Abstract
Cellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a phylogenetically conserved, ubiquitous enzyme that plays an indispensable role in energy metabolism. Although a wealth of information is available on cellular GAPDH, there is a clear paucity of data on its extracellular counterpart (i.e., the secreted or extracellular GAPDH). Here, we show that the extracellular GAPDH in human serum is a multimeric, high-molecular-weight, yet glycolytically active enzyme. The high-molecular-weight multimers of serum GAPDH were identified by immunodetection on one- and two-dimensional gel electrophoresis using multiple antibodies specific for various epitopes of GAPDH. Partial purification of serum GAPDH by DEAE Affigel affinity/ion exchange chromatography further established the multimeric composition of serum GAPDH. In vitro data demonstrated that human cell lines secrete a multimeric, high-molecular-weight enzyme similar to that of serum GAPDH. Furthermore, LC–MS/MS analysis of extracellular GAPDH from human cell lines confirmed the presence of unique peptides of GAPDH in the high-molecular-weight subunits. Furthermore, data from pulse-chase experiments established the presence of high-molecular-weight subunits in the secreted, extracellular GAPDH. Taken together, our findings demonstrate the presence of a high-molecular-weight, enzymatically active secretory GAPDH in human serum that may have a hitherto unknown function in humans.
Keywords: serum GAPDH, extracellular GAPDH, multimer, 2D gel electrophoresis, DEAE Affigel Blue column chromatography
Graphical Abstract

INTRODUCTION
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is a multifunctional glycolytic enzyme that plays a pivotal role in cellular energy metabolism.1,2 The clinical significance of GAPDH is also increasingly evidenced by its role in cell pathology,3 cancer,4,5 and neuronal disorders.6 Although cellular GAPDH has been extensively characterized, little is known about its extracellular counterpart, the secreted or serum GAPDH. Sporadic reports have indicated that GAPDH activity is detectable in human serum, albeit at various levels under normal and pathological conditions (e.g., myocardial infarction7 or liver cirrhosis8). Intriguingly, until now there has been no experimental evidence or documentation of the source of GAPDH in serum, including whether it is a consequence of (a) lytic release from dying or diseased cells or (b) secretion into the circulatory system. Moreover, as data on serum GAPDH remain obscure, there is also a lacuna in understanding the biochemical distinction between cellular GAPDH and extracellular or serum GAPDH, if any. The ramifications of the knowledge gained on this fundamental question could greatly impact our current understanding of GAPDH’s functions and its clinical relevance.
Here, we investigated GAPDH in human serum using a multipronged approach involving antigenic determinants, proteomic analysis, and qualitative enzyme assays and validated the secretory nature by in vitro experiments. First, using GAPDH-antibodies specific for various epitopes (N-terminal, C-terminal domains) or full length (FL) protein, we established the identity of serum GAPDH. To validate the specificity of immunodetection, we used antibodies obtained from different manufacturers, and included antibodies of both monoclonal and polyclonal origins. Next, the enzymatic function of serum GAPDH was verified by both quantitative and qualitative assays. Finally, the secretory nature of serum GAPDH was established by the biochemical characterization of extracellular GAPDH from human cell lines in vitro. On the basis of our findings, we demonstrate the occurrence of a hitherto unknown multimeric, enzymatically active GAPDH in serum.
MATERIALS AND METHODS
Human Sera Sample Preparation
Human blood samples were obtained from patients and healthy individuals according to the protocol approved by the Johns Hopkins Institutional Review Board. Blood samples were allowed to coagulate at room temperature for 30 min, followed by centrifugation at 1200 rpm for 15 min at 4 °C. The clear serum was separated and stored at −80 °C until further use.
Nondenaturing Gel Electrophoresis and Immunoblotting
Unless otherwise indicated, all procedures were performed using ice-cold buffers or at 4 °C. Nondenaturing gel electrophoresis was performed in NuPAGE 3–8% Tris acetate gels (Invitrogen) using Tris–glycine running buffer (25 mM Tris and 192 mM glycine), pH ~8.3. In brief, samples were prepared on ice by mixing a known quantity of human serum protein with native sample buffer and 5% n-dodecyl-β-D-maltoside (DDM) followed by a brief centrifugation (for thorough mixing) before being applied onto the gel. Electrophoresis was performed at a constant 120 V current at 4 °C. At the end of electrophoresis, the gels were removed and subjected to either colloidal Coomassie brilliant blue (CBB) staining9 or electrophoretic transfer for immunoblotting using GAPDH-specific antibodies, according to the suppliers’ instructions.
SDS-PAGE, 2D Gel Electrophoresis, Immunoaffinity Purification, and Immunoblotting
The protein content of all the samples was determined using a 2D Quant kit (GE-Healthcare). For immunoblotting, serum samples diluted (1:10) in ice-cold PBS (pH 7.4) were used due to their high protein concentration. SDS-PAGE was performed using NuPAGE Bis–Tris 4–12% gels as reported earlier, followed by either colloidal CBB9 or silver staining (Bio-Rad, Hercules, CA). The 2D gel electrophoresis and immunoblotting were performed as described earlier.10 Immunoaffinity purification was performed by the immunoprecipitation of GAPDH using monoclonal antibody, followed by the elution of GAPDH using low pH buffer (1 M Tris), which was neutralized and diluted immediately to achieve neutral-optimal buffer concentration. After immunoblotting, the membranes were stored for reprobing if necessary.
Quantitative Assay of GAPDH Activity
GAPDH activity was assayed by following the method of Bergmeyer11 as described earlier.12 Briefly, the change in optical density of the reaction mixture in the presence of GAPDH samples, due to the rate of oxidation of NADH to NAD per minute at 25 °C under controlled assay conditions, was recorded at 340 nm using a Beckman Coulter DU530 UV–vis spectrophotometer (Fullerton, CA). Each assay was performed for multiple time points under various concentrations of each sample as well.
Qualitative In-Gel Assay of GAPDH Activity
Qualitative analysis of GAPDH activity was performed by the in-gel assay in which the formation of calcium phosphate at the end of the enzyme reaction can be visualized on the gel under a dark background. The assay is based on the principle outlined in Electrophoresis of Enzymes.13 In brief, serum samples and the standard (purified rabbit muscle GAPDH) were subjected to nondenaturing gel electrophoresis as described above. At the end of the run, gels were subjected to incubation and staining in the substrate buffer. At the end of the staining, the gels were viewed under a dark background for the visualization of precipitate and then stored in a buffer containing glycine–KOH (50 mM, pH 10, containing 0.5 mg/mL Ca2+ and antibacterial agent) at 4 °C until image acquisition.
