Abstract
Aims
Fetuin-A is a hepatic secretory protein that both promotes insulin resistance and inhibits arterial calcification. Previous studies have suggested that the association of fetuin-A with incident cardiovascular disease (CVD) might be modified by glycemic status.
Methods and Results
We conducted a case-cohort study of fetuin-A and incident non-fatal CVD nested in the Multi-Ethnic Study of Atherosclerosis with follow-up from 2000 – 2007. Fetuin-A concentrations were measured from baseline serum samples among 2,505 randomly selected subcohort members and 142 incident cases. In weighted multivariable Cox regression models, no association was observed between fetuin-A and incident CVD in the total study population (HR per SD = 1.01; 95% CI: 0.84, 1.23). Although associations with CVD events were not statistically significant within categories of glycemic status, our results tended to support the interaction with glycemic status observed in other studies, with a positive trend restricted to participants with impaired fasting glucose or diabetes (HR per SD = 1.20; 95% CI: 0.89, 1.63) and an inverse trend among normoglycemic individuals (HR = 0.89; 95% CI: 0.69–1.13) p-interaction = 0.04). In addition, we observed significant interaction between fasting glucose and fetuin-A when both were treated continuously in the subset of participants not using diabetes medication (p-interaction = 0.006).
Conclusion
Our results suggest that fetuin-A is not associated with an overall risk of CVD, but support prior evidence indicating that the association might be modified by glycemic status.
Keywords: cardiovascular disease, fetuin-A, blood glucose
INTRODUCTION
Fetuin-A is a hepatic secretory glycoprotein believed to be related to cardiovascular disease (CVD) risk. However, observational studies of the association between fetuin-A concentrations and CVD risk have been conflicting. The first observational studies of fetuin-A were conducted in participants with advanced kidney disease, where lower fetuin-A concentrations were associated with risk of stroke1 and cardiovascular mortality.2 However, studies conducted in more general population samples have generated mixed results, with one study finding no overall association3 and others reporting positive4 and inverse5 associations. Two studies have detected significant modification of the association of fetuin-A concentrations with CVD outcomes by diabetes status5, 6 and markers of dysglycemia in participants free of diabetes,6 indicating that higher fetuin-A levels are associated with higher CVD risk only in those with signs of insulin resistance.
The biological role of fetuin-A in both insulin signaling and arterial calcification supports these divergent findings. On the one hand, fetuin-A forms stable colloidal complexes with calcium and phosphorus, potentially reducing arterial calcification.7 On the other hand, fetuin-A promotes insulin resistance by inhibiting the insulin receptor tyrosine kinase,8 and by mediating free fatty acid inflammatory signaling through toll-like receptor 4.9 Associations would therefore be expected to differ based on the relative susceptibility of populations to these competing actions of fetuin-A. The protective role of fetuin-A would likely predominate in populations with a high prevalence of arterial calcification and may explain why results were consistently inverse among patients with kidney disease,1, 2 a condition prone to arterial calcification. In populations with elevated glucose levels, the role of fetuin-A in promoting insulin resistance may outweigh its beneficial effects on calcification inhibition, potentially explaining the positive associations among dysglycemic individuals5, 6 in prior studies.
We aimed to investigate the association between fetuin-A and risk of CVD in the Multi-Ethnic Study of Atherosclerosis (MESA), a cohort of men and women from four major racial/ethnic groups in the U.S. We specifically sought to examine whether glycemic status would modify the association of fetuin-A with CVD events in this multi-ethnic study.
METHODS
Study Population
Participants were enrolled in MESA, an ongoing prospective cohort study designed to study risk factors for the progression of cardiovascular disease.10 MESA began in 2000 – 2002 with the recruitment of 6,814 men and women aged 45–84 years of Caucasian, Chinese, African or Hispanic descent from six regions in the U.S. (Baltimore City and Baltimore County, MD, Chicago, IL, Forsyth County, NC, Los Angeles County, CA, New York, NY, and St. Paul, MN) who were free of clinical cardiovascular disease. Follow-up exams were conducted in 2002–2003 (Exam 2), 2004–2005 (Exam 3), and 2005–2007 (Exam 4). The study complies with the Declaration of Helsinki, and the institutional review board at each study site approved the study protocol. Informed consent was obtained from all study participants.
