Abstract
Background
Alcohol has particularly harmful health effects in HIV-infected patients; therefore, HIV clinics are an important setting for integration of brief alcohol intervention and alcohol pharmacotherapy to improve patient outcomes. Current practices of alcohol screening, counseling, and prescription of pharmacotherapy by HIV providers are unknown.
Methods
We conducted a cross-sectional survey of HIV providers from 8 HIV clinical sites across the United States. Surveys queried knowledge and use of alcohol screening, brief advice, counseling and pharmacotherapy, confidence and willingness to prescribe pharmacotherapy and barriers to their use of alcohol pharmacotherapy. We used multivariable logistic regression to examine provider factors associated with confidence and willingness to prescribe pharmacotherapy.
Results
Providers (N=158) were predominantly female (58%) and Caucasian (73%); almost half were infectious disease physicians and 31% had been in practice 10–20 years. Most providers (95%) reported always or usually screening for alcohol use, although only 10% reported using a formal screening tool. Over two-thirds never or rarely treated alcohol-dependent patients with pharmacotherapy themselves. Most (71%) referred alcohol-dependent patients for treatment. Knowledge regarding alcohol pharmacotherapy was low. The major barrier to prescribing pharmacotherapy was insufficient training on use of pharmacotherapy. Provider confidence ratings were positively correlated with their practice patterns.
Conclusions
HIV providers reported high rates of screening for alcohol use, though few used a formal screening tool. Most providers referred alcohol dependent patients to outside resources for treatment. Few reported prescribing alcohol pharmacotherapy. Increased training alcohol pharmacotherapy may increase confidence in prescribing and use of these medications in HIV care settings.
Keywords: HIV, Alcohol Screening, Education, Confidence, Knowledge
1. INTRODUCTION
Alcohol misuse includes a spectrum of severity, from hazardous/risky use, defined as a “quantity or pattern of alcohol consumption that places patients at risk for adverse health events” which includes binge drinking, to alcohol abuse and/or dependence that result in adverse physical and psychological health effects (Reid et al., 1999). Alcohol misuse is prevalent among HIV-infected individuals, and is associated with decreased antiretroviral therapy uptake, adherence, and virologic suppression (Galvan et al., 2002; Samet et al., 2004; Braithwaite et al., 2005; Chander et al., 2006, 2008). Unfortunately, patient engagement and retention in traditional alcohol treatment services is poor in both HIV-infected and uninfected persons. Across the life span, fewer than 15% of persons with alcohol misuse ever receive any kind of formal alcohol treatment (Office of Applied Studies, 2009). To address this gap, screening and brief alcohol intervention (SBI) has been developed and tested for delivery in primary care and emergency room settings and has been shown to reduce alcohol misuse and improve health-related outcomes (Kaner et al., 2009). Brief alcohol intervention and motivational interviewing based interventions have also been effective in reducing alcohol use in HIV care settings (Hasin et al., 2013; Chander et al., 2015).
In addition to SBI, there are several FDA-approved pharmacotherapies with demonstrated efficacy in reducing alcohol consumption. A recent systematic review and meta-analysis (Jonas et al., 2014) of 122 randomized controlled trials highlighted the effectiveness of these therapies. Yet fewer than one in five alcohol treatment clinics offers alcohol pharmacotherapy to their patients (Ducharme et al., 2006). To broaden engagement and retention in treatment, there has been considerable interest in trying to move alcohol services into main stream medical practice in a model similar to that adopted for treatment of depression. However, despite a good evidence base for both SBI and alcohol pharmacotherapy for the treatment of alcohol misuse, these interventions also remain underused in primary care settings (Jonas et al., 2014).
Given the particularly harmful effects of alcohol misuse in HIV-infected patients, HIV clinics are an important setting for integration of SBI and alcohol pharmacotherapy. They provide long-term care to their patients, integrate a variety of specialty services, frequently have expanded funding for prescription medications, and often provide intensive case management models that promote outreach to and retention of patients. However, there are barriers to integration of alcohol screening, counseling and pharmacotherapy into HIV care, including increased demands on already busy providers, and lack of training on and familiarity with alcohol pharmacotherapy.
Given the potential benefits of reducing alcohol misuse among HIV-infected patients, the aims of the current study were to: 1) characterize current practice patterns related to alcohol screening, advice, counseling and pharmacotherapy; 2) examine providers’ knowledge, attitudes and beliefs about these alcohol interventions; and 3) identify HIV provider barriers to prescribing alcohol pharmacotherapy for HIV infected patients. The goal of this study was to inform the development of strategies to facilitate implementation of alcohol interventions in HIV primary care clinics.
2. MATERIAL AND METHODS
2.1 Study design
This was a cross-sectional survey of HIV providers across the United States. The survey was administered between January, 2013 and March, 2014.
2.2 Participants
Participants included attending physicians, fellows, medical residents, nurse practitioners, and physician assistants working in 8 HIV clinics. The selected sites are part of the Center for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS), including University of Alabama, Birmingham; University of Washington, Seattle; University of California San Francisco; Harvard University Fenway Clinic, Boston; Johns Hopkins University, Baltimore; University of California, San Diego; Case Western Reserve Hospital, Cleveland; and University of North Carolina, Chapel Hill. A list of provider email contact information was provided by each clinic.
