Over the past seven years, there have been a number of retrospective studies demonstrating that the early and empiric use of fresh frozen plasma to patients in hemorrhagic shock and receiving a massive transfusion is beneficial.1–3 More recently a prospective observational multicenter massive transfusion study (PROMMTT), confirmed that increased ratios of plasma to red blood cells and platelets to red blood cells decreased early mortality from hemorrhage.4 A prospective randomized optimum platelet and plasma ratios (PROP:P:R) study was recently completed and currently under analysis. Results of these studies have dramatically changed the manner in which bleeding trauma patients are resuscitated5, though the mechanism of protection remains unclear. We hypothesized that central to plasma’s protection is the endothelium. The important role of the endothelium to the pathophysiology of hemorrhagic shock has been coined the endotheliopathy of trauma.6 Injury to the endothelium from trauma and hemorrhage results in alterations in coagulation, inflammation, vasoregulation, and organ-specific barrier integrity. This review will focus on the endothelium as a therapeutic target to mechanistically explain the protection provided by plasma to the endothelium.
GLYCOCALYX
Overview
The glycocalyx is a network of soluble plasma components that project from the cell surface of both epithelial and endothelial cells and is believed to play a key role in stabilization of membrane integrity. The glycocalyx is composed of both proteoglycans and glycoproteins. The proteoglycans are comprised of a protein core to which attach a variety of glycosaminoglycans, primarily heparan sulfate. The major cell surface proteoglycan is syndecan, a focus of the current review. Glycoproteins are important to coagulation and include antithrombin III, heparin cofactor II, and thrombomodulin.7 Other glycoproteins include cell adhesion molecules such as selectins and ICAMs. Shedding of the endothelial glycocalyx exposes adhesion receptors to circulating neutrophils, thus enhancing endothelial-neutrophil adhesion.8
Glycocalyx in different diseases
In models of cardiac ischemia, shedding of the glycocalyx was associated with vascular hyperpermeability, an effect mitigated by antithrombin, highlighting the interplay of the glycocalyx with coagulation.9 Alterations in the endothelial glycocalyx have also been reported to be responsible for vascular leakage and leukocyte adhesion after cardiac arrest.10 Finally, shedding of the syndecan-1 backbone and heparin sulfate moieties occurs in patients undergoing abdominal aortic aneurysm repair.11 A dysfunctional glycocalyx has also been implicated in sepsis, diabetes, and atherosclerosis as well as renal failure and hypervolemia (related to atrial natriuretic peptide).12–15
Role of the Glycocalyx After Hemorrhagic Shock
Alterations in the endothelial glycocalyx have only recently been recognized to occur after hemorrhagic shock and to be modulated by resuscitation. We showed in a rat model of pressure controlled resuscitation that the endothelial glycocalyx, imaged using electron microscopy in the small bowel mesentery, was virtually ablated two hours after hemorrhagic shock.16 Figure 1 illustrates the virtual absence of the endothelial glycocalyx after hemorrhagic shock compared to shams. Glycocalyx thickness after resuscitation by lactated Ringers was similar to shock alone, whereas plasma significantly restored thickness. In a similar study by Torres et al, the cremaster muscle was imaged by intravital microscopy in a volume controlled resuscitation model of hemorrhagic shock.17 Glycocalyx thickness after lactated Ringers was 50% lower than in shams or rats resuscitated with fresh frozen plasma.
Figure 1. Virtual absence of the endothelial glycocalyx following hemorrhagic shock.
