral artery. HR was counted by triggering from the blood pressure pulse. For RSNA recording, a small branch of the renal nerve was isolated and dissected from the surrounding tissue through a left flank incision via a retroperitoneal approach (Li et al., 2001). The nerve discharge signal was amplified (gain: 20,000–30,000) and filtered (bandpass 100–3000 Hz) with an alternating current amplifier (model P511; Grass Instruments, Warwick, RI). RSNA and ABP were recorded using a 1401-PLUS analog-to-digital converter and Spike2 system (Cambridge Electronic Design, Cambridge, UK) and displayed and stored on a computer. The background noise was determined after the rats were killed by an overdose of sodium pentobarbital at the end of each experiment. The RSNA was integrated after subtracting background noise. We integrated never activity (with subtraction of background noise) and set the basal level as to 100% and the percentage change in RSNA from the baseline value was calculated.
For the PVN microinjections, a glass pipette (tip diameter, 20–30 μm) was advanced into the PVN through a small hole drilled in the dorsal surface of the skull according to the following stereotactic coordinates: 1.6–2.0 mm caudal to the bregma, 0.5 mm lateral to the midline, and 7.0–7.5 mm ventral to the dura (Chen et al., 2003; Li and Pan, 2007a). Drugs were pressure-ejected using a calibrated microinjection system, and the injections were monitored using an operating microscope. The injection site in the PVN was verified by the depressor responses to microinjection of 5 nmol gamma-aminobutyric acid (20 nl, 250 mmol/l). The location of the pipette tip and diffusion of the injectant into the PVN was examined and confirmed histologically. The drug solutions contained 5% rhodamine-labeled fluorescent microspheres (0.04 μm) to estimate drug dispersion around the site of injection. At the completion of the experiment, the rat brain was rapidly removed, fixed, and sectioned to verify the injection site and area of diffusion of the fluorescent dye, according to the Paxinos and Watson atlas (Paxinos and Watson, 1998). Rats in which the pipette tip was misplaced outside the PVN were excluded from the data analysis.
2.3. Western blot analysis and immunocytochemical staining
Rats were anesthetized with 2–3% isoflurane. The midbrain was quickly removed and the PVN tissues were obtained by using the punch microdissection technique. Then, the PVN tissues were processed to obtain membrane and cytosolic proteins. The protein concentration in the supernatant was determined by using a bicinchoninic acid protein assay kit. An aliquot of 15–25 μg of protein from each sample was separated by using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred electrophoretically onto polyvinylidene difluoride membranes. The membrane was blocked and incubated with the primary antibodies against OX1R (rabbit anti-rat OX1R), OX2R (rabbit anti-rat OX2R), and GAPDH (rabbit anti-rat GAPDH). Finally, the membrane was developed with an enhanced chemiluminescence kit according to the manufacturer’s instructions. The amounts of OX1R and OX2R proteins were quantified by normalizing the optical density of their protein band to that of GAPDH in the same samples. The mean values of OX1R or OX2R proteins in LZRs were considered to be 1.
For the immunocytochemical staining, the rats were put under deep anesthesia induced with sodium pentobarbital (60 mg/kg, intraperitoneally), and the rat tissue was fixed by intracardiac perfusion of 4% paraformaldehyde. The hypothalamus was removed quickly, sectioned into slices, and processed for immunostaining, as described in our previous studies (Li and Pan, 2005; Li et al., 2008b). To determine whether the labeled PVN neurons expressed OX1R, were performed immunofluorescence labeling of OX1R using the primary antibody (goat anti-OX1R, dilution 1:100, Alomone labs, Israel). Subsequently, the sections were rinsed in phosphate-buffered saline (PBS) and incubated with secondary antibodies (Alexa Fluor-488-conjugated goat anti-rabbit IgG). Finally, the sections were rinsed, mounted on slides, dried, and coverslipped. The sections were viewed using a confocal microscope, and the areas of interest were photographed.
2.4. In vitro electrophysiological experiments
The PVN-spinal output neurons were retrogradely labeled by FluoSpheres, as described previously (Li and Pan, 2005; Li et al., 2008b). Briefly, with the rat anesthetized with 2–3% isoflurane, laminectomy was performed to expose the spinal cord at the T2–T4 level. FluoSpheres (0.04 μm; Invitrogen) were pressure ejected bilaterally (Nanojector II; Drummond Scientific) through a glass pipette (20–30 μm in tip diameter) placed into the intermediolateral region in the spinal cord in six separate 50 nl injections (Li and Pan, 2005; Li et al., 2008b). After injection, rats were treated prophylactically with an antibiotic (5 mg/kg enrofloxacin, s.c., daily for 3 days) and an analgesic (0.5 mg/kg buprenorphine, s.c., every 12 h for 2 days). The rats were then returned to their home cages for 3–5 days to permit the FluoSpheres to be transported to the PVN.
Hypothalamic slices were prepared from the FluoSphere-injected rats, as described previously (Li et al., 2008b). Briefly, the rats were decapitated under anesthesia with 2% isoflurane, and the brain was quickly removed and placed in ice-cold artificial cerebrospinal fluid (aCSF) containing the following (in mM): 124.0 NaCl, 3.0 KCl, 1.3 MgSO4, 2.4 CaCl2, 1.4 NaH2PO4, 10.0 glucose, and 26.0 NaHCO3, which was saturated with a mixture of 95% O2 and 5% CO2. A tissue block containing the PVN was trimmed and glued onto the stage of a vibrating microtome (VT1000s, Leica). Coronal hypothalamic slices (300 μm in thickness) were cut and pre-incubated in aCSF continuously gassed with a mixture of 95% O2 and 5% CO2 at 34 °C for 1 h before recording.
Whole-cell recordings were performed in FluoSphere-labeled PVN neurons in the hypothalamic slices. The brain slices were placed in the recording chamber continuously perfused with aCSF (saturated with 95% O2 and 5% CO2) at 3 ml/min at a temperature of 34 °C maintained by using an inline solution heater. The labeled PVN neurons were first identified by an upright microscope equipped with epifluorescence illumination and differential interference contrast optics. To record the firing activity and orexin A-induced currents, we used recording electrodes, which were pulled from borosilicate capillaries by a micropipette puller. The resistance of the electrode was 3–6 MΩ when it was filled with a solution containing (mM) 140.0 K gluconate, 2.0 MgCl2, 0.1 CaCl2, 10.0 HEPES, 1.1 EGTA, 0.3 Na2-GTP, and 2.0 Na2-ATP adjusted to pH 7.25 with 1 M KOH, 270–290 mOsm. Orexin A-induced current were recorded in voltage-clamp mode by applying slow depolarizing voltage ramps (−100 to 0 mV, 10 mV/s) in the presence of tetrodotoxin (1 μM) to block Na+ channels, along with 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 50 μM AP5 to block ionotropic glutamate receptors and 20 μM gabazine to blocking GABAA receptors. Miniature excitatory postsynaptic current were recorded at a holding potential of −60 mV in the presence of 50 μM gabazine. GDP-β-s (1 mM) was included in the recording solution to block the postsynaptic effect of orexin A. Signals were processed using the Multiclamp 700B amplifier and Digidata 1320A. The recording was abandoned if the input resistance changed more than 15% during the recording. In all cases, values were obtained and averaged during a 3- to 5-min recording period before and after drug application.
