Abstract
Chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL) are different manifestations of the same disease, which are managed in the same way. The advent of novel monoclonal antibodies (ofatumumab and obinutuzumab) led to the development of effective chemoimmunotherapy regimens. The recently approved small molecule kinase inhibitors (ibrutinib and idelalisib) are effective treatment options for CLL in elderly patients with decreased tolerance for aggressive regimens and in patients with poor prognostic features who do not benefit from conventional chemoimmunotherapy regimens. This portion of the NCCN Guidelines for Non-Hodgkin’s Lymphomas describes the recent specific to the incorporation of recently approved targeted therapies for the management of patients with newly diagnosed and relapsed or refractory CLL/SLL.
Chronic lymphocytic leukemia (CLL) remains the most prevalent adult leukemia in Western countries, but it is considered rare in regions such as East Asia. CLL/small lymphocytic lymphoma (SLL) constitutes approximately 7% of newly diagnosed cases of non-Hodgkin’s lymphoma (NHL).1 In 2015, an estimated 14,620 people will be diagnosed with CLL in the United States, and an estimated 4650 people will die of the disease.2 Morphologically, the leukemic cells appear as small, mature lymphocytes that may be found admixed with occasional larger or atypical cells, or prolymphocytes.3 CLL and SLL are different manifestations of the same disease and are managed in much the same way.4 CLL/SLL is characterized by progressive accumulation of these leukemic cells in the peripheral blood, bone marrow, and lymphoid tissues. The major difference is that in CLL, a significant number of the abnormal lymphocytes are also found in the bone marrow and blood, while in SLL the abnormal lymphocytes are predominantly found in the lymph nodes and bone marrow.
Diagnosis
The diagnosis of CLL requires the presence of at least 5000 clonal B cells/mcL (5 × 109/L) in the peripheral blood, which is established by flow cytometry quantification.3 The presence of fewer B cells in the absence of palpable lymphadenopathy or other clinical features characteristic of a lymphoproliferative disorder is defined as monoclonal B lymphocytosis (MBL). MBL is a relatively recent diagnostic category describing individuals who present with an abnormal B-cell population with the immunopheno-type of CLL but do not meet the diagnostic criteria for CLL.5 Favorable molecular lesions, mutated immunoglobulin heavy-chain variable region gene (IGHV) and chromosomal abnormality del(13q) or normal cytogenetics are commonly seen in individuals with MBL.6 The estimated rate of progression of MBL to CLL is 1.1% per year.6









The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) for NHL now include an initial stratification between CLL/SLL and MBL (absolute B-lymphocyte count of <5000/mm3, lymph nodes <1.5 cm, no thrombocytopenia or anemia). Observation is recommended for all individuals with MBL. (To view the most recent and complete version of these guidelines, visit NCCN.org.) The diagnosis of CLL requires the presence of at least 5000 monoclonal B lymphocytes/mcL (5 × 109/L) in peripheral blood and the clonality of B cells should be confirmed by flow cytometry. The diagnosis of SLL requires the presence of lymphadenopathy and/or splenomegaly, with less than 5000 B lymphocytes/mcL (5 × 109/L) in the peripheral blood.3 B cells with a CLL/SLL phenotype may be found in samples from patients with reactive lymph nodes; however, a diagnosis of SLL should only be made when effacement of the lymph node architecture is observed in biopsy samples.
Adequate immunophenotyping is essential to establish the diagnosis of CLL/SLL. Flow cytometry of peripheral blood is adequate for the diagnosis of CLL, and bone marrow biopsy is generally not required. A diagnosis of SLL should ideally be confirmed with the evaluation of lymph node biopsy samples. Cell surface markers for flow cytometric studies should include kappa/lambda, CD19, CD20, CD5, CD23, and CD10. If flow cytometry is used to establish a diagnosis, flow evaluation for cyclin D1 or fluorescence in situ hybridization (FISH) analysis for t(11;14) should also be included to rule out mantle cell lymphoma (MCL). Paraffin-section immunohistochemistry (IHC) on excisional or incisional lymph node biopsy materials can be performed if a diagnosis is not established by flow cytometry. Recommended IHC panels include CD3, CD5, CD10, CD20, CD23, and cyclin D1. These can be useful, particularly for diagnosing CLL/SLL type without circulating leukemic cells.
The typical immunophenotype for CLL/SLL is CD5+, CD10−, CD19+, and CD20 dim, surface immunoglobulin dim, CD23+, CD43+/−, and cyclin D1-. Distinguishing CLL/SLL from MCL is essential, as they are both CD5+ B-cell tumors. Although CD23 is often helpful, absence of cyclin D1 expression is critical in this differentiation of tumor types. Stimulated cytogenetics or FISH analysis for t(11;14) can help to distinguish MCL from CLL and should be performed if flow cytometry alone is used to evaluate immunophenotype. FISH for the detection of del(11q), del(13q), trisomy 12, and del(17p), and stimulated metaphase karyotype and molecular genetic analysis (by PCR or sequencing) to detect IGHV mutation status and TP53 mutations can provide useful prognostic information and may guide selection of therapy. Recent reports suggest that complex karyotype (≥3 unrelated chromosomal abnormalities in more than one cell on conventional karyotyping of stimulated CLL cells) is associated with an unfavorable prognosis.7–9 Cytogenetic abnormalities can evolve over time; therefore, reevaluation of FISH and karyotype is necessary to direct treatment options in patients with indications for treatment.
Conventional metaphase cytogenetics is difficult in CLL as a result of the very low in vitro proliferative activity of the leukemic cells. Therefore, interphase cytogenetic analysis with FISH is the standard method to detect chromosomal abnormalities that may have prognostic significance. However, FISH can only detect abnormalities specific to the probes used. Cytokine or CpG oligonucleotide stimulation was used to enhance metaphase analysis.10 Recent studies demonstrated that stimulation with CpG oligonucleotide and interleukin-2 is more effective than with 12-O-tetradecanoylphorbol-13-acetate (TPA) for the detection of chromosomal abnormalities in CLL.11,12 A prospective study conducted by the CLL Research Consortium confirmed that abnormal clones in CLL are more readily detected with CpG oligonucleotide stimulation than with traditional B-cell mitogens; moreover, the clonal abnormalities revealed by CpG stimulated metaphase cytogenetics are consistent with that detected by interphase FISH and are reproducible among different cytogenetic laboratories.13 However, the use of CpG stimulation for CLL cytogenetics is not yet universally available.
Prognostic Factors
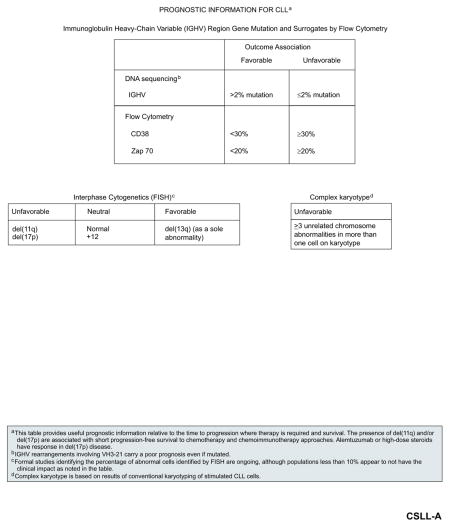
During the past decade, numerous factors were identified and evaluated in patients with CLL that may provide useful prognostic information beyond clinical staging (see “Staging,” page 350). These factors include serum markers such as thymidine kinase and beta-2 microglobulin, genetic markers including IGHV mutational status, and cytogenetic abnormalities detected by FISH (eg, del(13q), del(11q), del(17p)), CD38 expression, CD49d and zeta-associated protein 70 (ZAP-70) expression/methylation).14–26
IGHV mutational status is an important predictor of survival outcomes in CLL; unmutated IGHV (≥98% homology with germline gene sequence) is associated with poor prognosis and significantly decreased survival compared with mutated IGHV, irrespective of the stage of the disease.15,20 In addition, VH3-21 gene usage was associated with poor outcomes regardless of the mutation status (as defined by percent homology with germline sequence).27 Unmutated IGHV or the use of VH3-21 was shown to be independent predictors of shorter treatment-free interval and/or survival outcomes, even when high-risk genomic abnormalities were included in the multivariable regression models (see subsequent discuss of cytogenetic abnormalities detected by FISH).28–31
Expression of CD38 (≥7% of B lymphocytes) 15,16,22,29,30,32 and/or ZAP-70 (≥20% of B lymphocytes)14,23–25,33 were also associated with shorter progression-free survival (PFS) and overall survival (OS) outcomes. Among the flow cytometry–based prognostic assays (CD38, ZAP-70, and CD49d), CD49d appears to be the strongest prognostic parameter and is the only one that is independent of FISH and IGHV.26 Both CD38 and ZAP-70 positivity correlate with unmutated IGHV, and were suggested as potential surrogate markers for IGHV mutational status.14,15,25 However, discordant results between CD38 positivity and IGHV mutational status were seen in up to 28% of patients in one study; moreover, CD38 expression levels may vary over the course of the disease.21 Similarly, discordant results between ZAP-70 positivity and IGHV mutational status were reported in 20% to 25% of cases.24,30
In addition, ZAP-70 positivity was suggested to be a stronger predictor of outcomes (eg, time to first treatment) than IGHV mutational status or CD38 levels.24,33,34 ZAP-70 methylation analysis (which is closely associated with ZAP-70 expression and IGHV mutational status) was also reported to be a useful prognostic test for patients with CLL.35–37 CD38 and/or ZAP-70 expressions can be determined using IHC, flow cytometry, or methylation. However, standardization and reproducibility of ZAP-70 expression across laboratories remains a challenge. Evaluation of ZAP-70 protein expression is not recommended outside the context of clinical trials. Therefore, in clinical practice, IGHV mutation testing is recommended based on reproducibility and ready availability.
An elevated serum beta-2 microglobulin level was shown to be a strong independent prognostic indicator for treatment-free interval, response to treatment, and OS, including in patients treated with first-line chemoimmunotherapy regimens.38–40 One of the advantages of beta-2 microglobulin is that it is readily measured by standard laboratory evaluation of blood samples. However, it is influenced in a CLL disease–independent manner by renal dysfunction. Wierda et al41 developed a prognostic nomogram using clinical and laboratory parameters that are available in the routine clinical practice setting (age, beta-2 microglobulin, absolute lymphocyte count, sex, Rai stage, and number of involved lymph nodes); the nomogram was developed to estimate the median survival time, and the probability of 5-and 10-year survival.