2.3 Survey
The composition of the survey was largely based on prior research by Harris and Sun (2012), the Physician’s Competence in Substance Abuse Test (PCSAT) and Alford and colleagues (2008); items were added to specifically assess alcohol pharmacotherapy, a primary focus of our study. The survey addressed four areas of alcohol intervention: screening, brief advice, counseling and pharmacotherapy. Across these four areas, providers were asked to report on their current practice patterns and their confidence and willingness to engage in each intervention type. In addition, the survey included 17 knowledge items on alcohol interventions and 4 items specifically addressing potential barriers to their use of alcohol pharmacotherapy. Items were answered using Likert scales; scale anchors were modified according to the nature of the item. For example, practice items were rated on a 5-point Likert scale (i.e., never, rarely, sometimes, usually, always). Knowledge items were rated on a 7-point Likert scale from “strongly disagree” to “strongly agree”, with the center of the scale anchored by “Don’t Know.” Willingness and confidence items were rated on a 10-point Likert scale from not at all to extremely. Barriers to use of alcohol pharmacotherapy were rated on a 5-point scale from not a barrier to very major barrier.
This survey was designed and conducted prior to the launch of the American Psychiatric Association Diagnostic and Statistical Manual 5 (APA DSM 5), and therefore used the terms alcohol abuse and dependence, the diagnostic nomenclature of DSM IV. In the survey, items on referral to alcohol treatment and pharmacotherapy referenced patients with alcohol abuse or dependence. Items on the use of brief advice and counseling referenced patients with hazardous/risky drinking, that is, persons with a quantity or pattern of alcohol consumption that places patients at risk for adverse health events.
The survey was intended to be ten minutes in length and providers could elect to receive a $10 gift card for their participation. This study was approved by the JHU Institutional Review Board (IRB) and the IRBs at the study sites. The introduction to the survey assured participants that their responses were anonymous and data would not be provided back to the clinic directors. Completion of the survey served as consent for participation.
2.4 Survey Distribution
Within 2 weeks preceding survey distribution, clinic directors sent out an email to their provider network informing them of the upcoming survey and encouraging participation. An email inviting survey participation was sent to a total of 269 HIV/primary care providers across the 8 CNICS sites. Each provider’s email included a unique link to access an electronic survey hosted by Survey Monkey. Reminder emails were sent approximately 2, 4, and 6 weeks following the initial survey distribution.
2.5 Analysis Plan
We performed descriptive statistics to describe screening and treatment practices and confidence in screening, providing advice, counseling, and prescribing pharmacotherapy. We then conducted multivariable logistic regression analysis examining key provider characteristics associated with greater confidence (visual analog scale (VAS) >5)) and greater willingness (VAS >5) to prescribe pharmacotherapy. Provider characteristics included sex, provider type (i.e., Infectious Disease, Family Practice/Internist, and Physician Extender: Physician Assistant or Nurse Practitioner, Medical Resident/Fellow), years in practice, and hours of prior training in the management of alcohol use disorders (i.e., <10, ≥10, none/don’t remember) and study site. We then used multivariable logistic regression analysis to examine factors associated with self-reported prescription of pharmacotherapy for dependence, including provider type, confidence and willingness to prescribe, years in practice, prior training, and site. Several CNICS sites (n=6) use a tablet-based clinical assessment tool that includes the AUDIT-C; therefore, we also examined whether provider-reported use of a formal screening tool varied in clinics with and without the tool.
3. RESULTS
3.1 Provider characteristics
Of the 269 HIV providers who received an email invitation, 158 participants completed the survey. Response rates varied across CNICS sites, and ranged from 29% to 95% of distributed surveys. The overall electronic response rate across sites was 58.7%. Compared to the total sample who received a survey, respondents did not differ significantly by sex/gender or provider type.
As summarized in Table 1, providers reported a median age of 42, were predominantly female (58%), and Caucasian (73%). Over half of the respondents were attending physicians (63%) and 19% were physicians in residency or fellowship training. An additional 18% were nurse practitioners or physician assistants. Overall, this was a highly experienced group of practitioners, with 31% in practice 10 – 20 years and 23% in practice 21 years and more. Two-thirds of the providers (66%) reported less than 10 training hours related to alcohol problems during their medical/nursing/post-graduate education. Twenty eight percent of respondents were from the Mid-Atlantic, 34% from the West, 27% from the South, and the remaining 11% were from New England, and the Mid-West.
Table 1.