2.5. Drugs
Orexin A, N-(2-methyl-6-benzoxazolyl)-N-1,5-naphthyridin-4-yl urea (SB-334867), and (2S)-1-(3,4-dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)-3,3-dimethyl-2-[(4-pyridinylmethyl)amino]-1-butanone hydrochloride (TCS-OX2-29) were purchased from Tocris. CNQX, AP5, gabazine, and GDP-β-s were obtained from Sigma Aldrich. For the electrophysiological experiments, all reagents, except SB-334867, were dissolved in deionized water to form stock solutions and diluted with aCSF solution. SB-334867 was dissolved in dimethyl sulfoxide (DMSO), and the final concentration of DMSO in the bath solution was 0.05% which showed no effects on the firing activity of the vehicle control neurons.
2.6. Data analysis
For the in vivo data, the mean ABP was calculated as the diastolic pressure plus one-third of the pulse pressure. RSNA signals were rectified at a time constant of 1 s and integrated off-line by using Spike 5 (Version 5.0). The nerve activity was obtained by subtracting background noise, defined as the noise level after the rats were euthanized with an overdose of sodium phenobarbital. Control values of nerve activity were obtained by averaging the signal over a 60-s period immediately before each treatment. Response values following each intervention were averaged over 30 s when the maximal responses occurred. For the in vitro data, the firing activity and miniature excitatory postsynaptic currents (mEPSCs) were analyzed off-line with the peak detection program Mini-Analysis 6.0. Membrane potentials were determined when the neuron shows intermittent firing activity, the membrane potential was stable or the cell was silent. The orexin A-induced postsynaptic currents were analyzed by clampfit software. The firing rate, membrane potentials, and frequency and amplitude of mEPSCs were obtained by averaging these parameters before, during, and after drug application. To compare the responses of the ABP, RSNA, and HR to the agents microinjected within the group, we performed a repeated-measures analysis of variance with the Dunnett’s post hoc test. A two-way analysis of variance with the Bonferroni’s post hoc test was used to compare responses between LZRs and OZRs. P < 0.05 was considered statistically significant.
3. Results
We conducted our experiments in 51 OZRs and 52 LZRs. The OZRs displayed significantly higher basal mean ABP (116.9 ± 2.9 mmHg, P < 0.05) and heart rate (HR: 344 ± 6 bpm, P < 0.05) than LZRs (ABP: 87.8 ± 2.1 mm Hg; HR: 318 ± 6 bpm) under stable anesthesia conditions.
3.1. OX1R antagonism in the PVN decreased ABP and RSNA in OZRs but not in LZRs
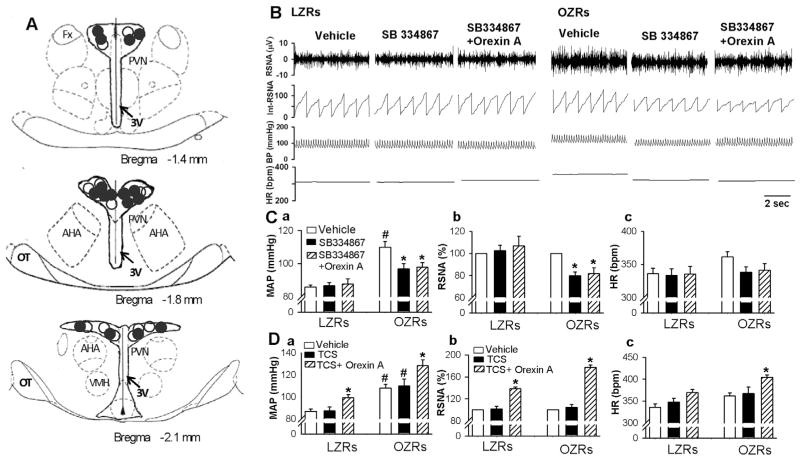
To determine the role of OX1R in the regulation of sympathetic vasomotor tone in obesity, we microinjected SB334867, an antagonist for OX1R (Shahid et al., 2012) into the PVN. Bilateral micro-injection of SB334867 (1 nmol in 50 nl aCSF) into the PVN did not significantly alter ABP, RSNA, and HR in 8 LZRs. In 8 OZRs, SB334867 significantly decreased the basal ABP from 109.8 ± 3.6 to 96.7 ± 3.1 mmHg (P < 0.05), RSNA from 100% to 79.7 ± 3.6% (P < 0.05), and HR from 361.5 ± 7.5 to 337.8 ± 8.5 bpm (P < 0.05, Fig. 1B and C). To determine if SB334867 effectively blocked OX1R, orexin A (30 pmol in 50 nl) was microinjected into the PVN following microinjection of SB334867. SB334867 plus orexin A had no effect on ABP, RSNA, and HR in LZRs but decreased ABP, RSNA, and HR in OZRs (Fig. 1B and C). In contrast, microinjection of the OX2R antagonist TSC-OX2-29 (100 pmol in 50 nl aCSF) into the PVN did not significantly change ABP, RSNA, or HR in either LZRs (n = 7 rats) or OZRs (n = 6 rats, Fig. 1D). In addition, following TCS-OX2-29 injection, microinjection of orexin A into the PVN still increased ABP, RSNA, and HR in both LZRs and OZRs.
Fig. 1.
Blockade of orexin 1 receptor (OX1R) in the PVN decreased ABP, RSNA, and HR in OZRs. A: Schematic drawings show the center of the microinjection sites in LZRs (○) and OZRs (●). B and C: representative tracings (B) and summary data (C) show that microinjection of SB334867 or SB334867 plus orexin A into the PVN decreased basal ABP and RSNA in OZRs (n = 8) but not in LZRs (n = 8). D, Summary data show that blocking OX2R did not affect the response of ABP (a), RSNA (b), and HR (c) to microinjection of orexin A into the PVN in LZRs (n = 7) and in OZRs (n = 6). Data are presented as means ± SEM. *P < 0.05, compared with the baseline control in each group. #P < 0.05, compared with the corresponding value in LZRs. TCS: TCS-OX2-29. Fx: fornix; AH, anterior hypothalamus; 3V, third ventricle.