DEAE Affigel Blue Chromatography
Partial purification of serum GAPDH was performed by affinity/ion exchange chromatography using DEAE Affigel Blue, and the fractions were eluted in phosphate buffer (50 mM, pH 7.1) with a NaCl gradient (0.1–1 M). In brief, the matrix was prewashed on a Buchner funnel with wash buffer (0.1 M acetic acid, pH 3.0, and 1.4 M NaCl, 40% isopropanol) equivalent to 5 times the bed volume to remove excess dye from the gel. Following the prewash, the matrix was washed and equilibrated with either a running or an application buffer (20 mM K2HPO4, pH 8.0, and 0.02 NaN3) to lower the ionic strength. A human serum sample (500 μL) was mixed with 500 μL of ice-cold phosphate buffer and concentrated using an Amicon Ultra centrifugal concentrator (MW 10 kDa) at 10000g for 10 min at 4 °C. The final concentrated sample (500 μL) was mixed with the application buffer (phosphate buffer 50 mM, pH 7.1) and applied to the column with a sample and bed volume ratio of 1:10. The elution was carried out at a gravity flow rate of 1 mL/min starting with the application buffer and the gradient application buffer with NaCl. Thus, fractions 1–15 had eluants from the phosphate buffer (50 mM, pH 7.1), fractions 16–25 had eluants from the phosphate buffer with NaCl (0.1 M), and fractions 26–37 had eluants from the phosphate buffer with NaCl (0.2 M), with every subsequent 12 fractions having an increasing molarity of NaCl (0.3, 0.4, 0.5, 0.6, and 1 M) in the phosphate buffer. Fractions were briefly stored at 4 °C until further analysis.
Analysis of Animal Sera
Animal sera from different species such as goat, horse, pig, rabbit, and rat were purchased from Thermo Scientific Co. (Rockford, IL). Prior to analysis, serum samples of the animals were subjected to the removal of the abundant protein, albumin, using the Pierce albumin depletion kit (Thermo Scientific Co.). Following the albumin depletion, sera were subjected to protein quantification and SDS-PAGE analysis for the further immunoblotting for GAPDH protein. The immunoblotting protocol, including the antibodies used, was followed as described elsewhere.
Cell Culture, Antibodies, and Chemicals
Human HCC cell lines SK-Hep1, HepG2, Hep3B, and the breast cancer cell line MCF-7 were obtained from ATCC (Manassas, VA) and cultured in MEM medium (Invitrogen Corp., Carlsbad, CA) and supplemented with Hyclone 10% fetal bovine serum (FBS) (Thermo Scientific Inc., Logan, UT), 100 000 units/L of penicillin (Invitrogen), and 100 mg/L of streptomycin (Invitrogen) at a pH of 7.4. For MCF-7, the medium also contained 0.01 mg/mL of bovine insulin. The Huh7 cell line was a kind gift from the Department of Medicine, Gastroenterology (Johns Hopkins), and was cultured in RPMI 1640 medium supplemented with 10% FBS, penicillin, and streptomycin as described above. The cells were incubated in a humidified atmosphere with 5% CO2 at 37 °C.
Unless otherwise mentioned, all chemicals including purified rabbit muscle GAPDH, protease and phosphatase inhibitor cocktails, and the reagents for GAPDH activity assay were purchased from Sigma Chemical Co. (St. Louis, MO). GAPDH antibodies against FL protein or N-terminal and internal domains (Santa Cruz Biotechnology, Santa Cruz, CA) or C-terminal domain (Cell Signaling Technologies, Danvers, MA) were purchased from respective suppliers. PVDF membranes for the transfer during immunoblotting were procured from Bio-Rad (Hercules, CA). The ECL Plus detection reagent and the necessary materials for the chemiluminescent detection of immunoblots were procured from GE-Healthcare (Piscataway, NJ).
Preparation of Conditioned Media
The extracellular or secreted GAPDH was analyzed in the conditioned medium of different cell lines such as SK-Hep1, HepG2, Hep3B, Huh7, and MCF-7. In brief, cells were cultured in their respective growth media until they were 60–70% confluent. Then, the media were replaced with serum-free media (i.e., in respective media without FBS) and the cells were maintained 12–15 h to allow the accumulation of secreted proteins. Next, the media were collected and centrifuged at 1000g for 5 min to remove any particulate or cellular debris. The clear supernatant media was concentrated using Millipore’s Amicon Ultra centrifugal filters (MWCO 10 kDa). The protein concentration was determined using a 2D Quant kit as described above and subjected to immunoblotting for GAPDH under either nondenaturing or denaturing conditions.
Pulse-Chase Experiment
The metabolic labeling of cellular proteins was performed using the Easy-Tag Express protein labeling mix, 35S-methionine and 35S-cysteine (PerkinElmer Co.) with a modification of the earlier protocol.14 On the day of the experiment, cells confluent at 70–80% were used. In brief, culture medium was removed from respective cell lines and replaced with DMEM media [devoid of methionine, cysteine, and FBS, but containing L-glutamine (2 mM), glucose (4.5 g/L), and Hepes buffer at a final concentration of 25 mM]. Cells were maintained for 1 h, following which 35S-methione and 35S-cysteine were added to the culture and maintained for an additional 1 h. Next, the media containing 35S-amino acids were removed, replaced with complete growth medium, and allowed to culture for 6 h. At the end of 6 h, the media were collected and concentrated as described elsewhere and stored at −80 °C until further analysis.
Total cellular protein from all the cell lines was prepared in RIPA buffer as described elsewhere. The cell lines were washed with ice-cold PBS (pH7.4) and lysed in ice-cold RIPA buffer (Sigma), containing protease and phosphatase inhibitors, by a Dounce homogenizer. The lysates were centrifuged at 10000g for 15 min at 4 °C. The clear supernatant was separated and stored at −80 °C until further analysis. For immunoprecipitation experiments, the total cell lysates or conditioned media from different cell lines were precleared, incubated with the GAPDH-specific antibody for 2 h at 4 °C on a rotator shaker, added to Protein A/G Plus agarose beads (Santa Cruz Biotechnology) and then gently mixed overnight at 4 °C on a rotator shaker. The immunocomplexes were separated by a brief centrifugation (1000g, 30 s at 4 °C). After being stringently washed with RIPA buffer, the immunoprecipitates were resolved on SDS-PAGE, incubated in radioactive “Amplify” solution (GE-Healthcare) prior to vacuum drying, and then exposed to X-ray film (GE-Healthcare) to obtain the images. Authorized personnel performed all procedures involving handling of radioactive samples. Appropriate radioactive decontaminations and containments were followed strictly according to the Johns Hopkins Radiation Safety rules and regulations.