Provider Demographics Characteristics (N=158)
| Variable | n (%)* |
|---|---|
| Age | |
| Median | 42 |
| Interquartile Range | 36–53 |
|
| |
| Sex | |
| Female | 91 (58) |
| Male | 66 (42) |
| Not Reported | 1 (0) |
|
| |
| Race | |
| African American or Black | 5 (3) |
| Caucasian | 115 (73) |
| Asian | 19 (12) |
| Other | 6 (4) |
| Not Reported | 13 (8) |
|
| |
| Ethnicity | |
| Hispanic or Latino/a | 10 (6) |
| Not Hispanic or Latino/a | 134 (85) |
| Not Reported | 14 (9) |
|
| |
| Provider Type | |
| Family Practice Internal Medicine Attending | 23 (15) |
| Physician Extenders** | 29 (18) |
| Resident/Fellow | 30 (19) |
| Infectious Disease Attending | 76 (48) |
|
| |
| Years in Practice | |
| Less than 10 | 59 (37) |
| 10–20 | 49 (31) |
| More than 20 | 37 (23) |
| Not Reported | 13 (8) |
|
| |
| Training hours for alcohol problems in post-graduate school? | |
| Less than 10 hours | 104 (66) |
| More than 10 hours | 18 (11) |
| Do Not Remember/Not Reported | 36 (23) |
|
| |
| Geographic Location | |
| New England | 13 (8) |
| Mid-Atlantic | 44 (28) |
| South | 43 (27) |
| West | 53 (34) |
| Mid-West | 5 (3) |
|
| |
| Practice Location | |
| Urban | 126 (80) |
| Suburban | 19 (12) |
| Rural | 1 (1) |
| Not Reported | 12 (8) |
Percentages may not add to 100% due to rounding.
Physician Extenders are Nurse Practitioners and Physician Assistants.
3.2 Current practice behaviors
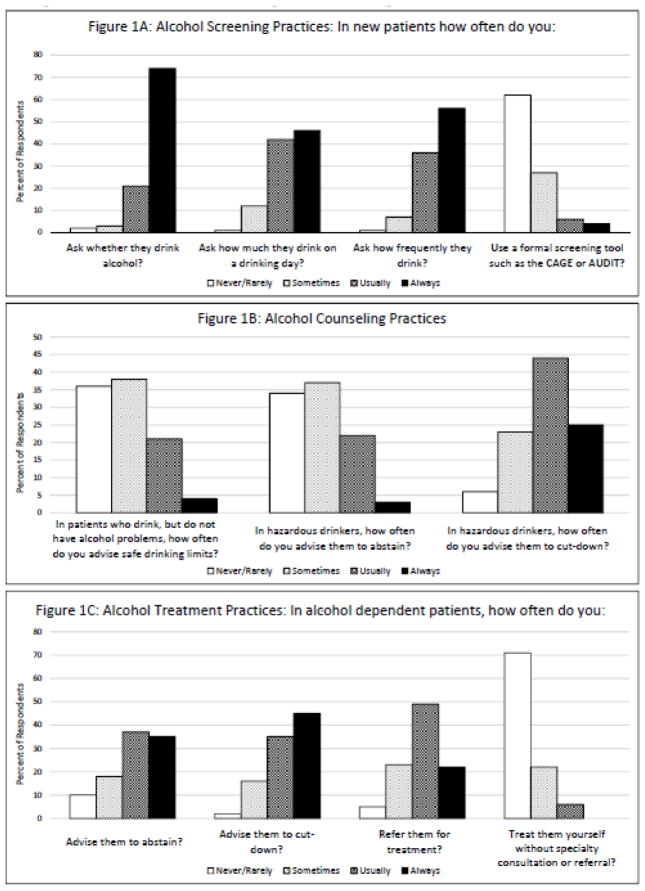
As shown in Figure 1A, the majority of providers reported usually or always screening for alcohol use among new patients. Specifically, 74% of providers reported always and an additional 21% reported usually asking new patients whether they drink alcohol. Approximately half of providers reported always asking new patients about their drinking frequency (56%) and intensity (46%) (drinks per drinking day), and an additional one-third or more of providers reported usually asking these questions. Few providers (10%) reported always or usually using a formal screening tool to assess alcohol use. This estimate did not differ between providers practicing at clinic sites using tablet-based clinical assessments (n=109), and those that did not (n=49).
Figure 1.
Figure 1a, b, c. Alcohol Screening, Counseling and Treatment Practices.
In addition to screening for alcohol use, providers also reported providing advice about drinking (Figure 1B). One-quarter always or usually provided advice on safer drinking limits to patients who drink but do not have alcohol problems. For patients with hazardous drinking, over two-thirds (69%) of providers reported always or usually recommending that the patient cut-down and 25% recommended alcohol abstinence. For patients with alcohol dependence, 80% of providers always or usually recommended cutting down and 72% recommended abstinence (Figure 1C). While providers tended to provide advice on drinking reduction to patients, they were less likely to treat those with dependence. Over two-thirds of providers (71%) reported rarely or never treating alcohol dependent patients themselves, and instead reported referring their alcohol-dependent patients for treatment. Three-quarters had not prescribed a medication to support alcohol abstinence in the past year (data not shown).
3.3 Willingness and confidence as a function of intervention type
Most providers expressed confidence (VAS > 5) in their skills at taking an alcohol history (90%), assessing a patient’s risk for developing alcohol-related problems (74%), using a formal alcohol screening tool (69%), advising patients regarding safe drinking limits (83%), and counseling patients about hazardous alcohol use (86%).