We injected a fluorescent dye into the drug injection site at the end of the experiment. The distribution of microinjection sites within the PVN did not differ between LZRs and OZRs (Fig. 1A). The spread of the dye neither penetrated to the third ventricular ependymal lining nor was consistently observed in an area outside of the PVN. Two LZRs and 2 OZRs were excluded from the data analysis because the micropipette was misplaced and the fluorescent dye spread outside of the PVN.
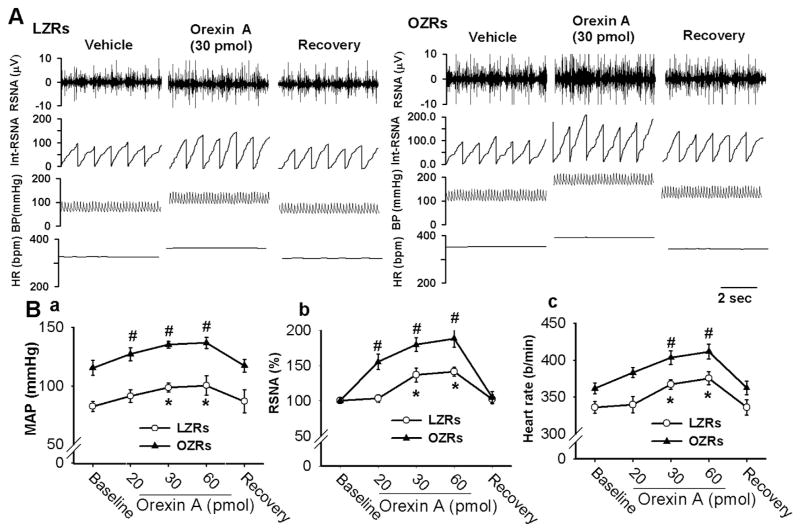
We then compared the effects of microinjection of orexin A into the PVN on sympathetic vasomotor tone in LZRs and OZRs. Bilateral microinjection of orexin A (20.0–60.0 pmol in 50 nl artificial cerebrospinal fluid, aCSF) into the PVN significantly increased ABP, RSNA, and HR in a dose-dependent manner in LZRs (n = 7). The onset latency of increased ABP, RSNA, and HR in response to orexin A injection was 0.8 ± 0.1 min, and the peak response appeared 4.3 ± 0.8 min after orexin A injection. The increases in the ABP, RSNA, and HR elicited by orexin A microinjections were significantly greater in OZRs (n = 8 rats) compared with those in LZRs (Fig. 2). The ABP, RSNA, and HR returned to basal levels 30–35 min after orexin A injection. The mean recovery times for ABP, RSNA, and HR did not differ significantly between LZRs and OZRs.
Fig. 2.
Microinjection of orexin A into the PVN produced a greater sympathoexcitatory response in OZRs than in LZRs. A, Original tracings show the responses of ABP, RSNA, and HR to bilateral microinjection orexin A (30 pmol/50 nl) into the PVN in 1 LZR (A) and 1 OZR (B) rats. B, Summary data show the value (B) of mean ABP (a), RSNA (b), and HR (c) in response to microinjection of different doses of orexin A (20, 30, and 60 pmol/50 nl) into the PVN, and the recovery (R) period in LZRs (n = 7) and OZRs (n = 8). *P < 0.05, compared with the baseline control in each group. #P < 0.05, compared with the corresponding value in the LZR group.
3.2. OX1R expression levels were increased in the PVN in OZRs
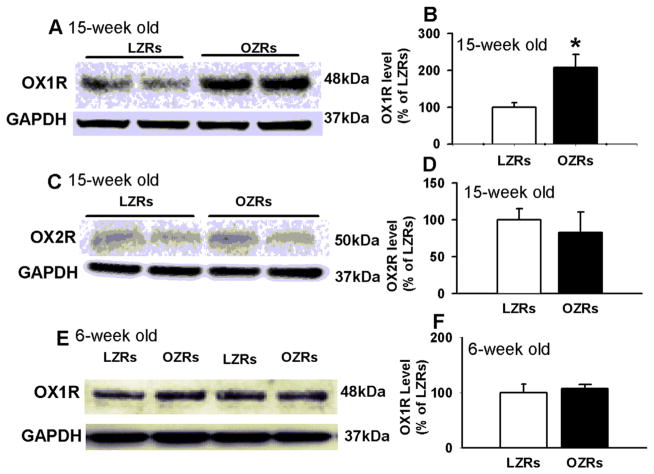
We next performed Western blot analysis to measure OX1R and OX2R protein levels in micropunched PVN tissues from 4 LZRs and 4 OZRs. Only a single protein band was displayed on the gel using OX1R or OX2R primary antibodies (48 kD for OX1R and 50 kD for OX2R). The intensity of the OX1R band was significantly higher in OZRs than in LZRs (n = 4, P < 0.05, Fig. 3A and B). However, the band intensity of OX2R in OZRs did not significantly differ from that of LZRs (100% in OZRs and 82.7 ± 12.3% in LZRs, Fig. 3C and D).
Fig. 3.
OX1R was upregulated in the PVN in OZRs. A and B, Original gel images (A) and summary data (B) show OX1R protein levels in the PVN were increased in OZRs compared with in LZRs. C and D: Gel images (C) and summary data (D) depict OX2R protein level in the PVN tissue obtained from LZRs and OZRs. E and F, Gel images (E) and summary data (F) showing OX1R expression levels in young (6-week-old) LZRs and OZRs. Each group contained 4 samples and each sample contained PVN tissues from 1 rat and the molecular weight are indicated on the right by each band. The OX1R and OX2R protein amounts were quantified by normalizing the density of their protein band to that of GAPDH in the same samples. The mean values of OX1R or OX2R proteins in LZRs were considered to be 1. Data are presented as means ± SEM. *P < 0.05 compared with LZRs.
To determine whether upregulation of OX1R in the PVN in OZRs is associated with high blood pressure or obesity, we measured the OX1R expression levels in the PVN in 6-week-old LZRs and OZRs. The ABP did not differ between LZRs and OZRs (tail-cuff measurement of systolic blood pressure: 148.9 ± 4 mmHg for LZRs and 149.1 ± 6 mmHg for OZRs; n = 4 for each group, P > 0.05). The body weight of OZRs (168.2 ± 3.5 g) was higher than that of LZRs (129.8 ± 7.6 g, P < 0.05). However, OX1R expression levels were similar in 6-week old LZRs (n = 4) and OZRs (n = 4) (Fig. 3E and F).
