Abstract
Objective:
To explore the possibility of using interleukin-17 (IL-17) production by CD4+ T cells in the CSF as a potential biomarker for cerebral vasculitis in stroke patients.
Methods:
In this consecutive case study, we performed prospective analysis of CSF and blood in patients admitted to a university medical center with symptoms of stroke and suspected cerebral vasculitis. Flow cytometry was performed for intracellular detection of inflammatory cytokines in peripheral blood lymphocytes and expanded T cells from CSF.
Results:
CSF CD4+ lymphocytes from patients with cerebral vasculitis showed significantly higher levels of the proinflammatory cytokine IL-17 compared to patients with stroke not due to vasculitis or with other, noninflammatory neurologic diseases. There was no difference in the production of interferon-γ in the CSF and no overall differences in the relative frequencies of peripheral immune cells.
Conclusions:
Intracellular IL-17 in CSF cells is potentially useful in discriminating cerebral vasculitis as a rare cause in patients presenting with ischemic stroke.
Classification of evidence:
This study provides Class II evidence that an increased proportion of IL-17-producing CD4+ cells in CSF of patients presenting with stroke symptoms is indicative of cerebral vasculitis (sensitivity 73%, 95% confidence interval [CI] 39–94%; specificity 100%, 95% CI 74%–100%).
Cerebral vasculitis (CV) is one important cause of stroke, especially in younger adults, and it remains difficult to differentiate this condition from other causes of ischemic brain injury.1–3 Gold standard for the diagnosis of CV is a biopsy of an affected brain vessel, but a negative result does not rule out the disease. Other diagnostic tools such as MRI, magnetic resonance angiography, or CSF analysis have a high sensitivity (close to 100%) but low specificity (around 30%).2 Due to the different therapy approaches, it is crucial to distinguish CV from other causes of cerebral ischemia or inflammatory brain disease.
Proinflammatory cytokines such as interferon-γ (IFN-γ) and interleukin-17 (IL-17) are potent mediators of chemokine release, activation of endothelial surfaces, and recruitment of inflammatory cells. In particular, IL-17 is known to play an important role in the pathogenesis of anti–neutrophil cytoplasmic autoantibody–associated vasculitis (AAV) and giant cell vasculitis.4 Here, we focused on the production of IFN-γ and IL-17 by T lymphocytes in the CSF of patients with a cerebral ischemic event as a potential biomarker for cerebral vasculitis. Our primary research goal was to determine whether IL-17 could serve as a biomarker for cerebral vasculitis within an unselected sample of patients presenting with stroke-like symptoms.
METHODS
Patients and diagnostic criteria.
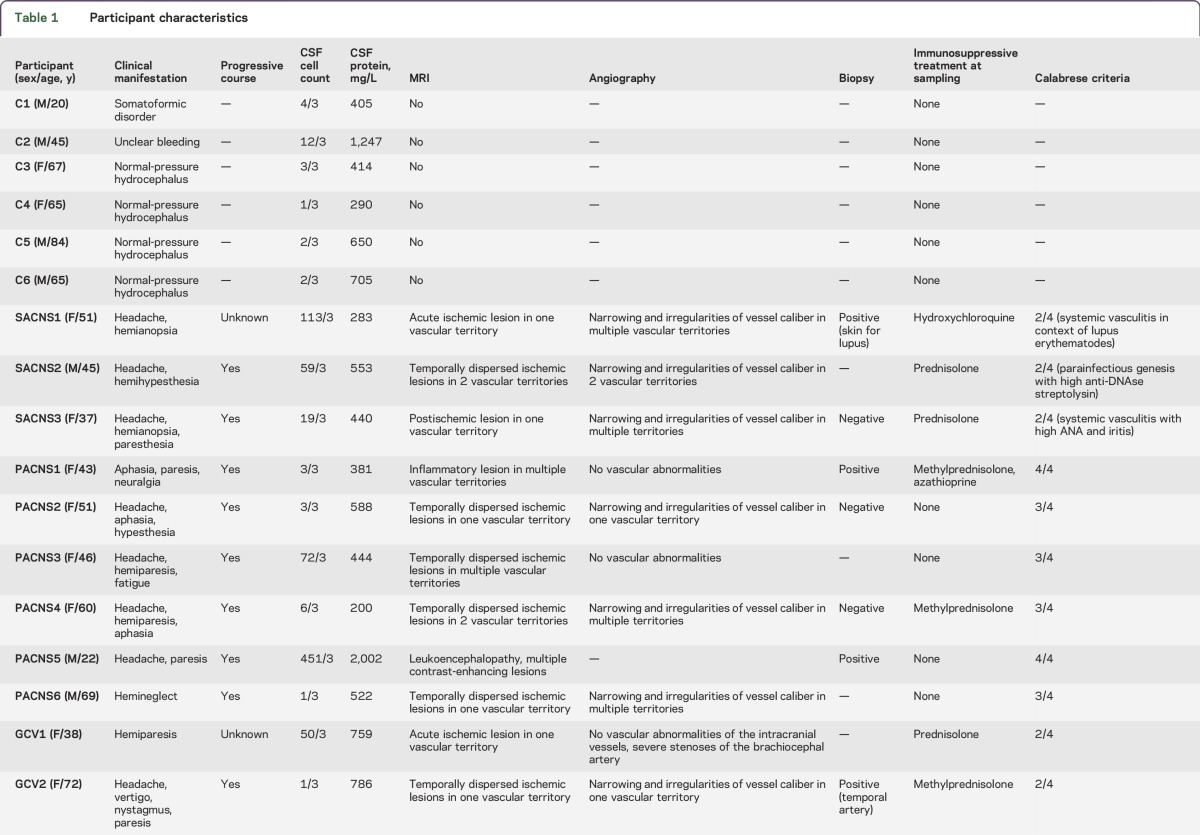