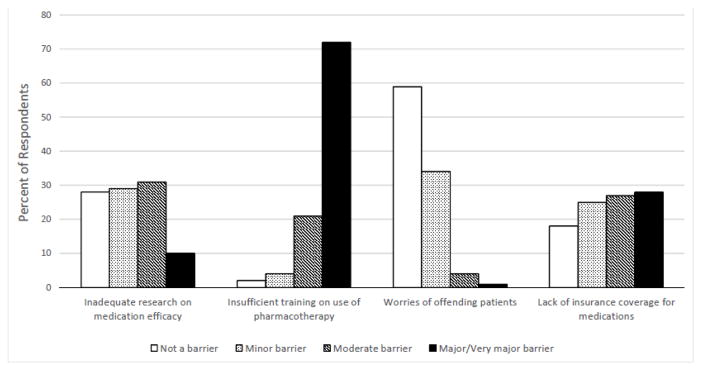
In contrast, only 16% reported confidence in prescribing alcohol pharmacotherapy for their patients with alcohol dependence; although one-third of providers indicated a willingness (VAS > 5) to prescribe these medications. Providers rated four potential barriers to their use of alcohol pharmacotherapy (Figure 2). The only item rated by most providers (72%) as a major or very major barrier was insufficient training on how to use medications in individuals with alcohol dependence. In contrast, 10% of providers rated inadequate research on medication efficacy, 1% rated concerns about offending patients, and 29% rated lack of insurance as major or very major barriers to their use of pharmacotherapy.
Figure 2.
Provider Barriers to the Use of Alcohol Pharmacotherapy
3.4 Knowledge items on alcohol interventions
Responses on knowledge items were evaluated for incorrect and don’t know responses. Overall, providers were familiar with definitions of binge drinking for men and women (>80% correctly identifying ≥4 drinks/occasion in women and ≥5 drinks/occasion in men as binge drinking), brief advice and counseling; however, the majority of participants were not knowledgeable about alcohol pharmacotherapy. Eighty-eight percent of respondents answered “don’t know” (65%) or incorrectly (23%) to a statement indicating that acamprosate was contraindicated in individuals with liver impairment, and 76% of respondents marked “don’t know” to a statement indicating that there was no benefit to naltrexone if initial pharmacotherapy with acamprosate was not successful. Finally, 34% of providers answered don’t know (27%) or incorrectly (7%) to a statement indicating that disulfiram is the preferred treatment if a patient wants to continue drinking but at reduced levels. After combining these three pharmacotherapy knowledge questions, 8% of individuals answered all three questions correctly.
3.5 Relationship between provider characteristics and alcohol pharmacotherapy ratings
In multivariable analysis (Table 2), family practitioners and general internists reported greater confidence in prescribing alcohol medications than infectious disease providers (OR=8.18, (95% CI=2.26 –29.58)). Providers with 10 – 20 years in practice reported higher confidence in prescribing alcohol medications than those with fewer than 10 years in practice (OR=5.72 p=0.041 (95% CI=1.08 – 30.35)). There also was a trend for hours of alcohol-related training to predict confidence, with those reporting more than 10 training hours reporting greater confidence than providers with fewer than 10 training hours (OR= 3.67 p= 0.078 (95% CI=0.89 – 15.05)). There were no differences in confidence ratings across sites or for male and female providers.
Table 2.
Multivariable analysis of factors associated with confidence in and willingness to prescribe alcohol pharmacotherapy.
| Provider Characteristics | Provider Confidence (VAS >5) | Provider Willingness (VAS >5) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Odds Ratio | 95% Confidence Interval | P-Value | Odds Ratio | 95% Confidence Interval | P-Value | |
| Provider Sex | ||||||
| Male | 1.10 | 0.39–3.11 | 0.85 | 1.25 | 0.61–2.57 | 0.54 |
| Female | 1.0 (ref) | 1.0 (ref) | ||||
|
| ||||||
| Provider Type | ||||||
| Family Practice Internal Medicine Attending | 8.19 | 2.27–29.59 | 0.00 | 2.16 | 0.78–5.97 | 0.14 |
| Physician Extender | 2.99 | 0.78–11.45 | 0.11 | 2.30 | 0.87–6.11 | 0.09 |
| Resident/Fellow | 2.14 | 0.20–22.97 | 0.53 | 1.20 | 0.33–4.33 | 0.78 |
| Infectious Disease Attending | 1.0 (ref) | 1.0 (ref) | ||||
|
| ||||||
| Years in Practice | ||||||
| Not Reported | 4.30 | 0.22–83.19 | 0.33 | 3.89 | 0.72–21.10 | 0.12 |
| >20 | 3.79 | 0.67–21.40 | 0.13 | 1.55 | 0.53–4.50 | 0.42 |
| 10–20 years | 5.72 | 1.08–30.36 | 0.04 | 2.40 | 0.89–6.48 | 0.08 |
| <10 years | 1.0 (ref) | 1.0 (ref) | ||||
|
| ||||||
| Hours of Prior Alcohol Training | ||||||
| Not reported/Does not remember | 0.42 | 0.08–2.09 | 0.29 | 0.65 | 0.23–1.84 | 0.42 |
| ≥10 hours | 3.67 | 0.89–15.05 | 0.07 | 2.68 | 0.91–7.91 | 0.08 |
| <10 hours | 1.0 (ref) | 1.0 (ref) | ||||
Adjusted for all variables above and by site
There were no significant differences in willingness to prescribe pharmacotherapy by provider type, sex, years in practice or prior training in the treatment of AUD. However, though not statistically significant, providers with 10 – 20 hours of alcohol-related training reported greater willingness to prescribe compared to those with less than 10 hours (OR= 2.67, p=0.07 (95% CI=0.90 – 7.91)).