Protein Identification by Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS)
Protein samples from either gel bands or immunoprecipitation followed by the elution of antigen were proteolyzed with trypsin as described previously.10,15–17 Digested peptides were extracted and subjected to vacuum drying in a Speedvac followed by reconstitution in 5 μL of 2% acetonitrile/0.1% formic acid for further analysis by liquid chromatography/tandem mass spectrometry (LC–MS/MS) using LTQ Orbitrap Velos (2) MS (Thermo Fisher Scientific, http://www.thermofisher.com). For data analysis, tandem mass spectra were extracted and the charge state deconvoluted and deisotoped by Proteome Discoverer v1.3 (Thermo Fisher Scientific). All MS/MS spectra were analyzed with Mascot v.2.2 (Matrix Science, London, U. K.) using the RefSeq Complete 2012 Database for mammalian species and Homo sapiens, with acquired raw MS/MS data, trypsin as enzyme, missed cleavage 1, precursor mass tolerance 10 or 12 ppm, fragment mass tolerance 0.03 Da, y and b ions, and oxidation on methionine and carbamidomethylation as the variable modifications. For each sample, the Mascot search result *.dat files for nodes with or without extract were processed in Scaffold (http://www.proteomesoftware.com) combined as a MUDPIT experiment to validate the protein and peptide identifications. The criteria for protein identification was as follows: Scaffold version Scaffold_4.2.0 (Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 95.0% probability by the Peptide Prophet algorithm18 with Scaffold delta mass correction. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm.18 In the database, at least 47 5687 entries were searched. Proteins that contained similar peptides and could not be differentiated on the basis of MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Isoelectofocusing (IEF) and Immunoblotting
Isoelectrofocusing on vertical gels was performed using IEF gels with pH 3–10 (Invitrogen). In brief, serum samples were mixed with IEF sample buffer, pH 3–10, and subjected to gel electrophoresis using prechilled Novex cathode buffer and Novex anode buffer. The initial electrophoresis was performed at a constant current of 100 V for 1 h followed by 200 and 250 V each for an additional 1 h as per the supplier’s instructions. At the end of electrophoresis, the gels were subjected to either CBB staining or immunoblotting. For colloidal CBB staining, the gels were fixed in 12% trichloroacetate (TCA) for 30 min, rinsed three times, and then washed for 5 min in distilled water. Following the fixing, further steps of the staining procedures were followed as described earlier. For immunoblotting, the electrotransfer of proteins was performed using prechilled 0.7% acetic acid (pH 3.0). Prior to transfer, the gels were equilibrated with prechilled 0.7% acetic acid for 10 min. The transfer setup was modified from the conventional electrotransfer. The gel membrane sandwich was oriented so as to retain the gel toward the anode (+) and the membrane toward the cathode (−), a reversed or inverted position from the conventional transfer (in which the transfer buffer will have a pH of 8–9). At pH 3.0, as the current passed from the anode to the cathode resulting in protein transfer, the transfer of proteins from pI gel to the membrane was achieved. The electrotransfer was performed at a constant 10 V overnight at 4 °C. Following the transfer, membranes were removed and subjected to immunodetection for GAPDH with specific antibodies as per the suppliers’ instructions.
RESULTS
Serum GAPDH as a High-Molecular-Weight Protein
To characterize human serum GAPDH, we first validated its molecular identity by immunodetection using multiple antibodies specific for various epitopes of GAPDH in human sera collected from patients and healthy individuals (Table 1). Figure 1A shows the CBB-stained gel of human sera under native (nondenaturing, nonreducing) conditions. Immunodetection of serum GAPDH under native conditions revealed it as a high-molecular-weight protein, as evidenced by the molecular weight markers and the low (electrophoretic) mobility (Figure 1B). The higher molecular size was consistent in multiple serum samples. The identity of GAPDH was also confirmed by the anti-GAPDH antibody specific for the C-terminal domain (Figure 1C). Immunodetection of rabbit muscle GAPDH under native conditions identical to those of the human sera experiment validated the specificity of the anti-GAPDH antibody and confirmed the known molecular size of native cellular GAPDH (<200 kDa) (Figure 1D).
Table 1.
Demographic and Clinical Characteristics of Individuals
| total number | 60 |
| male | 44 |
| female | 16 |
| health condition | |
| hepatocellular carcinoma (HCC) | 31 |
| fibrolamellar HCC | 3 |
| cholangiocarcinoma | 6 |
| liver metastasis | 13 |
| neuroendocrine metastasis | 5 |
| healthy | 2 |
Figure 1.
Serum GAPDH, a high-molecular-weight protein. (A) CBB-stained native gel of human serum showing the overall protein profile. Immunodetection of serum GAPDH as a high-molecular-weight protein under native, nondenaturing conditions by antibodies specific for (B) FL GAPDH and (C) C-terminal domains. (D) Rabbit muscle GAPDH, shown as a reference, representative of cellular GAPDH.
Serum GAPDH as a Multimeric Protein
The immunoblotting of serum GAPDH under denaturing (SDS-PAGE) conditions showed multiple proteins besides the known ~37 kDa (Figure 2). In fact, both antibodies (which were specific for FL and for the C-terminal domain of GAPDH) strongly and consistently recognized high-molecular-weight proteins (~50–75 kDa) along with some proteins with weak signals (Figure 2). The data were consistent in serum samples from 60 individuals. Next, a 2D gel immunoblotting of serum GAPDH showed the recognition of several subunits as GAPDH by multiple antibodies (Figure 3). The subunits were of varying sizes (~25 and 50–75 kDa) besides ~37 kDa (Figure 3). The 2D gel immunoblot data also confirmed that the high-molecular-weight proteins detected by SDS-PAGE are GAPDH (Figure 2A,B). Although the signal intensity of individual subunits varied with different antibodies, the subunits were commonly recognized by the antibodies. As shown in Figure 3, the majority of the subunits (except the high-molecular-weight subunits, ~50–75 kDa) were within the range of neutral pH (≥7). Collectively, the data from Figures 2 and 3 establish that serum GAPDH is a multimer consisting of several high-molecular-weight subunits besides the ~37 kDa subunit.
Figure 2.
Serum GAPDH, a multimeric protein. Immunoblots of 60 human serum samples, probed with (A) a specific-FL antibody and (B) a C-terminal antibody, showing the recognition of multiple proteins besides the ~37 kDa protein band.
Figure 3.
Serum GAPDH with multiple high-molecular-weight subunits. Immunoblots of serum GAPDH on 2D gel electrophoresis showing that GAPDH antibodies specific for (A) FL protein, (B) C-terminal, (C) N-terminal, and (D) internal domains detect multiple high-molecular-weight subunits. Note that the majority of subunits are identified at or above neutral pH (>pI 7.0).
Multimeric Serum GAPDH and Enzymatic Activity
Intrigued by the multimeric, higher-molecular-size serum GAPDH, we next investigated its catalytic capacity. Functional analysis of serum GAPDH by quantitative and qualitative enzyme assays demonstrated that the GAPDH in circulation (that is, in serum) is enzymatically active (Figure 4). However, the level of activity showed a marked difference among individuals (Figure 4A). An in-gel enzyme assay confirmed qualitatively that serum GAPDH retains its catalytic capacity and exhibits concentration-dependent kinetics (Figure 4B). Evidently, the enzymatic function of serum GAPDH remained unaffected by the multimers. To gain further insight, we partially purified the serum GAPDH by affinity/ion exchange chromatography using DEAE Affigel Blue matrix (Figure 5A). The eluted fractions from DEAE Affigel chromatography were assayed for GAPDH activity. Although two major peaks showed GAPDH activity, the second peak (fractions 15–20) showed higher specific activity, indicating its purification or isolation (Figure 5A). Immunoblots of fraction 18 confirmed the presence of high-molecular-size GAPDH and multiple proteins under native and SDS-PAGE conditions, respectively (as shown in the insert in Figure 5A). A silver-stained 2D gel and the corresponding immunoblot of the partially purified serum GAPDH (fraction 18) confirmed the multimeric nature of human serum GAPDH (Figure 5B).