In addition, based on the sum of points assigned to the 6 parameters used for the nomogram, a more simplified prognostic index was developed to help stratify untreated patients with CLL into 3 different risk groups (low, intermediate, and high).41 The estimated median survival was not reached for the low-risk group. The median survival times for intermediate-and high-risk groups were 10 and 5 years, respectively. The 5-year survival rates were 97% for low-risk, 80% for intermediate-risk, and 55% for high-risk groups; the 10-year survival rates were 80%, 52%, and 26%, respectively.41 It should be noted that sufficient data were not available for recently identified prognostic factors (eg, IGHV mutational status, ZAP-70, cytogenetic abnormalities detected by FISH) to be incorporated into this version of the prognostic model. Nevertheless, several studies independently confirmed the utility of this prognostic index in estimating both survival probability and time to first treatment in previously untreated patients with CLL, including in patients with early-stage (Rai stage 0) disease.42,43
Cytogenetic abnormalities that can be detected by FISH are present in more than 80% of patients with previously untreated CLL. FISH is categorized according to the highest-risk abnormality present, according to a hierarchical categorization. The most common abnormality is del(13q) (55%) as a sole finding, followed by del(11q) (18%), trisomy 12 (16%), del(17p) (7%), and del(6q) (7%).17 As a sole abnormality, del(13q) is associated with favorable prognosis and the longest median survival (133 months), whereas del(11q) is often associated with extensive lymphadenopathy, disease progression, and shorter median survival (79 months).17,44 Among patients with del(11q), those with a complete loss of ATM function might have impaired response to irradiation or cytotoxic drugs, resulting in poor clinical outcome.45 Recent studies showed that previously untreated patients with del(11q) respond well to combination therapy with fludarabine and cyclophosphamide (FC), suggesting that the addition of an alkylating agent to fludarabine may help to overcome the adverse prognostic significance of del(11q) in patients with CLL.30,46 Del(17p), which reflects the loss of the TP53 gene and is frequently associated with mutations in the remaining TP53 allele, is associated with worst outcomes, with short treatment-free interval, short median survival (32 months), and poor response to chemotherapy.17
The phase III randomized CLL8 study of the German CLL Study Group (GCLLSG; first-line FC vs rituximab combined with FC [FCR]) showed that both del(17p) and unmutated IGHV were significant independent predictors of poor survival outcomes, irrespective of the treatment arm.47 Although FCR was associated with significantly improved PFS among patients with del(17p), the 3-year PFS rate was only 18% in this subgroup. In addition, OS outcomes in patients with del(17p) were similar between the FCR and FC arms (3-year OS, 38% and 37%, respectively).47 The prognostic importance of del(17p) may be dependent on the proportion of malignant cells with this abnormality. In the UK LRF CLL4 trial (comparing first-line therapy with chlorambucil vs fludarabine vs FC), similar outcomes were seen between patient subgroups with 5% to 10% of cells with TP53 deletion (ie, del(17p13.1)) and the subgroup without TP53 deletion (deletion in <5% of cells); patients with 10% to 20% TP53 deletion had outcomes similar to those with more than 20% TP53 deletion.30,48 Patients with 10% or more cells with TP53 deletion had a poor outcome, with a 29% response rate (6% complete or nodular partial response) and a median survival of less than 6 months.30 The finding that del(17p) is more frequently seen in treated than in untreated patients suggests that treatment-driven clonal selection may occur during therapy. Indeed, acquisition and/or expansion of CLL clones with del(17p) were observed during the course of treatment.49
A prognostic nomogram for estimating time to first treatment was developed based on a multivariable model that included both traditional clinical and laboratory parameters and newer prognostic factors (eg, FISH cytogenetics, IGHV mutational status, and ZAP-70 expression levels).50 The following factors were identified as independent predictors of shorter time to first treatment and were included in a weighted model to estimate the probability of treatment (at 2 and 4 years) and time to first treatment: increased size of cervical lymph nodes, 3 involved nodal sites, del(17p) or del(11q), unmutated IGHV status, and elevated serum LDH levels.50 This nomogram may help to identify newly diagnosed patients at high risk for disease progression who may require earlier intervention. More recently, the GCLLSG developed a comprehensive prognostic index to stratify patients into 4 risk groups based on OS. In this model, sex, age, ECOG status, del(17p), del(11q), IGHV mutation status, serum beta-2 microglobulin, and serum thymidine kinase were identified as independent predictors of OS in newly diagnosed patients.51
Abnormalities of TP53 can be observed in the absence of del(17p).52,53 Studies with fludarabine-based regimens identified TP53 mutations as an independent predictor of decreased survival and resistance to chemotherapy.52–55 Resistance to chemotherapy was attributed to the presence of mutation in the remaining TP53 allele.53 Thus, the presence of TP53 mutation predicts for poor survival outcomes independent of 17p chromosome status.52 In an analysis from the CLL8 study, mutation in TP53 was associated with significantly decreased PFS and OS outcomes regardless of treatment with FCR or FC.53
The impact of these prognostic factors on the clinical outcome of patients was examined in large prospective randomized studies. In the long-term follow up from the CALGB 9712 study (first-line therapy with concurrent vs sequential fludarabine and rituximab), unmutated IGHV was a significant independent predictor for shorter PFS and OS, while poor-risk cytogenetic abnormalities (ie, del(17p) or del(11q)) remained an independent predictor for shorter survival.56 In the UK LRF CLL4 trial, TP53 loss was found to be the strongest predictor of poor outcomes.30,54 Among the subgroup of patients without TP53 loss, unmutated IGHV (or VH3-21 usage) and elevated beta-2 microglobulin (>4 mg/L) were significant independent predictors for both PFS and OS outcomes.30 In addition, del(11q) and treatment allocation were independent predictors for PFS, and age was an independent predictor for OS. In the German CLL8 trial (first-line FC vs FCR), mutated TP53, del(17p), unmutated IGHV, and treatment arm were significant independent prognostic factors for both PFS and OS outcomes.53
During the past few years, recurrent mutations in NOTCH1, SF3B1, and BIRC3 genes with prognostic implications in CLL were identified.53,57–65 NOTCH1 mutation was also independently associated with Richter’s transformation.66,67 The impact of these mutations relative to treatment with newer targeted therapies is uncertain. Collectively, the previously discussed studies suggest that the prognostic significance of these mutations may vary depending on the patient population, treatment regimens, and clinical outcomes being evaluated. Although these prognostic factors may provide useful prognostic information, treatment initiation or selection of treatment options should not be driven by these factors. Moreover, in the general clinical practice setting, prognostic factors should not determine treatment choices, with the exception of del(17p) or del(11q).
Workup
The workup for CLL/SLL is similar to that for other lymphoid neoplasms. Quantitative immunoglobulins may be informative in patients with recurrent infections. Measurement of beta-2 microglobulin may provide useful prognostic information.39,41 Though the pattern of bone marrow involvement (diffuse vs nodular) had prognostic significance classically, this is no longer a factor when one uses more reliable prognostic markers such as IGHV mutational status and cytogenetic abnormalities determined by FISH, all of which can be obtained by analysis of circulating lymphocytes. Thus, bone marrow biopsy is no longer considered a required part of the diagnostic evaluation of patients with suspected CLL, though it remains useful to evaluate the etiology of cytopenias.
CT scans may be useful to follow and monitor disease progression in patients with new symptoms when peripheral adenopathy is not present. For asymptomatic patients, serial CT scans are not recommended. For patients with anemia, reticulocyte counts and a direct Coombs’ test should be performed to evaluate for the possibility of hemolysis and pure red aplasia. PET scan is generally not useful in CLL, but it can assist in directing nodal biopsy if Richter’s transformation is suspected.68,69 Bone marrow biopsy with or without aspirate could be useful in ceratin circumstances before starting treatment.
Staging
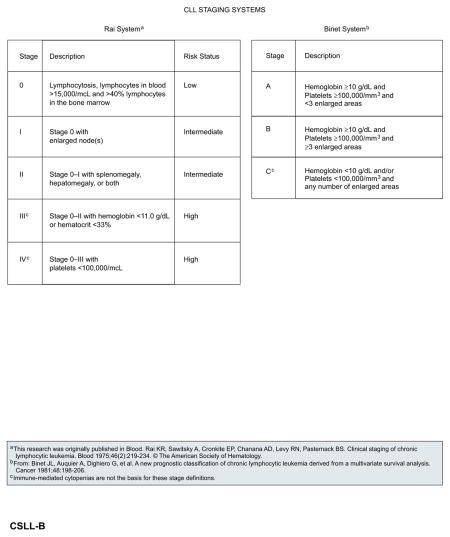
Two staging systems, Rai and Binet, are currently used worldwide in the evaluation of patients with CLL in both routine practice and clinical trial settings.70,71 Both staging systems rely solely on physical examination (presence of lymph node involvement, enlarged spleen and/or liver) and blood parameters (presence of anemia or thrombocytopenia) to assess the degree of tumor burden. The modified Rai classification stratifies patients into 3 risk groups (low, intermediate, high). Survival of patients with low-risk disease (Rai stage 0; median survival, 150 months) is essentially the same as the survival rate of age-matched controls. Patients with intermediaterisk disease (Rai stage, I–II; median survival, 71–101 months) have shorter survival, particularly when other adverse factors coexist, such as a lymphocyte doubling time of less than 1 year. Patients with high-risk features (Rai stage, III–IV; median survival, 19 months) have poor prognosis.70 The Binet staging system is based on the number of involved areas and the level of hemoglobin and platelets. Similar to the Rai staging system, it provides meaningful correlation with clinical outcome.71 The nearly universal involvement of bone marrow and peripheral blood in CLL/SLL limits the utility of the Ann Arbor staging system.
Response Criteria
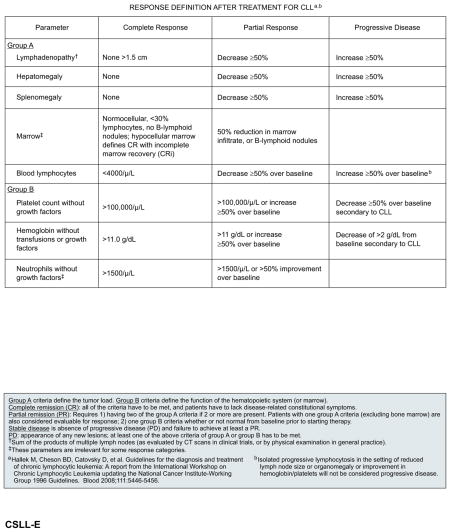
The NCI-sponsored Working Group (NCI-WG) on CLL published guidelines for the diagnosis and management of CLL in 1988 and 1996, primarily to facilitate consistency in the design and conduct of clinical trials. Most clinical trials of CLL reporting response outcomes have, until very recently, used the response criteria set forth in the 1996 NCI-WG guidelines.72 In 2008, the NCI-WG guidelines were revised to reflect recent advances in the understanding of newer prognostic markers, diagnostic parameters, and treatments.3 In particular, the 2008 guidelines provide further recommendations on the evaluations and response assessments appropriate for the general clinical practice setting versus for clinical trials.3
In the clinical practice setting, response assessment involves both physical examination and evaluation of blood parameters. For a complete response (CR), all of the following criteria must be met (at least 2 months after treatment completion): peripheral blood lymphocyte counts less than 4 × 109/L; absence of lymphadenopathy (ie, palpable nodes must be ≤1.5 cm in diameter); absence of splenomegaly or hepatomegaly; absence of constitutional symptoms (ie, weight loss, significant fatigue, fevers, night sweats); and normalization of blood counts without growth factor support (ie, neutrophils >1.5 × 109/L, platelets >100 × 109/L, hemoglobin >11 g/dL).3 For a partial response (PR), at least 2 of the following criteria must be met for at least 2 months: at least 50% reductions from baseline in peripheral blood lymphocyte counts, lymphadenopathy (based on sum of the products of multiple affected nodes), hepatomegaly, and/or splenomegaly; in addition, at least 1 of the blood counts should be normalized or increased by 50% or more from baseline for at least 2 months duration.
Progressive disease comprises any of the following: at least 50% increase from baseline in lymphocyte counts, lymphadenopathy, hepatomegaly, or splenomegaly, and appearance of any new lesions or occurrence of cytopenias attributable to disease (ie, ≥50% decrease from baseline in platelet count, >2 g/dL decrease from baseline in hemoglobin levels).3 Patients who do not have progressive disease but do not meet the criteria for a CR or PR are considered to have stable disease. Relapse is defined as evidence of disease progression 6 months or more after an initial CR or PR. Refractory disease is defined as failure to experience a response or experiencing disease progression within 6 months of the last treatment.3
CT scans are desirable in clinical trials for evaluation of adenopathy and organ involvement and to select patients outside of trials. In addition, a bone marrow evaluation should be conducted to confirm a CR (<30% lymphocytes, normocellular morphology, absence of lymphoid nodules) if all other criteria for clinical CR (as defined previously) are met. Patients who fulfill the criteria for CR (including evaluation of the bone marrow) but present with persistent cytopenias due to treatment-related toxicities should be considered to have experienced a CR with incomplete marrow recovery (CRi).3
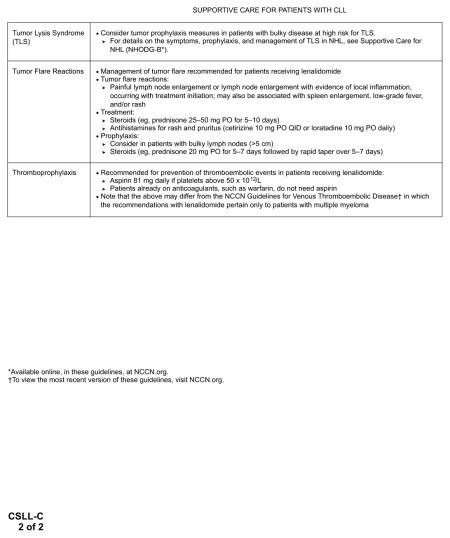
These response criteria were recently revised to more precisely predict outcomes for patients with CLL treated with immunomodulating agents and small molecule kinase inhibitors.73 Treatment with immunomodulating agents such as lenalidomide results in a tumor flare reaction characterized by painful enlargement of lymph nodes and lymphocytosis, rash, and bone pain. Tumor flare reaction was correlated with clinical response in patients with CLL treated with lenalidomide.74 Similarly, the use of small molecule kinase inhibitors (ibrutinib and idelalisib) was known to result in an initial transient increase in lymphocytosis resulting from the redistribution or release of leukemic cells from the lymph node compartment to the peripheral blood.75,76 In most patients treated with ibrutinib, lymphocytosis resolves within 8 months, but in a subgroup of patients lymphocytosis lasts for more than 12 months. Prolonged lymphocytosis after ibrutinib treatment was reported to represent the persistence of a quiescent clone and does not predict a subgroup of patients likely to relapse early.77
Considering these findings, for patients receiving idelalisib and ibrutinib, the revised response criteria recently proposed by Cheson et al73 allow for a new response category, “PR with lymphocytosis,” to include patients with a clinical response (reduction in lymph nodes and splenomegaly) with persistent lymphocytosis (in the absence of other indicators of progressive disease).73
Minimal residual disease (MRD) negativity determined in the peripheral blood after the end of treatment is emerging as an important predictor of treatment efficacy. In the combined analysis of 2 phase III GCLLSG studies, among patients who achieved CR, there was a statistically significant difference in PFS between MRD-negative and MRD-positive patients (69.2 vs 40.4 months; P=.001).78 The persistence of posttreatment splenomegaly as a sole abnormality in MRD-negative patients had no negative influence on PFS. These results support the use of MRD for response evaluation.
