Abstract
Legionella pneumophila is a facultative intracellular bacterium that lives in aquatic environments where it parasitizes amoeba. However, upon inhalation of contaminated aerosols it can infect and replicate in human alveolar macrophages, which can result in Legionnaires’ disease, a severe form of pneumonia. Upon experimental airway infection of mice, L. pneumophila is rapidly controlled by innate immune mechanisms. Here we identified, on a cell-type specific level, the key innate effector functions responsible for rapid control of infection. In addition to the well-characterized NLRC4-NAIP5 flagellin recognition pathway, tumor necrosis factor (TNF) and reactive oxygen species (ROS) are also essential for effective innate immune control of L. pneumophila. While ROS are essential for the bactericidal activity of neutrophils, alveolar macrophages (AM) rely on neutrophil and monocyte-derived TNF signaling via TNFR1 to restrict bacterial replication. This TNF-mediated antibacterial mechanism depends on the acidification of lysosomes and their fusion with L. pneumophila containing vacuoles (LCVs), as well as caspases with a minor contribution from cysteine-type cathepsins or calpains, and is independent of NLRC4, caspase-1, caspase-11 and NOX2. This study highlights the differential utilization of innate effector pathways to curtail intracellular bacterial replication in specific host cells upon L. pneumophila airway infection.
Author Summary
Legionella pneumophila is a motile gram-negative bacterium found mainly in fresh water environments where it replicates in amoeba. It uses a molecular syringe to inject effector molecules into these predatory host cells, reprograming them to support L. pneumophila growth. Upon inhalation of contaminated aerosols, L. pneumophila uses the same approach to replicate in human alveolar macrophages, which can result in a severe pneumonia known as Legionnaires’ disease. However, L. pneumophila is normally controlled by the innate immune system, and the key mechanisms and cells involved in this immune response remain unclear. Here we show that tumor necrosis factor (TNF) and reactive oxygen species (ROS) play a dominant role in the clearance of L. pneumophila from the lung. Neutrophils kill L. pneumophila using ROS, while alveolar macrophages are activated by TNF produced by neutrophils and monocytes that are recruited to the lung. TNF-activated alveolar macrophages kill L. pneumophila by recruiting lysosomes and acidifying L. pneumophila containing vacuoles. Caspases other than caspase-1 and 11 are involved in this mechanism, with a minor contribution from cysteine-type cathepsins or calpains. This study deepens our understanding of the mechanisms by which TNF contributes to the control of intracellular pathogens, and highlights the key elements of the innate immune response to L. pneumophila lung infection.
Introduction
L. pneumophila is a Gram-negative bacterium with global distribution in freshwater environments, where it replicates intracellularly mainly in amoebae [1–3]. L. pneumophila commonly causes community acquired and nosocomial pneumonia. Although it is normally controlled by the innate immune response, L. pneumophila has the potential to cause a severe pneumonia known as Legionnaires' disease with mortality rates of up to 30% if early bacterial replication is not controlled [4–6]. Infection occurs through inhalation of L. pneumophila contaminated aerosols, mostly generated by manmade technologies such as cooling towers, air conditioners or even car windshield wipers [7–9]. In the lung L. pneumophila initially exclusively infects alveolar macrophages (AM), using a type IV secretion system (T4SS) to inject over 300 effector proteins into the cytosol [7,10–12]. These effectors block phagosomal maturation and fusion with lysosomes, thus preventing L. pneumophila degradation, and promoting the establishment of a Legionella containing vacuole (LCV), the intracellular niche in which L. pneumophila replicates [13–16].
Though critical for L. pneumophila replication, the T4SS also potently induces the innate immune response by several mechanisms (reviewed in [17]). AM sense the action of the T4SS and respond by secreting IL-1α, inducing the secretion of chemokines by airway epithelial cells (AECs), resulting in the rapid recruitment of neutrophils and monocytes to the lung [10,18,19]. Neutrophils are known to be critical for the clearance of L. pneumophila lung infection, as evidenced by neutrophil depletion studies [18,20,21], in vivo blockade of CXCR2 [22] and studies examining the role of IL1R signaling [18,19,23]. However, the mechanisms by which neutrophils contribute to the resolution of L. pneumophila lung infection remain incompletely understood.