Figure 4.
Enzymatic activity of multimeric serum GAPDH. (A) Human serum GAPDH showing different levels of enzyme activity among individuals, irrespective of the background and disease condition. (B) An in-gel activity assay demonstrating the glycolytic capacity of human serum GAPDH.
Figure 5.
Partial purification of serum GAPDH. (A) The partial purification of serum GAPDH by DEAE Affigel column chromatography shows the eluted fractions of human serum and the GAPDH activity in respective fractions. Peak 2, representing fractions 17–19, had a higher specific activity of GAPDH, indicating GAPDH purification or isolation. The insert shows immunoblots of fraction 18 that demonstrate the presence of high-molecular-size GAPDH and of multiple proteins under nondenaturing and denaturing conditions, respectively. (B) The silver-stained gel and corresponding immunoblot of eluted fraction 18 (which shows that the majority of silver-stained peptide spots are detected by the FL-GAPDH antibody) indicate the isolation of serum GAPDH by DEAE Affigel and the multimeric subunit composition.
GAPDH in Animal Sera as a Multimeric Protein
The immunoblotting of GAPDH in the albumin-depleted sera of goat, horse, pig, rabbit, and rat under denaturing (SDS-PAGE) conditions showed multiple proteins (Figure 6A,B). GAPDH antibodies that were specific for FL protein as well as for the C-terminal domain strongly and consistently recognized multiple protein bands (~25, 50–75 kDa) along with some proteins with weak signals (Figure 6A,B). The data were consistent in serum samples from five different species. Interestingly, the popularly known ~37 kDa protein, which is responsible for the catalytic/glycolytic function, was either less abundant (rabbit and rat) or undetectable (goat, horse, and pig) (Figure 6A,B).
Figure 6.

Serum GAPDH in animal species, also a multimeric protein. Immunoblots of serum samples from five different animal species, probed with (A) specific-FL antibody and (B) C-terminal antibody, showing the recognition of multiple proteins.
Extracellular, Secreted GAPDH as a High-Molecular-Weight Enzyme
Having identified the enzymatically active high-molecular-size GAPDH in serum, we then investigated whether such circulating GAPDH is secreted by human cells. In vitro, the secreted GAPDH [referred herein as extracellular GAPDH] was analyzed in the “conditioned medium” (CM), also known as “spent medium”, of multiple human cancer cell lines such as SK-Hep1, HepG2, Hep3B, Huh7, and MCF-7. The immuno-detection of extracellular GAPDH under nondenaturing, native conditions revealed that the secreted GAPDH is also a high-molecular-weight protein (Figure 7). Although the CBB-stained gel showed multiple proteins in the CM of different cell lines, antibodies recognized GAPDH by selective binding, validating the specificity of the antibodies used. Besides the primary strong immunodetection signal, an additional band was also recognized by the antibodies (Figure 7). Verification of a representative gel band (indicated by asterisks [*]), from the CBB-stained native gel, by mass spectrometry analysis also confirmed the occurrence of GAPDH proteins in these high-molecular-weight proteins of CM (Table 2).
Figure 7.

Extracellular GAPDH, a high-molecular-weight protein. A CBB-stained nondenaturing (native) gel and corresponding immunoblots showing that the extracellular GAPDH in the conditioned media (CM) of human cell lines is a high-molecular-weight protein, as confirmed by specific antibodies. Note that although multiple proteins were observed in the CBB-stained gel, the antibodies selectively detect the target (GAPDH), indicating their specificity. The asterisks (*) indicated in the gel show the protein band subjected to LC–MS/MS analysis for the verification of the GAPDH proteins. The human cancer cell lines indicated are SK (SK-Hep1), G2 (HepG2), 3B (Hep3B), H7 (Huh7), and M7 (MCF-7).
Table 2.
LC–MS/MS Analysis of the Protein Band (Indicated by Asterisks in Figure 7) from the CBB-Stained Native Gel Showing the Presence of GAPDH Peptides
| protein name |
accession number |
number of unique peptides |
sequence coverage (%) |
peptide sequence | probability | mascot ion score |
modifications | observed (m/z) |
actual mass |
charge |
|---|---|---|---|---|---|---|---|---|---|---|
| GAPDH (Homo sapiens) | gi| 7669492 | 4 | 16.7 | (R)119 VIISAPS ADAPMFVMGVNHE K139(Y) | 100% | 85.45 | 738.37 | 2212.1 | 3 | |
| (R)VIISAPSADAPmFVMGVNHE K(Y) | 100% | 60.75 | oxidation (+16) | 743.70 | 2228.1 | 3 | ||||
| (K)67 LVINGNPITIFQER 80(D) | 100% | 79.35 | 807.45 | 1612.8 | 2 | |||||
| (K)228 LTGMAFR 234(V) | 100% | 48.21 | 398.21 | 794.4 | 2 | |||||
| (K)LTGMAFR(V) | 100% | 40.42 | oxidation (+16) | 398.21 | 794.4 | 2 | ||||
| (K)LTGmAFR(V) | 100% | 36.27 | 406.21 | 810.4 | 2 | |||||
| (K)310 LISWYDNEFGYSNR 323(V) | 100% | 74.16 | 882.40 | 1762.8 | 2 |
Next, the immunoblotting of extracellular GAPDH under denaturing conditions also demonstrated the presence of several protein bands in addition to the ~37 kDa protein, as confirmed by GAPDH antibodies (Figure 8). Like serum GAPDH, extracellular (CM) GAPDH has high-molecular-weight subunits that were detected predominantly within the range of ~50–75 kDa in addition to a peptide with a weak signal at ~250 kDa. The immunoprecipitation of extracellular GAPDH followed by immunoblotting also established the presence of multiple high-molecular-weight subunits (Figure 8). Mass spectrometry analysis of the immunoprecipitated extracellular GAPDH also verified the identity of the protein in CM as GAPDH (Table 3). Next, a 2D gel immunoblotting of CM from the HepG2 cell line demonstrated that extracellular GAPDH is composed of multiple subunits of higher molecular mass (Figure 9A). Notably, a comparative analysis of cellular GAPDH under identical conditions did not show any high-molecular-weight subunits corresponding to the extracellular GAPDH, indicating a clear distinction between cellular and extracellular GAPDH (Figure 9B). The results were verified in the SK-Hep1 cell line as well (data not shown). Interestingly, unlike serum GAPDH, the high-molecular-mass subunits of extracellular GAPDH were detected at a lower pH (<7.0) than that of the ~37 kDa subunit (pI ~ 8.4) (Figure 3 vs Figure 9A).