Treatment Options
During the past several decades, therapeutic options for CLL have evolved from the use of alkylating agent monotherapy to purine analog-based combination regimens. The advent of monoclonal antibodies that target cell surface antigens (eg, CD20, CD52) and immunomodulating agents (eg, lenalidomide) led to the development of new and effective combination chemoimmunotherapy regimens. More recently, a number of critical signaling pathways, including the B-cell receptor (BCR), CXCR4/5, CD40, integrin, and IL-6, that signal via phosphatidylinositol 3-kinase (PI3K), Bruton’s tyrosine kinase (BTK), and/or spleen tyrosine kinase are implicated in the pathogenesis of CLL.79,80 Novel small molecule inhibitors targeting these kinases have been evaluated in clinical trials for the treatment of patients with CLL.
This section describes the role of recently approved targeted therapies for the management of patients with CLL/SLL.
First-Line Therapy
Obinutuzumab is a glycoengineered, humanized type II antibody targeted against CD20. The safety and efficacy of obinutuzumab in combination with chlorambucil for previously untreated CLL in patients with coexisting conditions was evaluated in the phase III randomized trial (CLL11 trial).81 In this trial, 781 patients with comorbidities (defined as Cumulative Illness Rating Score [CIRS] >6 or an estimated creatinine clearance [CrCl] of 30 to 69 mL/min) were randomized to receive chlorambucil (n=118), obinutuzumab plus chlorambucil (n=333) or rituximab plus chlorambucil (n=330). The combinations of obinutuzumab-chlorambucil and of rituximab-chlorambucil resulted in significant improvement in the median PFS compared with chlorambucil alone (26.7, 16.3, and 11.1 months, respectively; P<.001). The survival benefit was seen in all of the subgroups except in patients with del(17p). The combination of obinutuzumab plus chlorambucil also resulted in higher overall response rate (ORR, 78.4% vs 65.1%) and CR rate (20.7% vs 7.0%) and significantly prolonged median PFS (26.7 vs 15.2 months; P<.001) compared with rituximab plus chlorambucil.81 The most frequent grade 3 or higher toxicities with obinutuzumab-chlorambucil included neutropenia (35%), infusion-related reactions (21%), thrombocytopenia (11%), and infections (11%).81 The most frequent grade 3 or higher toxicities with rituximab plus chlorambucil included neutropenia (28%) and infections (14%). The results of the CLL11 study established obinutuzumab plus chlorambucil as the new standard of care for both elderly patients and for patients with comorbid conditions lacking del(17p).81 Based on the results of this study, obinutuzumab in combination with chlorambucil was FDA approved for the treatment of untreated CLL/SLL.
The efficacy of obinutuzumab monotherapy in patients with untreated CLL was demonstrated in the phase II GAGE study.82 In this trial, 80 patients with intact organ function and ECOG performance status less than 3 were stratified to 2 different obinutuzumab doses (1000 vs 2000 mg). The median age was 67 years. Obinutuzumab at 2000 mg resulted in higher ORR (assessed at 2 months after treatment according to the International Workshop on CLL criteria) than obinutuzumab at 1000 mg (67% and 49%, respectively; P=.08).82 Infusion-related reaction was the most frequent grade 3 or 4 adverse event in both treatment arms. Additional studies are warranted to determine the durability of response and long-term adverse effects of obinutuzumab monotherapy in patients with untreated CLL.
Ofatumumab, a fully human CD20 monoclonal antibody, initially approved for the treatment of CLL refractory to fludarabine and alemtuzumab, was also evaluated as a first-line treatment for patients with untreated CLL who were considered inappropriate for fludarabine-based therapy due to advanced age and/or comorbidities.83 In a multicenter open-label phase III study, 447 patients were randomized to receive ofatumumab plus chlorambucil or chlorambucil monotherapy. With a median follow-up of 29 months, the PFS was significantly longer for patients treated with ofatumumab plus chlorambucil compared with chlorambucil monotherapy (22.4 vs 13.1 months; P<.001).83 Ofatumumab plus chlorambucil also resulted in higher ORR (82% vs 69%; P=.001) and superior CR rate (12% vs 1%) compared with chlorambucil alone. The median OS was reached in both arms. Based on the results of this study, the FDA approved ofatumumab plus chlorambucil for the treatment of previously untreated patients with CLL for whom fludarabine-based therapy is considered inappropriate.
Ibrutinib is a covalent-binding, irreversible inhibitor of BTK, initially approved for relapsed or refractory CLL. It was also evaluated in patients with untreated CLL, including those with del(17p).84–86 In an open-label multicenter phase Ib/II study of patients over the age of 65 (n=31; median age, 71 years [range, 65–84]; 74% of patients were ≥70 years of age, ibrutinib (420 mg) resulted in an ORR of 71% (13% CR, 3% nodular PR, and 55% PR).84 The median follow-up was 22 months. The responses were independent of the presence of high-risk features; however, the frequency of patients with del(17p), del(11q), or elevated beta-2 microglobulin was relatively low in this study.84 The ORR of 84% as reported in an independent evaluation of efficacy data at 3 years after the start of therapy confirmed the durability of responses with ibrutinib in untreated CLL.85
In another open-label study of 29 patients (15 treatment-naïve patients) with del (17p), ibrutinib resulted in a nodal response rate of 82% at 6 months in treatment-naïve patients.86 The median follow-up was 9 months. Grade 3 or higher non-hematologic toxicities were reported in 14% of patients. Ibrutinib was recently approved for first-line treatment of patients with del(17p). Unlike chemotherapy, ibrutinib causes early mobilization of lymphocytes into the blood, which in the setting of clinical improvement should not be mistaken for progressive disease.72,77 Although lymphocytosis can sometimes be profound, clinical consequence (ie, leukostasis) is extremely rare, and in general therapy should be continued. Slow or incomplete resolution of lymphocytosis does not appear to impact outcome as measured by PFS.77 (See “Special Considerations for the Use of B-Cell Receptor Inhibitors (Ibrutinib and Idelalisib),” available online, in these guidelines, at NCCN.org [NHODG-E]).
Idelalisib (the isoform-selective oral inhibitor of PI3K-delta) in combination with rituximab is approved for the treatment of relapsed or refractory CLL. The efficacy of idelalisib monotherapy or in combination with rituximab was also shown in older patients (≥65 years) with previously untreated CLL and adverse risk factors.87,88 In the preliminary analysis of a phase II study (n=37; median age, 70 years), idelalisib had substantial monotherapy activity resulting in an ORR of 81% (33% PR and 48% PR with lymphocytosis).87 The most frequent grade 3 or higher treatment-related adverse events were rash (3%), diarrhea (3%), pneumonia (5%), transaminase elevation (8%), anemia (5%), and neutropenia (20%). In another phase II study (n = 64; median age, 71 years), the combination of idelalisib and rituximab induced an ORR in 97% of patients (78% PR and 19% CR).88 Diarrhea/colitis (42%), pneumonia (19%), rash (13%), dehydration (8%), dyspnea (5%), and respiratory failure (5%) were the most frequent grade 3 or higher treatment-related adverse events. Similar early lymphocytosis as described with ibrutinib can occur with idelalisib and should be managed similarly.76,89 (See “Special Considerations for the Use of B-Cell Receptor Inhibitors (Ibrutinib and Idelalisib),” available online, in these guidelines, at NCCN.org [NHODG-E])
Relapsed or Refractory Disease
The current standards of care for relapsed or refractory CLL are ibrutinib monotherapy and idelalisib plus rituximab.
Ibrutinib showed remarkable monotherapy activity with favorable toxicity profile in patients with relapsed/refractory B-cell malignancies.90 The safety and efficacy of ibrutinib in relapsed or refractory CLL/SLL was first evaluated in a phase Ib/II study (n=85; 51 patients received 420 mg and 34 patients received 840 mg).75 Most patients were considered to have high-risk features (advanced-stage disease, del(17p), and del(11q) were present in 65%, 33%, and 36% of patients, respectively). The ORR was the same (71%) in the 2 dose groups. Among the subgroup of 28 patients with del(17p), the ORR was 68% (CR in 3.5%). PR with lymphocytosis was seen in 20% and 15% of patients in the 2 dose groups (420 and 840 mg, respectively). The estimated PFS and OS at 26 months were 75% and 83%, respectively. The most common grade 3 or 4 adverse events included neutropenia (15%), pneumonia (12%), sinusitis (5%), and hypertension (5%).
In the subsequent phase III randomized study (RESONATE), 391 patients with previously treated CLL were randomized to monotherapy with ibrutinib (420 mg once daily) or ofatumumab.91 Most patients had advanced-stage disease and high-risk features, including del(17p), del(11q), or elevated beta-2 microglobulin level (>3.5 mg/L). At a median follow-up of 9.4 months, ibrutinib significantly prolonged PFS (median not reached vs 8.1 months for ofatumumab) and OS (hazard ratio for death in the ibrutinib group was 0.43; P=.005; 57% reduction in the risk of death). Among patients with del(17p), median PFS was not reached with ibrutinib, compared with a median PFS of 5.8 months with ofatumumab. At 12 months, the OS rate was 91% and 81%, respectively, for ibrutinib and ofatumumab.91 The ORR was also significantly higher with ibrutinib (42% vs 4%; P<.001). The most frequent nonhematologic adverse events were mild (grade 1–2) diarrhea, fatigue, pyrexia, and nausea in the ibrutinib group and fatigue, infusion-related reactions, and cough in the ofatumumab group. The updated results of this study also confirmed that ibrutinib significantly improved PFS, OS, and ORR compared with ofatumumab in patients with CLL/SLL who had received at least one prior therapy.92 At a median follow-up of 16 months, ibrutinib showed significantly better ORR (90% vs 25%; P<.0001), median PFS (not reached vs 8.1 months for ofatumumab; P<.0001), and OS rates (18-month OS, 85% vs 78%, respectively). The results of the phase II study, RESONATE-17, further confirmed the efficacy of ibrutinib in patients with relapsed or refractory CLL with del(17p).93 At a median follow-up of 13 months, the ORR and PFS rate were 82.6% and 79.3%, respectively.
Ibrutinib was approved by the FDA for the treatment of patients with CLL who received at least one previous therapy and for first-line therapy in patients with del(17p) CLL.
Idelalisib, the isoform-selective oral inhibitor of PI3K-delta, demonstrated promising clinical activity in phase I–II studies in patients with relapsed/refractory CLL, both as monotherapy and in combination with rituximab.76,89 In a multicenter phase III randomized study, 220 patients with relapsed CLL were randomized to receive rituximab with either idelalisib (150 mg) or placebo.76 Most of the patients (78%) were aged 65 years or older, 40% had moderate renal dysfunction (CrCl, <60 mL/min), 35% had poor bone marrow function (grade 3 or higher cytopenias), and 85% had a CIRS score greater than 6. At the first planned interim analysis, the study was stopped early due to the overwhelming efficacy of idelalisib plus rituximab.76 At 24 weeks, the PFS rates were 93% and 46% in the idelalisib group and placebo group, respectively. Among patients with relapsed CLL with coexisting conditions, idelalisib plus rituximab significantly improved ORR (81% vs 13%; P<.001), PFS (not reached in the idelalisib group vs 5.5 months in the placebo group), and OS at 12 months (92% vs 80%; P=.02), compared with rituximab plus placebo. Grade 3 or 4 adverse events (pneumonia, pyrexia, and febrile neutropenia) were reported in 40% of patients in the idelalisib group and 35% in the placebo group.