Blood and CSF were obtained from patients consecutively admitted to the University Medical Center Hamburg-Eppendorf with stroke-like symptoms between 2013 and 2015 (table 1). These participants were divided into patients with a diagnosis of primary angiitis of the CNS (PACNS) according to the Calabrese diagnostic criteria2 (n = 6), secondary angiitis of the CNS (SACNS, n = 3), giant cell vasculitis (GCV, n = 3), and ischemic stroke where vasculitis was ruled out (n = 15). Classification of the patients was done by senior clinicians (C.G., T.M.) blinded to the results of the laboratory test. Finally, 6 patients with noninflammatory neurologic disease from whom CSF was taken during diagnostic procedures were included in the study as controls (table 1).
Table 1.
Participant characteristics
Patient consents.
All procedures were approved by the local ethics committee and informed consent was obtained from all patients or their legal representatives.
Expansion of CSF cells.
After centrifugation of the CSF, cells were stimulated with 2.5 μg/mL phytohemagglutinin in RPMI1640 medium containing 10% human serum, antibiotics, and in the presence of irradiated (36 Gy) feeder cells.
IL-2 (equivalent to 20 U/mL) was added to the cultures every 3–4 days together with 50% fresh medium. After 2 weeks, cells were harvested and used directly for cytokine determination or restimulated for further expansion. In about 10% of the samples, expansion was not successful.
Flow cytometry.
For intracellular cytokine detection, expanded CSF cells were stimulated with 250 ng/mL PMA and 1 μg/mL ionomycin. A total of 100 μL heparin-blood was stimulated in parallel with 5-fold concentrations of PMA and ionomycin. Cells and blood were cultured for 5 hours in serum-free medium (X-Vivo15) in the presence of 10 μg/mL Brefeldin A, as described.5 Cells were subsequently harvested, permeabilized, and stained for lineage markers CD3, CD4, CD8, and TCRγδ, and with anti-IFN-γ and anti-IL-17. Dead cells were discriminated using LIVE/DEAD stain (Invitrogen, Carlsbad, CA). In approximately 10% of the patients, immunocytochemistry from the peripheral blood failed due to unsuccessful stimulation. Samples were analyzed in a LSR Fortessa (BD Biosciences, East Rutherford, NJ) flow cytometer and results were evaluated using FlowJo software.
Cytokine determination in serum and CSF.
Multiplex determination of IL-17, IFN-γ, IL-1β, IL-6, tumor necrosis factor–α, and IL-12/23 was performed using the high-resolution Meso Scale Discovery (MSD) (Rockville, MD) platform.
Statistical analysis.
The primary research question of this study was the determination of sensitivity and specificity of IL-17 as a biomarker for cerebral vasculitis within an unselected sample of patients presenting with stroke-like symptoms in order to provide Class II evidence for IL-17 as a biomarker for cerebral vasculitis.
For classification of the IL-17 values, a cutoff of 7% was used since it was located at the middle of an interval that separated normal and increased values of IL-17. The samples were analyzed retrospectively.