IL-1 is closely linked to the induction of TNF in a broad spectrum of unrelated models of inflammation, and these cytokines are known to have synergistic effects in vivo [24–26]. Indeed, anti-TNF therapy is a recognized risk factor for Legionnaire's disease, suggesting a role for TNF in the immune response to L. pneumophila [27–31]. Previous work has established that TNF is produced in response to L. pneumophila in a T4SS-dependent and flagellin-independent manner [32,33] and can limit replication in macrophages [34–36]. Furthermore, it was shown that TNF contributes to immune defense against L. pneumophila in vivo [37–39]. However, the mechanisms by which TNF contributes to innate immune control of L. pneumophila and the cells upon which it acts in vivo have yet to be elucidated.
Macrophages from C57BL/6 mice are not permissive for L. pneumophila replication due to the intracellular sensor NAIP5 which binds cytosolic flagellin and recruits NLRC4, resulting in inflammasome assembly and the activation of Caspase-1 [40,41]. Active caspase-1 can initiate a pro-inflammatory form of cell death known as pyroptosis, the secretion of IL-1β and IL-18, as well as activate Caspase-7, which induces the fusion of lysosomes with LCVs, resulting in bacterial degradation [42,43]. Murine macrophages missing key components in this pathway are permissive to L. pneumophila replication, including NAIP5-/-, NLRC4-/-, Caspase-1-/- and Caspase-7-/- macrophages [42]. NLRC4 also restricts L. pneumophila via caspase-1 independent mechanisms [44]. Similarly, it has been shown that human NAIP (hNAIP), the only NAIP protein identified in humans, can mediate inflammasome assembly and L. pneumophila restriction when overexpressed in murine macrophages, and that L. pneumophila replication is enhanced in human macrophages when hNAIP is silenced [45,46]. Furthermore, primary human macrophages sense L. pneumophila flagellin via hNAIP and activate caspase-1 [47,48].
Macrophages from A/J mice are permissive to L. pneumophila replication due to an allelic variation in the NAIP5 gene, resulting in 14 amino acid (aa) differences as compared to C57BL/6 mice [49,50]. A/J macrophages are able to activate Caspase-1 in response to L. pneumophila infection [51], but fail to activate caspase-7, suggesting that at least some of the 14 aa are involved in promoting caspase-1 and caspase-7 interactions [40,42]. Other mouse strains also display partial susceptibility to L. pneumophila infection and replication, including FvB/N, C3H/HeJ, BALB/cJ and 129S1 mice [49]. In this paper we make use of mice with the 129S1 NAIP5 allele (NAIP5129S1) that have a targeted TNF deletion in macrophages, monocytes and neutrophils (MN-TNF NAIP5129S1 mice) [52] to examine the role of TNF derived from macrophages, monocytes and neutrophils in L. pneumophila lung infection in the absence of strong NAIP5 signaling.
In the present study, we demonstrate that TNF and reactive oxygen species (ROS) are essential for the effective innate immune control of L. pneumophila, and that in vivo TNF can compensate for the well characterized NLRC4-NAIP5 flagellin pathway. While ROS are essential for the bactericidal activity of neutrophils, TNF produced by neutrophils and monocytes is required to enhance AM-mediated restriction of L. pneumophila via TNFR1 in vivo. This TNF-mediated antibacterial mechanism is independent of NLRC4, caspase-1 and 11, but involves other caspases with a minor contribution from cysteine-type cathepsins or calpains, and also the fusion of LCVs with lysosomes and their acidification. The striking susceptibility of MN-TNF NAIP5129S1 mice to L. pneumophila lung infection suggests that TNF is a key component of innate immunity to L. pneumophila lung infection, especially when NAIP5-NLRC4 mediated responses are dampened.