Figure 8.

Extracellular GAPDH, a multimeric protein. (A) Immunoblots showing that GAPDH antibodies specific to different epitopes detect multiple subunits besides the ~37 kDa subunit. (B) The immunoblotting of immunoaffinity-purified extracellular GAPDH also confirmed the presence of multiple high-molecular-weight subunits. The asterisk indicates mass spectrometry analysis of IP samples that further confirmed the presence of GAPDH peptides. Arrows indicate additional protein bands besides the known ~37 kDa band. The human cancer cell lines indicated are SK (SK-Hep1), G2 (HepG2), 3B (Hep3B), H7 (Huh7), and M7 (MCF-7).
Table 3.
LC–MS/MS Analysis of the Immunoprecipitated GAPDH from the CM of SK-Hepl Cells (Indicated by Asterisks in Figure 8B) Showing the Presence of GAPDH Peptides
| protein name | accession number |
number of unique peptides |
sequence coverage (%) |
peptide sequence | probability | mascot ion score |
modifications | observed (m/z) |
actual mass | charge |
|---|---|---|---|---|---|---|---|---|---|---|
| GAPDH (Homo sapiens) | gi|7669492 | 5 | 21 | (R)119 VIISAPSADAPmFVmGVNHEK 139(Y) | 99% | 34.78 | oxidation (+16) | 749.03 | 2244.09 | 3 |
| (R)201 GALQNIIPASTGAAK 215(A) | 100% | 71.19 | 706.39 | 1410.78 | 2 | |||||
| (R)GALQNIIPASTGAAK(A) | 100% | 66.33 | 1411.79 | 1410.78 | 1 | |||||
| (R)GALQNIIPASTGAAK(A) | 100% | 58.85 | 706.39 | 1410.78 | 2 | |||||
| (R)GALQNIIPASTGAAK(A) | 100% | 57.05 | 706.39 | 1410.78 | 2 | |||||
| (R)GALQNIIPASTGAAK(A) | 100% | 60.05 | 1411.79 | 1410.78 | 1 | |||||
| (R)GALQNIIPASTGAAK(A) | 100% | 51.71 | 706.39 | 1410.78 | 2 | |||||
| (K)228 LTGMAFR 234(V) | 100% | 37.96 | 398.21 | 794.4113 | 2 | |||||
| (K)LTGMAFR(V) | 100% | 38.04 | 398.21 | 794.4113 | 2 | |||||
| (R) 235 VPTANVSVVDLTcR 248(L) | 100% | 92.75 | methylthio (+46) | 1519.76 | 1518.75 | 1 | ||||
| (R)VPTANVSVVDLTcR(L) | 100% | 82.71 | methylthio (+46) | 760.38 | 1518.75 | 2 | ||||
| (R)VPTANVSVVDLTcR(L) | 100% | 76 | methylthio (+46) | 760.38 | 1518.75 | 2 | ||||
| (K)LISWYDNEFGYSNR(V) | 100% | 86.31 | 1763.80 | 1762.79 | 1 | |||||
| (K)310 LISWYDNEFGYSNR 323(V) | 100% | 70.64 | 882.40 | 1762.79 | 2 | |||||
| (K)LISWYDNEFGYSNR(V) | 100% | 59.52 | 882.40 | 1762.79 | 2 |
Figure 9.
Extracellular GAPDH has multiple high-molecular-weight subunits. (A) Immunoblots of 2D gels showing that the extracellular GAPDH from HepG2 cells have multiple high-molecular-weight subunits, as evident by specific antibodies. (B) Corresponding immunoblots for cellular GAPDH (from rabbit muscle) are shown as a reference to demonstrate the absence of high-molecular-weight subunits. Note: the majority of subunits are in the acidic pH (≤pI 7.0). The human cancer cell lines indicated are SK (SK-Hep1), G2 (HepG2), 3B (Hep3B), H7 (Huh7), and M7 (MCF-7).
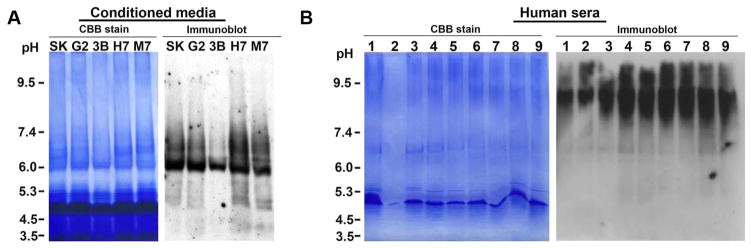
Autoradiograms of a pulse-chase experiment, in which the cellular proteins were metabolically labeled with 35S-methionine and 35S-cysteine, demonstrated the presence of multiple proteins besides ~37 kDa protein in the GAPDH immunoprecipitated from cell lysate as well as CM (Figure 10A). With multiple lines of evidence demonstrating that the extracellular GAPDH is a high-molecular-weight protein, we next examined its catalytic capacity. Similar to that of serum GAPDH, the functional analysis of extracellular GAPDH by quantitative and qualitative enzyme assays also confirmed that the secreted GAPDH preserved its enzymatic function (Figure 10B,C). Immunoblotting of human sera and extracellular GAPDH under a pH gradient gel showed a prominent difference in their pI values (Figure 11). The GAPDH from CM showed a pI value of <7.0, whereas the pI value of serum GAPDH was between pH 8.0 and 8.5. Data from the current study clearly show that the extracellular GAPDH detected in the CM of various cell lines is a secreted high-molecular-weight isoform of GAPDH.
Figure 10.

Pulse-Chase experiment showing multiple high-molecular-weight proteins of GAPDH. (A) Autoradiograms of immunoprecipitated cellular and extracellular GAPDH from human cancer cell lines subjected to metabolic labeling (Pulse-Chase) using 35S-methionine and 35S-cysteine demonstrate the presence of multiple high-molecular-weight (>37 kDa) subunits. N: neat lysate. IP: GAPDH immunoprecipitation. (B) GAPDH activity assay in the CM of human cancer cell lines demonstrated that extracellular GAPDH is enzymatically active. (C) Qualitative analysis of the glycolytic efficiency of extracellular GAPDH by in-gel assay. Rabbit (Rb) muscle GAPDH and red blood cell (RBC) GAPDH were used as references. The human cancer cell lines indicated are SK (SK-Hep1), G2 (HepG2), 3B (Hep3B), H7 (Huh7), and M7 (MCF-7).