We used SPSS (Chicago, IL) version 22.0 for statistical calculations. Paired Student t test was used to compare peripheral blood and CSF data. To compare multiple groups, we used analysis of variance. Differences were considered significant if p ≤ 0.05.
RESULTS
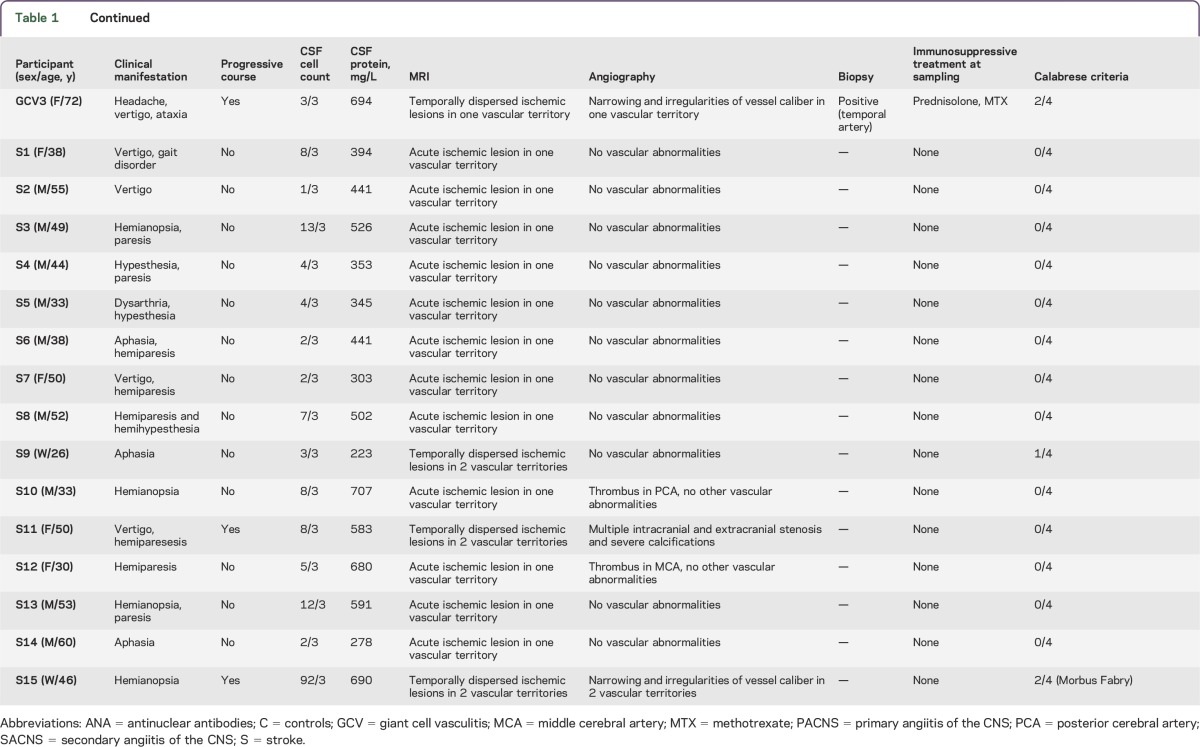
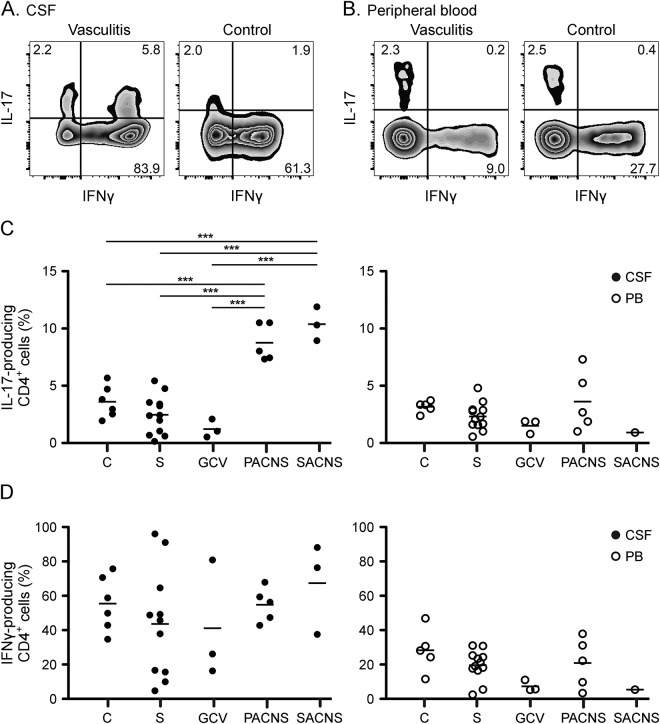
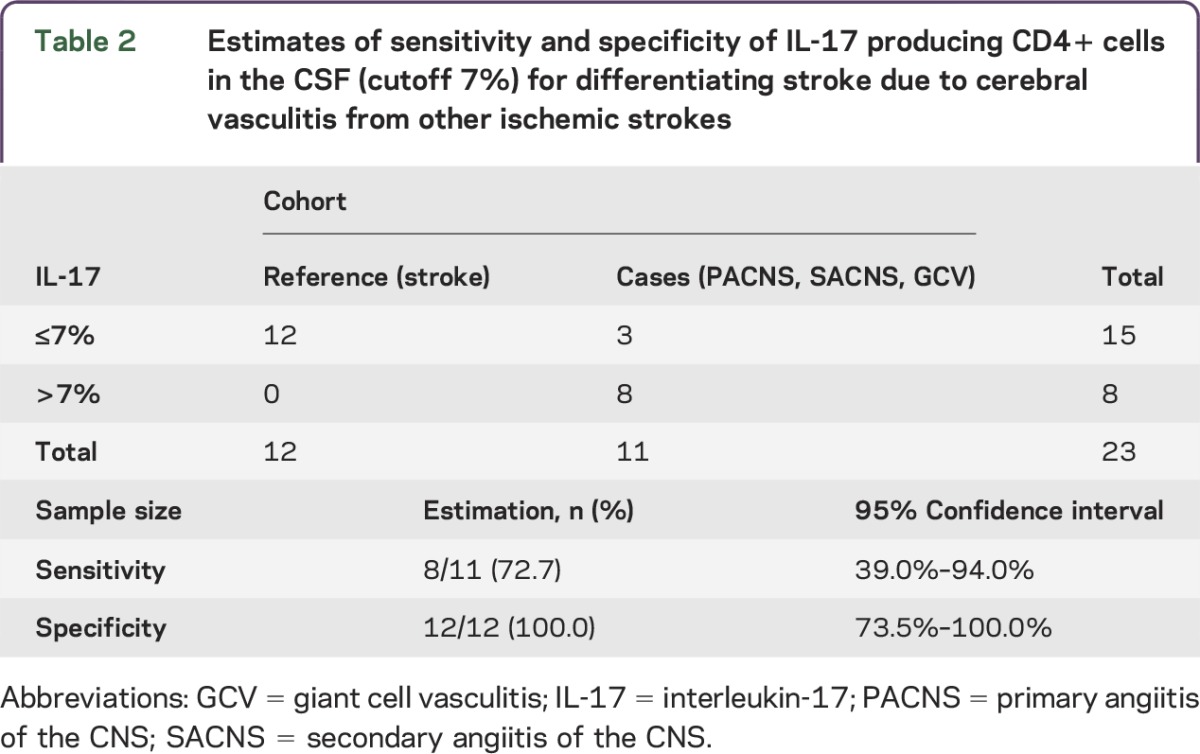
Considering the inflammatory nature of cerebral vasculitis, and in search of putative biomarkers, we performed a phenotypical characterization of peripheral immune cells in our cohort. We could not find any differences in the frequency of all immune cell subsets or in the expression of activation or maturation markers (not shown). To assess lymphocytes close to the target organ, we expanded cells from the CSF until enough cells were available for cell surface phenotyping and intracellular cytokine analysis, typically 2–4 weeks. After expansion, in all patient groups CD4+ cells were the dominant population, while the rest were CD8+ and Tγδ lymphocytes. We observed a higher percentage of CD4+ cells producing IL-17 exclusively in patients with cerebral vasculitis (figure 1). Interestingly, all samples with CSF cells analyzed of patients with PACNS and SACNS groups showed high levels of IL-17-producing CD4+ cells (average 8.8%, range 7.3%–10.5%, for PACNS; average 10.4%, range 8.9%–11.9%, for SACNS). In both cases, comparison to the IL-17 production by CSF cells in stroke patients was highly significant (p < 0.001). The GCV patients showed rather low IL-17 production (1.2%, range 0.5%–2.1%), comparable to the other groups (2.5%, range 0.14%–5.4% in stroke patients; and 3.6%, range 1.9%–5.7% in the control group). With respect to the primary research question, we set a cutoff at 7% and considered stroke patients as reference cohort and the PACNS, SACNS, and GCV as case cohort (table 2). We found that an increased proportion of IL-17-producing CD4+ cells in CSF accurately identifies patients with cerebral vasculitis with a sensitivity of 73% (95% CI 39%–94%) and a specificity of 100% (95% CI 74%–100%).