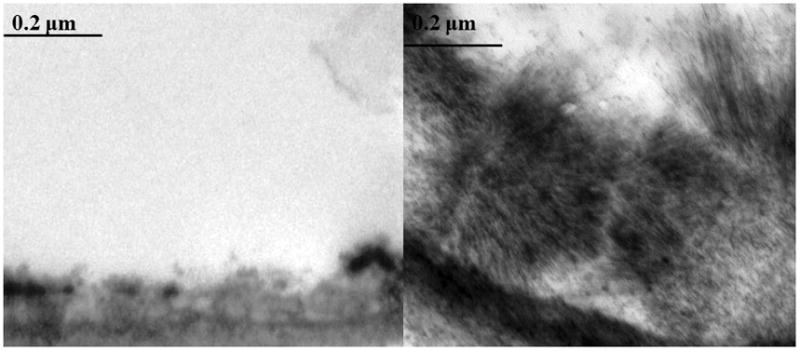
Rats were subjected to 90 minutes of hemorrhagic shock then 2 hours of reperfusion. The small bowel mesentery was harvested and perfused with uranyl acetate and lead citrate to stain the glycocalyx as we have described16. Representative images of post-capillary venules are shown. The left image is following hemorrhagic shock alone and illustrates the virtual absence of the gycocalyx compared to the sham in the right image. Magnification is 20,000x.
SYNDECAN-1
Syndecan’s are a family of heparin sulfate proteoglycans expressed on both epithelial and endothelial cells. They are transmembrane proteins with an extracellular domain that may be shed in response to a variety of stimuli. There are four members of the syndecan family, but syndecan-1has been the focus of most laboratory and clinical studies.
Syndecan-1 Ectodomain Shedding
Ectodomain shedding is an important post-translational mechanism that modulates diverse pathophysiologic processes that are not well understood.18 In rodent models of sepsis, syndecan-1 shedding protects against Gram-positive toxic shock by inhibiting dysfunctional inflammation.19 Shedding facilitates resolution of inflammation by binding to chemokines to aide in removal of pro-inflammatory mediators.20 More recently, Johansson et al demonstrated an association between the sympathoadrenal activation, fibrinolysis, and syndecan-1 shedding in a small clinical study in septic patients, suggesting the catecholamine surge of sepsis may lead to endothelial damage.21
Oxidative stress-induced shedding caused neutrophil chemotaxis and aberrant wound healing in a model of pulmonary fibrosis.22,23 Hemorrhagic shock-induced shedding appears to be injurious, resulting in the exposure of the injured endothelium to pro-inflammatory mediators and in alterations to the structural integrity of the endothelium with resultant hyperpermeability. We have shown in a small pilot study that syndecan-1 is shed at the time of injury in severely injured patients in hemorrhagic shock.24 Our data also suggested that patients with higher post resuscitation syndecan-1 levels had a higher mortality (survivors144±141 ng/ml vs nonsurvivors 289±226 ng/ml, p=0.15). Shed syndecan-1 negatively correlated with three proinflammatory cytokines, interferonγ, fractaline, and interleukin-1β, while IL-10 was positively correlated. These four cytokines interestingly all play a role in endothelial integrity. More recently, Johansson et al demonstrated increased mortality in patients with high admission shed syndecan-1 levels and that shedding correlated with inflammation and coagulopathy.25 To begin to understand the mechanisms underlying the pathologic role of shedding in injured patients in shock and importantly the mechanisms responsible for plasma’s protective role, we used an in vitro model of shock, hypoxia-reoxygenation, to examine endothelial integrity.24 Hyperpermeability induced by shock was mitigated by fresh frozen plasma (FFP) but not lactated Ringers. Additionally, adherens junction protein vascular endothelial cadherin (VE-cadherin), which is responsible of endothelial integrity, was similarly disrupted by shock and lessened by FFP but not lactated Ringers. Using a rat pressure controlled model of hemorrhagic shock and the lung as an end-organ damaged after injury, we next demonstrated that lung histopathology was present after shock, not improved by lactated Ringers resuscitation, but significantly lessened by plasma.16 Pulmonary syndecan-1 mRNA and cell surface syndecan-1 immunostaining were similarly increased by plasma but not lactated Ringers. These findings were expanded in a coagulopathic mouse model of volume controlled resuscitation. Hemorrhagic shock led to systemic shedding of syndecan-1 and lessened pulmonary syndecan-1 immunostaining, changes which correlated with lung hyperpermeability and inflammation.26 FFP compared to lactated Ringers abrogated these pathologic changes. Changes in permeability and inflammation by FFP were mimicked by spray-dried plasma.27