3.6 Predictors of alcohol pharmacotherapy prescribing practices
Twenty four percent of the sample reported prescribing pharmacotherapy for alcohol use in the prior 12 months (naltrexone, acamprosate, disulfiram, or ondansetron). Family physicians and internists were more likely to prescribe pharmacotherapy compared to infectious disease doctors in multivariable analysis (OR: 3.20; 95% CI: 1.12–9.15, p=0.030) adjusted for sex, years in practice, prior training in the management of alcohol use and site. In separate multivariable analyses including either confidence or willingness to prescribe pharmacotherapy, confidence (VAS >5) (OR:33.7; 95% CI:8.89–128.21, p<0.001) and willingness to prescribe pharmacotherapy (VAS >5) (OR: 6.69;95% CI:2.70–16.58, p=0 <0.001) were most strongly associated with self-reported prescription of pharmacotherapy after adjusting for provider type, years in practice, prior training and site.
4. DISCUSSION
In this sample of HIV providers, 95% reported always or usually screening new patients for alcohol use, and over 2/3 offered advice to their hazardous drinking patients. In contrast, providers in general reported low knowledge of alcohol pharmacotherapy; few providers reported prescribing alcohol pharmacotherapy in the past year, and most referred their alcohol dependent patients to outside resources for treatment. Provider confidence ratings were positively correlated with their practice patterns; that is, participants rated their confidence high in those practice areas that they engaged in most frequently. In the area of alcohol pharmacotherapy, providers reported very low confidence and perceived a high need for additional training in this area.
Findings for this sample of HIV providers are in line with those reported for providers in other types of medical settings in terms of successful integration of alcohol screening and advice for hazardous/risky drinkers, but lower rates of implementation of alcohol counseling and pharmacotherapy for alcohol dependent patients (Mark et al., 2009a; Oslin et al., 2014). This likely reflects the perception of primary care providers that alcohol dependence requires subspecialty treatment, as this has been the predominant model of care to date.
While providers reported routinely screening for alcohol use, it was notable that few reported using a formal screening tool for identifying alcohol misuse. This has important implications for the management of alcohol misuse in HIV clinical settings as the use of a screening tool such as the AUDIT-C has been found to be more sensitive to detection of alcohol use compared to quantity/frequency questions (Fiellin et al., 2000). Furthermore, in the Veteran’s Administration (VA) setting, where universal screening has been systematically implemented, patient self-administered AUDIT-C improved reporting of alcohol use, compared to provider/staff administered AUDIT-C (Hawkins et al., 2007). This was explored in a follow-up qualitative study, where investigators found that when asking the questions verbally, AUDIT-C questions were adapted, suggested responses were implied, or questions were omitted (Williams et al., 2015). Use of the single item screen (Smith et al., 2009), “How many times in the past year have you had five (four for women) or more drinks in a day” or the AUDIT-C verbatim, may increase detection of alcohol misuse and result in more opportunities to intervene in HIV clinics. In addition, the AUDIT-C can provide normative data on severity of use, which may further guide treatment decisions.
In this sample, provider knowledge of alcohol pharmacotherapy was low, with the majority of providers answering “don’t know” to questions related to the use of naltrexone and acamprosate for alcohol dependence. These findings are consistent with past studies of alcohol pharmacotherapy, where low provider knowledge has been noted to be a significant barrier to use of these medications (Mark et al., 2003; Harris et al., 2013). In the current study, several provider characteristics were related to self-reported confidence to prescribe alcohol pharmacotherapy. Specifically, both higher years in practice and hours of alcohol-related training were associated with greater confidence to prescribe. Family practitioners and general internists were more confident than ID providers about prescribing alcohol pharmacotherapy but provider type did not influence self-reported willingness to prescribe.
These findings are of high importance. Training hours is a modifiable characteristic and thus may represent the primary target for changing providers’ attitudes and behaviors. Alcohol pharmacotherapy training materials are already available through a variety of resources and in a variety of formats. The other provider characteristic that was related to confidence and willingness to prescribe was years in practice. The more senior providers may represent practitioners in the HIV care setting who would be most likely to help lead change in prescribing patterns within the practice. For example, research has supported the effectiveness of identifying change and/or opinion leaders as a strategy for facilitating changes in provider practices (Flodgren et al., 2011; Mostofian et al., 2015).