Figure 1. CD4+ CSF cells of patients with cerebral vasculitis, but not stroke patients, show a strong Th17 profile.
Flow cytometry dot plots depict interleukin-17 (IL-17) and interferon-γ (IFN-γ) expression by expanded CSF CD4+ cells (A) and peripheral blood (PB) CD4+ cells (B) after PMA/ionomycin stimulation from a patient with primary angiitis of the CNS (PACNS) (left) and a control (right). Numbers in plots indicate the frequencies of gated CD4+ cells in each quadrant. Frequencies of IL-17-producing (C) and IFN-γ-producing (D) cells in expanded CSF CD4+ cells (left, closed symbols) and in PB CD4+ cells (right, open symbols) in patients with noninflammatory neurologic disease (C), giant cell vasculitis (GCV), PACNS, secondary angiitis of the CNS (SACNS), and stroke (S). ***p < 0.001.
Table 2.
Estimates of sensitivity and specificity of IL-17 producing CD4+ cells in the CSF (cutoff 7%) for differentiating stroke due to cerebral vasculitis from other ischemic strokes
We also assessed soluble IL-17 in the CSF of our patients using the highly sensitive MSD platform. All values were under detection levels, except for one patient where CSF was taken shortly before he died of a fulminant vasculitis (PACNS5; data not shown). In this patient, cells were not available for analysis. In contrast to IL-17, IFN-γ production by expanded CSF cells was very high, up to 90% of the cells, but similar in all groups (figure 1). We found no differences in cytokine production by peripheral CD4, CD8, or Tγδ cells (figure 1 and not shown).
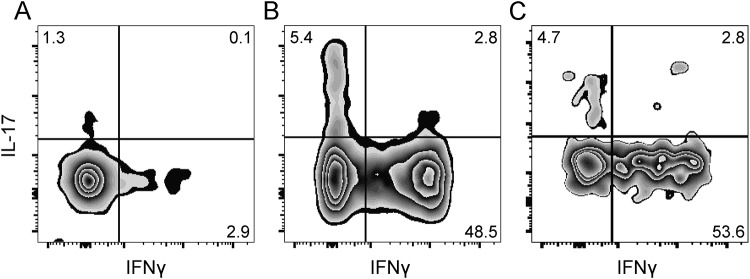
In one case (patient SACNS1), we had enough CSF cells to directly analyze cytokine production ex vivo, without expansion. We found that CD4+ cells also produced high amounts of IL-17 (8.2%) and IFN-γ (51.3%) compared to peripheral blood cells (1.3% and 2.9%, respectively). Of note, we found that the percentages of cytokine-producing T cells in the CSF of the same patient after the 2-week expansion period (7.5% IL-17 and 56.4% IFN-γ) were similar to the ex vivo cells (figure 2). This suggests that our expansion protocol did not substantially alter the cytokine profile of the CSF cells.
Figure 2. T-cell expansion did not alter the cytokine profile of CD4 cells.
Flow cytometry dot plots show interleukin-17 (IL-17) and interferon-γ (IFN-γ) production by CD4+ T cells in a patient (SACNS1) for whom we could analyze peripheral blood CD4+ cells (A), CSF cells ex vivo (B), and expanded CSF CD4+ cells (C). Numbers in plots indicate frequencies of gated cells within each quadrant.
DISCUSSION
Even with modern imaging techniques and improved detection methods, the diagnosis of cerebral vasculitis remains uncertain. There is no information available on biomarkers associated with CV. Here, we report on intracellular IL-17 in expanded T cells from the CSF of patients with cerebral ischemia as potential diagnostic tool to help distinguish patients with cerebral vasculitis from patients with stroke due to other causes.