Results
TNF and ROS are important for the clearance of L. pneumophila in vivo
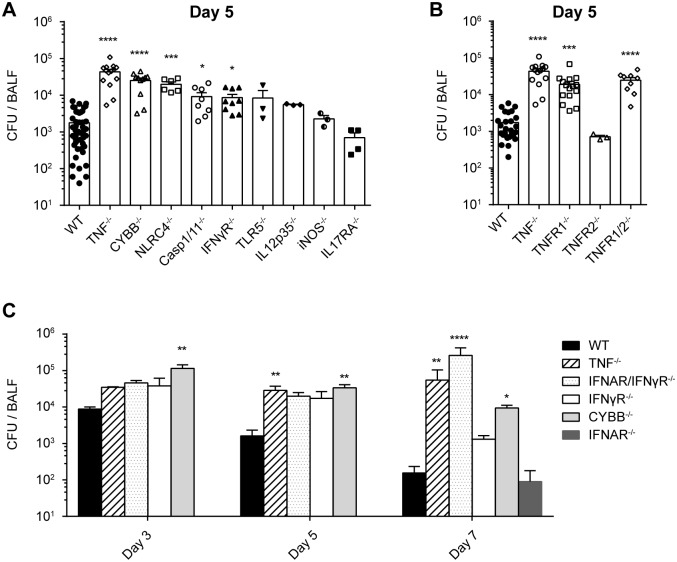
Many host immune factors have been shown to be involved in L. pneumophila control in vitro, whereas relatively few studies have assessed their impact in vivo. We therefore used an intranasal mouse infection model to identify crucial innate immune effector molecules and pathways that have been implicated in the clearance of L. pneumophila lung infection, by assessing their relative impact on bacterial burden in the lung 3–7 days p.i.. As has been previously demonstrated, we found that while IFNγR-/- and IFNAR-/- mice showed limited susceptibility to infection, double deficiency for IFNAR/IFNγR dramatically increased bacterial loads, in particular by day 7 post infection (Fig 1A and 1C, [53]). Similarly, by day 5 and 7 p.i., TNF deficiency resulted in severely increased bacterial burden, and deficiency in the phagocyte NADPH oxidase NOX2/gp91phox (CYBB-/- mice) resulted in potent impairment in bacterial control from day 3 through to day 7 (Fig 1A and 1C). In contrast, NLRC4, caspase-1/11, TLR5, IL-12, iNOS and IL17RA seem to play a less dominant role in controlling L. pneumophila lung infection (Fig 1A). These results show that TNF and ROS, as well as the combined action of Type I and II IFN signaling are crucial for the innate immune response to L. pneumophila lung infection.
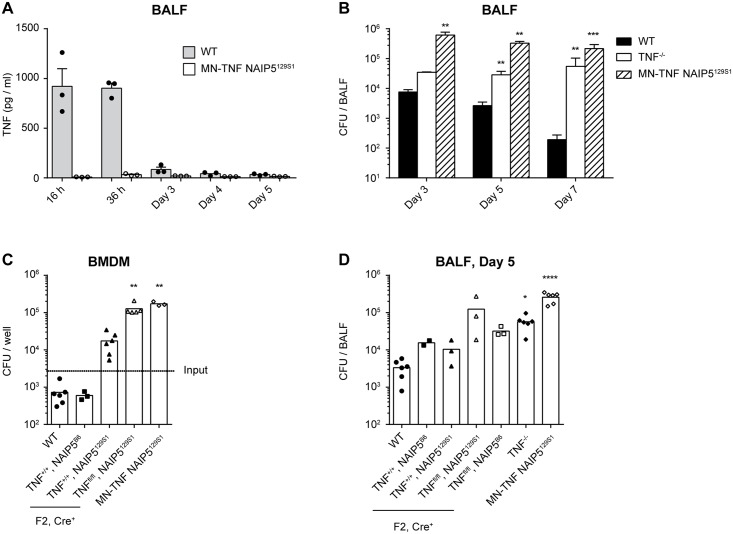
Fig 1. TNF / TNFR1 and ROS are important for clearance of L. pneumophila in vivo.