3.3. OX1R was expressed in PVN-spinal neurons
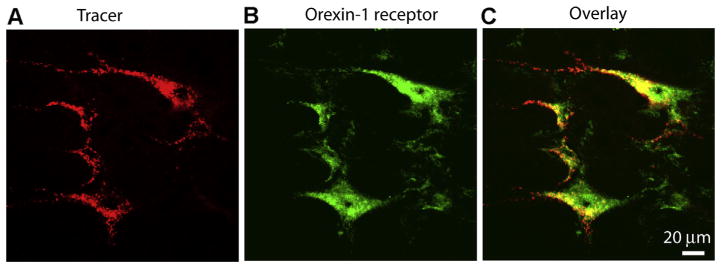
We next determined the spatial relationship between OX1R and PVN-spinal neurons. PVN-spinal neurons were retrogradely labeled by FluoSpheres injected into the intermediolateral cell column in the spinal cord. Brain slices containing FluoSphere-labeled PVN neurons were immunostained with antibody against OX1R. All negative controls displayed no detectable staining. Confocal images revealed that FluoSphere-labeled PVN neurons (red) were positive for OX1R immunoreactivity (green, Fig. 4). Almost all FluoSphere-labeled PVN neurons were OX1R-positive in LZRs (LZRs: 97.7%, 172 of 176 neurons, OZRs: 98.4%, 181 of 184 neurons). On the other hand, a percentage of OX1R-positive neurons (green) in the PVN are spinally projecting (red) were 47.3% (172 of 364 neurons) in LZRs and 46.5% (181 of 389 neurons) in OZRs.
Fig. 4.
OX1R immunoreactivity is positive on spinally projecting PVN neurons. Confocal images showing the spatial relationship of spinally projecting PVN neurons and OX1R-positive neurons in PVN. A: FluoSphere-labeled PVN neurons (red). B: OX1R immunoreactivity (green). C: merged images from A and B. The color change (yellow) indicates a colocalization of FluoSphere and OX1R immunoreactivity. Magnification, ×400. Images are single confocal optical sections. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.4. Orexin A caused greater excitation of PVN-spinal neurons in OZRs than in LZRs
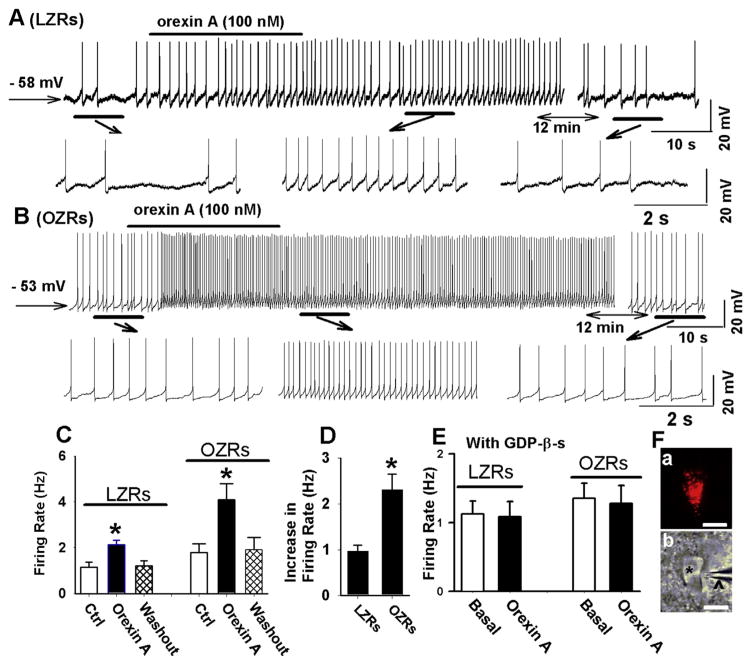
To determine the acute effect of orexin A on the firing activity of PVN-spinal neurons, we applied orexin A (100 nM) to the perfusion solution. The retrogradely labeled PVN-spinal neurons were first identified (Fig. 5F). Bath application of 100 nM orexin A depolarized the membrane potential of labeled PVN neurons in both LZRs (from −58.8 ± 2.1 to −54.8 ± 1.8 mV, n = 9 neurons, P < 0.05) and OZRs (from −55.1 ± 2.3 to −51.8 ± 2.8 mV, n = 11 neurons, P < 0.05). Furthermore, orexin A significantly increased the firing rate from 1.1 ± 0.2 to 2.0 ± 0.2 Hz (n = 9, P < 0.05, Fig. 5A, C, and D) in LZRs. Orexin A induced a significantly greater increase in the firing rate in OZRs (n = 11 neurons) than in LZRs (n = 9 neurons) (Fig. 5B, C, and D).
Fig. 5.
Orexin A induced a greater increase in the firing activity of PVN-spinal neurons in OZRs than in LZRs. A and B, Raw traces show that orexin A induced a depolarization and increased the firing rate of a labeled PVN neuron from one LZR (A) and one OZR (B). C, Summary data showing that orexin A increased the firing activity of PVN neurons in both LZRs (n = 9 neurons) and OZRs (n = 11 neurons). D, Summary data showing that orexin A induced a greater increase in the firing activity of PVN neurons in OZRs (n = 11 neurons) than in LZRs (n = 9 neurons). E, Summary data show that intracellular dialysis of GDP-β-s (1 mM) eliminated the excitatory effect of orexin A on the firing activity of PVN neurons in both LZRs (n = 6 neurons) and OZRs (n = 7 neurons). F, A FluoSphere-labeled PVN neuron in a slice viewed with fluorescence illumination (a) and in a photomicrograph (b). The neuron (red) in Fa was the same neuron shown in Fb (*) with an attached recording electrode (Λ) in the slice viewed with differential interference contrast optics. Data are presented as mean ± SEM. *P < 0.05 compared with the basal values in each group. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To further determine if postsynaptic mechanisms mediated the effect of orexin A on PVN-spinal neurons, GDP-β-s (1 mM) was included in the internal recording solution to block the G-protein coupled signaling pathways in the postsynaptic neurons because orexin receptors are G protein-coupled receptors (Sakurai et al., 1998). With inclusion of GDP-β-s in the recording solution, basal firing rate of PVN neurons in LZRs (1.1 ± 0.2 Hz, n = 6 neurons) did not significantly differ from that in OZRs (1.3 ± 0.2 Hz, n = 7 neurons, P > 0.05). Furthermore, GDP-β-s eliminated the orexin A-induced excitatory effect on the firing activity in PVN neurons in both LZRs (n = 6 neurons) and OZRs (n = 7 neurons, Fig. 5E).