Participants were selected for fetuin-A measurement in 2009 using a case-cohort designed for the primary purpose of studying fetuin-A in relation to changes in coronary artery calcium (CAC), as measured by cardiac computed tomography (CT) scans. CT scans were performed on all MESA participants at baseline and by random assignment at either Exam 2 or 3. The original participant selection for analyses of CAC included all individuals who received their second CT scan at Exam 3, and thus participants eligible for our case-cohort study of incident CVD were required to survive until Exam 3. As our study selection criteria precluded the inclusion of fatal cases prior to Exam 3, we restricted our primary endpoint to non-fatal CVD, with cases occurring between Exam 1 and the end of follow-up in May 2007 included in our analyses (Fig. 1). In sensitivity analyses, we included 18 fatal CVD cases that occurred between Exam 3 and May 2007.
Figure 1.
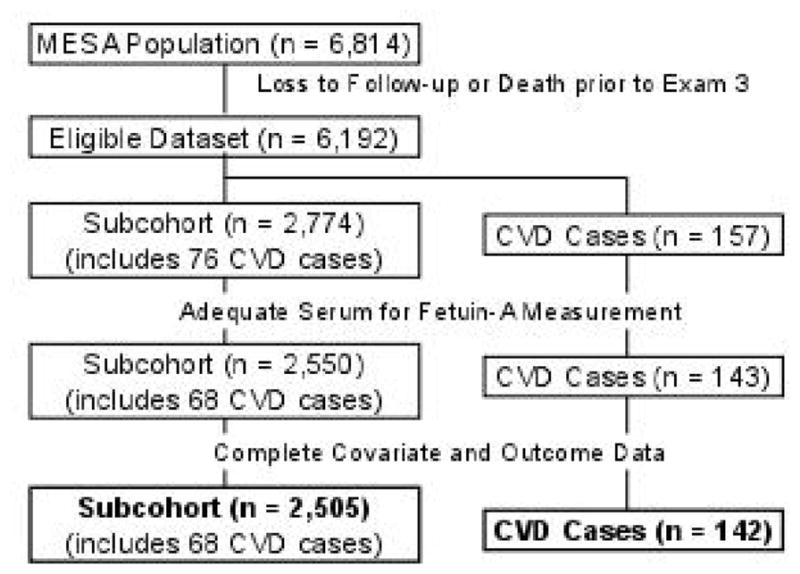
Selection of cases and subcohort for analyses of fetuin-A and incident non-fatal cardiovascular disease (CVD) in the Multi-Ethnic Study of Atherosclerosis (MESA)
Our case-cohort sample included 2,774 subcohort members and 157 cases (76 of whom belonged to the subcohort) (Fig. 1A). After excluding participants who had insufficient serum for fetuin-A measurement (n = 224) and missing CVD or covariate data (n = 45), 2,505 subcohort members and 142 nonfatal CVD cases (68 within the subcohort) remained for analysis (Fig. 1A and B). Thus, the final sample size for this analysis was 2,579 individuals. In our sensitivity analyses that additionally included fatal events after Exam 3, we had a total of 160 CVD events.
Fetuin-A measurements
Baseline serum fetuin-A concentrations were measured in 2009 by the clinical laboratory at the University of Maryland using a human enzyme linked immunosorbent assay kit (Epitope Diagnostics, San Diego, CA). Samples were run in duplicate, and values were averaged. The intra- and inter-assay coefficients of variation for fetuin-A measurements were 2.1–3.4% and 5.7–6.8%, respectively.
Assessment of cardiovascular events
Participants were queried about hospital admissions and CVD outpatient diagnoses during follow-up examinations, and by telephone interviews completed every 9–12 months.10 In this study, CVD events included myocardial infarction (MI), resuscitated cardiac arrest, and stroke. The diagnosis of MI was based on clinical, electrocardiographic and biochemical findings. Stroke events included any documented rapid onset focal neurologic deficit that was not related to other nonvascular causes, and lasted at least 24 hours, unless also accompanied by a clinically relevant lesion on brain imaging. Two physicians from the MESA study events committee independently reviewed participants’ medical records to identify incident cases of CVD.10
Assessment of covariates and effect modifiers
At baseline, participant information was collected through questionnaires and physical examinations. Information collected included age, sex, racial/ethnic group, field center, smoking (never, former, current), alcohol intake (never, former, current with <1 drink per day, current with 1–2 drinks per day, current with >2 drinks per day), body mass index (BMI) (normal weight: <25.0 kg/m2, overweight: 25.0–29.9 kg/m2, obese: ≥30.0 kg/m2), systolic blood pressure, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol, triglycerides, family history of MI or stroke (yes, no, don’t know), education (less than high-school, high-school up to technical or associate degree, bachelor’s degree or more), annual income (<$25,000, $25–49,999, ≥$50,000, not stated), and physical activity (moderate-vigorous in MET-min/wk).