The second interim analysis of this study confirmed the superior safety and efficacy of idelalisib plus rituximab in terms of ORR, PFS, and OS.94 Idelalisib plus rituximab also retained efficacy in patients with high-risk features such as del(17p) or TP53 mutations, unmutated IGHV, ZAP-70 and CD38 expression, and beta-2 microglobulin (>4 mg/L).95 Idelalisib in combination with rituximab was recently approved for the treatment of relapsed CLL in patients for whom rituximab monotherapy would be considered inappropriate due to the presence of other comorbidities (reduced renal function as measured by CrCl <60 mL/min, or NCI CTCAE grade ≥3 neutropenia or thrombocytopenia resulting from myelotoxic effects of prior therapy with cytotoxic agents).
Ofatumumab is a fully human CD20 monoclonal antibody with activity in patients with CLL refractory to fludarabine and alemtuzumab or in patients for whom alemtuzumab is contraindicated due to bulky lymphadenopathy.96 In the final analysis from the pivotal international clinical trial, which included data from 206 patients with either fludarabine-and alemtuzumab-refractory (FA-ref; n=95) CLL or fludarabine-refractory CLL with bulky lymphadenopathy (BF-ref; n=111), ofatumumab therapy resulted in an ORR of 51% in the FA-ref and 44% in the BF-ref patients.97 The median PFS was 5.5 months for both groups, and the median OS was 14 and 17 months for the FA-ref and the BF-ref groups, respectively. The most common grade 3 or higher adverse events were infections (24%) and neutropenia (12%). An ad hoc retrospective analysis of patients with FA-ref CLL (n=96) and BF-ref CLL (n=111) showed that ofatumumab was also effective and well tolerated in patients with FA-ref CLL and previous rituximab exposure.98 The ORRs were 43%, 44%, and 53%, respectively, for CLL with previous rituximab exposure, rituximab-refractory CLL, and rituximab-naive CLL. The median PFS times were 5.3, 5.5, and 5.6 months, respectively, and median OS times were 15.5, 15.5, and 20.2 months, respectively. Ofatumumab is approved for the treatment of patients with CLL refractory to fludarabine and alemtuzumab.
Obinutuzumab has monotherapy activity in patients with heavily pretreated relapsed/refractory CLL. In a phase II study of 20 patients (GAUGIN study), obinutuzumab at a fixed dose of 1000 mg resulted in a best ORR of 30%; median PFS and duration of response were 10.7 and 8.9 months, respectively.99 A subset analysis of the CLL11 study showed that the combination of obinutuzumab plus chlorambucil was also active in patients with CLL refractory to prior treatment with chlorambucil.100 Among the 30 patients who crossed over to obinutuzumab plus chlorambucil, clinical response was seen in 87% (77% PR, 7% CR, and 3% incomplete CR). The median PFS from start of crossover treatment was 17.2 months.
Allogeneic hematopoietic stem cell transplant (HSCT) was evaluated to improve the prognosis in patients with advanced disease and those with poor-risk features.101–107 Available evidence from nonrandomized clinical studies suggest that allogeneic HSCT may be an effective treatment option for patients with disease refractory to chemoimmunotherapy or who develop recurrence within 12 months after treatment with purine analogs.108
Assessment of Functional Status and Comorbidity
CLL is diagnosed mainly in older adults, with a median age of 72 years at diagnosis. Approximately 70% of patients are diagnosed at age 65 years or older, and 40% of patients are diagnosed at age 75 years or older.109 Comorbidities are frequently present in older patients. In addition, organ function and bone marrow reserve also decline with advancing age.
Although chemoimmunotherapy is now considered the standard of care for younger or fit older patients, it is often not well tolerated in older patients due to decline in organ function, reduced bone marrow reserve, and/or the presence of comorbidities.110 In the first phase III randomized study (CLL5) that evaluated first-line chlorambucil versus fludarabine in a cohort of older patients (≥65 years) with untreated CLL, 65% of patients presented with at least one comorbidity, and approximately a third of patients had 2 or more comorbidities at the time of enrollment.111 In this trial, the presence of multiple comorbidities was a negative prognostic factor independent of disease stage or age. In multivariate analysis, elevated serum beta-2 microglobulin and the presence of 2 or more comorbidities were significant independent predictors of shorter PFS and OS.111 Several retrospective studies also reported on the negative impact of comorbidities on patient outcomes in CLL.112–114 The most common comorbidities reported include hypertension (19%–53%), coronary artery disease (7%–24%), hyperlipidemia or lipometabolic disease (16%–38%), and diabetes mellitus (10%–21%).112–114
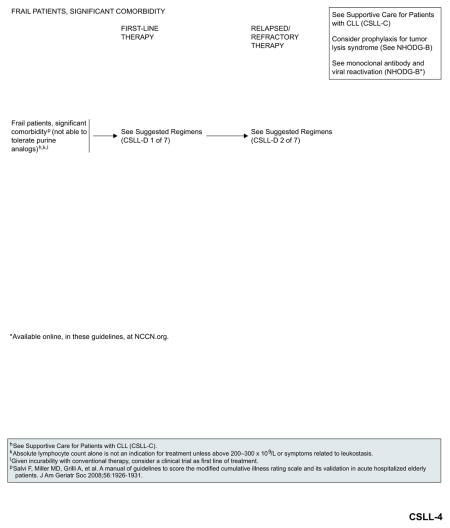
These findings underscore the need to assess comorbidities, in addition to patient age and performance status, before treatment selection. The tolerability of a treatment regimen relative to a patient’s physical fitness is an important consideration in the management of CLL. The CIRS, Charlson comorbidity index, and NCI comorbidity index are some of the scoring systems that can be used to assess comorbidities in patients with CLL. CIRS in combination with CrCl was used by the GCLLSG to assess the overall fitness of patients enrolled in clinical trials.47,115
NCCN Recommendations
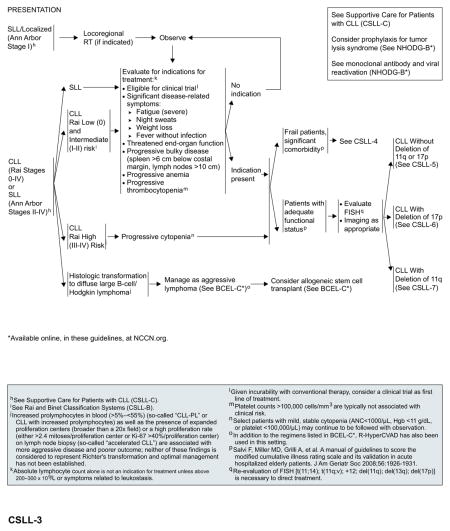
Localized SLL (Ann Arbor Stage I)
Locoregional radiation therapy (RT) is an appropriate induction therapy for patients with symptomatic localized disease. In rare patients, RT may be contraindicated or may be a suboptimal therapy due to the presence of comorbidities or the potential for long-term toxicity. Patients with localized SLL that has progressed after initial RT should be treated as described subsequently for patients with SLL (Ann Arbor stage II–IV).
SLL (Ann Arbor Stages II–IV) or CLL (Rai Stages 0–IV)
Early-stage disease in some patients may have an indolent course and in others may progress rapidly to advanced disease requiring immediate treatment. A “watch and wait” approach is often appropriate for patients with early-stage, low-risk disease (Rai stage 0; Binet A) in the absence of disease symptoms.
Patients with Binet B or intermediate-risk disease (Rai stage I or II) may benefit from therapy if they show evidence of progressive disease or become symptomatic.3 Patients with advanced-stage or high-risk CLL (Binet C; Rai stage III–IV) with progressive cytopenia require immediate treatment. Selected patients with mild, stable cytopenia may continue to be observed.
Absolute lymphocyte count alone is not an indication for treatment unless it is greater than 200 to 300 × 109/L or symptoms related to leukostasis occur. Standard indications for initiating treatment include the following3: significant disease related constitutional symptoms including severe fatigue, weight loss, night sweats and fever without infection; threatened end-organ function; progressive bulky disease (enlarged spleen or lymph nodes); progressive anemia or thrombocytopenia; or autoimmune anemia/thrombocytopenia unresponsive to corticosteroids. Asymptomatic patients should be observed until such indications (as mentioned previously) become apparent.
Given the incurability of the disease, the NCCN Guidelines recommend enrollment in clinical trials, when locally available, as the preferred option for all patients with indications for treatment. In the absence of suitable clinical trials, the treatment recommendations included in the NCCN Guidelines are based on patient’s age or functional status (comorbidity index/performance status) and the presence or absence of del(17p) and/or del(11q).
Management of Frail Patients With Significant Comorbidity
Obinutuzumab plus chlorambucil (category 1),81 ofatumumab plus chlorambucil,83 and rituximab plus chlorambucil116,117 are the preferred treatment options for frail patients with significant comorbidities that preclude treatment with purine analogs. Obinutuzumab monotherapy is included as an alternative option with a category 2B recommendation.82 See “Suggested Treatment Regimens: Frail Patient, Significant Comorbidity (not able to tolerate purine analogs)” (CSLL-D, 1 of 7; page 339) for a list of other suggested regimens.
Management of Patients With Adequate Functional Status
Patients with adequate functional status can be treated with more active or intensive therapies and should be evaluated for cytogenetic abnormalities by FISH. Patient age and the presence or absence of del(17p) and/or del(11q) should then help to direct treatment options, as shown in later sections.
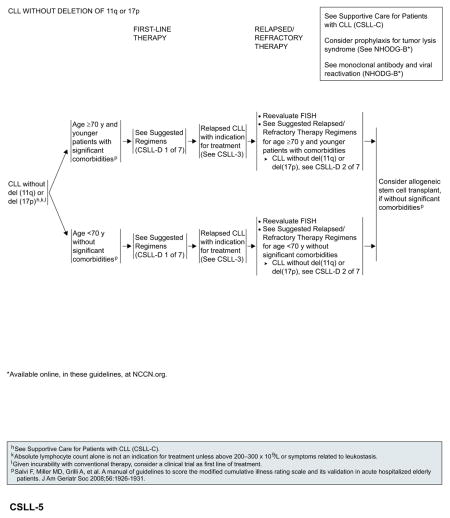
CLL Without del(17p) or del(11q)
For patients aged 70 years or older and younger patients with significant comorbidities, obinutuzumab plus chlorambucil (category 1)81 is the preferred first-line regimen, followed by ofatumumab plus chlorambucil.83 Obinutuzumab monotherapy is included as an alternative option with a category 2B recommendation.82 In patients younger than 70 years without significant comorbidities, fludarabine-based chemoimmunotherapy has emerged as the standard of care.47,56,118 Bendamustine plus rituximab is a reasonable alternative for patients aged 70 years or older who are otherwise eligible for chemoimmunotherapy.118,119
Based on the recent FDA approvals, ibrutinib (category 1)91 and idelalisib with or without rituximab76,120 are included as preferred options for patients with relapsed or refractory disease, regardless of their age and comorbidities. Monotherapy with ofatumumab or obinutuzumab are included as alternative options. Allogeneic HSCT can be considered for select patients (without significant comorbidities) after reinduction of remission.
See “Suggested Treatment Regimens: CLL Without del(11q) or del(17p)” (CSLL-D, 1 and 2 of 7; pages 339 and 340) for a list of other suggested regimens
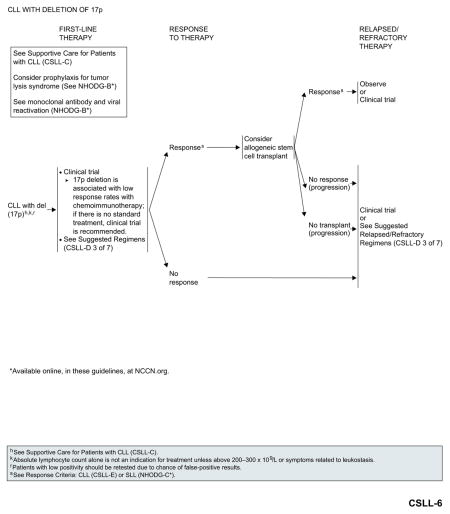
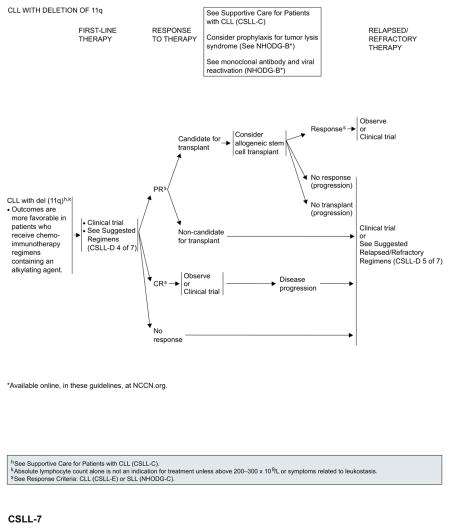
CLL With del(17p)
Outcomes remain poor with currently available chemoimmunotherapy regimens. Enrollment in an appropriate clinical trial is recommended for patients with del(17p). In the absence of appropriate clinical trials in the patient’s local area, based on the recent FDA approval, ibrutinib is included as an option for first-line therapy.