(A-C) WT or knockout mice were infected intranasally with WT L. pneumophila, and 5 days p.i. (A) or 3–7 days p.i. (C) BALF CFU were quantified on CYE agar plates. Data in panel A, B and C are from 15, 8, and 2 pooled experiments, respectively. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to WT by Kruskal-Wallis test with Dunn's post test.
TNF acts via TNFR1 to control L. pneumophila infection
To identify the receptor through which TNF exerts its protective effect, WT, TNF-/-, TNFR1-/-, TNFR2-/- and TNFR1/2-/- mice were infected intranasally with WT L. pneumophila and CFUs were compared in the BALF 5 days p.i.. Bacterial clearance was delayed to a similar extent in TNF-/-, TNFR1-/- and TNFR1/2-/- but not TNFR2-/- mice compared to WT mice, showing that TNF mediates its antibacterial effect via TNFR1 in vivo (Fig 1B).
TNF / TNFR1 signaling contributes to AM but not neutrophil-mediated killing of L. pneumophila in vivo
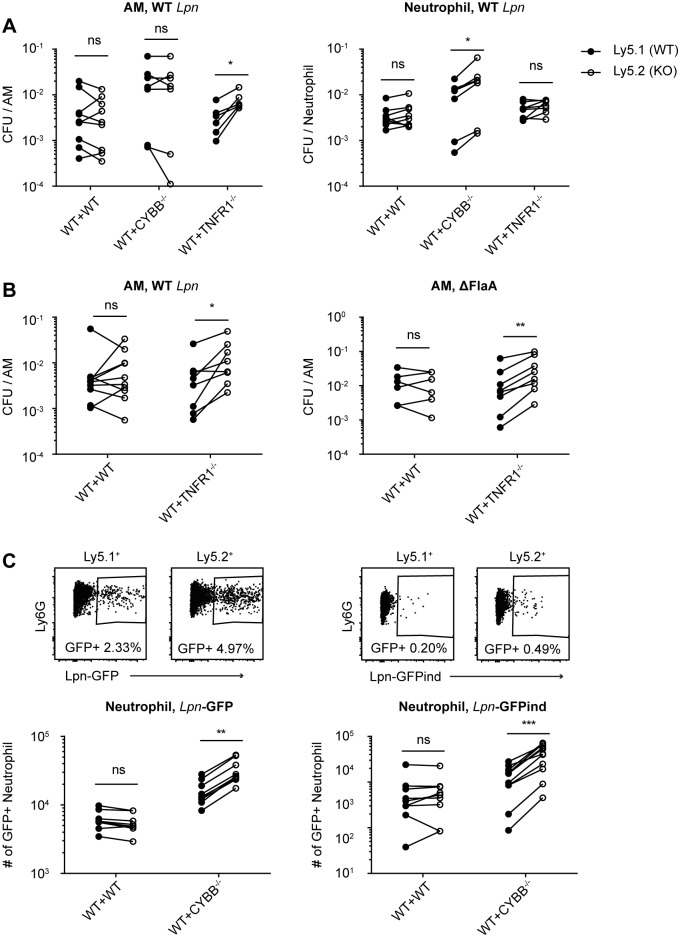
A recent study using a T4SS-based reporter system has demonstrated that AM and neutrophils are the primary targets for L. pneumophila in vivo, with L. pneumophila replication having been demonstrated in AM [10]. We therefore examined the impact of TNF on AM and neutrophil-mediated killing of L. pneumophila in vivo. To circumvent the problem that TNFR1-/- mice have greater bacterial burdens in the lung than WT mice and allow for the direct comparison of AM and neutrophil bacterial loads in WT and TNFR1-/- cells within a single mouse, we used a mixed chimera approach. Mixed bone marrow (BM) chimeric mice were generated with a mix of 50% Ly5.1+ WT BM and either 50% Ly5.2+ WT or Ly5.2+ TNFR1-/- BM. After 8 weeks of reconstitution, WT:WT and WT:TNFR1-/- mice were inoculated intranasally with WT L. pneumophila, and 2 days p.i. Ly5.1+ and Ly5.2+ AM and neutrophils were sorted from the BALF, and cells were plated on CYE plates to quantify viable L. pneumophila. Significantly more CFU / AM were recovered from TNFR1-/- AM than from WT AM, indicating that TNF signaling via TNFR1 promotes the killing of L. pneumophila by AM in vivo (Fig 2A and 2B). In contrast, there was no difference in the number of viable L. pneumophila / neutrophil recovered from WT vs. TNFR1-/- neutrophils, indicating that TNF signaling does not contribute to neutrophil-mediated killing of L. pneumophila (Fig 2A). The killing of L. pneumophila lacking flagellin was also impaired in TNFR1-/- AM compared to WT AM, demonstrating that the antibacterial mechanism mediated in AM by TNF / TNFR1 is independent of the NAIP5-NLRC4 flagellin recognition pathway (Fig 2B). These results highlight that TNF / TNFR1 signaling mediates a non-redundant antibacterial mechanism that contributes to L. pneumophila killing in AM but not in neutrophils in vivo.
Fig 2. TNF / TNFR1 signaling contributes to AM-mediated killing of L. pneumophila, while ROS are required for efficient neutrophil-mediated killing in vivo.
(A-C) Mixed BM chimeric mice reconstituted with 50% Ly5.1+ WT BM, and either 50% Ly5.2+ WT, TNFR1-/- or CYBB-/- BM were generated. (A) Chimeras were infected with WT L. pneumophila, and 2 days p.i. BALF was harvested and Ly5.1+ and Ly5.2+ AM and neutrophils were sorted. Cells were lysed and CFU were quantified on CYE agar plates. (B) Chimeras were infected with WT or ΔFlaA L. pneumophila, and CFU were quantified in AM as in A). (C) Chimeras were infected with Lpn-GFP or Lpn-GFPind (with IPTG induction) and BALF was analyzed by flow cytometry 38 hr p.i.. GFP+ neutrophils were normalized for the number of Ly5.1+ and Ly5.2+ neutrophils, respectively. Data are from 2–4 pooled experiments. *p<0.05, **p<0.01, ***p<0.001 by Wilcoxon test.
ROS are required for efficient neutrophil but not AM-mediated killing of L. pneumophila in vivo
To analyze the impact of ROS on AM and neutrophil-mediated killing of L. pneumophila, we generated BM chimeric mice with a mix of 50% Ly5.1+ WT BM and either 50% WT Ly5.2+ or Ly5.2+ CYBB-/- BM. 2 days p.i. we observed that while sorted CYBB-/- AM did not contain more viable L. pneumophila / AM than WT AM, sorted CYBB-/- neutrophils contained more viable L. pneumophila / neutrophil than did WT neutrophils from the same mouse (Fig 2A). This indicates that in contrast to TNF, NOX2-derived ROS play a non-redundant role in neutrophil-mediated killing of L. pneumophila but not AM-mediated killing of L. pneumophila in vivo.