Plasma glucose (Vitros analyzer, Johnson & Johnson Clinical Diagnostics) and insulin were measured at baseline and hemoglobin A1c (HbA1c) was measured at Exam 2 from fasting blood samples. American Diabetes Association (ADA) criteria were used to define glycemic status according to fasting plasma glucose levels and HbA1c. Fasting plasma glucose was categorized as normoglycemic (fasting glucose <100 mg/dL), impaired fasting glucose (IFG) (fasting glucose 100–125 mg/dL), and diabetes (fasting glucose ≥126 mg/dL or use of diabetes medications)11, and HbA1c as normal (<5.7%), elevated diabetes risk (5.7–6.4%), and diabetes (≥6.5%)14. HOMA-IR (homeostasis model assessment of insulin resistance) was calculated as insulin (mU/L) × (glucose [mg/dL] × 0.055)/22.5.
Statistical analyses
The distribution of baseline characteristics was examined among CVD cases and the random subcohort. Hazard ratios (HRs) and 95% confidence intervals (95% CI) for the association between fetuin-A concentrations and incident non-fatal CVD were estimated by Cox proportional hazards regression, with Kalbfleisch and Lawless weights and the robust variance estimator12 used to account for the case-cohort design.
We observed no deviations from linearity using restricted cubic splines to evaluate the functional form of fetuin-A, and we therefore modeled fetuin-A concentrations per standard deviation (SD) (0.10 g/L). We additionally evaluated associations according to tertiles of fetuin-A based on the distribution in the subcohort. Participants contributed person-time from baseline until the date of an event, death, or end of follow-up (May 2007), whichever occurred first. Cases were assigned a weight of 1, irrespective of whether the case was in the random subcohort, and subcohort non-cases were assigned a weight equal to the inverse of the probability of being in the subcohort.12 Multivariable models included age, sex, racial/ethnic group, field center, smoking status, alcohol intake, BMI, systolic blood pressure, LDL cholesterol, HDL cholesterol and triglyceride concentrations, family history of CVD, education, income, and physical activity. Additional adjustment for waist circumference did not affect the multivariable-adjusted estimates. No violations of the proportional hazards assumption were observed via analysis of martingale residuals.13 As associations did not appear to vary by sex and race, we performed all analyses in the total study population.
We examined whether the association of fetuin-A concentrations with CVD risk varied in the three defined strata of glycemic status (normoglycemic, IFG, and diabetes). In addition, we tested the interaction between continuously measured fetuin-A and fasting glucose levels, with this analysis restricted to participants not on diabetes medication at baseline to avoid an influence of medication use on glucose levels. In a post-hoc analysis, we pooled the IFG and diabetes groups and compared the association of fetuin-A and CVD events in this combined group with fasting glucose ≥100 mg/dL to that among normoglycemic individuals. In secondary analyses, we examined the interaction between fetuin-A and glycemic status defined by HbA1c (<5.7%, 5.7–6.4%, ≥6.5%). To investigate whether findings might extend to individuals without high fasting glucose but with other underlying metabolic risk factors, we explored whether associations varied by BMI (<30, ≥30 kg/m2) and HOMA-IR (<median, ≥ median [1.9 units]) among normoglycemic participants. The likelihood ratio test was used to compare models with and without multiplicative interaction terms for all potential effect modifiers. All statistical tests were performed using SAS 9.4 (SAS Institute; Cary, NC), and p-values <0.05 were considered statistically significant.
RESULTS
Participants were followed for a median of 6.0 years. Compared with subcohort members, participants who subsequently developed CVD were older and more likely to be Caucasian and male and were more likely to currently smoke and to have diabetes and higher systolic blood pressure (Table 1).