Our results are similar to those recently reported by Montague and colleagues (Montague et al., 2015). In their study, HIV providers (N=159) also reported moderate to high levels of confidence in their abilities to assess and monitor patients with heavy/hazardous drinking patterns, and lower confidence in their ability to effectively manage alcohol treatment needs on-site in the HIV clinic. A strength of the current report is that providers were sampled nationwide, whereas Montague et al limited their sample to providers in New England. Thus our study extends findings outside of the New England region, to the Mid-Atlantic, South and West. In addition, survey response rate was higher in this current study than in the Montague study (58% vs. 3%).
A variety of Federal and professional agencies, including the U.S. Preventive Services Task Force (USPTF), have recommended routine alcohol screening in adult patients and the provision of brief behavioral counseling for persons identified with heavy or hazardous alcohol use (Moyer, 2013). This has led to fairly wide-spread adoption of these practices across a variety of health care settings, including primary care sites, emergency departments, and Obstetrics/Gynecology services. The Affordable Care Act requires coverage for evidence based preventive services set forth by the USPSTF that have an evidence grade A or B thus integration of screening and brief intervention may increase further in HIV and other primary care settings. In contrast, prescription of alcohol medications by primary care physicians has lagged. In 2006, approximately half of all retail prescriptions for FDA-approved alcohol pharmacotherapies were written by psychiatrists and roughly a quarter were written by general practitioners (Mark et al., 2009a). This low uptake is surprising given the wide spread prescription of antidepressants by primary care clinicians. Indeed, approximately 3/4 of all antidepressant prescriptions are now written by non-psychiatrists compared to ¼ of alcohol pharmacotherapies (Mark et al., 2009b; Mojtabai and Olfson, 2011). Furthermore, in 2007, 720,000 retail prescriptions were filled for alcohol medications compared with 226 million antidepressant prescriptions (Mark et al., 2009a). This is striking given that there is less than a two-fold difference in the 12-month prevalence of depression (6.9%; Substance Abuse and Mental Health Services Administration, 2013) and alcohol dependence (4%; Hasin et al., 2013) in the United States.
The importance of integration of effective alcohol treatments into primary care settings is highlighted by results of a recent randomized controlled clinical trial by Oslin and colleagues (2014) comparing a primary-care based Alcohol Care Management (ACM) program focused on pharmacotherapy and psychosocial support with standard care in specialty outpatient addiction treatment services. Participants randomized to ACM were significantly more likely to remain in care over the 26-week trial and to reduce heavy drinking days, although no group difference in abstinence was observed. Results strongly support the potential benefits of moving alcohol care out of specialty settings and into mainstream health care delivery. Integrating alcohol treatment into HIV primary care settings also has the potential to overcome patient and system level barriers to treatment, including waiting time to treatment entry, lack of treatment availability, privacy and alcohol dependence-related stigma (Redko et al., 2006; Rapp et al., 2006; Carr et al., 2008; Schomerus et al., 2011).
There are limitations to this study. First, responses were based on self-report, and may reflect some social desirability bias, though all respondents were all informed that surveys were confidential. Second, though our survey was based on the Physicians’ Competence in Substance Abuse Test (P-CSAT), a measure with established reliability and validity, we modified the P-CSAT by adding items on pharmacotherapy without conducting further psychometric assessment of the revised instrument. Third, our results may underestimate use of a screening tool. Low reported use of formal alcohol screening may be due in part to the providers’ ability to access the tablet- based clinical assessments which are available 6 of the 8 sites. Finally, though we sampled providers from several sites across the United States, these sites our not representative of all sites of HIV care. Furthermore, our response rate was 58%, and thus findings may not be representative of all HIV providers and may be subject to non-respondent bias.
Our findings indicate that overall alcohol screening and advice has been integrated into HIV care settings; however, wide spread adoption of the use of alcohol pharmacotherapy is more challenging as a result of providers’ lack of knowledge, training and confidence. Our findings strongly support the need for additional provider training as well as other provider support strategies and systems to encourage use of alcohol medications.
Highlights.
We surveyed HIV providers on their alcohol screening and treatment practices
The majority of HIV providers report routinely screening for alcohol use, though fewer reported using a formal screening tool
Fewer providers provide pharmacotherapy for alcohol use
Lack of knowledge is cited as a barrier to the use of pharmacotherapy in HIV clinics
Acknowledgments
Role of Funding Source: This research was funded by the National Institute of Alcohol Abuse and Alcoholism NIAAA U24AA020801 (McCaul, Chander, Monroe, Hutton), NIAAA U01 (Saag, Cropsey) and NIAAA U01 (Crane, Kitahata), K23MH105284-01 (Monroe) and the CFAR Network of Integrated Clinical Systems (R24 AI0667039)
The authors would like to acknowledge Dr. Richard Saitz who provided survey materials used in the development of this project.
Footnotes
Conflict of Interest: No conflicts declared.