Cellular immunity plays a major role in the pathogenesis of systemic vasculitis. For example, a chronic CD4 T-cell activation can be found in peripheral blood from patients with AAV with the degree of CD4+ T-cell activation correlating with the disease severity.6 In patients with AAV, T cells are biased towards Th17 cells following in vitro stimulation and increased levels of Th17 cells can persist even during remission,4,7 which argues in favor of Th17 cells being present in all disease stages. In addition, serum IL-17A and IL-23 levels are elevated in acute disease stages of patients with AAV but also in a proportion of convalescent patients, while IFN-γ production returns to control levels.8 In agreement with these observations, we also found persistent Th17 cells in the CSF of patients who were in remission and in patients with normal CSF cell count (table 1), while we did not see a difference in the production of IFN-γ in our expanded T-cell population compared to controls.
Interestingly, in the 3 patients with a proven GCV, we did not detect an increased IL-17 in the expanded CSF cells. In untreated patients with GCV, Th17 cells can be found in the blood and are part of the cellular infiltrates in the arteries.9 However, arteries from treated patients contain few Th17 cells, which is a clear difference from AAV. We would argue that the reason for the absence of IL-17-producing T cells in the CSF of patients with GCV can be explained by 2 possibilities: (1) GCV only affects large vessels, which do not come in contact with the CSF, or (2) Th17 cells already disappeared after the steroid treatment.
The presence of Th17 cells and the role of IL-17 can be generalized to other autoimmune diseases such as Churg-Strauss vasculitis, rheumatoid arthritis, and systemic lupus erythematosus, making it a target for novel therapeutic interventions. Humanized anti-IL-17 antibodies neutralizing the biologic activity of IL-17 induce clinically relevant responses in patients with psoriasis and rheumatoid arthritis.10 Anti-IL-17 is now on trial for AAV and might constitute a future therapeutic option for cerebral vasculitis.
The levels of IL-17 in the CSF have proven too low for detection, even using a highly sensitive method. Detection of IL-17 produced by the cellular component is feasible, but due to the low number of T cells in the CSF, it is unavoidable to expand the cells before analysis, and this manipulation could bias the results. However, in 2 patients (one of them with a neurosarcoidosis, and therefore not included in the study), we were able to collect enough cells to stain IL-17 and IFN-γ directly and after the expansion with similar results, arguing that our expansion protocol reflects the in vivo situation. In addition, recent publications demonstrate cytokine memory for T-helper cells.11,12 Taken together, it is likely that in systemic autoimmune disease, particularly vasculitis, persistent Th17 cells can be found. Compartments such as the CSF are spaces with close contact to small vessels and can reflect inflammatory vessel pathology. Thus, CSF Th17 cells might be a much more specific biomarker than cell count or protein elevation.
Limitations.
As the choice of the cutoff was data-driven and sensitivity and specificity resulted from an in-sample evaluation, an optimistic bias of the result cannot be excluded. Therefore, an independent validation of the cutoff and the diagnostic accuracy in a dataset of appropriate size is recommended.
ACKNOWLEDGMENT
The authors thank the patients and healthy blood donors for their cooperation; the FACS Core Facility at the UKE for technical help; and Dr. Vettorazzi, Department of Medical Biometry and Epidemiology, for help with the statistical analysis.
GLOSSARY
- AAV
anti–neutrophil cytoplasmic autoantibody–associated vasculitis
- CV
cerebral vasculitis
- GCV
giant cell vasculitis
- IFN-γ
interferon-γ
- IL-17
interleukin-17
- MSD
Meso Scale Discovery
- PACNS
primary angiitis of the CNS
- SACNS
secondary angiitis of the CNS
AUTHOR CONTRIBUTIONS
Vivien Thom: study concept and design, acquisition of data, analysis and interpretation. Sabrina Schmid: acquisition of data. Mathias Gelderblom: study concept and design, critical revision of the manuscript for important intellectual content. Manuela Kolster: acquisition of data. Romy Hackbusch: acquisition of data. Simon Schuster: critical revision of the manuscript for important intellectual content. Götz Thomalla: critical revision of the manuscript for important intellectual content. Oliver Keminer: acquisition of data. Ole Pless: critical revision of the manuscript for important intellectual content. Christian Bernreuther: acquisition of data, critical revision of the manuscript for important intellectual content. Markus Glatzel: acquisition of data, critical revision of the manuscript for important intellectual content. Karl Wegscheider: analysis and interpretation. Christian Gerloff: critical revision of the manuscript for important intellectual content, study supervision. Tim Magnus: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Eva Tolosa: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision.