Table 1.
Baseline characteristics in a random sub-cohort and participants who developed non-fatal cardiovascular disease events in the Multi-Ethnic Study of Atherosclerosis
| Variable | Subcohort (n = 2505) | Incident cases (n = 142)* |
|---|---|---|
| Mean fetuin-A, g/L (SD) | 0.48 (0.10) | 0.47 (0.11) |
| Mean age, years (SD) | 62 (10) | 68 (10) |
| Male, N (%) | 1198 (48) | 91 (64) |
| Caucasian, N (%) | 994 (40) | 69 (48) |
| Chinese-American, N (%) | 315 (13) | 7 (5) |
| African-American, N (%) | 684 (27) | 35 (25) |
| Hispanic, N (%) | 512 (20) | 31 (22) |
| Mean body mass index, kg/m2 (SD) | 28.3 (5.4) | 28.5 (4.5) |
| Current smoker, N (%) | 290 (12) | 25 (18) |
| Diabetes medication use, N (%) | 226 (9) | 29 (20) |
| Median triglycerides, mg/dL (IQR) | 110 (77, 156) | 133 (96, 196) |
| Mean LDL-cholesterol, mg/dL (SD) | 116 (31) | 121 (32) |
| Median HDL-cholesterol, mg/dL (IQR) | 48 (41, 59) | 45 (36, 54) |
| Mean SBP, mmHg (SD) | 126 (21) | 137 (24) |
| eGFR (ml/min/1.73m2), mean (SD) | 78 (16) | 72 (19) |
| Median fasting glucose, mg/dL (IQR) | 89 (82, 99) | 91 (85, 107) |
Abbreviations: SD = standard deviation, IQR = interquartile range, LDL = low-density lipoprotein, HDL = high-density lipoprotein, SBP = systolic blood pressure, eGFR = estimated glomerular filtration rate
68 cases that occurred within the subcohort are included in both case and subcohort number.
We observed no overall association between fetuin-A and incident CVD (HR per SD = 1.01; 95% CI: 0.84, 1.23). Although associations were of only borderline statistical significance across baseline glycemic status (global p-value for 3-level interaction = 0.09), suggestive positive associations were only observed among those with IFG and prevalent diabetes (Table 2). The HR per SD was 1.56 (95% CI: 0.84, 2.89) in participants with IFG and 1.27 (95% CI: 0.85, 1.90) among those with diabetes. In contrast, each SD (0.10 g/L) higher fetuin-A was associated with a slightly lower risk of CVD (HR=0.89, 95% CI: 0.69, 1.13) in normoglycemic individuals. When participants with either IFG or diabetes were combined, the HR per SD was 1.20 (95% CI: 0.89, 1.63), which was statistically significantly different from the estimate among normoglycemic individuals (p-interaction = 0.04). We also observed a significant interaction between continuous fasting glucose and fetuin-A (p-interaction=0.006) among participants not taking diabetes medication.
Table 2.
Hazard ratio and 95% confidence intervals for non-fatal cardiovascular disease events* across tertiles of fetuin-A, stratified by glycemic status in the Multi-Ethnic Study of Atherosclerosis
| Tertiles (g/L) | Continuous per SD** |
|||
|---|---|---|---|---|
| 0.20 – 0.43 | >0.43 – 0.51 | >0.51 – 0.94 | ||
| Normoglycemic | ||||
| Cases | 40 | 24 | 27 | 91 |
| Multivariable HR† (95% CI) | 1.0 (ref) | 0.64 (0.38–1.10) | 0.67 (0.38–1.18) | 0.89 (0.69–1.13) |
| Impaired fasting glucose | ||||
| Cases | 5 | 4 | 11 | 20 |
| Multivariable HR (95% CI) | 1.0 (ref) | 1.25 (0.17, 9.13) | 5.64 (0.92, 34.59) | 1.56 (0.84, 2.89) |
| Diabetes | ||||
| Cases | 9 | 11 | 11 | 31 |
| Multivariable HR (95% CI) | 1.0 (ref) | 3.19 (1.00–10.14) | 1.90 (0.63, 5.76) | 1.27 (0.85, 1.90) |
CI, confidence interval, HR, hazard ratio, SD, standard deviation
Normoglycemic: fasting glucose <100 mg/dL; impaired fasting glucose: fasting glucose 100 – 125 mg/dL; diabetes: fasting glucose ≥126 mg/dL or the use medications for diabetes
Non-fatal CVD events include acute myocardial infarction, resuscitated cardiac arrest, and stroke
SD = 0.10 g/L; p-interaction = 0.09
Adjusted for age, sex, racial/ethnic group, field center, smoking status, alcohol intake, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglyceride concentrations, family history of cardiovascular disease, education, income, and physical activity.