Patients with response to first-line therapy should be considered for allogeneic HSCT, if they are eligible. However, the role of allogeneic HSCT in the up-front setting is evolving with the introduction of new therapies such as ibrutinib. Patients with a response after allogeneic HSCT can either be observed or enrolled in clinical trials. Patients with no response to first-line therapy, patients who respond to first-line therapy but are not eligible for allogenic HSCT, and patients with no response to allogenic HSCT should be enrolled in clinical trials or be treated with second-line therapy for relapsed or refractory disease.
Ibrutinib and idelalisib with or without rituximab are the preferred options for relapsed or refractory disease.84–86,91 The efficacy of ibrutinib in patients with relapsed or refractory CLL with del(17p) exceeds the results of alternative regimens in the upfront setting and should be considered as the best choice in the absence of a contraindication to give this treatment.
See “Suggested Treatment Regimens: CLL with del(17p)” (CSLL-D, 3 of 7; page 341) for a list of other suggested regimens.
CLL With del(11q)
Outcomes are more favorable for patients treated with alkylating agent–based chemoimmunotherapy regimens. For patients younger than 70 years without significant comorbidities, first-line treatment options include FCR, bendamustine with or without rituximab, or PCR. For patients aged 70 years or older and younger patients with comorbidities, preferred first-line treatment options include obinutuzumab plus chlorambucil (category 1),81 followed by ofatumumab plus chlorambucil.83
Patients who experienced CR to first-line therapy can either be observed until disease progression or enrolled in clinical trials. Patients with PR to first-line therapy should be considered for allogeneic HSCT, if they are eligible. However, the role of allogeneic HSCT is evolving with the introduction of new targeted therapies. After transplantation, treatment options are similar to those described for patients with del(17p).
Patients with no response to first-line therapy and patients with PR to first-line therapy but who are not eligible for allogenic HSCT should be enrolled in clinical trials or can be treated with secondline therapy for relapsed or refractory disease. Ibrutinib and idelalisib with or without rituximab are the preferred options for relapsed or refractory disease.
See “Suggested Treatment Regimens: CLL with del(11q)” (CSLL-D, 4 and 5 of 7; pages 342 and 343) for a list of other suggested regimens based on the patient’s age and the presence or absence of significant comorbidities.
Histologic Transformation
Approximately 2% to 10% of patients with CLL will develop Richter’s transformation (histologic transformation to diffuse large B-cell lymphoma or Hodgkin lymphoma) during the course of their disease and treatment.121–123 The incidence of histologic transformation increases with the number of prior regimens. Recent reports identified inactivation of NOTCH1 and disruption of TP53 and CDKN2A/B as possible genetic pathways involved in the pathogenesis of Richter’s transformation.124,125
Patients with Richter’s transformation should be treated with chemoimmunotherapy regimens initially developed for diffuse large B-cell lymphoma.126,127 OFAR and hyper-CVAD with rituximab were also used for the treatment of patients with Richter’s transformation.128–130 Allogeneic HSCT can also be considered after a response to initial therapy in patients with Richter’s transformation. In a nonrandomized comparative analysis, the estimated cumulative 3-year survival rate was significantly higher (75%) for patients who underwent allogeneic HSCT after achieving CR or PR to initial therapy compared with those who responded to initial therapy but did not undergo allogeneic HSCT, or who underwent allogeneic HSCT for relapsed or refractory Richter’s transformation (75% vs 27% and 21%, respectively; P=.019).126 High-dose therapy/autologous stem cell rescue may also be an appropriate therapy for patients with Richter’s transformation who have a response to initial therapy but are not a candidate for allogeneic HSCT due to age, comorbidities, or lack of a suitable donor.131 Patients with Hodgkin histology should receive a standard regimen used for the treatment of Hodgkin lymphoma.
Supportive Care for Patients With CLL
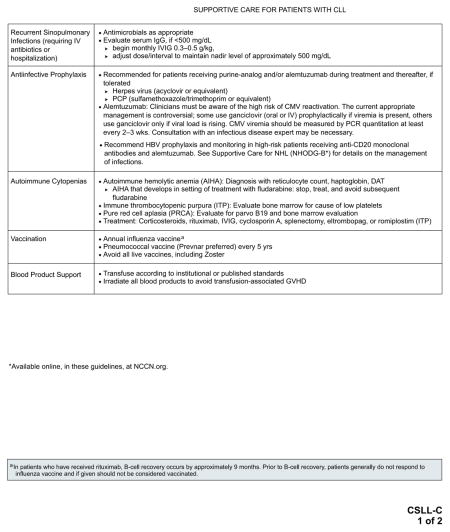
Management of disease-specific complications is an integral part of the management of patients with CLL/SLL. See “Supportive Care for Patients With CLL” (CSLL-C; pages 337 and 338) for specific recommendations.
Summary
CLL is diagnosed mainly in older adults, who often present with multiple comorbidities. Cytogenetic abnormalities identified in most patients also have an impact on the clinical outcome. In addition to the disease stage and patient age, treatment options for patients with symptomatic disease should be based the presence or absence of comorbid conditions and/or poor prognostic features. Obinutuzumab plus chlorambucil is the new standard of care for both elderly patients and patients with comorbid conditions, lacking del(17p). Ibrutinib or idelalisib with rituximab are effective treatment options for patients with relapsed or refractory CLL. In addition, ibrutinib monotherapy is also an option for first-line therapy in patients with del(17p). Supportive care for the management of disease-specific complications should also be an integral part in the management of patients with CLL.
Table 1.
Individual Disclosures of the NCCN Non-Hodgkin’s Lymphomas Panel
| Panel Member | Clinical Research Support/Data Safety Monitoring Board | Scientific Advisory Boards, Consultant, or Expert Witness | Promotional Advisory Boards, Consultant, or Speakers Bureau | Date Completed |
|---|---|---|---|---|
| Jeremy S. Abramson, MD | Celgene Corporation; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals, Inc.; Constellation, Inc.; Gilead Sciences, Inc.; Seattle Genetics, Inc.; and Pharmacyclics, Inc. | Amgen Inc.; Jannsen Pharmaceutica Products, LP; Millennium Pharmaceuticals, Inc.; Gilead Sciences, Inc.; and Infinity Pharmaceuticals | Jannsen Pharmaceutica Products, LP; and Millennium Pharmaceuticals, Inc. | 10/9/14 |
| Ranjana H. Advani, MD | Celgene Corporation; Genentech, Inc.; Jannsen Pharmaceutica Products, LP; Millennium Pharmaceuticals, Inc.; Agensys, Inc.; Allos Therapeutics; Idera Pharmaceuticals; Regeneron Pharmaceuticals, Inc.; Seattle Genetics, Inc.; and Pharmacyclics, Inc. | Genentech, Inc.; Clarient Diagnostic Services, Inc.; and Seattle Genetics, Inc. | None | 1/9/15 |
| C. Babis Andreadis, MD, MSCEa | GlaxoSmithKline; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals, Inc.; and AVEO Pharmaceuticals, Inc. | Bristol-Myers Squibb Company; Millennium Pharmaceuticals, Inc.; and Seattle Genetics, Inc. | None | 1/7/15 |
| Nancy Bartlett, MD | AstraZeneca Pharmaceuticals LP; Celgene Corporation; Genentech, Inc.; Jannsen Pharmaceutica Products, LP; MedImmune Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; Seattle Genetics, Inc.; Pfizer Inc.; and Pharmacyclics, Inc. | None | Gilead Sciences, Inc.; and Seattle Genetics, Inc. | 1/26/15 |
| John C. Byrd, MD | None | None | Genentech, Inc.; and Pharmacyclics, Inc. | 9/8/14 |
| Myron S. Czuczman, MD | Celgene Corporation; Onyx Pharmaceuticals, Inc.; and Gilead Sciences, Inc. | Boehringer Ingelheim GmbH; Celgene Corporation; Millennium Pharmaceuticals, Inc.; Algeta; Gilead Sciences, Inc.; MorphoSys AG; Mundipharma International Ltd; TG Therapeutics, Inc.; and Teva Pharmaceutical Industries Ltd. | None | 5/22/14 |
| Luis E. Fayad, MD | Centocor, Inc.; Eisai Inc.; Eli Lilly and Company; Genentech, Inc.; GlaxoSmithKline; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Roche Laboratories, Inc.; sanofi-aventis U.S.; and Wyeth Pharmaceuticals | None | None | 10/9/14 |
| Richard I. Fisher, MD | None | Celgene Corporation; Johnson & Johnson; and MorphoSys AG | None | 12/18/14 |
| Martha J. Glenn, MD | Amgen Inc.; Institutional DSMC; and sanofi-aventis U.S. | None | None | 12/15/14 |
| Leo I. Gordon, MD | Jannsen Pharmaceutica Products, LP | None | None | 12/16/14 |
| Thomas M. Habermann, MD | None | None | None | 10/31/14 |
| Nancy Lee Harris, MD | Pharmacyclics, Inc. | None | None | 1/26/15 |
| Richard T. Hoppe, MD | None | None | None | 12/15/14 |
| Steven M. Horwitz, MD | Celgene Corporation; Millennium Pharmaceuticals, Inc.; Infinity Pharmaceuticals; Kyowa Hakko Kirin Co., Ltd.; Seattle Genetics, Inc.; and Spectrum Pharmaceuticals, Inc. | Amgen Inc.; Celgene Corporation; Millennium Pharmaceuticals, Inc.; Actelion Pharmaceuticals Ltd.; Kyowa Hakko Kirin Co., Ltd.; Seattle Genetics, Inc.; and Spectrum Pharmaceuticals, Inc. | Celgene Corporation | 1/6/15 |
| Christopher R. Kelsey, MD | Varian Medical Systems, Inc. | None | None | 1/30/15 |
| Youn H. Kim, MD | Eisai Inc.; Millennium Pharmaceuticals, Inc.; Kyowa Hakko Kirin Co., Ltd.; Seattle Genetics, Inc.; and Shape Pharmaceuticals, Inc. | Celgene Corporation; Eisai Inc.; Millennium Pharmaceuticals, Inc.; Actelion Pharmaceuticals Ltd.; Galderma S.A.; and OncoSec Medical Inc. | None | 12/15/14 |
| Susan Krivacic, MPAff | None | None | None | 12/29/14 |
| Ann S. LaCasce, MD | None | GlaxoSmithKline | None | 12/19/14 |
| Auayporn Nademanee, MD | Jannsen Pharmaceutica Products, LP; and Seattle Genetics, Inc. | Gilead Sciences, Inc. | None | 1/7/15 |
| Pierluigi Porcu, MD | Infinity Pharmaceuticals; OncoMed Pharmaceuticals, Inc.; and Seattle Genetics, Inc. | Celgene Corporation; Actelion Pharmaceuticals Ltd.; and Kyowa Hakko Kirin Co., Ltd. | Celgene Corporation; and Actelion Pharmaceuticals Ltd. | 9/19/14 |
| Oliver Press, MD, PhDb | Genentech, Inc.; and Roche Laboratories, Inc. | Adaptive Biotechnologies Corp. | None | 1/22/15 |
| Rachel Rabinovitch, MD | RTOG Data Safety and Monitoring Board | Accuray Incorporated | None | 1/26/15 |
| Nishitha Reddy, MD | Celgene Corporation | Jannsen Pharmaceutica Products, LP | None | 10/9/14 |
| Erin Reid, MD | Bristol-Myers Squibb Company; Jannsen Pharmaceutica Products, LP; Millennium Pharmaceuticals, Inc.; Abbvie Pharmaceuticals; AIDS Malignancy Consortium; CALGB/CTSU; Isis Pharmaceuticals, Inc.; Pharmacyclics, Inc.; and Takeda Pharmaceuticals North America, Inc. | None | None | 10/24/14 |
| Ayman A. Saad, MD | None | ICARE; and IMS Consulting Group | None | 12/18/14 |
| Lubomir Sokol, MD, PhD | None | Alexion Pharmaceuticals, Inc.; Celgene Corporation; and Spectrum Pharmaceuticals, Inc | Jannsen Pharmaceutica Products, LP; and Onyx Pharmaceuticals, Inc. | 12/15/14 |
| Lode J. Swinnen, MB, ChB | Abbott Laboratories | None | None | 10/10/14 |
| Christina Tsien, MD | None | None | None | 5/27/14 |
| Julie M. Vose, MD, MBA | Bristol-Myers Squibb Company; Celgene Corporation; Genentech, Inc.; GlaxoSmithKline; Jannsen Pharmaceutica Products, LP; Onyx Pharmaceuticals, Inc.; Incyte Corporation; US Biotest, Inc.; Pharmacyclics, Inc.; and sanofi-aventis U.S. | None | None | 12/15/14 |
| William G. Wierda, MD, PhD | Abbott Laboratories; and GlaxoSmithKline | Celgene Corporation; Genentech, Inc.; and Roche Laboratories, Inc. | None | 10/8/14 |
| Lynn Wilson, MD, MPHa,b | None | None | None | 9/18/14 |
| Joachim Yahalom, MD | None | None | None | 8/18/14 |
| Nadeem Zafar, MD | None | None | None | 1/7/15 |
| Andrew D. Zelenetz, MD, PhD | Abbott Laboratories; Boehringer Ingelheim GmbH; Celgene Corporation; Genentech, Inc.; Jannsen Pharmaceutica Products, LP; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals, Inc.; Constellation Pharmaceuticals; Curis, Inc.; Gilead Sciences, Inc.; Infinity Pharmaceuticals; and Seattle Genetics, Inc. | Amgen Inc.; Celgene Corporation; Genentech, Inc.; Adaptive Biotechnologies Corp.; Alissa Pharma; Gilead Sciences, Inc.; Roche Laboratories, Inc.; and sanofi-aventis U.S. | None | 1/26/15 |
The following have disclosed a Spouse/Domestic Partner/Dependent Potential conflict: C. Babis Andreadis, MD, MSCE: Genentech, Inc. and Roche Laboratories, Inc. Lynn Wilson, MD: Amgen Inc.; Biogen Idec; Bristol-Myers Squibb Company; Celgene Corporation; GlaxoSmithKline; Merck & Co., Inc.; Novartis Pharmaceuticals Corporation; ImmunoGen, Inc.; Isis Pharmaceuticals, Inc.; Teva Pharmaceutical Industries Ltd.; UCAN; UnitedHealthcare; and Vertex Pharmaceuticals Incorporated.