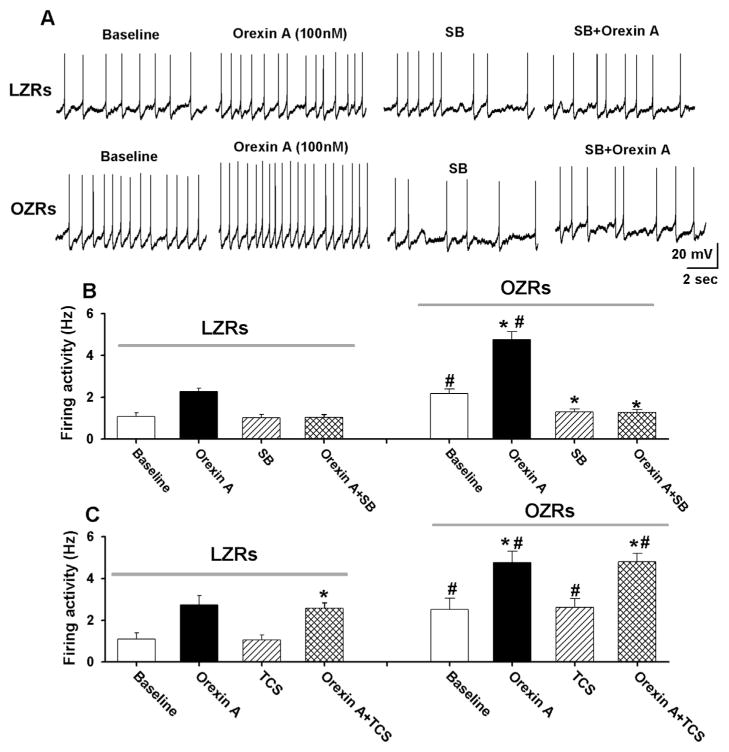
We next determined the role of OX1R in the regulation of the firing activity of PVN-spinal neurons. After testing the initial effect of orexin A on the firing activity in labeled PVN neurons, the OX1R antagonist SB334867 (10 μM) (Huang et al., 2010) was bath applied to the recording chamber. Subsequent application of orexin A failed to increase the firing activity of labeled PVN neurons in both LZRs (n = 7 neurons) and OZRs (n = 9 neurons, Fig. 6A and B). SB334867 (10 μM) alone had no significant effect on the basal firing rate of labeled PVN neurons in LZRs (baseline: 0.9 ± 0.1 SB334867: 1.0 ± 0.1 Hz, P > 0.05). However, SB334867 significantly hyper-polarized the membrane potential (from −54.6 ± 2.5 to −58.9 ± 1.8 mV, P < 0.05) and decreased the firing rate from 2.2 ± 0.2 to 1.3 ± 0.1 Hz (P < 0.05, Fig. 6B) of labeled PVN neurons in OZRs. We also determined whether OX2R is involved in mediating the effect of orexin A on the firing activity in PVN-spinal neurons. Bath application of the OX2R antagonist TCS-OX2-29 (10 μM) (Huang et al., 2010) alone did not change the basal firing activity of labeled PVN neurons in either LZRs (n = 7 neurons) or OZRs (n = 6 neurons). Subsequent application of orexin A (100 nM) still increased the firing activity of the PVN neurons in the presence of 10 μM TCS-OX2-29 in LZRs and OZRs (Fig. 6C).
Fig. 6.
OX1R, but not OX2R, mediated the effect of orexin A on the firing activity of PVN neurons. A and B, Raw tracings (A) and summary data (B) show that SB334867 blocked the orexin A-induced increase in the firing activity of PVN-spinal neurons in LZRs (n = 7 neurons) and OZRs (n = 9 neurons). C, Summary data showing that the OX2R antagonist TCS-OX2-29 had no effect on the orexin A-induced increase in firing activity of PVN-spinal neurons in LZRs (n = 7 neurons) or OZRs (n = 6 neurons). Data are presented as mean ± SEM. *P < 0.05 compared with the basal values in each control group. SB: SB334867. TCS: TCS-OX2-29.
3.5. Orexin A induced greater currents in PVN-spinal neurons in OZRs than in LZRs
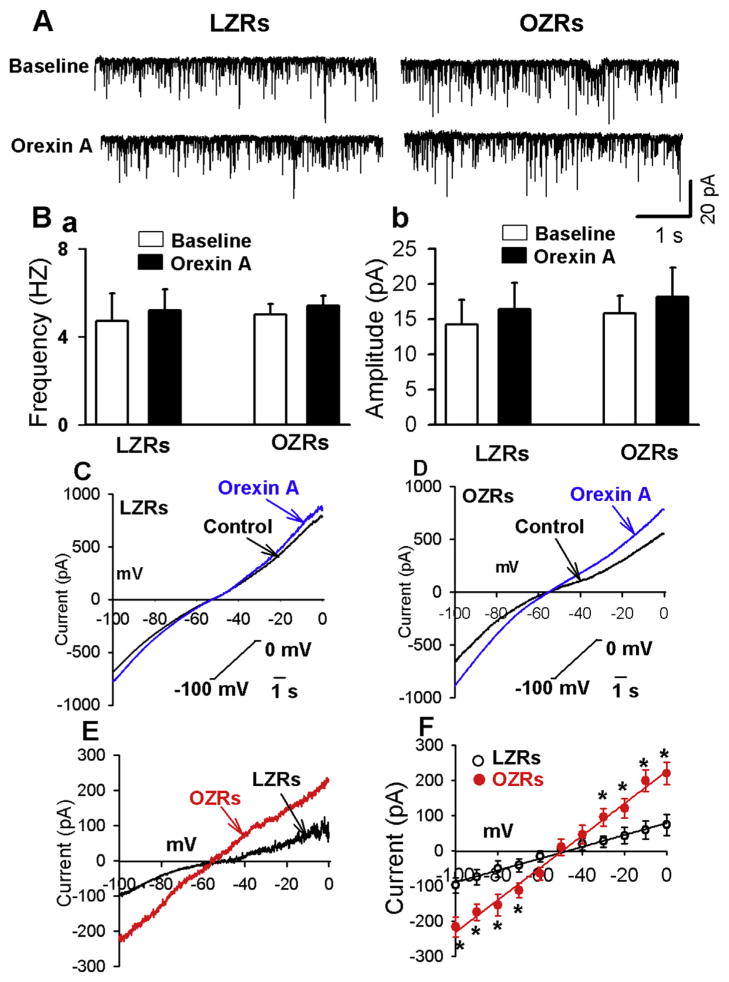
Orexin A stimulates neuronal activity through either increasing synaptic inputs or enhancing postsynaptic conductance (Follwell and Ferguson, 2002; Yang and Ferguson, 2002, 2003; Yang et al., 2003). We first determined the effect of orexin A on glutamatergic miniature excitatory postsynaptic currents (EPSCs) in PVN-spinal neurons. GDP-β-s (1 mM) was included in the recording solution to block the postsynaptic effect of orexin A. Bath application of orexin A (100 nM) had no significant effect on the frequency and amplitude of miniature EPSCs in either LZRs (n = 6 neurons) or OZRs (n = 6 neurons, Fig. 7A and B). The miniature EPSCs were completely blocked by application of non-NMDA receptor blocker CNQX (20 μM, n = 6 neurons in LZRs and n = 6 neurons in OZRs).