Divergent associations of fetuin-A were also observed when HbA1c was used to define glycemic status. Fetuin-A was suggestively positively associated with CVD events among participants with high (≥6.5%) or moderately elevated HbA1c (5.7–6.4%). HRs per SD were 1.18 (95% CI: 0.73, 1.91) and 1.28 (95% CI: 0.93, 1.75), respectively, whereas fetuin-A was inversely associated among those with the lowest HbA1c levels (HbA1c <5.7%; HR = 0.81; 95% CI: 0.60, 1.09) (p-interaction = 0.006) (Table 3). Similar to the results for the combined group with IFG or diabetes, a positive association was observed when the upper HbA1c categories were collapsed (HR, HbA1c ≥5.7% = 1.27; 95% CI: 0.97, 1.66) (p-interaction, HbA1c ≥5.7% vs. HbA1c <5.7% = 0.001).
Table 3.
Hazard ratio and 95% confidence intervals for non-fatal cardiovascular disease events across tertiles of fetuin-A, stratified by HbA1c
| Tertiles (g/L) | Continuous per SD* |
|||
|---|---|---|---|---|
| 0.20 – 0.43 | >0.43 – 0.51 | >0.51 – 0.94 | ||
| HbA1c <5.7% | ||||
| Cases | 35 | 22 | 23 | 80 |
| Multivariable HR** (95% CI) | 1.0 (ref) | 0.65 (0.36, 1.16) | 0.62 (0.33, 1.18) | 0.81 (0.60, 1.09) |
| HbA1c 5.7 – 6.4% | ||||
| Cases | 12 | 11 | 16 | 39 |
| Multivariable HR (95% CI) | 1.0 (ref) | 1.50 (0.58, 3.88) | 1.65 (0.71, 3.87) | 1.28 (0.93, 1.75) |
| HbA1c ≥6.5% | ||||
| Cases | 7 | 6 | 10 | 23 |
| Multivariable HR (95% CI) | 1.0 (ref) | 1.32 (0.23, 7.47) | 1.21 (0.32, 4.58) | 1.18 (0.73, 1.91) |
SD = 0.10 g/L
Adjusted for age, sex, racial/ethnic group, field center, smoking status, alcohol intake, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglyceride concentrations, family history of cardiovascular disease, education, income, and physical activity.
Among participants without impaired fasting plasma glucose or diabetes, associations of fetuin-A similarly diverged according to the presence of metabolic risk factors (Table 4). In this normoglycemic subset, obese individuals had a non-significantly higher risk of diabetes (HR per SD = 1.32; 95% CI: 0.86, 2.03), whereas the association was inverse among non-obese individuals (HR= 0.77; 95% CI: 0.56, 1.06). The association was also suggestively positive among normoglycemic participants with HOMA-IR levels above the median (HR = 1.28; 95% CI: 0.87, 1.90) and inverse among those with HOMA-IR below the median (HR = 0.68; 95% CI: 0.48, 0.97). Differences were significant by both obesity (p-interaction = 0.049) and HOMA-IR (p-interaction = 0.02).
Table 4.