Available evidence suggests that the injurious changes to the endothelium after hemorrhagic shock are due to loss of the syndecan-1 backbone and encompassing glycocalyx.16, 24–26 In uninjured vessels an intact glycocalyx harbors adhesion molecules within its protective structure. With pathologic stimuli such as shock, exposure of the now injured endothelium to pathologic neutrophils can occur. Chappell et al have shown in models of ischemia/reperfusion that protection of the glycocalyx reduced both leukocyte and platelet adhesion and subsequent hyperpermeability and inflammation.28,29 Additional investigations using syndecan-1 loss in vitro or in vivo have confirmed a pro-inflammatory phenotype in response to shear stress.30 However, studies specifically examining sequalea of endothelial loss of syndecan-1 using syndecan-1 null mice may be hampered. Savery et al have shown that the thickness of the endothelial glycocalyx in null mice was only slightly less than that of wild type mice, suggesting that either synedcan-1 was not essential or that these null mice may adapt to loss of syndecan-1 by increasing expression of other proteoglycans.31 Endothelial cells also contain both syndecan-2 and syndecan-431 and our preliminary data suggest that syndecan-1 null mice have increased levels of syndecan-2 (unpublished data). Studies to evaluate the role of syndecan-1 using syndecan-1 null mice in models of hemorrhagic shock are underway.
Potential Mechanisms by Which Plasma Reconstitutes Syndecan-1
Syndecan-1 shedding is controlled by outside-in signaling which initiates the proteolytic cleavage of the syndecan-1 ectodomain.18 Little is known about the intracellular processes that lead to shedding. Hayashida et al have shown that shedding agonists stimulate dissociation of GTPase Rab5 from the cytoplasmic domain of syndecan-1, which then exposes syndecan-1 to sheddases.32 The metalloproteinases of the A Disintegrin And Metalloproteinase (ADAM) family is the largest group of sheddases. It is possible that hemorrhagic shock is a pathologic stimuli that activates MMPs or ADAMs and that plasma reduces their activation. In addition to direct effects on MMPs and ADAMs, plasma could also reduce the activities of these enzymes by increasing the expression of endogenous inhibitors such as the Tissue Inhibitor of Metalloproteinase (TIMP) family of glycoproteins.33 In particular, TIMP3 has been shown to be a critical regulator of ADAM17 activity, with the balance of TIMP3 and ADAM17 being important for the regulation of TNFα-induced inflammation.34 TNFα is increased early after trauma 35 and plasma is known to contain TIMP3 (unpublished data). The potential role of plasma’s inhibition of sheddases and activation of inhibitiors of sheddases is currently being investigated.
In summary, hemorrhagic shock causes shedding of the syndecan-1 ectodomain which is associated with organ damage and worsened outcomes. Recent evidence suggests that resuscitation with plasma reduces shedding and reconstitutes the endothelial glycoclayx. Regulation of syndecan-1 shedding post-injury and/or reconstitution of syndecan-1 and the glycocalyx could potentially serve as viable therapeutic targets for novel drug discovery. As plasma is composed of thousands of circulating proteins, investigation of the specific component(s) of plasma responsible for its protective effects may warrant further investigation.
Acknowledgments
This study was supported in part by the National Institutes of Health grants RO1GM107482 and P50GM038529 and the Department of DefenseW81XWH-11-2-006
Footnotes
The authors have no conflicts of interest to report.
The opinions or assertions contained herein are the private views of the authors and are not construed as official or reflecting the views if the US Department of Defense or the US Government.
Contributor Information
Rosemary A. Kozar, Email: rkozar@umm.edu, Shock Trauma Center, University of Maryland, Baltimore, MD.
Shibani Pati, Email: Shibani.Pati@bloodsystems.org, Blood Systems Research Institute and the University of California; San Francisco, California.