Fig. 7.
Orexin A induced greater currents in PVN-spinal neurons in OZRs than in LZRs. A and B, Raw recordings and summary data (B) showing that orexin A did not alter the frequency (a) nor amplitude (b) of miniature EPSCs in PVN-spinal neurons in both LZRs (n = 6 neurons) and OZRs (n = 6 neurons). GDP-β-s (1 mM) was included in the recording solution. C and D, Raw recordings showing currents obtained by applying slow depolarizing voltage ramps (−100 to 0 mV, 10 mV/s) to labeled PVN neurons from LZRs (C) and OZRs (D) before and during exposure brain slice to orexin A (100 nM). E, Orexin A-sensitive currents in PVN-spinal neurons in LZRs and OZRs. Orexin A-sensitive currents were obtained by subtracting the control current from the current recorded during orexin A application. F: orexin A-induced currents displayed linear I–V relationship in OZRs (n = 7 neurons) than in LZRs (n = 8 neurons). Data are presented as mean ± SEM. *P < 0.05 compared with the values at the same holding potentials in LZR group.
We next compared orexin A-induced currents in PVN-spinal neurons in LZRs and OZRs. After blocking Na+ channels with 1 μM tetrodotoxin, ionotropic glutamate receptors with 20 μM CNQX and 50 μM AP5, and GABAA receptors with 20 μM gabazine, a slow depolarizing voltage ramps (−100 to 0 mV, 10 mV/s) was applied to the recorded neurons in voltage-clamp configuration. Orexin A (100 nM) was added to the perfusion solution. Orexin A-sensitive currents were obtained by subtracting the control currents from those obtained during application of orexin A (Fig. 7E). These currents displayed linear relationship with voltages in the holding potential range of −100 to 0 mV (R2, 0.93 for LZRs and 0.98 for OZRs). Furthermore, orexin A-sensitive currents in labeled PVN neurons were significantly larger in OZRs (n = 7 neurons) than in LZRs (n = 8 neurons) in both negative and positive membrane potentials (Fig. 7E and F). The reversal potentials of the orexin A-sensitive current (−46.1 ± 2.1 mV) in LZRs did not significantly differ from that in OZRs (−45.8 ± 1.5 mV, P > 0.05, Fig. 7F).
4. Discussion
This is the first study to determine the role of orexin receptors in the regulation of sympathetic vasomotor tone and firing activity of presympathetic PVN neurons in obesity. The PVN is a critical brain region containing neurons that provide excitatory drive to vasomotor neurons in the brainstem and sympathetic preganglionic neurons in the spinal cord (Pyner and Coote, 2000; Ranson et al., 1998). Consistent with previous studies, we found that the basal ABP and RSNA were significantly higher in OZRs than in LZRs (D’Angelo et al., 2006; Morgan et al., 1995). Furthermore, we found that the increases in ABP and RSNA induced by microinjection of orexin A into the PVN were significantly greater in OZRs than in LZRs. This enhanced sympathoexcitatory response indicates that the receptor mediating the effect of orexin A on sympathetic vasomotor tone is upregulated in OZRs. We also determined the receptor subtype mediating the sympathoexcitatory effect of orexin A. We found that microinjection of the OX1R receptor antagonist SB334867, but not the OX2R receptor antagonist TCS-OX2-29, prior to administration of orexin A into the PVN abolished the orexin A-induced sympathoexcitatory response in both LZRs and OZRs. This finding indicates that OX1R is the receptor subtype mediating the effect of orexin A in the PVN on sympathetic vasomotor tone. In contrast, in other brain regions such as the rostral ventrolateral medulla, both OX1R and OX2R mediate the effect of orexin A on sympathetic vasomotor tone (Huang et al., 2010; Shahid et al., 2012; Xiao et al., 2013).
Strikingly, blocking OX1R with SB334867 in the PVN decreased basal ABP and RSNA in OZRs but did not alter sympathetic vasomotor tone in LZRs. These data suggest that OX1R is tonically activated in the PVN to support the elevated sympathetic vasomotor tone in OZRs. Tonic activation of OX1R could be due to increases in the orexin levels or upregulation of OX1R in the PVN in OZRs. However, both orexin A immunoreactivity and preproorexin A mRNA levels in the hypothalamus are not significantly different between LZRs and OZRs (Taheri et al., 2001) and may even be lower in OZRs (Beck et al., 2001). The mRNA level of preproorexin in the hypothalamus was significantly reduced in high fat diet-induced obesity in mice (Tanno et al., 2013). We found that OX1R protein expression levels in PVN tissues were significantly higher in OZRs than in LZRs, whereas OX2R protein expression levels in the PVN did not differ between LZRs and OZRs. However, the OX1R expression level in the PVN did not differ between 6-week-old LZRs and OZRs. The body weight of 6-week-old OZRs was higher than age-matched LZRs, but the ABP of OZRs did not differ between LZRs and OZRs at the age of 6-week old. Together with data showing the OX1R level was significantly higher in 15-wk-old OZRs, which display both obesity and hypertension Thus, it is likely that upregulation of OX1R is associated with hypertension but not obesity.