The following have disclosed an Employment/Governing Board, Patent, Equity, or Royalty conflict: Oliver Press, MD, PhD: Emergent Biosolutions Lynn Wilson, MD: Amgen Inc.; Biogen Idec; Bristol-Myers Squibb Company; Celgene Corporation; GlaxoSmithKline; Merck & Co., Inc.; Novartis Pharmaceuticals Corporation; ImmunoGen, Inc.; Isis Pharmaceuticals, Inc.; Teva Pharmaceutical Industries Ltd.; UCAN; UnitedHealthcare; and Vertex Pharmaceuticals Incorporated. The NCCN Guidelines staff have disclosed that they have no conflicts of interest.
NCCN Non-Hodgkin’s Lymphomas Panel Members
*Andrew D. Zelenetz, MD, PhD/Chair†Þ
Memorial Sloan Kettering Cancer Center
*Leo I. Gordon, MD/Co-Vice Chair‡
Robert H. Lurie Comprehensive Cancer Center of
Northwestern University
*William G. Wierda, MD, PhD/Co-Vice Chair†‡
The University of Texas MD Anderson Cancer Center
Jeremy S. Abramson, MD†‡
Massachusetts General Hospital Cancer Center
Ranjana H. Advani, MD†
Stanford Cancer Institute
C. Babis Andreadis, MD, MSCE‡
UCSF Helen Diller Family Comprehensive Cancer Center
Nancy Bartlett, MD†
Siteman Cancer Center at Barnes-Jewish Hospital and
Washington University School of Medicine
John C. Byrd, MD‡Þ
The Ohio State University Comprehensive Cancer Center –
James Cancer Hospital and Solove Research Institute
Myron S. Czuczman, MD†‡
Roswell Park Cancer Institute
Luis E. Fayad, MD‡Þ
The University of Texas MD Anderson Cancer Center
Richard I. Fisher, MD‡
Fox Chase Cancer Center
Martha J. Glenn, MD†‡Þ
Huntsman Cancer Institute at the University of Utah
Thomas M. Habermann, MD‡
Mayo Clinic Cancer Center
Nancy Lee Harris, MD≠
Massachusetts General Hospital Cancer Center
Richard T. Hoppe, MD§
Stanford Cancer Institute
Steven M. Horwitz, MD†Þ
Memorial Sloan Kettering Cancer Center
Christopher R. Kelsey, MD§
Duke Cancer Institute
Youn H. Kim, MDϖ
Stanford Cancer Institute
Susan Krivacic, MPAff¥
Consultant
Ann S. LaCasce, MD†
Dana-Farber/Brigham and Women’s Cancer Center
Auayporn Nademanee, MD†‡§
City of Hope Comprehensive Cancer Center
Pierluigi Porcu, MD‡Þ
The Ohio State University Comprehensive Cancer Center –
James Cancer Hospital and Solove Research Institute
Oliver Press, MD, PhD†‡
Fred Hutchinson Cancer Research Center/Seattle Cancer Care Alliance
Rachel Rabinovitch, MD§
University of Colorado Cancer Center
Nishitha Reddy, MD‡§
Vanderbilt-Ingram Cancer Center
Erin Reid, MD‡
UC San Diego Moores Cancer Center
Ayman A. Saad, MD‡§
University of Alabama at Birmingham
Comprehensive Cancer Center
Lubomir Sokol, MD, PhD†‡Þ§
Moffitt Cancer Center
Lode J. Swinnen, MB, ChB‡
The Sidney Kimmel Comprehensive Cancer Center at
Johns Hopkins
Christina Tsien, MD§
University of Michigan Comprehensive Cancer Center
Julie M. Vose, MD, MBA‡≠
Fred & Pamela Buffett Cancer Center
Lynn Wilson, MD, MPH§
Yale Cancer Center/Smilow Cancer Hospital
Joachim Yahalom, MD§
Memorial Sloan Kettering Cancer Center
Nadeem Zafar, MD≠
St. Jude Children’s Research Hospital/The University of Tennessee Health Science Center
NCCN Staff: Mary Dwyer, MS, and Hema Sundar, PhD
KEY:
*Writing Committee Member
Specialties: †Medical Oncology; ‡Hematology/Hematology Oncology; §Radiotherapy/Radiation Oncology; §Bone Marrow Transplantation; ≠Pathology; ÞInternal Medicine; ϖDermatology; ¥Patient Advocacy
Footnotes
NCCN Categories of Evidence and Consensus
Category 1: Based upon high-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2A: Based upon lower-level evidence, there is uniform NCCN consensus that the intervention is appropriate.
Category 2B: Based upon lower-level evidence, there is NCCN consensus that the intervention is appropriate.
Category 3: Based upon any level of evidence, there is major NCCN disagreement that the intervention is appropriate.
All recommendations are category 2A unless otherwise noted.
Clinical trials: NCCN believes that the best management for any cancer patient is in a clinical trial. Participation in clinical trials is especially encouraged.
The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) are a statement of consensus of the authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult the NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The National Comprehensive Cancer Network ® (NCCN®) makes no representation or warranties of any kind regarding their content, use, or application and disclaims any responsibility for their applications or use in any way. The full NCCN Guidelines for Non-Hodgkin’s Lymphomas are not printed in this issue of JNCCN but can be accessed online at NCCN.org.
© National Comprehensive Cancer Network, Inc. 2015, All rights reserved. The NCCN Guidelines and the illustrations herein may not be reproduced in any form without the express written permission of NCCN.
Disclosures for the NCCN Non-Hodgkin’s Lymphomas Panel
At the beginning of each NCCN Guidelines panel meeting, panel members review all potential conflicts of interest. NCCN, in keeping with its commitment to public transparency, publishes these disclosures for panel members, staff, and NCCN itself.
Individual disclosures for the NCCN Non-Hodgkin’s Lymphomas Panel members can be found on page 362. (The most recent version of these guidelines and accompanying disclosures are available on the NCCN Web site at NCCN.org.)
These guidelines are also available on the Internet. For the latest update, visit NCCN.org.
References
- 1.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma: the Non-Hodgkin’s Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsimberidou AM, Wen S, O’Brien S, et al. Assessment of chronic lymphocytic leukemia and small lymphocytic lymphoma by absolute lymphocyte counts in 2,126 patients: 20 years of experience at the University of Texas MD Anderson Cancer Center. J Clin Oncol. 2007;25:4648–4656. doi: 10.1200/JCO.2006.09.4508. [DOI] [PubMed] [Google Scholar]
- 5.Rawstron AC. Monoclonal B-cell lymphocytosis. Hematology Am Soc Hematol Educ Program. 2009:430–439. doi: 10.1182/asheducation-2009.1.430. [DOI] [PubMed] [Google Scholar]
- 6.Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359:575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 7.Baliakas P, Iskas M, Gardiner A, et al. Chromosomal translocations and karyotype complexity in chronic lymphocytic leukemia: a systematic reappraisal of classic cytogenetic data. Am J Hematol. 2014;89:249–255. doi: 10.1002/ajh.23618. [DOI] [PubMed] [Google Scholar]
- 8.Woyach JA, Ruppert AS, Lozanski G, et al. Association of disease progression on ibrutinib therapy with the acquisition of resistance mutations: a single-center experience of 267 patients [abstract] J Clin Oncol. 2014;32(15_suppl) Abstract 7010. [Google Scholar]
- 9.Thompson PA, Wierda WG, Ferrajoli A, et al. Complex karyotype, rather than del(17p), is associated with inferior outcomes in relapsed or refractory CLL patients treated with ibrutinib-based regimens [abstract] Blood. 2014;124 doi: 10.1002/cncr.29566. Abstract 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dicker F, Schnittger S, Haferlach T, et al. Immunostimulatory oligonucleotide-induced metaphase cytogenetics detect chromosomal aberrations in 80% of CLL patients: a study of 132 CLL cases with correlation to FISH, IgVH status, and CD38 expression. Blood. 2006;108:3152–3160. doi: 10.1182/blood-2006-02-005322. [DOI] [PubMed] [Google Scholar]
- 11.Put N, Konings P, Rack K, et al. Improved detection of chromosomal abnormalities in chronic lymphocytic leukemia by conventional cytogenetics using CpG oligonucleotide and interleukin-2 stimulation: a Belgian multicentric study. Genes Chromosomes Cancer. 2009;48:843–853. doi: 10.1002/gcc.20691. [DOI] [PubMed] [Google Scholar]
- 12.Struski S, Gervais C, Helias C, et al. Stimulation of B-cell lymphoproliferations with CpG-oligonucleotide DSP30 plus IL-2 is more effective than with TPA to detect clonal abnormalities. Leukemia. 2009;23:617–619. doi: 10.1038/leu.2008.252. [DOI] [PubMed] [Google Scholar]
- 13.Heerema NA, Byrd JC, Dal Cin PS, et al. Stimulation of chronic lymphocytic leukemia cells with CpG oligodeoxynucleotide gives consistent karyotypic results among laboratories: a CLL Research Consortium (CRC) study. Cancer Genet Cytogenet. 2010;203:134–140. doi: 10.1016/j.cancergencyto.2010.07.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 15.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 16.Del Poeta G, Maurillo L, Venditti A, et al. Clinical significance of CD38 expression in chronic lymphocytic leukemia. Blood. 2001;98:2633–2639. doi: 10.1182/blood.v98.9.2633. [DOI] [PubMed] [Google Scholar]
- 17.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 18.Hallek M, Langenmayer I, Nerl C, et al. Elevated serum thymidine kinase levels identify a subgroup at high risk of disease progression in early, nonsmoldering chronic lymphocytic leukemia. Blood. 1999;93:1732–1737. [PubMed] [Google Scholar]
- 19.Hallek M, Wanders L, Ostwald M, et al. Serum beta(2)-microglobulin and serum thymidine kinase are independent predictors of progression-free survival in chronic lymphocytic leukemia and immunocytoma. Leuk Lymphoma. 1996;22:439–447. doi: 10.3109/10428199609054782. [DOI] [PubMed] [Google Scholar]
- 20.Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–1854. [PubMed] [Google Scholar]
- 21.Hamblin TJ, Orchard JA, Ibbotson RE, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood. 2002;99:1023–1029. doi: 10.1182/blood.v99.3.1023. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim S, Keating M, Do KA, et al. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood. 2001;98:181–186. doi: 10.1182/blood.v98.1.181. [DOI] [PubMed] [Google Scholar]
- 23.Orchard JA, Ibbotson RE, Davis Z, et al. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. 2004;363:105–111. doi: 10.1016/S0140-6736(03)15260-9. [DOI] [PubMed] [Google Scholar]
- 24.Rassenti LZ, Huynh L, Toy TL, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 25.Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101:4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 26.Bulian P, Shanafelt TD, Fegan C, et al. CD49d is the strongest flow cytometry-based predictor of overall survival in chronic lymphocytic leukemia. J Clin Oncol. 2014;32:897–904. doi: 10.1200/JCO.2013.50.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobin G, Thunberg U, Johnson A, et al. Somatically mutated Ig V(H)3–21 genes characterize a new subset of chronic lymphocytic leukemia. Blood. 2002;99:2262–2264. doi: 10.1182/blood.v99.6.2262. [DOI] [PubMed] [Google Scholar]
- 28.Krober A, Bloehdorn J, Hafner S, et al. Additional genetic high-risk features such as 11q deletion, 17p deletion, and V3–21 usage characterize discordance of ZAP-70 and VH mutation status in chronic lymphocytic leukemia. J Clin Oncol. 2006;24:969–975. doi: 10.1200/JCO.2005.03.7184. [DOI] [PubMed] [Google Scholar]
- 29.Krober A, Seiler T, Benner A, et al. V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood. 2002;100:1410–1416. [PubMed] [Google Scholar]
- 30.Oscier D, Wade R, Davis Z, et al. Prognostic factors identified three risk groups in the LRF CLL4 trial, independent of treatment allocation. Haematologica. 2010;95:1705–1712. doi: 10.3324/haematol.2010.025338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oscier DG, Gardiner AC, Mould SJ, et al. Multivariate analysis of prognostic factors in CLL: clinical stage, IGVH gene mutational status, and loss or mutation of the p53 gene are independent prognostic factors. Blood. 2002;100:1177–1184. [PubMed] [Google Scholar]