Hazard ratio and 95% confidence intervals for non-fatal cardiovascular disease events across tertiles of fetuin-A among normoglycemic participants, stratified by metabolic risk factors
| Tertiles (g/L) | Continuous per SD* |
|||
|---|---|---|---|---|
| 0.20 – 0.43 | >0.43 – 0.51 | >0.51 – 0.94 | ||
| BMI <30 | ||||
| Multivariable HR** (95% CI) | 1.0 (ref) | 0.52 (0.27, 1.00) | 0.50 (0.25, 0.99) | 0.77 (0.56, 1.06) |
| BMI ≥30 | ||||
| Multivariable HR (95% CI) | 1.0 (ref) | 1.93 (0.52, 7.16) | 1.89 (0.43, 8.30) | 1.32 (0.86, 2.03) |
| HOMA-IR <median† | ||||
| Multivariable HR (95% CI) | 1.0 (ref) | 0.50 (0.23, 1.10) | 0.26 (0.10, 0.67) | 0.68 (0.48, 0.97) |
| HOMA-IR ≥median | ||||
| Multivariable HR (95% CI) | 1.0 (ref) | 0.88 (0.29, 2.67) | 2.25 (0.80, 6.37) | 1.28 (0.87, 1.90) |
SD = 0.10 g/L
Adjusted for age, sex, racial/ethnic group, field center, smoking status, alcohol intake, body mass index, systolic blood pressure, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol and triglyceride concentrations, family history of cardiovascular disease, education, income, and physical activity.
Median in subcohort (1.9)
Upon addition of the fatal CVD cases that occurred after Exam 3, main associations of fetuin-A with CVD risk were similar to those among non-fatal cases alone for individuals with normoglycemia (HR = 0.81; 95% CI: 0.64, 1.02) and IFG or diabetes (HR = 1.13; 95% CI: 0.84, 1.51) (p-interaction = 0.03).
DISCUSSION
In this multi-ethnic study of U.S. men and women, we found no overall association between fetuin-A levels and incident CVD, but our results supported the reported interaction observed in other studies,5, 6 with a positive trend restricted to participants with impaired fasting glucose or diabetes.
Prior prospective studies of fetuin-A and CVD risk, conducted in predominantly Caucasian populations, have been conflicting. We have previously observed an interaction with diabetes in two studies of older adults,5, 6 where risk of CVD was elevated with higher fetuin-A only among participants with diabetes. In contrast, a case-cohort study within the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study found higher fetuin-A concentrations associated with greater CVD risk among all participants regardless of their glycemic status,4 and a case-control study nested in the Nurses’ Health Study (NHS) observed no overall association of fetuin-A and CVD events and no effect modification by diabetes status.3 Results have also been mixed for Mendelian randomization analyses examining whether the relationship between fetuin-A and risk of CVD may be causal. While higher genetically predicted fetuin-A levels were associated with a marginally lower risk of CVD in a recent analysis in the Cardiovascular Health Study15, a positive association was observed in EPIC-Potsdam16, and no association was found in a pooled analysis of 7 large prospective cohorts15.
The dual roles of fetuin-A in promoting insulin resistance and inhibiting coronary artery calcification may explain these seemingly contradictory findings. In vitro, fetuin-A inhibits insulin signaling by binding the insulin receptor in muscle and fat8. Supporting this mechanism, mice treated with exogenous fetuin-A rapidly develop insulin resistance17, while fetuin-A knockout mice show improved insulin sensitivity and are less susceptible to weight gain18. In human studies, fetuin-A has been associated with cardiometabolic risk factors (e.g., elevated LDL and triglyceride levels, higher BMI, and insulin resistance) in cross-sectional studies19, 20, and an independent association between higher levels of fetuin-A and risk of diabetes has now been demonstrated in a number of prospective cohort studies21–25. Thus, fetuin-A may contribute to a higher risk of CVD among individuals susceptible to the development of insulin resistance. In agreement with this explanation, we observed a positive association between fetuin-A and CVD events only among MESA participants with IFG and diabetes, with similar positive associations present for corresponding categories of HbA1c. Moreover, similar to our findings in the Cardiovascular Health Study6, participants with normal glucose levels but with obesity or high HOMA-IR also appeared to be at higher risk, suggesting that the role of fetuin-A in enhancing insulin resistance may predominate in individuals with metabolic risk factors but without overt dysglycemia.