Figure 11.
pH difference between extracellular GAPDH and serum GAPDH. (A) Colloidal CBB-stained IEF gel and its corresponding immunoblot, probed with FL antibody, showing that the isoelectric point of extracellular GAPDH is below neutral pH (<pH 7.0). (B) CBB-stained IEF gel and its corresponding immunoblot, probed with FL antibody, showing that the isoelectric point of serum GAPDH is above neutral pH (>pH 7.0). Note: On the CBB-stained IEF gel, although an abundant protein band is visualized at pH 4–5, the corresponding immunoblot has no nonspecific binding of GAPDH antibody at pH 4–5, reiterating that the immunoblot signals are specific for GAPDH. The human cancer cell lines indicated are SK (SK-Hep1), G2 (HepG2), 3B (Hep3B), H7 (Huh7), and M7 (MCF-7).
DISCUSSION
Data from the current report convincingly demonstrate that both serum GAPDH and extracellular GAPDH (in vitro) differ significantly from the cellular counterpart in subunit composition as well as overall molecular size. Further, the detection of extracellular GAPDH in the CM by immunoblotting, immunoprecipitation, and autoradiography (pulse-chase labeling) ascertains that the high-molecular-weight GAPDH is secreted by human cells. Thus, it is plausible that serum GAPDH, which is also distinct from cellular GAPDH, could be the secreted, extracellular GAPDH in circulation whose biological significance remains to be characterized.
The validity of our findings has been strongly supported by careful experimental approaches. To validate the specificity of immunodetection, we used GAPDH-specific antibodies in this study that were obtained from various sources, and the study included both monoclonal and polyclonal antibodies. Similarly, during in vitro experiments involving the analysis of CM, several precautions were taken to eliminate any interference or contamination of cellular GAPDH with the CM. For example, the CM was collected from cells growing in log phase (60–70% confluent) within ~12–16 h of the addition of fresh medium. Also, during the period of CM collection (12–16 h) care was taken to avoid fetal bovine serum (FBS) in the media (i.e., FBS-free media) to prevent any unwanted or unexpected effects of abundant proteins (e.g., albumin) on the secreted GAPDH.
Immunodetection of multiple bands under denaturing conditions (SDS-PAGE) indicates that the increase in molecular weight witnessed under native, nondenaturing conditions is not due to any aggregation or binding of multiple proteins but rather the assembly of all different subunits of the same protein. This is further substantiated by the occurrence of multiple high-molecular-weight subunits in 2D immunoblots. Nonetheless, though multiple antibodies of GAPDH recognized high-molecular-weight subunits yet the signal intensities were not similar. It remains to be known whether the difference in the signal intensity of selective subunits is related to any biochemical modifications of corresponding epitopes or not. Future investigations could reveal whether multiple forms of serum GAPDH (due to post-translational modifications) exist in circulation, given that such modifications in cellular GAPDH have been identified.19 Next, it is intriguing to understand if the presence of the high-molecular-weight isoform of GAPDH is human-specific. Preliminary results from immunoblot analysis of albumin-depleted serum samples from various animal species such as goat, horse, pig, rabbit and rat also showed multiple subunits as detected by GAPDH-specific antibodies. Thus, the multimeric high-molecular-weight GAPDH is serum-specific and probably conserved (at least in higher mammals), underscoring its biological significance.
Noteworthy, although both serum GAPDH and extracellular GAPDH differed from cellular GAPDH in size and subunit composition, biochemical differences between serum and extracellular GAPDH were also witnessed. For example, in serum GAPDH under denaturing conditions, immunodetection of a ~25 kDa protein was consistently observed with multiple antibodies. However, in the extracellular GAPDH the prominent occurrence of ~25 kDa was not observed. Similarly, a marked difference in the pH was also found between serum GAPDH and extracellular GAPDH. Immunodetection of extracellular GAPDH and serum GAPDH under one-dimensional isoelectrofocusing revealed that the former has a pI value of <7.0 whereas the later has a pI value between pH 8.0 and 8.5. It remains to be known if it can be attributed to the pH of serum (in vivo) or the pH of the culture medium (in vitro).
Several reports unravel the participation of cellular GAPDH in pathways that are cross-linked with disease-related phenotypes. GAPDH has been known to interact with the nucleic acids of Hepatitis B20,21 and C22 viruses that cause hepatitis, a major contributing factor for hepatocarcinogenesis. Similarly, GAPDH binding with nucleic acids of influenza virus and Japanese encephalitis virus have also been reported although the regulatory role remains to be completely characterized.23,24 In cancer, accumulating data indicate a strong link between GAPDH up-regulation and tumorigenic potential. GAPDH has been known to bind with the mRNA of colony-stimulating factor-1 (CSF-1), a factor known to play a pivotal role in several malignancies,25–27 resulting in its increased stability.28 Similarly, samples from liver cancer patients revealed that the incidence of GAPDH up-regulation in human HCC strongly correlates with c-jun, a proto-oncogene that has long been known to be involved in liver tumorigenesis.29 Although it is not known whether GAPDH up-regulation is sufficient or necessary for tumorigenesis, substantial data unequivocally demonstrate the existence of an association between GAPDH overexpression and pro-survival mechanisms30 and chemoresistance31 in cancer cells. Metabolically, the increased expression of cellular GAPDH is invariably associated with increased glycolytic capacity32,33 facilitating tumor progression. Recently, a hitherto unknown role of GAPDH in the regulation of mammalian target of rapamycin (mTOR)-complex1 (mTOR-C1) signaling pathway has been documented.34 The mTOR pathway is a growth signaling mechanism that has been active during hepatocarcinogenesis.35,36 Thus, in addition to its role in glycolysis and energy production, cellular GAPDH also signals and regulates other pathways like mTOR, depending upon cellular requirements. Hence, GAPDH is one of the very few earliest known metabolic enzymes to exert such pleiotropic effects on energy metabolism and related signaling pathways.
Arguably, the occurrence of GAPDH in serum could be a consequence of either a regulated secretory process or a random release during cell renewal or lysis. The possibility of a later process cannot be ruled out, as a similar glycolytic enzyme, LDH, has been known to be released to the blood during lytic processes.37,38 Another hypothesis for the presence of high-molecular-weight GAPDH emanates from recent reports in which the cross-linking of GAPDH protein by tissue trans-glutaminase has been demonstrated.39,40 This is particularly important as GAPDH has been known to consist of glutamine-reactive lysine residues.39 However, further investigations are required as such cross-linking of GAPDH protein cannot explain the presence of a hitherto unknown low-molecular-weight subunit (25 kDa) that has been witnessed in human serum as well as animal sera.