- 32.Gentile M, Mauro FR, Calabrese E, et al. The prognostic value of CD38 expression in chronic lymphocytic leukaemia patients studied prospectively at diagnosis: a single institute experience. Br J Haematol. 2005;130:549–557. doi: 10.1111/j.1365-2141.2005.05659.x. [DOI] [PubMed] [Google Scholar]
- 33.Del Principe MI, Del Poeta G, Buccisano F, et al. Clinical significance of ZAP-70 protein expression in B-cell chronic lymphocytic leukemia. Blood. 2006;108:853–861. doi: 10.1182/blood-2005-12-4986. [DOI] [PubMed] [Google Scholar]
- 34.Rassenti LZ, Jain S, Keating MJ, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112:1923–1930. doi: 10.1182/blood-2007-05-092882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corcoran M, Parker A, Orchard J, et al. ZAP-70 methylation status is associated with ZAP-70 expression status in chronic lymphocytic leukemia. Haematologica. 2005;90:1078–1088. [PubMed] [Google Scholar]
- 36.Claus R, Lucas DM, Stilgenbauer S, et al. Quantitative DNA methylation analysis identifies a single CpG dinucleotide important for ZAP-70 expression and predictive of prognosis in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:2483–2491. doi: 10.1200/JCO.2011.39.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Claus R, Lucas DM, Ruppert AS, et al. Validation of ZAP-70 methylation and its relative significance in predicting outcome in chronic lymphocytic leukemia. Blood. 2014;124:42–48. doi: 10.1182/blood-2014-02-555722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tam CS, O’Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsimberidou AM, Tam C, Wierda W, et al. Beta-2 microglobulin (B2M) is an independent prognostic factor for clinical outcomes in patients with CLL treated with frontline fludarabine, cyclophosphamide, and rituximab (FCR) regardless of age, creatinine clearance (CrCl) [abstract] J Clin Oncol. 2007;25 Abstract 7034. [Google Scholar]
- 40.Wierda WG, O’Brien S, Wang X, et al. Characteristics associated with important clinical end points in patients with chronic lymphocytic leukemia at initial treatment. J Clin Oncol. 2009;27:1637–1643. doi: 10.1200/JCO.2008.18.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wierda WG, O’Brien S, Wang X, et al. Prognostic nomogram and index for overall survival in previously untreated patients with chronic lymphocytic leukemia. Blood. 2007;109:4679–4685. doi: 10.1182/blood-2005-12-051458. [DOI] [PubMed] [Google Scholar]
- 42.Shanafelt TD, Jenkins G, Call TG, et al. Validation of a new prognostic index for patients with chronic lymphocytic leukemia. Cancer. 2009;115:363–372. doi: 10.1002/cncr.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molica S, Mauro FR, Callea V, et al. The utility of a prognostic index for predicting time to first treatment in early chronic lymphocytic leukemia: the GIMEMA experience. Haematologica. 2010;95:464–469. doi: 10.3324/haematol.2009.011767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neilson JR, Auer R, White D, et al. Deletions at 11q identify a subset of patients with typical CLL who show consistent disease progression and reduced survival. Leukemia. 1997;11:1929–1932. doi: 10.1038/sj.leu.2400819. [DOI] [PubMed] [Google Scholar]
- 45.Austen B, Skowronska A, Baker C, et al. Mutation status of the residual ATM allele is an important determinant of the cellular response to chemotherapy and survival in patients with chronic lymphocytic leukemia containing an 11q deletion. J Clin Oncol. 2007;25:5448–5457. doi: 10.1200/JCO.2007.11.2649. [DOI] [PubMed] [Google Scholar]
- 46.Tsimberidou AM, Tam C, Abruzzo LV, et al. Chemoimmunotherapy may overcome the adverse prognostic significance of 11q deletion in previously untreated patients with chronic lymphocytic leukemia. Cancer. 2009;115:373–380. doi: 10.1002/cncr.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 48.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 trial): a randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 49.Stilgenbauer S, Sander S, Bullinger L, et al. Clonal evolution in chronic lymphocytic leukemia: acquisition of high-risk genomic aberrations associated with unmutated VH, resistance to therapy, and short survival. Haematologica. 2007;92:1242–1245. doi: 10.3324/haematol.10720. [DOI] [PubMed] [Google Scholar]
- 50.Wierda WG, O’Brien S, Wang X, et al. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J Clin Oncol. 2011;29:4088–4095. doi: 10.1200/JCO.2010.33.9002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pflug N, Bahlo J, Shanafelt TD, et al. Development of a comprehensive prognostic index for patients with chronic lymphocytic leukemia. Blood. 2014;124:49–62. doi: 10.1182/blood-2014-02-556399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 53.Stilgenbauer S, Schnaiter A, Paschka P, et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood. 2014;123:3247–3254. doi: 10.1182/blood-2014-01-546150. [DOI] [PubMed] [Google Scholar]
- 54.Gonzalez D, Martinez P, Wade R, et al. Mutational status of the TP53 gene as a predictor of response and survival in patients with chronic lymphocytic leukemia: results from the LRF CLL4 trial. J Clin Oncol. 2011;29:2223–2229. doi: 10.1200/JCO.2010.32.0838. [DOI] [PubMed] [Google Scholar]
- 55.Rossi D, Cerri M, Deambrogi C, et al. The prognostic value of TP53 mutations in chronic lymphocytic leukemia is independent of Del17p13: implications for overall survival and chemorefractoriness. Clin Cancer Res. 2009;15:995–1004. doi: 10.1158/1078-0432.CCR-08-1630. [DOI] [PubMed] [Google Scholar]
- 56.Woyach JA, Ruppert AS, Heerema NA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol. 2011;29:1349–1355. doi: 10.1200/JCO.2010.31.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fabbri G, Rasi S, Rossi D, et al. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med. 2011;208:1389–1401. doi: 10.1084/jem.20110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang L, Lawrence MS, Wan Y, et al. SF3B1 and other novel cancer genes in chronic lymphocytic leukemia. N Engl J Med. 2011;365:2497–2506. doi: 10.1056/NEJMoa1109016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quesada V, Conde L, Villamor N, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44:47–52. doi: 10.1038/ng.1032. [DOI] [PubMed] [Google Scholar]
- 61.Rossi D, Fangazio M, Rasi S, et al. Disruption of BIRC3 associates with fludarabine chemorefractoriness in TP53 wild-type chronic lymphocytic leukemia. Blood. 2012;119:2854–2862. doi: 10.1182/blood-2011-12-395673. [DOI] [PubMed] [Google Scholar]
- 62.Messina M, Del Giudice I, Khiabanian H, et al. Genetic lesions associated with chronic lymphocytic leukemia chemo-refractoriness. Blood. 2014;123:2378–2388. doi: 10.1182/blood-2013-10-534271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rossi D, Rasi S, Spina V, et al. Integrated mutational and cytogenetic analysis identifies new prognostic subgroups in chronic lymphocytic leukemia. Blood. 2013;121:1403–1412. doi: 10.1182/blood-2012-09-458265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oscier DG, Rose-Zerilli MJ, Winkelmann N, et al. The clinical significance of NOTCH1 and SF3B1 mutations in the UK LRF CLL4 trial. Blood. 2013;121:468–475. doi: 10.1182/blood-2012-05-429282. [DOI] [PubMed] [Google Scholar]
- 65.Schnaiter A, Paschka P, Rossi M, et al. NOTCH1, SF3B1, and TP53 mutations in fludarabine-refractory CLL patients treated with alemtuzumab: results from the CLL2H trial of the GCLLSG. Blood. 2013;122:1266–1270. doi: 10.1182/blood-2013-03-488197. [DOI] [PubMed] [Google Scholar]
- 66.Rossi D, Rasi S, Spina V, et al. Different impact of NOTCH1 and SF3B1 mutations on the risk of chronic lymphocytic leukemia transformation to Richter syndrome. Br J Haematol. 2012;158:426–429. doi: 10.1111/j.1365-2141.2012.09155.x. [DOI] [PubMed] [Google Scholar]
- 67.Villamor N, Conde L, Martinez-Trillos A, et al. NOTCH1 mutations identify a genetic subgroup of chronic lymphocytic leukemia patients with high risk of transformation and poor outcome. Leukemia. 2013;27:1100–1106. doi: 10.1038/leu.2012.357. [DOI] [PubMed] [Google Scholar]
- 68.Conte MJ, Bowen DA, Wiseman GA, et al. Use of positron emission tomography-computed tomography in the management of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma. 2014;55:2079–2084. doi: 10.3109/10428194.2013.869801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falchi L, Keating MJ, Marom EM, et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood. 2014;123:2783–2790. doi: 10.1182/blood-2013-11-536169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46:219–234. [Google Scholar]
- 71.Binet J, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48:198–206. doi: 10.1002/1097-0142(19810701)48:1<198::aid-cncr2820480131>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 72.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 73.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:2820–2822. doi: 10.1200/JCO.2012.43.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chanan-Khan A, Miller KC, Lawrence D, et al. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukemia predicts clinical response. Cancer. 2011;117:2127–2135. doi: 10.1002/cncr.25748. [DOI] [PubMed] [Google Scholar]
- 75.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123:1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kovacs G, Boettcher S, Bahlo J, et al. Value of minimal residual disease (MRD) negative status at response evaluation in chronic lymphocytic leukemia (CLL): combined analysis of two phase III studies of the German CLL Study Group (GCLLSG) [abstract] Blood. 2014;124 Abstract 23. [Google Scholar]
- 79.Burger JA, Montserrat E. Coming full circle: 70 years of chronic lymphocytic leukemia cell redistribution, from glucocorticoids to inhibitors of B-cell receptor signaling. Blood. 2013;121:1501–1509. doi: 10.1182/blood-2012-08-452607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fowler N, Davis E. Targeting B-cell receptor signaling: changing the paradigm. Hematology Am Soc Hematol Educ Program. 2013;2013:553–560. doi: 10.1182/asheducation-2013.1.553. [DOI] [PubMed] [Google Scholar]
- 81.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1111. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 82.Flynn JM, Byrd JC, Kipps TJ, et al. Obinutuzumab (GA101) 1,000 mg versus 2,000 mg in patients with chronic lymphocytic leukemia (CLL): results of the phase II GAGE (GAO4768g) trial [abstract] J Clin Oncol. 2014;32(Suppl 15) Abstract 7083. [Google Scholar]
- 83.Robak T, Janssens A, Govindbabu K, et al. Ofatumumab + chlorambucil versus chlorambucil alone in patients with untreated chronic lymphocytic leukemia (CLL): results of the phase III study complement 1 (OMB110911) [abstract] Blood. 2013;122 Abstract 528. [Google Scholar]
- 84.O’Brien S, Furman RR, Coutre SE, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15:48–58. doi: 10.1016/S1470-2045(13)70513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Brien SM, Furman RR, Coutre SE, et al. Independent evaluation of ibrutinib efficacy 3 years post-initiation of monotherapy in patients with chronic lymphocytic leukemia/small lymphocytic leukemia including deletion 17p disease [abstract] J Clin Oncol. 2014;32(15_suppl) Abstract 7014. [Google Scholar]