In addition to its adverse role in CVD pathogenesis via effects on insulin sensitivity, fetuin-A may protect against the development of CVD by inhibiting CAC formation. In vitro experiments indicate that fetuin-A prevents the formation of calcium and phosphorous deposits in human serum26, and fetuin-A knockout mice, although less prone to insulin resistance18, are more susceptible to ectopic calcification26. While evidence from human studies for an inhibitory effect of fetuin-A on CAC is limited, previous investigations in MESA have found an inverse association between fetuin-A and arterial calcification only among participants without diabetes27. Thus, we hypothesized that among primarily normoglycemic individuals, fetuin-A may act mainly via the inhibition of arterial calcification, obscuring any adverse effects of fetuin-A and potentially reducing risk of CVD. Consistent with this explanation, we observed positive associations only among those with IFG and diabetes in the present study. However, further research is needed on the potential effects of fetuin-A on calcification metabolism, particularly in advanced atherosclerosis where calcification may paradoxically have a protective role in enhancing plaque stability28.
Other explanations for discrepancies across studies may be differences in sample sizes and follow-up time, differing CVD endpoints, or variation in the assays used for the measurement of fetuin-A. Differences in diabetes case ascertainment and the prevalence of diabetes medication use may have also contributed to discrepancies in diabetes subgroup analyses, as some studies may have captured more severe diabetes cases in whom the effects of fetuin-A on insulin resistance may have varied.
Our study has many notable strengths, including a comprehensive assessment of potential confounding factors for CVD risk and careful adjudication of CVD events. This is the first study of fetuin-A and incident CVD in an ethnically diverse population where we were also able to evaluate the role of baseline glycemia. However, several important limitations warrant consideration. As CVD case numbers were modest, it is difficult to determine whether the lack of significant associations was due to low power or true lack of effect, either overall or in subgroups. Due to our study design, we were limited to mostly non-fatal outcomes in the current study, and we had insufficient case numbers to evaluate the association separately for coronary heart disease and stroke. However, similar differences by glycemic status were observed when later fatal events were included, as also reported in the Rancho Bernardo Study and across CVD endpoints in CHS, providing some reassurance that our results were not unique to our combined CVD endpoint. We also measured fetuin-A at only one point in time, although fetuin-A has previously been shown to be stable over several years.3
In conclusion, our results lend further support to prior studies suggesting that positive associations between fetuin-A concentration and incident CVD may be limited to individuals with insulin resistance. Further study is needed to better understand the complex interrelationships between fetuin-A, arterial calcification, and glycemic status in the development of CVD.
Fetuin-A and risk of CVD was prospectively examined in a multi-ethnic population.
No overall association between serum fetuin-A and CVD events was found.
The association of fetuin-A with risk of CVD was found to depend on glycemic status.
Fetuin-A tended to be inversely associated with CVD events in normoglycemic persons.
Fetuin-A was directly associated in those with impaired fasting glucose or diabetes.
Acknowledgments
This research was supported by grants R01 HL071739 and R21 HL091217 from the National Heart, Lung, and Blood Institute (NHLBI), T32 DK 007703 from the National Institute of Diabetes and Digestive and Kidney Diseases, UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources, and by contracts N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from NHLBI.
The results of this study were presented as an abstract at the American Heart Association Scientific Sessions, Chicago, IL, November 2014.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen HY, Chiu YL, Hsu SP, et al. Low serum fetuin A levels and incident stroke in patients with maintenance haemodialysis. European journal of clinical investigation. 2013;43:387–396. doi: 10.1111/eci.12057. [DOI] [PubMed] [Google Scholar]