More than three decades ago, Arcari et al.41,42 provided evidence for the presence of more than one form of GAPDH-mRNA in humans. Similarly, data from the genetic analysis of GAPDH indicate the prevalence of pseudogenes that can be attributed to the occurrence of a high-molecular-weight GAPDH in serum. GAPDH has been known as the only glycolytic enzyme with a maximum number of pseudogenes (>60).43 Recent research on the emerging roles of GAPDH as a moonlighting protein, associated with several nonglycolytic functions, perhaps necessitates multiple forms or isoforms of GAPDH. It is unclear if the pseudogenes contribute to the generation of GAPDH isforms considering the fact that pseudogenes are generally nonfunctional and are not transcribed.44
Given that cellular GAPDH displays the unique feature of being functionally active in various cellular compartments such as the nucleus, cytoplasm, and plasma membrane, it is plausible that extracellular GAPDH in serum is associated with a hitherto unknown function. For instance, cellular GAPDH’s interaction with VDAC-1 promotes pro-apoptotic membrane permeabilization of mitochondria45 and binding with a telomere31,46 has been known to impact the genetic regulation of Oct-1 by participating in the OCA-s complex. Such a multifaceted role for a single protein (GAPDH) would nevertheless require complex structural organization and regulation under dynamic physiological conditions. In this context the role of post-translational modification (PTM) of GAPDH has been critically analyzed. It has been well documented that GAPDH undergoes one or more modifications such as acetylation, O-GlcNAcylation, S-nitrosylation, thiolation, and “siah-1-binding” depending upon its cellular function. All of these PTMs have already been known to affect the enzymatic function of GAPDH.47 In other words, PTMs that underlie GAPDH’s nonglycolytic roles eventually affects its glycolytic function.
A solitary report on human cell lines suggested that exogenous addition of cellular GAPDH inhibits cell spreading in vitro.48 However, these findings are related to the presence of cellular GAPDH (the tetrameric protein) in the extracellular space and are not associated with the secreted, extracellular GAPDH. Until now there exists a lacuna in understanding any biochemical distinction between the cellular and extracellular GAPDH. Here, we document that the serum (or extracellular) GAPDH differs significantly from cellular GAPDH in multimeric high molecular size. Furthermore, both serum and extracellular GAPDH, despite their multimeric large size, demonstrated the preservation of catalytic function, the necessity or significance of which remains to be determined. Variations in cellular GAPDH in phyletically diverse organisms have been known.49 However, until now the sperm-specific GAPDH50 has been the only other isoform of GAPDH known in humans. Here, we provide evidence for the occurrence of a hitherto unknown form of multimeric yet enzymatically active GAPDH in serum. Given the multitude of functions of cellular GAPDH besides metabolism, future studies could reveal the biological/clinical significance of this novel serum GAPDH.
Acknowledgments
The authors would like to acknowledge the Mass Spectrometry and Proteomics Core Facility of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, supported by NIH grant P30 CA006973. This work was supported by the Abdulrahman Abdulmalik Research Fund and the Charles Wallace Pratt Research Fund. The authors thank Norman Barker and Jon Christofersen of the Department of Pathology for the digital photography of gel images. While this manuscript was under revision, the first author, Dr. Rani Kunjithapatham, passed away. Rani’s exemplary contribution, meticulous approach, and unparalleled enthusiasm for science made this manuscript possible.
Footnotes
The authors declare no competing financial interest.
References
- 1.Sirover MA. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: biochemical mechanisms and regulatory control. Biochim Biophys Acta. 2011;1810:741–751. doi: 10.1016/j.bbagen.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Seidler NW. GAPDH and intermediary metabolism. Adv Exp Med Biol. 2013;985:37–59. doi: 10.1007/978-94-007-4716-6_2. [DOI] [PubMed] [Google Scholar]
- 3.Sirover MA. Role of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in normal cell function and in cell pathology. J Cell Biochem. 1997;66:133–140. [PubMed] [Google Scholar]
- 4.Colell A, Green DR, Ricci JE. Novel roles for GAPDH in cell death and carcinogenesis. Cell Death Differ. 2009;16:1573–1581. doi: 10.1038/cdd.2009.137. [DOI] [PubMed] [Google Scholar]
- 5.Ganapathy-Kanniappan S, Kunjithapatham R, Geschwind JF. Glyceraldehyde-3-phosphate dehydrogenase: a promising target for molecular therapy in hepatocellular carcinoma. Oncotarget. 2012;3:940–953. doi: 10.18632/oncotarget.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SB, Kim CK, Lee KH, Ahn JY. S-nitrosylation of B23/nucleophosmin by GAPDH protects cells from the SIAH1-GAPDH death cascade. J Cell Biol. 2012;199:65–76. doi: 10.1083/jcb.201205015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karliner JS, Gander MP, Sobel BE. Elevated serum glyceraldehyde phosphate dehydrogenase activity following acute myocardial infarction. Chest. 1971;60:318–323. doi: 10.1378/chest.60.4.318. [DOI] [PubMed] [Google Scholar]
- 8.Shibuya A, Ikewaki N. High serum glyceraldehyde-3-phosphate dehydrogenase levels in patients with liver cirrhosis. Hepatol Res. 2002;22:174–179. doi: 10.1016/s1386-6346(01)00134-6. [DOI] [PubMed] [Google Scholar]
- 9.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 10.Ganapathy-Kanniappan S, et al. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is pyruvylated during 3-bromopyruvate mediated cancer cell death. Anticancer Res. 2009;29:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 11.Bergmeyer HU, Bergmeyer J, Grassl M. Methods of Enzymatic Analysis. Verlag Chemie; Weinheim/Deerfield Beach, FL: 1983, 1986. [Google Scholar]
- 12.Ganapathy-Kanniappan S, et al. Human hepatocellular carcinoma in a mouse model: assessment of tumor response to percutaneous ablation by using glyceraldehyde-3-phosphate dehydrogenase antagonists. Radiology. 2012;262:834–845. doi: 10.1148/radiol.11111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothe G. Electrophoresis of Enzymes: Laboratory Methods. Vol. 307 Springer-Verlag; New York: 1994. [Google Scholar]
- 14.Ganapathy-Kanniappan S, et al. 3-Bromopyruvate induces endoplasmic reticulum stress, overcomes autophagy and causes apoptosis in human HCC cell lines. Anticancer Res. 2010;30:923–935. [PubMed] [Google Scholar]
- 15.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 16.Kunjithapatham R. Systemic administration of 3-bromopyruvate reveals its interaction with serum proteins in a rat model. BMC Res Notes. 2013;6:277. doi: 10.1186/1756-0500-6-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller JH, et al. Determination of hyperforin in mouse brain by high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2003;75:6084–6088. doi: 10.1021/ac034520z. [DOI] [PubMed] [Google Scholar]
- 18.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 19.Epner DE, Partin AW, Schalken JA, Isaacs JT, Coffey DS. Association of glyceraldehyde-3-phosphate dehydrogenase expression with cell motility and metastatic potential of rat prostatic adenocarcinoma. Cancer Res. 1993;53:1995–1997. [PubMed] [Google Scholar]