We performed similar experiments in which WT:WT and WT:CYBB-/- BM chimeric mice were inoculated with either L. pneumophila constitutively expressing GFP (Lpn-GFP), or with L. pneumophila containing a plasmid on which GFP expression can be induced by the addition of IPTG (Lpn-GFPind), thereby identifying metabolically active bacteria (Fig 2C). Neutrophils were analyzed by flow cytometry 38 hours p.i., and in the case of Lpn -GFPind infected mice, IPTG was administered intranasally at 35 hours p.i., resulting in the induction of GFP in all viable L. pneumophila. In line with the results of the BM chimera sort and plating experiments, there were more GFP+ CYBB-/- neutrophils than GFP+ WT neutrophils in WT:CYBB-/- BM chimeric mice, both with Lpn-GFP infection and with Lpn-GFPind infection (Fig 2C). In the case of Lpn-GFP infection this indicates that there were more NOX2-deficient neutrophils that contained dead or viable L. pneumophila than WT neutrophils, and in the case of Lpn-GFPind infection it indicates that there were more NOX2-deficient neutrophils that contained viable L. pneumophila than WT neutrophils in the same mouse. These data support the hypothesis that neutrophils require ROS to kill and degrade L. pneumophila in vivo.
In vivo, neutrophils but not AM produce ROS in response to L. pneumophila infection
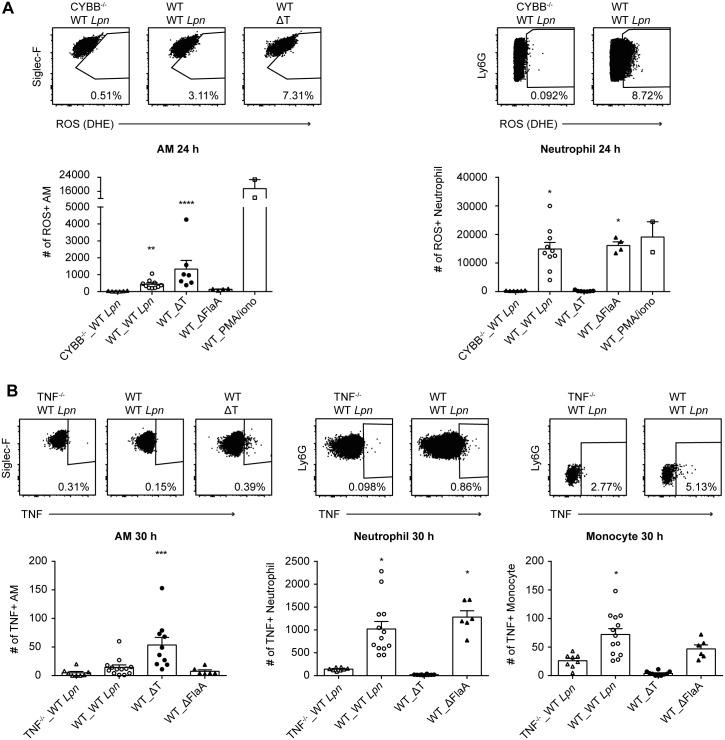
Having established that NOX2-dependent mechanisms are involved in neutrophil-mediated killing of L. pneumophila, we sought to determine if neutrophils actively produce ROS in response to L. pneumophila. We infected WT and CYBB-/- mice with WT, T4SS deficient (ΔT) and ΔFlaA L. pneumophila and stained neutrophils and AM with a flow cytometry based ROS detection reagent (Dihydroethidium) 24 h p.i.. We observed that neutrophils but not AM produced ROS in response to WT and ΔFlaA L. pneumophila 24 h p.i., suggesting that ROS could have direct bactericidal effects in L. pneumophila containing neutrophils (Fig 3A). Since we did not observe neutrophil ROS production in response to ΔT L. pneumophila, our results suggest this ROS production is T4SS-dependent and flagellin independent (Fig 3A). Conversely, AM produced very little ROS in response to WT L. pneumophila, but more in response to ΔT L. pneumophila, in line with a publication suggesting that L. pneumophila actively inhibits ROS production in macrophages via T4SS-dependent effector molecules (Fig 3A, [54]).
Fig 3. In vivo, neutrophils but not AM produce ROS and TNF in response to L. pneumophila infection.