- 86.Wiestner A, Farooqui M, Valdez J, et al. Single agent ibrutinib (PCI-32765) is highly effective in chronic lymphocytic leukaemia patients with 17p deletion [abstract] Hematol Oncol. 2013;31(Suppl 1):98. Abstract 008. [Google Scholar]
- 87.Zelenetz AD, Lamanna N, Kipps TJ, et al. A phase 2 study of idelalisib monotherapy in previously untreated patients ≥65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) [abstract] Blood. 2014;124 Abstract 1986. [Google Scholar]
- 88.O’Brien S, Lamanna N, Kipps TJ, et al. Update on a phase 2 study of idelalisib in combination with rituximab in treatment-naïve patients ≥65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) [abstract] Blood. 2014;124 Abstract 1994. [Google Scholar]
- 89.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110delta, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123:3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. J Clin Oncol. 2013;31:88–94. doi: 10.1200/JCO.2012.42.7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Byrd JC, Brown JR, O’Brien S, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213–223. doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown JR, Hillmen P, O’Brien S, et al. Updated efficacy including genetic and clinical subgroup analysis and overall safety in the phase 3 RESONATETM trial of ibrutinib versus ofatumumab in previously treated chronic lymphocytic leukemia/small lymphocytic lymphoma [abstract] Blood. 2014;124 Abstract 3331. [Google Scholar]
- 93.O’Brien S, Jones JA, Coutre S, et al. Efficacy and safety of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic leukemia with 17p deletion: results from the phase II RESONATE™-17 trial [abstract] Blood. 2014;124 Abstract 327. [Google Scholar]
- 94.Sharman JP, Coutre SE, Furman RR, et al. Second interim analysis of a phase 3 study of idelalisib (ZYDELIG®) plus rituximab (R) for relapsed chronic lymphocytic leukemia (CLL): efficacy analysis in patient subpopulations with del(17p) and other adverse prognostic factors [abstract] Blood. 2014;124 Abstract 330. [Google Scholar]
- 95.Sharman JP, Coutre SE, Furman RR, et al. Efficacy of idelalisib in CLL subpopulations harboring del(17p) and other adverse prognostic factors: results from a phase 3, randomized, double-blind, placebo-controlled trial [abstract] J Clin Oncol. 2014;32(15_suppl) Abstract 7011. [Google Scholar]
- 96.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wierda WG, Kipps TJ, Mayer J, et al. Final analysis from the international trial of single-agent ofatumumab in patients with fludarabine-refractory chronic lymphocytic leukemia [abstract] Blood. 2010;116 Abstract 921. [Google Scholar]
- 98.Wierda WG, Padmanabhan S, Chan GW, et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase II international study. Blood. 2011;118:5126–5129. doi: 10.1182/blood-2011-04-348656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cartron G, de Guibert S, Dilhuydy MS, et al. Obinutuzumab (GA101) in relapsed/refractory chronic lymphocytic leukemia: final data from the phase 1/2 GAUGUIN study. Blood. 2014;124:2196–2202. doi: 10.1182/blood-2014-07-586610. [DOI] [PubMed] [Google Scholar]
- 100.Goede V, Engelke A, Fischer K, et al. Salvage therapy with obinutuzumab (GA101) plus chlorambucil (Clb) after treatment failure of Clb alone in patients with chronic lymphocytic leukemia (CLL) and comorbidities: results of the CLL11 study [abstract] Blood. 2014;124 Abstract 3327. [Google Scholar]
- 101.Gribben JG, Zahrieh D, Stephans K, et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood. 2005;106:4389–4396. doi: 10.1182/blood-2005-05-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khouri IF, Keating MJ, Saliba RM, Champlin RE. Long-term follow-up of patients with CLL treated with allogeneic hematopoietic transplantation. Cytotherapy. 2002;4:217–221. doi: 10.1080/146532402320219736. [DOI] [PubMed] [Google Scholar]
- 103.Sorror ML, Storer BE, Sandmaier BM, et al. Five-year follow-up of patients with advanced chronic lymphocytic leukemia treated with allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. J Clin Oncol. 2008;26:4912–4920. doi: 10.1200/JCO.2007.15.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khouri IF, Saliba RM, Admirand J, et al. Graft-versus-leukaemia effect after non-myeloablative haematopoietic transplantation can overcome the unfavourable expression of ZAP-70 in refractory chronic lymphocytic leukaemia. Br J Haematol. 2007;137:355–363. doi: 10.1111/j.1365-2141.2007.06591.x. [DOI] [PubMed] [Google Scholar]
- 105.Moreno C, Villamor N, Colomer D, et al. Allogeneic stem-cell transplantation may overcome the adverse prognosis of unmutated VH gene in patients with chronic lymphocytic leukemia. J Clin Oncol. 2005;23:3433–3438. doi: 10.1200/JCO.2005.04.531. [DOI] [PubMed] [Google Scholar]
- 106.Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26:5094–5100. doi: 10.1200/JCO.2008.16.2982. [DOI] [PubMed] [Google Scholar]
- 107.Dreger P, Dohner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–2447. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]
- 108.Tsimberidou AM, Keating MJ. Treatment of fludarabine-refractory chronic lymphocytic leukemia. Cancer. 2009;115:2824–2836. doi: 10.1002/cncr.24329. [DOI] [PubMed] [Google Scholar]
- 109.National Cancer Institute. SEER Stat Fact Sheets: Chronic Lymphocytic Leukemia. Bethesda, MD: 2012. [Accessed August 2012]. Available at: http://seer.cancer.gov/statfacts/html/clyl.html. [Google Scholar]
- 110.Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50:171–178. doi: 10.1080/10428190802688517. [DOI] [PubMed] [Google Scholar]
- 111.Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114:3382–3391. doi: 10.1182/blood-2009-02-206185. [DOI] [PubMed] [Google Scholar]
- 112.Thurmes P, Call T, Slager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49:49–56. doi: 10.1080/10428190701724785. [DOI] [PubMed] [Google Scholar]
- 113.Goede V, Cramer P, Busch R, et al. Interactions between comorbidity and treatment of chronic lymphocytic leukemia: results of German Chronic Lymphocytic Leukemia Study Group trials. Haematologica. 2014;99:1095–1100. doi: 10.3324/haematol.2013.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Satram-Hoang S, Reyes C, Hoang KQ, et al. Treatment practice in the elderly patient with chronic lymphocytic leukemia-analysis of the combined SEER and Medicare database. Ann Hematol. 2014;93:1335–1344. doi: 10.1007/s00277-014-2048-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Salvi F, Miller MD, Grilli A, et al. A manual of guidelines to score the modified cumulative illness rating scale and its validation in acute hospitalized elderly patients. J Am Geriatr Soc. 2008;56:1926–1931. doi: 10.1111/j.1532-5415.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 116.Hillmen P, Gribben JG, Follows GA, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: final analysis of an open-label phase II study. J Clin Oncol. 2014;32:1236–1241. doi: 10.1200/JCO.2013.49.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Foa R, Giudice ID, Cuneo A, et al. Chlorambucil plus rituximab with or without maintenance rituximab as first-line treatment for elderly chronic lymphocytic leukemia patients. Am J Hematol. 2014;89:480–486. doi: 10.1002/ajh.23668. [DOI] [PubMed] [Google Scholar]
- 118.Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30:3209–3216. doi: 10.1200/JCO.2011.39.2688. [DOI] [PubMed] [Google Scholar]
- 119.Eichhorst B, Fink AM, Busch R, et al. Frontline chemoimmunotherapy with fludarabine (F), cyclophosphamide (C), and rituximab (R) (FCR) shows superior efficacy in comparison to bendamustine (B) and rituximab (BR) in previously untreated and physically fit patients (pts) with advanced chronic lymphocytic leukemia (CLL): final analysis of an international, randomized study of the German CLL Study Group (GCLLSG) (CLL10 Study) [abstract] Blood. 2014;124 Abstract 19. [Google Scholar]
- 120.Gopal AK, Kahl BS, de Vos S, et al. PI3Kdelta inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsimberidou AM, Keating MJ. Richter syndrome: biology, incidence, and therapeutic strategies. Cancer. 2005;103:221–228. doi: 10.1002/cncr.20773. [DOI] [PubMed] [Google Scholar]
- 122.Tsimberidou AM, O’Brien S, Kantarjian HM, et al. Hodgkin transformation of chronic lymphocytic leukemia: the M D Anderson Cancer Center experience. Cancer. 2006;107:1294–1302. doi: 10.1002/cncr.22121. [DOI] [PubMed] [Google Scholar]
- 123.Rossi D, Gaidano G. Richter syndrome: molecular insights and clinical perspectives. Hematol Oncol. 2009;27:1–10. doi: 10.1002/hon.880. [DOI] [PubMed] [Google Scholar]
- 124.Chigrinova E, Rinaldi A, Kwee I, et al. Two main genetic pathways lead to the transformation of chronic lymphocytic leukemia to Richter syndrome. Blood. 2013;122:2673–2682. doi: 10.1182/blood-2013-03-489518. [DOI] [PubMed] [Google Scholar]
- 125.Fabbri G, Khiabanian H, Holmes AB, et al. Genetic lesions associated with chronic lymphocytic leukemia transformation to Richter syndrome. J Exp Med. 2013;210:2273–2288. doi: 10.1084/jem.20131448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsimberidou AM, O’Brien S, Khouri I, et al. Clinical outcomes and prognostic factors in patients with Richter’s syndrome treated with chemotherapy or chemoimmunotherapy with or without stem-cell transplantation. J Clin Oncol. 2006;24:2343–2351. doi: 10.1200/JCO.2005.05.0187. [DOI] [PubMed] [Google Scholar]
- 127.Tadmor T, Shvidel L, Goldschmidt N, et al. Hodgkin’s variant of Richter transformation in chronic lymphocytic leukemia; a retrospective study from the Israeli CLL study group. Anticancer Res. 2014;34:785–790. [PubMed] [Google Scholar]
- 128.Tsimberidou AM, Kantarjian HM, Cortes J, et al. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin, and dexamethasone plus rituximab and granulocyte-macrophage-colony stimulating factor (GM-CSF) alternating with methotrexate and cytarabine plus rituximab and GM-CSF in patients with Richter syndrome or fludarabine-refractory chronic lymphocytic leukemia. Cancer. 2003;97:1711–1720. doi: 10.1002/cncr.11238. [DOI] [PubMed] [Google Scholar]
- 129.Tsimberidou AM, Wierda WG, Plunkett W, et al. Phase I–II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter’s syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008;26:196–203. doi: 10.1200/JCO.2007.11.8513. [DOI] [PubMed] [Google Scholar]
- 130.Tsimberidou AM, Wierda WG, Wen S, et al. Phase I–II clinical trial of oxaliplatin, fludarabine, cytarabine, and rituximab therapy in aggressive relapsed/refractory chronic lymphocytic leukemia or Richter syndrome. Clin Lymphoma Myeloma Leuk. 2013;13:568–574. doi: 10.1016/j.clml.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cwynarski K, van Biezen A, de Wreede L, et al. Autologous and allogeneic stem-cell transplantation for transformed chronic lymphocytic leukemia (Richter’s syndrome): a retrospective analysis from the chronic lymphocytic leukemia subcommittee of the chronic leukemia working party and lymphoma working party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2211–2217. doi: 10.1200/JCO.2011.37.4108. [DOI] [PubMed] [Google Scholar]