Contributors: Geetanjali Chander, M.D. participated in obtaining the funding for the study. She designed the study and the final survey instrument, obtained IRB approval for the study, participated in writing the manuscript, and approved the final manuscript for publication. Anne Monroe, M.D. participated in the design of the study and the final survey instrument, participated in writing the manuscript, and approved the final manuscript for publication. Mary E. McCaul, Ph.D. obtained the funding for the study, participated in the design of the study and the final survey instrument, participated in writing the manuscript, and approved the final manuscript for publication. Michael Saag, M.D. participated in obtaining the funding for the study, obtained local IRB approval, facilitated the acquisition of the sample, and approved the final manuscript for publication. Heidi Crane, M.D. participated in obtaining the funding for the study, obtained local IRB approval, facilitated the acquisition of the sample, and approved the final manuscript for publication. Karen Cropsey, PsyD participated in obtaining funding for the study, in the design of the final survey instrument, and approved the final manuscript for publication. Site Clinic Directors (Elvin Geng, MD; Joseph Eron, MD; Stephen Boswell, MD; William Christopher Mathews, MD; Benigno Rodrigues, MD; E. Byrd Quinlivan, MD, Mari Kitahata, MD, Richard D. Moore, MD) facilitated the acquisition of the sample and approved the final manuscript for publication. Heidi Hutton, Ph.D. participated in obtaining funding for the study, in the design of the final survey instrument, and approved the final manuscript for publication. Megan Ellison, M.S. participated in data management and analyses for the study, in writing the manuscript, and approved the final manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Geetanjali Chander, Email: Gchande1@jhmi.edu.
Anne K. Monroe, Email: amonroe4@jhmi.edu.
Heidi M. Crane, Email: hcrane@uw.edu.
Heidi E. Hutton, Email: hhutton@jhmi.edu.
Michael S. Saag, Email: msaag@uab.edu.
Karen Cropsey, Email: kcropsey@uab.edu.
Joseph J. Eron, Email: joseph_eron@med.unc.edu.
E. Byrd Quinlivan, Email: ebq@med.unc.edu.
Elvin Geng, Email: genge@php.ucsf.edu.
William Christopher Mathews, Email: cmathews@ucsd.edu.
Stephen Boswell, Email: sboswell@fenwayhealth.org.
Megan Ellison, Email: Ellison@jhmi.edu.
Mari M. Kitahata, Email: kitahata@uw.edu.
Richard D. Moore, Email: rdmoore@jhmi.edu.
Mary E. McCaul, Email: mmccaul1@jhmi.edu.
References
- Alford DP, Richardson JM, Chapman SE, Dubé CE, Schadt RW, Saitz R. A web-based Alcohol Clinical Training (ACT) curriculum: Is in-person faculty development necessary to affect teaching? BMC Med Educ. 2008;8:11. doi: 10.1186/1472-6920-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, Cook RL, Gordon A, Bridges MW, Seiler JF. A temporal and dose- response association between alcohol consumption and medication adherence among veterans in care. Alcohol Clin Exp Res. 2005;29:1190–1197. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- Carr CJ, Xu J, Redko C, Lane DT, Rapp RC, Goris J, Carlson RG. Individual and system influences on waiting time for substance abuse treatment. J Subst Abuse Treat. 2008;34:192–201. doi: 10.1016/j.jsat.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander G, Josephs J, Fleishman J, Korthuis P, Gaist P, Hellinger J, Gebo K. Alcohol use among HIV- infected persons in care: results of a multi-site survey. HIV Med. 2008;9:196–202. doi: 10.1111/j.1468-1293.2008.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander G, Lau B, Moore RD. Hazardous alcohol use: a risk factor for non-adherence and lack of suppression in HIV infection. J Acquir Immune Defic Syndr. 2006;43:411. doi: 10.1097/01.qai.0000243121.44659.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander G, Hutton HE, Lau B, Xu X, McCaul ME. Brief Intervention decreases drinking frequency in HIV-infected, heavy drinking women: tesults of a randomized controlled trial. J Acquir Immune Defic Syndr. 2015;70:137–145. doi: 10.1097/QAI.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM. Trends in the adoption of medications for alcohol dependence. J Clin Psychopharmacol. 2006;26(Suppl 1):S13–19. doi: 10.1097/01.jcp.0000246209.18777.14. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Reid MC, O’Connor PG. Screening for alcohol problems in primary care: a systematic review. Arch Intern Med. 2000;160:1977–1989. doi: 10.1001/archinte.160.13.1977. [DOI] [PubMed] [Google Scholar]
- Flodgren G, Parmelli E, Doumit G, Gattellari M, O’Brien MA, Grimshaw J, Eccles MP. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2011:CD000125. doi: 10.1002/14651858.CD000125.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Harris AH, Ellerbe L, Reeder RN, Bowe T, Gordon AJ, Hagedorn H, Oliva E, Lembke A, Kivlahan D, Trafton JA. Pharmacotherapy for alcohol dependence: perceived treatment barriers and action strategies among Veterans Health Administration service providers. Psychol Serv. 2013;10:410. doi: 10.1037/a0030949. [DOI] [PubMed] [Google Scholar]