STUDY FUNDING
No targeted funding.
DISCLOSURES
V. Thom and S. Schmid report no disclosures. M. Gelderblom served on the scientific advisory board for Merz Pharmaceuticals, received travel funding and/or speaker honoraria from Merz Pharmaceuticals, received research support from Merz Pharmaceuticals and Allergan and is on the editorial board for PLOSone. R. Hackbusch, M. Kolster, and S. Schuster report no disclosures. G. Thomalla received travel funding and/or speaker honoraria from Bayer, Boheringer Ingelheim, Daichii Sankyo, and Bristol Myer Squibb/Pfizer, consulted for Acandis and GlaxoSmithKline, and received research support from Bayer, European Union, and Deutsche Forschungsgesellschaft. O. Keminer, O. Pless, and C. Bernreuther report no disclosures. M. Glatzel received research support from Janssen Pharmaceuticals. K. Wegscheider served on the scientific advisory board for Resmed and Biotronik, and received research support from Resmed, Biotronik, BMBF, and DFG. C. Gerloff serves on the scientific advisory board for Bayer Vital, Boehringer Ingelheim, EBS Technologies, and Silk Road Medical, received travel funding and/or speaker honoraria from Bayer Vital, Boehringer Ingelheim, Biogen Idec, ev2/Covidien, GlaxoSmithKline, Grifols, Inomed, Lundbeck, Nexstim, Pfizer, Sanofi Aventis, UCB, and Merck Serono, is on the editorial board for INFO Neurology Psychatrie, and Aktuelle Neurologie, is editor of the textbook Therapie und Verlauf neurologischer Erkrankungen (Kohlhammer Verlag), has consulted for EBS Technologies and Silk Road Medical, and received research support from Merz Pharmaceuticals, Allergan, Novartis, NeuroConn, DFG, BMBF/DFG, EU FP7, and Wegener Foundation. T. Magnus received travel funding and/or speaker honoraria from Novartis, Merck-Serono, Grifols, Boehringer Ingelheim, and CSL Behring, and received research support from Novartis, ERANET, DFG, and Werner Otto Society. E. Tolosa received research support from DFG, Wissenschaftsstiftung Hamburg, and Werner-Otto Foundation. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Berlit P, Kraemer M. Cerebral vasculitis in adults: what are the steps in order to establish the diagnosis? Red flags and pitfalls. Clin Exp Immunol 2014;175:419–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajj-Ali RA, Calabrese LH. Diagnosis and classification of central nervous system vasculitis. J Autoimmun 2014;48-49:149–152. [DOI] [PubMed] [Google Scholar]
- 3.Salvarani C, Brown RD, Jr, Hunder GG. Adult primary central nervous system vasculitis. Lancet 2012;380:767–777. [DOI] [PubMed] [Google Scholar]
- 4.Kallenberg CG. Pathophysiology of ANCA-associated small vessel vasculitis. Curr Rheumatol Rep 2010;12:399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain 2009;132:3329–3341. [DOI] [PubMed] [Google Scholar]
- 6.Marinaki S, Kalsch AI, Grimminger P, et al. Persistent T-cell activation and clinical correlations in patients with ANCA-associated systemic vasculitis. Nephrol Dial Transplant 2006;21:1825–1832. [DOI] [PubMed] [Google Scholar]
- 7.Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. Skewed distribution of Th17 lymphocytes in patients with Wegener's granulomatosis in remission. Arthritis Rheum 2008;58:2196–2205. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira E, Hamour S, Sawant D, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant 2010;25:2209–2217. [DOI] [PubMed] [Google Scholar]
- 9.Weyand CM, Goronzy JJ. Immune mechanisms in medium and large-vessel vasculitis. Nat Rev Rheumatol 2013;9:731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hueber W, Patel DD, Dryja T, et al. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci Transl Med 2010;2:52ra72. [DOI] [PubMed] [Google Scholar]
- 11.Becattini S, Latorre D, Mele F, et al. T cell immunity: functional heterogeneity of human memory CD4(+) T cell clones primed by pathogens or vaccines. Science 2015;347:400–406. [DOI] [PubMed] [Google Scholar]
- 12.Helmstetter C, Flossdorf M, Peine M, et al. Individual T helper cells have a quantitative cytokine memory. Immunity 2015;42:108–122. [DOI] [PMC free article] [PubMed] [Google Scholar]