Hypothalamic PVN is a heterogeneous nucleus containing many types of neurons, including PVN-spinal neurons. It has been shown that elevated sympathetic vasomotor tone is attributed to hyper-activity of presympathetic PVN neurons (Li et al., 2008a,b). The upregulation of OX1R in PVN tissue may not represent the increased OX1R levels in presympathetic neurons in the PVN. Because OX1R immunoreactivity was positive in retrogradely labeled PVN-spinal neurons, we examined the effect of orexin A on the firing activity of these PVN-spinal neurons. We found that the basal firing activity of PVN-spinal neurons was higher in OZRs than in LZRs. It is likely that the hyperactivity of PVN-spinal neurons leads to elevated sympathetic vasomotor tone in OZRs. Blocking OX1R reduced the basal firing activity of PVN-spinal neurons in OZRs, suggesting that OX1Rs are tonically activated in PVN-spinal neurons and may lead to hyperactivity of these neurons in obesity. Furthermore, we found that orexin A produced a greater excitatory effect on the firing activity of PVN-spinal neurons in OZRs than in LZRs, an effect was blocked by the antagonist for OX1R, but not for OX2R. These data suggested the OX1R is upregulated in the PVN-spinal neuron in OZRs and upregulation of OX1R may contributes to hyperactivity of PVN presympathetic neurons in obesity. Adult OZRs also display insulin resistance and hyperglycemia, which are the characteristics of the type 2 diabetes. It has been shown that OX1R deficiency ameliorates hyperglycemia and hyperinsulinemia (Funato et al., 2009). Furthermore, OX1R antagonist reduces plasma insulin and glucose levels (Haynes et al., 2002). It is possible that upregulation of OX1R in the PVN contributes to insulin resistance and hyperglycemia in OZRs. Thus, OX1R is a potential target for the treatment of type 2 diabetes.
Orexin A increases neuronal activity through a variety of mechanisms. For example, orexin A enhances glutamatergic synaptic input to stimulate magnocellular neurons in the PVN, while enhancing postsynaptic cation conductance to excite parvocellular neurons in the PVN (Follwell and Ferguson, 2002), serotoninergic neurons (Liu et al., 2002), and locus coeruleus neurons (Taheri et al., 2001). Because orexin receptors are G protein-coupled receptors (Sakurai et al., 1998), we blocked the postreceptor signaling by including GDP-β-s in the recording internal solution. We found that inclusion of GDP-β-s eliminated the orexin A-induced excitatory effect on the firing activity of PVN neurons. Furthermore, orexin A had no effect on miniature EPSCs recorded in the labeled PVN neurons. These data suggest that postsynaptic mechanisms mediate the excitatory effect of orexin A on PVN-spinal neurons, although we cannot completely rule out the effect of orexin A on presynaptic neurotransmitter release.
In addition, we found that orexin A induced linear I–V relationship current within the voltage range tested in the PVN neurons in both LZRs and OZRs. These orexin A-induced currents were insensitive to tetradotoxin, DNQX, and AP5. Also, the reversal potentials of orexin A-induced currents in PVN neurons from LZRs did not differ from those in ORZs. Furthermore, we found that orexin A induced significantly greater inward currents in negative membrane potentials in the PVN-spinal neuron in OZRs than in LZRs. This may be the reason that orexin A induced greater depolarization of PVN-spinal neurons in OZRs than in LZRs. Similarly, Orexin A induces non-selective cation currents in neurons in different brain regions (Follwell and Ferguson, 2002; Liu et al., 2002; Murai and Akaike, 2005; Yang and Ferguson, 2003). Although Ca2+ currents display a similar reversal potential as of orexin A-induced current, Ca2+ currents are inward at membrane potentials more depolarizing than −50 mV. However, it is possible that Ca2+ is one of the carriers of orexin A-induced currents. It is not clear the ionic components of the orexin A-included currents in the PVN neurons. Previous studies have shown that both Na+ and K+ are involved in orexin-induced currents in locus coeruleus neurons (Murai and Akaike, 2005) and amygdala neurons (Bisetti et al., 2006). Cl− is another potential components of orexin A-induced current. Thus, future studies are deserved to determine the exact component of the orexin A-induced current.
In summary, our findings in this study indicate that hypothalamic OX1R is upregulated in obesity and this upregulation of OX1R leads to hyperactivity of PVN-spinal neurons and elevated sympathetic vasomotor tone in obesity. These findings provide a rational that hypothalamic OX1R may represent a new target to reduce the heightened sympathetic vasomotor tone in obesity-related hypertension.
Acknowledgments
This work was supported by the National Institutes of Health (Grants MH096086 to D-P. L.), National Natural Science Foundation of China (31271223 and 31071002), National Basic Research Development Program of China (2012CB518200), and the Natural Science Foundation of Hebei Province (C2012206001).
Nonstandard abbreviation
- PVN
paraventricular nucleus
- ABP
arterial blood pressure
- RSNA
renal sympathetic nerve activity
- OZRs
obese Zucker rats
- LZRs
lean Zucker rats
- OX1R
Orexin receptor 1
- OX2R
Orexin receptor 2
- aCSF
artificial cerebrospinal fluid
- EPSCs
excitatory postsynaptic currents
Footnotes
Authorship contribution statement
Zhou J: Data acquisition and analysis, interpretation of finding. Drafting the manuscripts.
Yuan F: Data acquisition and analysis, interpretation of finding.
Zhang Y: Study design, data analysis, interpretation of data and revising the manuscript.
Li D-P: Study design, data analysis, interpretation of data and revising the manuscript.
Conflict of interest and disclosure
The authors have nothing to disclose for this study.
Contributor Information
Jing-Jing Zhou, Email: zhyhenry@hotmail.com.
De-Pei Li, Email: dpli@mdanderson.org.