- Harris JM, Sun H. The Physicians’ Competence in Substance Abuse Test (P-CSAT): a multidimensional educational measurement tool for substance abuse training programs. Drug Alcohol Depend. 2012;122:236–240. doi: 10.1016/j.drugalcdep.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Aharonovich E, O’Leary A, Greenstein E, Pavlicova M, Arunajadai S, Waxman R, Wainberg M, Helzer J, Johnston B. Reducing heavy drinking in HIV primary care: a randomized trial of brief intervention, with and without technological enhancement. Addiction. 2013;108:1230–1240. doi: 10.1111/add.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins EJ, Kivlahan DR, Williams EC, Wright SM, Craig T, Bradley KA. Examining quality issues in alcohol misuse screening. Subst Abuse. 2007;28:53–65. doi: 10.1300/J465v28n03_06. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311:1889–1900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Kaner EF, Dickinson HO, Beyer F, Pienaar E, Schlesinger C, Campbell F, Saunders JB, Burnand B, Heather N. The effectiveness of brief alcohol interventions in primary care settings: Aasystematic review. Drug Alcohol Rev. 2009;28:301–323. doi: 10.1111/j.1465-3362.2009.00071.x. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kassed CA, Vandivort-Warren R, Levit KR, Kranzler HR. Alcohol and opioid dependence medications: prescription trends, overall and by physician specialty. Drug Alcohol Depend. 2009a;99:345–349. doi: 10.1016/j.drugalcdep.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Song X, Bransberger P, Poole VH, Crosse S. Physicians’ opinions about medications to treat alcoholism. Addiction. 2003;98:617–626. doi: 10.1046/j.1360-0443.2003.00377.x. [DOI] [PubMed] [Google Scholar]
- Mark TL, Levit KR, Buck JA. Psychotropic drug prescriptions by medical specialty. Psychiatr Serv. 2009b;60:1167. doi: 10.1176/ps.2009.60.9.1167. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M. Proportion of antidepressants prescribed without a psychiatric diagnosis is growing. Health Aff. 2011;30:1434–1442. doi: 10.1377/hlthaff.2010.1024. [DOI] [PubMed] [Google Scholar]
- Montague BT, Kahler CW, Colby SM, McHugh RK, Squires D, Fitzgerald B, Operario D, Gallagher D, Monti PM, Mayer KH. Attitudes and training needs of New England HIV care and addiction treatment providers: opportunities for better integration of HIV and alcohol treatment services. Addict Disord Their Treat. 2015;14:16–28. doi: 10.1097/ADT.0000000000000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofian F, Ruban C, Simunovic N, Bhandari M. Changing physician behavior: what works? Am J Manag Care. 2015;21:75–84. [PubMed] [Google Scholar]
- Moyer VA. Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:210–218. doi: 10.7326/0003-4819-159-3-201308060-00652. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies. Alcohol Treatment: Need, Utilization, and Barriers. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2009. [Google Scholar]
- Oslin DW, Lynch KG, Maisto SA, Lantinga LJ, McKay JR, Possemato K, Ingram E, Wierzbicki M. A randomized clinical trial of alcohol care management delivered in Department of Veterans Affairs primary care clinics versus specialty addiction treatment. J Gen Intern Med. 2014;29:162–168. doi: 10.1007/s11606-013-2625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp RC, Xu J, Carr CA, Lane DT, Wang J, Carlson R. Treatment barriers identified by substance abusers assessed at a centralized intake unit. J Subst Abuse Treat. 2006;30:227–235. doi: 10.1016/j.jsat.2006.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redko C, Rapp RC, Carlson RG. Waiting time as a barrier to treatment entry: perceptions of substance users. J Drug Issues. 2006;36:831–852. doi: 10.1177/002204260603600404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MC, Fiellin DA, O’Connor PG. Hazardous and harmful alcohol consumption in primary care. Arch Intern Med. 1999;159:1681–1689. doi: 10.1001/archinte.159.15.1681. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV- infected persons with alcohol problems. Alcohol: Clin Exp Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Schomerus G, Lucht M, Holzinger A, Matschinger H, Carta MG, Angermeyer MC. The stigma of alcohol dependence compared with other mental disorders: a review of population studies. Alcohol Alcohol. 2011;46:105–112. doi: 10.1093/alcalc/agq089. [DOI] [PubMed] [Google Scholar]
- Smith PC, Schmidt SM, Allensworth-Davies D, Saitz R. Primary care validation of a single-question alcohol screening test. J Gen Intern Med. 2009;24:783–788. doi: 10.1007/s11606-009-0928-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2012 National Survey on Drug use and Health: Mental Health Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, Ludman EJ, Berger D, Bradley KA. Factors underlying quality problems with alcohol screening prompted by a clinical reminder in primary care: a multi-site qualitative study. J Gen Intern Med. 2015;30:1125–1132. doi: 10.1007/s11606-015-3248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]