- 2.Stenvinkel P, Wang K, Qureshi AR, et al. Low fetuin-A levels are associated with cardiovascular death: Impact of variations in the gene encoding fetuin. Kidney international. 2005;67:2383–2392. doi: 10.1111/j.1523-1755.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 3.Sun Q, Jimenez MC, Townsend MK, et al. Plasma Levels of Fetuin-A and Risk of Coronary Heart Disease in US Women: The Nurses’ Health Study. Journal of the American Heart Association. 2014;3 doi: 10.1161/JAHA.114.000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weikert C, Stefan N, Schulze MB, et al. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 5.Laughlin GA, Cummins KM, Wassel CL, et al. The association of fetuin-A with cardiovascular disease mortality in older community-dwelling adults: the Rancho Bernardo study. Journal of the American College of Cardiology. 2012;59:1688–1696. doi: 10.1016/j.jacc.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen MK, Bartz TM, Mukamal KJ, et al. Fetuin-A, type 2 diabetes, and risk of cardiovascular disease in older adults: the cardiovascular health study. Diabetes care. 2013;36:1222–1228. doi: 10.2337/dc12-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith ER, Hanssen E, McMahon LP, et al. Fetuin-A-containing calciprotein particles reduce mineral stress in the macrophage. PloS one. 2013;8:e60904. doi: 10.1371/journal.pone.0060904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathews ST, Chellam N, Srinivas PR, et al. Alpha2-HSG, a specific inhibitor of insulin receptor autophosphorylation, interacts with the insulin receptor. Molecular and cellular endocrinology. 2000;164:87–98. doi: 10.1016/s0303-7207(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 9.Pal D, Dasgupta S, Kundu R, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nature medicine. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 10.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 11.Expert Committee on the Diagnosis Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 12.Petersen L, Sorensen TI, Andersen PK. Comparison of case-cohort estimators based on data on premature death of adult adoptees. Statistics in medicine. 2003;22:3795–3803. doi: 10.1002/sim.1672. [DOI] [PubMed] [Google Scholar]
- 13.Lin DY, Wei LJ, Ying Z. Checking the Cox model with cumulative sums of martingale-based residuals. Biometrika. 1993;80:557–572. [Google Scholar]
- 14.American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes care. 2014;37(Suppl 1):S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 15.Laugsand LE, Ix JH, Bartz TM, et al. Fetuin-A and risk of coronary heart disease: A Mendelian randomization analysis and a pooled analysis of AHSG genetic variants in 7 prospective studies. Atherosclerosis. 2015;243:44–52. doi: 10.1016/j.atherosclerosis.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisher E, Stefan N, Saar K, et al. Association of AHSG gene polymorphisms with fetuin-A plasma levels and cardiovascular diseases in the EPIC-Potsdam study. Circ Cardiovasc Genet. 2009;2:607–613. doi: 10.1161/CIRCGENETICS.109.870410. [DOI] [PubMed] [Google Scholar]
- 17.Hennige AM, Staiger H, Wicke C, et al. Fetuin-A induces cytokine expression and suppresses adiponectin production. PloS one. 2008;3:e1765. doi: 10.1371/journal.pone.0001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathews ST, Singh GP, Ranalletta M, et al. Improved insulin sensitivity and resistance to weight gain in mice null for the Ahsg gene. Diabetes. 2002;51:2450–2458. doi: 10.2337/diabetes.51.8.2450. [DOI] [PubMed] [Google Scholar]
- 19.Ix JH, Shlipak MG, Brandenburg VM, et al. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stefan N, Hennige AM, Staiger H, et al. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes care. 2006;29:853–857. doi: 10.2337/diacare.29.04.06.dc05-1938. [DOI] [PubMed] [Google Scholar]
- 21.Ix JH, Biggs ML, Mukamal KJ, et al. Association of fetuin-a with incident diabetes mellitus in community-living older adults: the cardiovascular health study. Circulation. 2012;125:2316–2322. doi: 10.1161/CIRCULATIONAHA.111.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laughlin GA, Barrett-Connor E, Cummins KM, et al. Sex-specific association of fetuin-A with type 2 diabetes in older community-dwelling adults: the Rancho Bernardo study. Diabetes care. 2013;36:1994–2000. doi: 10.2337/dc12-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ix JH, Wassel CL, Kanaya AM, et al. Fetuin-A and incident diabetes mellitus in older persons. Jama. 2008;300:182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefan N, Fritsche A, Weikert C, et al. Plasma fetuin-A levels and the risk of type 2 diabetes. Diabetes. 2008;57:2762–2767. doi: 10.2337/db08-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Q, Cornelis MC, Manson JE, et al. Plasma levels of fetuin-A and hepatic enzymes and risk of type 2 diabetes in women in the U.S. Diabetes. 2013;62:49–55. doi: 10.2337/db12-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafer C, Heiss A, Schwarz A, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ix JH, Katz R, de Boer IH, et al. Fetuin-A is inversely associated with coronary artery calcification in community-living persons: the Multi-Ethnic Study of Atherosclerosis. Clinical chemistry. 2012;58:887–895. doi: 10.1373/clinchem.2011.177725. [DOI] [PubMed] [Google Scholar]
- 28.Criqui MH, Denenberg JO, Ix JH, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. Jama. 2014;311:271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]