References
- Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- Alonso-Galicia M, Brands MW, Zappe DH, Hall JE. Hypertension in obese Zucker rats. Role of angiotensin II and adrenergic activity. Hypertension. 1996;28:1047–1054. doi: 10.1161/01.hyp.28.6.1047. [DOI] [PubMed] [Google Scholar]
- Beck B, Richy S, Dimitrov T, Stricker-Krongrad A. Opposite regulation of hypothalamic orexin and neuropeptide Y receptors and peptide expressions in obese Zucker rats. Biochem Biophys Res Commun. 2001;286:518–523. doi: 10.1006/bbrc.2001.5420. [DOI] [PubMed] [Google Scholar]
- Bisetti A, Cvetkovic V, Serafin M, Bayer L, Machard D, Jones BE, Muhlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142:999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension. 2000;35:403–408. doi: 10.1161/01.hyp.35.1.403. [DOI] [PubMed] [Google Scholar]
- Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–731. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- D’Angelo G, Mintz JD, Tidwell JE, Schreihofer AM, Pollock DM, Stepp DW. Exaggerated cardiovascular stress responses and impaired beta-adrenergic-mediated pressor recovery in obese Zucker rats. Hypertension. 2006;48:1109–1115. doi: 10.1161/01.HYP.0000247306.53547.d4. [DOI] [PubMed] [Google Scholar]
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545:855–867. doi: 10.1113/jphysiol.2002.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, Yanagisawa M. Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab. 2009;9:64–76. doi: 10.1016/j.cmet.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimaraes PS, Huber DA, Campagnole-Santos MJ, Schreihofer AM. Development of attenuated baroreflexes in obese Zucker rats coincides with impaired activation of nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2014;306:R681–R692. doi: 10.1152/ajpregu.00537.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes AC, Chapman H, Taylor C, Moore GB, Cawthorne MA, Tadayyon M, Clapham JC, Arch JR. Anorectic, thermogenic and anti-obesity activity of a selective orexin-1 receptor antagonist in ob/ob mice. Regul Pept. 2002;104:153–159. doi: 10.1016/s0167-0115(01)00358-5. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Huang SC, Dai YW, Lee YH, Chiou LC, Hwang LL. Orexins depolarize rostral ventrolateral medulla neurons and increase arterial pressure and heart rate in rats mainly via orexin 2 receptors. J Pharmacol Exp Ther. 2010;334:522–529. doi: 10.1124/jpet.110.167791. [DOI] [PubMed] [Google Scholar]
- Kasiske BL, O’Donnell MP, Keane WF. The Zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension. 1992;19:I110–I115. doi: 10.1161/01.hyp.19.1_suppl.i110. [DOI] [PubMed] [Google Scholar]
- Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591:4237–4248. doi: 10.1113/jphysiol.2013.256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Averill DB, Pan HL. Differential roles for glutamate receptor subtypes within commissural NTS in cardiac-sympathetic reflex. Am J Physiol Regul Integr Comp Physiol. 2001;281:R935–R943. doi: 10.1152/ajpregu.2001.281.3.R935. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Angiotensin II attenuates synaptic GABA release and excites paraventricularrostral ventrolateral medulla output neurons. J Pharmacol Exp Ther. 2005;313:1035–1045. doi: 10.1124/jpet.104.082495. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension. 2007a;49:916–925. doi: 10.1161/01.HYP.0000259666.99449.74. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Ther. 2007b;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL. Plasticity of pre- and postsynaptic GABAB receptor function in the paraventricular nucleus in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2008a;295:H807–H815. doi: 10.1152/ajpheart.00259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Yang Q, Pan HM, Pan HL. Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J Physiol. 2008b;586:1637–1647. doi: 10.1113/jphysiol.2007.149732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, van den Pol AN, Aghajanian GK. Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 2002;22:9453–9464. doi: 10.1523/JNEUROSCI.22-21-09453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Morgan DA, Anderson EA, Mark AL. Renal sympathetic nerve activity is increased in obese Zucker rats. Hypertension. 1995;25:834–838. doi: 10.1161/01.hyp.25.4.834. [DOI] [PubMed] [Google Scholar]
- Murai Y, Akaike T. Orexins cause depolarization via nonselective cationic and K+ channels in isolated locus coeruleus neurons. Neurosci Res. 2005;51:55–65. doi: 10.1016/j.neures.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic; New York: 1998. [DOI] [PubMed] [Google Scholar]
- Pyner S, Coote JH. Identification of branching paraventricular neurons of the hypothalamus that project to the rostroventrolateral medulla and spinal cord. Neuroscience. 2000;100:549–556. doi: 10.1016/s0306-4522(00)00283-9. [DOI] [PubMed] [Google Scholar]
- Ranson RN, Motawei K, Pyner S, Coote JH. The paraventricular nucleus of the hypothalamus sends efferents to the spinal cord of the rat that closely appose sympathetic preganglionic neurones projecting to the stellate ganglion. Exp Brain Res. 1998;120:164–172. doi: 10.1007/s002210050390. [DOI] [PubMed] [Google Scholar]
- Rauch M, Riediger T, Schmid HA, Simon E. Orexin A activates leptin-responsive neurons in the arcuate nucleus. Pflugers Arch. 2000;440:699–703. doi: 10.1007/s004240000342. [DOI] [PubMed] [Google Scholar]
- Sainz N, Barrenetxe J, Moreno-Aliaga MJ, Martinez JA. Leptin resistance and diet-induced obesity: central and peripheral actions of leptin. Metabolism. 2015;64:35–46. doi: 10.1016/j.metabol.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Mandel DA, Mobley SC, Stepp DW. Impairment of sympathetic baroreceptor reflexes in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2007;293:H2543–H2549. doi: 10.1152/ajpheart.01201.2006. [DOI] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol. 2012;165:2292–2303. doi: 10.1111/j.1476-5381.2011.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaka T, Miyahara S, Kunitake T, Jin QH, Kato K, Takasaki M, Kannan H. Orexin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1114–R1118. doi: 10.1152/ajpregu.2001.281.4.R1114. [DOI] [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Taheri S, Gardiner J, Hafizi S, Murphy K, Dakin C, Seal L, Small C, Ghatei M, Bloom S. Orexin A immunoreactivity and preproorexin mRNA in the brain of Zucker and WKY rats. Neuroreport. 2001;12:459–464. doi: 10.1097/00001756-200103050-00008. [DOI] [PubMed] [Google Scholar]
- Tanno S, Terao A, Okamatsu-Ogura Y, Kimura K. Hypothalamic preproorexin mRNA level is inversely correlated to the non-rapid eye movement sleep level in high-fat diet-induced obese mice. Obes Res Clin Pract. 2013;7:e251–257. doi: 10.1016/j.orcp.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Xiao F, Jiang M, Du D, Xia C, Wang J, Cao Y, Shen L, Zhu D. Orexin A regulates cardiovascular responses in stress-induced hypertensive rats. Neuropharmacology. 2013;67:16–24. doi: 10.1016/j.neuropharm.2012.10.021. [DOI] [PubMed] [Google Scholar]
- Yang B, Ferguson AV. Orexin-A depolarizes dissociated rat area postrema neurons through activation of a nonselective cationic conductance. J Neurosci. 2002;22:6303–6308. doi: 10.1523/JNEUROSCI.22-15-06303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Ferguson AV. Orexin-A depolarizes nucleus tractus solitarius neurons through effects on nonselective cationic and K+ conductances. J Neurophysiol. 2003;89:2167–2175. doi: 10.1152/jn.01088.2002. [DOI] [PubMed] [Google Scholar]
- Yang B, Samson WK, Ferguson AV. Excitatory effects of orexin-A on nucleus tractus solitarius neurons are mediated by phospholipase C and protein kinase C. J Neurosci. 2003;23:6215–6222. doi: 10.1523/JNEUROSCI.23-15-06215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]