- 20.Zang WQ, Fieno AM, Grant RA, Yen TS. Identification of glyceraldehyde-3-phosphate dehydrogenase as a cellular protein that binds to the hepatitis B virus posttranscriptional regulatory element. Virology. 1998;248:46–52. doi: 10.1006/viro.1998.9255. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, et al. Role of glyceraldehyde-3-phosphate dehydrogenase binding to hepatitis B virus posttranscriptional regulatory element in regulating expression of HBV surface antigen. Arch Virol. 2009;154:519–524. doi: 10.1007/s00705-009-0326-8. [DOI] [PubMed] [Google Scholar]
- 22.Petrik J, Parker H, Alexander GJ. Human hepatic glyceraldehyde-3-phosphate dehydrogenase binds to the poly(U) tract of the 3′ non-coding region of hepatitis C virus genomic RNA. J Gen Virol. 1999;80(Pt 12):3109–3113. doi: 10.1099/0022-1317-80-12-3109. [DOI] [PubMed] [Google Scholar]
- 23.De BP, Gupta S, Zhao H, Drazba JA, Banerjee AK. Specific interaction in vitro and in vivo of glyceraldehyde-3-phosphate dehydrogenase and LA protein with cis-acting RNAs of human parainfluenza virus type 3. J Biol Chem. 1996;271:24728–24735. doi: 10.1074/jbc.271.40.24728. [DOI] [PubMed] [Google Scholar]
- 24.Yang SH, Liu ML, Tien CF, Chou SJ, Chang RY. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) interaction with 3′ ends of Japanese encephalitis virus RNA and colocalization with the viral NS5 protein. J Biomed Sci. 2009;16:40. doi: 10.1186/1423-0127-16-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto S, et al. Granulocyte-colony-stimulating-factor-producing hepatocellular carcinoma. J Gastroenterol. 1999;34:640–644. doi: 10.1007/s005350050387. [DOI] [PubMed] [Google Scholar]
- 26.Araki K, et al. Hepatocellular carcinoma producing a granulocyte colony-stimulating factor: report of a resected case with a literature review. Liver Int. 2007;27:716–721. doi: 10.1111/j.1478-3231.2007.01468.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhu XD, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707–2716. doi: 10.1200/JCO.2007.15.6521. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, et al. The multifunctional protein glyceraldehyde-3-phosphate dehydrogenase is both regulated and controls colony-stimulating factor-1 messenger RNA stability in ovarian cancer. Mol Cancer Res. 2008;6:1375–1384. doi: 10.1158/1541-7786.MCR-07-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eferl R, et al. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 30.Colell A, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129:983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 31.Demarse NA, et al. Direct binding of glyceraldehyde 3-phosphate dehydrogenase to telomeric DNA protects telomeres against chemotherapy-induced rapid degradation. J Mol Biol. 2009;394:789–803. doi: 10.1016/j.jmb.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hugo-Wissemann D, Anundi I, Lauchart W, Viebahn R, de Groot H. Differences in glycolytic capacity and hypoxia tolerance between hepatoma cells and hepatocytes. Hepatology. 1991;13:297–303. [PubMed] [Google Scholar]
- 33.Gong Y, Cui L, Minuk GY. Comparison of glyceraldehyde-3-phosphate dehydrogenase and 28s-ribosomal RNA gene expression in human hepatocellular carcinoma. Hepatology. 1996;23:734–737. doi: 10.1002/hep.510230413. [DOI] [PubMed] [Google Scholar]
- 34.Lee MN, et al. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Mol Cell Biol. 2009;29:3991–4001. doi: 10.1128/MCB.00165-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villanueva A, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135(6):1972–83. doi: 10.1053/j.gastro.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho C, et al. AKT (v-akt murine thymoma viral oncogene homolog 1) and N-Ras (neuroblastoma ras viral oncogene homolog) coactivation in the mouse liver promotes rapid carcinogenesis by way of mTOR (mammalian target of rapamycin complex 1), FOXM1 (forkhead box M1)/SKP2, and c-Myc pathways. Hepatology. 2012;55:833–845. doi: 10.1002/hep.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cohen JA, Brecher ME, Bandarenko N. Cellular source of serum lactate dehydrogenase elevation in patients with thrombotic thrombocytopenic purpura. J Clin Apher. 1998;13:16–19. doi: 10.1002/(sici)1098-1101(1998)13:1<16::aid-jca3>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.Kato GJ, et al. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orru S, et al. Identification of tissue transglutaminase-reactive lysine residues in glyceraldehyde-3-phosphate dehydrogenase. Protein Sci. 2002;11:137–146. doi: 10.1110/ps.17102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruoppolo M, Orru S, Francese S, Caputo I, Esposito C. Structural characterization of transglutaminase-catalyzed cross-linking between glyceraldehyde 3-phosphate dehydrogenase and polyglutamine repeats. Protein Sci. 2003;12:170–179. doi: 10.1110/ps.0216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arcari P, Martinelli R, Salvatore F. The complete sequence of a full length cDNA for human liver glyceraldehyde-3-phosphate dehydrogenase: evidence for multiple mRNA species. Nucleic Acids Res. 1984;12:9179–9189. doi: 10.1093/nar/12.23.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arcari P, Martinelli R, Salvatore F. Human glyceraldehyde-3-phosphate dehydrogenase pseudogenes: molecular evolution and a possible mechanism for amplification. Biochem Genet. 1989;27:439–450. doi: 10.1007/BF02399673. [DOI] [PubMed] [Google Scholar]
- 43.Liu YJ. Comprehensive analysis of the pseudogenes of glycolytic enzymes in vertebrates: the anomalously high number of GAPDH pseudogenes highlights a recent burst of retrotrans-positional activity. BMC Genomics. 2009;10:480. doi: 10.1186/1471-2164-10-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seidler NW. Basic biology of GAPDH. Adv Exp Med Biol. 2013;985:1–36. doi: 10.1007/978-94-007-4716-6_1. [DOI] [PubMed] [Google Scholar]
- 45.Tarze A, et al. GAPDH, a novel regulator of the pro-apoptotic mitochondrial membrane permeabilization. Oncogene. 2007;26:2606–2620. doi: 10.1038/sj.onc.1210074. [DOI] [PubMed] [Google Scholar]
- 46.Sundararaj KP, et al. Rapid shortening of telomere length in response to ceramide involves the inhibition of telomere binding activity of nuclear glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 2004;279:6152–6162. doi: 10.1074/jbc.M310549200. [DOI] [PubMed] [Google Scholar]
- 47.Tristan C, Shahani N, Sedlak TW, Sawa A. The diverse functions of GAPDH: views from different subcellular compartments. Cell Signalling. 2011;23:317–323. doi: 10.1016/j.cellsig.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaji R, et al. Glyceraldehyde-3-phosphate dehydrogenase in the extracellular space inhibits cell spreading. Biochim Biophys Acta. 2005;1726:261–271. doi: 10.1016/j.bbagen.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Lebherz HG, Rutter WJ. Glyceraldehyde-3-phosphate dehydrogenase variants in phyletically diverse organisms. Science. 1967;157:1198–1200. doi: 10.1126/science.157.3793.1198. [DOI] [PubMed] [Google Scholar]
- 50.Miki K, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci U S A. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]