References
- 1.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal L, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248:447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 3.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Niles SE, McLaughlin DF, Wade CE, Holcomb JB. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;642:S69–S78. doi: 10.1097/TA.0b013e318160ba2f. [DOI] [PubMed] [Google Scholar]
- 4.Holcomb JB, del Junco DJ, Fox EE, et al. for the PROMMTT Study Group. The Prospective, Observational, Multicenter, Massive Transfusion Study, PROMMTT: Comparative Effectiveness of a Time-varying Treatment and Competing Risks. JAMA Surg. 2013;148(2):127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duchesne JC, Heaney J, Guidry C, McSwain N, Meade P, Cohen M, Schreiber M, Inaba K, Skiada D, Demetriades D, et al. Diluting the benefits of hemostatic resuscitation: a multi-institutional analysis. J Trauma Acute Care Surg. 2013 Jul;75(1):76–82. doi: 10.1097/TA.0b013e3182987df3. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins DH, Rappold JF, Badloe JF, Berséus O, Blackbourne L, Brohi KH, Butler FK, Cap AP, Cohen MJ, Davenport R, et al. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation: definitions, current practice, and knowledge gaps. Shock. 2014;41(Suppl 1):3–12. doi: 10.1097/SHK.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69(7):777–84. doi: 10.1111/anae.12661. [DOI] [PubMed] [Google Scholar]
- 8.Lipowsky HL. The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Ann Biomed Eng. 2012;40(4):840–848. doi: 10.1007/s10439-011-0427-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chappell D, Jacob M, Hofmann-Kiefer K, Rehm M, Welsch U, Conzen P, Becker BF. Antithrombin reduces shedding of the endothelial glycocalyx following ischaemia/reperfusion. Cardiovasc Res. 2009;83(2):388–96. doi: 10.1093/cvr/cvp097. [DOI] [PubMed] [Google Scholar]
- 10.Grundmann S, Fink K, Rabadzhieva L, Bourgeois N, Schwab T, Moser M, Bode C, Busch HJ. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83(6):715–20. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Bruno R, et al. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- 12.Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2008;83:536–545. doi: 10.1189/jlb.0607373. [DOI] [PubMed] [Google Scholar]
- 13.Drake-Holland AJ, Nobel MI. Update on the important new drug target in cardiovascular medicine- the vascular glycocalyx. Cardiovasc Hematol Disord Drug Targets. 2012;12(1):76–81. doi: 10.2174/187152912801823183. [DOI] [PubMed] [Google Scholar]
- 14.Padberg JS, Wiesinger A, di Marco GS, Reuter S, Grabner A, Kentrup D, Lukasz A, Oberleithner H, Pavenstädt H, Brand M, Kümpers P. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234(2):335–343. doi: 10.1016/j.atherosclerosis.2014.03.016. Epub 2014 Mar 29. [DOI] [PubMed] [Google Scholar]
- 15.Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol. 2013;108(3):347. doi: 10.1007/s00395-013-0347-z. Epub 2013 Apr 6. [DOI] [PubMed] [Google Scholar]
- 16.Kozar RA, Peng Z, Zhang R, Holcomb JB, Pati S, Park P, Ko TC, Paredes A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112:1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torres LN, Sondeen JL, Ji L, Dubick MA, Torres Filho I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J Trauma Acute Care Surg. 2013;75(5):759–66. doi: 10.1097/TA.0b013e3182a92514. [DOI] [PubMed] [Google Scholar]
- 18.Nam EJ, Park PW. Shedding of cell membrane-bound proteoglycans. Methods Mol Biol. 2012;836:291–305. doi: 10.1007/978-1-61779-498-8_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashida K, Chen Y, Bartlett AH, Park PW. Syndecan-1 is an in vivo suppressor of Gram-positive toxic shock. J Biol Chem. 2008;283(29):19895–903. doi: 10.1074/jbc.M801614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson PI, Haase N, Perner A, Ostrowski SR. Association between sympathoadrenal activation, fibrinolysis, and endothelial damage in septic patients: a prospective study. J Crit Care. 2014;29(3):327–33. doi: 10.1016/j.jcrc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Raats CJ, Bakker MA, van den Born J, Berden JH. Hydroxyl radicals depolymerize glomerular heparan sulfate in vitro and in experimental nephrotic syndrome. J Biol Chem. 1997;272(42):26734–26741. doi: 10.1074/jbc.272.42.26734. [DOI] [PubMed] [Google Scholar]
- 23.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, Rosas I, Oury TD. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haywood-Watson RJ, Holcomb JB, Gonzalez EA, Peng Z, Pati S, Park PW, Wang WW, Zaske AM, Menge T, Kozar RA. Modulation of syndecan-1 shedding after hemorrhagic shock and resuscitation. PLoS ONE. 2011;6(8):e23530. doi: 10.1371/journal.pone.0023530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, Protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 26.Peng Z, Pati S, Potter D, Brown R, Holcomb JB, Grill R, Wataha K, Park PW, Xue H, Kozar RA. Fresh frozen plasma lessens pulmonary endothelial inflammation and hyperpermeability after hemorrhagic shock and is associated with loss of syndecan-1. Shock. 2013;40(3):195–202. doi: 10.1097/SHK.0b013e31829f91fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wataha K, Menge T, Deng X, Shah A, Bode A, Holcomb JB, Potter D, Kozar RA, Spinella PC, Pati S. Spray-dried plasma and fresh frozen plasma modulate permeability and inflammation in vitro in vascular endothelial cells. Transfusion. 2013;53(Suppl 1):80S–90S. doi: 10.1111/trf.12040. [DOI] [PubMed] [Google Scholar]
- 28.Chappell D, Dorfler N, Jacob M, Rehm M, Welsch U, Conzen P, Becker BF. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock. 2010;34(2):133–139. doi: 10.1097/SHK.0b013e3181cdc363. [DOI] [PubMed] [Google Scholar]
- 29.Chappell D, Brettner F, Doerfler N, Jacob M, Rehm M, Bruegger D, Conzen P, Jacob B, Becker BF. Protection of glycocalyx decreases platelet adhesion after ischaemia/reperfusion: An animal study. Eur J Anaesthesiol. 2014;31(9):474–481. doi: 10.1097/EJA.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 30.Voyvodic PL, Min D, Liu R, Williams E, Chitalia V, Dunn AK, Baker AB. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem. 2014;289(14):9547–9559. doi: 10.1074/jbc.M113.541573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savery MD, Jiang JX, Park PW, Damiano ER. The endothelial glycocalyx in syndecan-1 deficient mice. Microvascular Research. 2013;87:83–91. doi: 10.1016/j.mvr.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashida K, Stahl PD, Park PW. Syndecan-1 ectodomain shedding is regulated by the small GTPase Rab5. J Biol Chem. 2008;283:35435–35444. doi: 10.1074/jbc.M804172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomain is regulated by multiple signaling pathways and mediated by a TIMP-3 sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cesaro A, Abakar-Mohamat A, Brest P, Lassalle S, Selva E, Filippi J, Hebuterne X, Hugot JP, Doglio A, Galland F, Naquet P, Vouret-Craviari V, Mograbi B, Hofman PM. Differential expression and regulation of ADAM17 and TIMP3 in acute inflamed intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296(6):G1332–43. doi: 10.1152/ajpgi.90641.2008. [DOI] [PubMed] [Google Scholar]
- 35.Jastrow K, Gonzalez EA, McGuire MF, Suliburk JW, Kozar RA, Iyengar S, Motschall DA, McKinley BA, Moore FA, Mercer DW. Early cytokine production risk stratifies trauma patients for multiple organ failure. J Am Coll Surg. 2009;209:320–331. doi: 10.1016/j.jamcollsurg.2009.05.002. [DOI] [PubMed] [Google Scholar]