(A) WT and CYBB-/- mice were infected intranasally with WT, ΔT or ΔFlaA L. pneumophila and BALF cells were harvested 24 hr p.i.. AM, neutrophils and monocytes were stained for ROS with Dihydroethidium (DHE) and analyzed by flow cytometry. (B) WT and TNF-/- mice were infected intranasally with WT, ΔT or ΔFlaA L. pneumophila and BALF cells were harvested 30 hr p.i.. AM, neutrophils and monocytes were stained for TNF and analyzed by flow cytometry. Data are from 2–3 pooled experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to CYBB-/- or TNF-/- mice by Kruskal-Wallis test with Dunn's post test.
TNF mediates an antibacterial effect in macrophages via TNFR1, which is independent of NLRC4 and NOX2
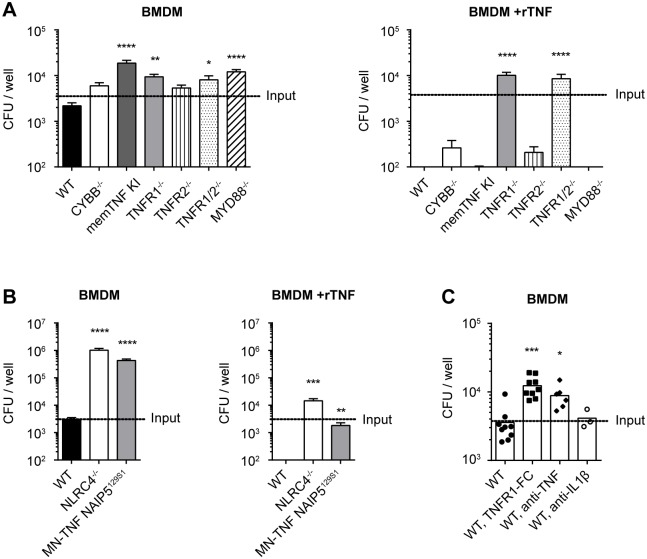
The in vivo results presented in Fig 2A in combination with the observation that in vitro, TNFR1-/- and TNFR1/2-/- but not TNFR2-/- bone marrow derived macrophages (BMDM) were more permissive to L. pneumophila replication than WT BMDM, suggest that TNF directly inhibits L. pneumophila replication in macrophages via signaling through TNFR1 (Fig 4A). Furthermore, the addition of recombinant TNF (rTNF) to BMDM abrogated L. pneumophila growth in all of the genotypes with a functional TNFR1, including NLRC4-/- and CYBB-/- BMDM (Fig 4A). These data show that TNF-mediates an antibacterial mechanism in BMDM via TNFR1, which is independent of NOX2-derived ROS and the NAIP5-NLRC4 flagellin recognition pathway. The flagellin independence of this mechanism was further shown by the TNF-mediated abrogation of ΔFlaA L. pneumophila replication in WT BMDM but not TNFR1-/- BMDM (S3B Fig). Importantly, three day exposure to 100 ng/ml rTNF did not induce BMDM cell death (S1 Fig), suggesting an active antibacterial mechanism mediated by TNF rather than the induction of cell death. Membrane TNF knock-in (memTNF KI) BMDM, which are only able to make membrane bound but not secreted TNF, were also more susceptible than WT BMDM, suggesting that TNF signals as a soluble molecule on BMDM in vitro (Fig 4A). To consolidate this observation, we added a neutralizing anti-TNF antibody or TNFR1 fused to the Fc portion of human IgG1 (TNFR1-Fc) to WT BMDM infected with L. pneumophila, in order to neutralize soluble TNF secreted by the BMDM. This resulted in the sensitization of WT BMDM to L. pneumophila infection to a similar level as that observed for TNFR1-/- BMDM, suggesting that the difference in susceptibility between WT and TNFR1-/- BMDM is due to endogenously secreted TNF in response to L. pneumophila infection (Fig 4A and 4C). Also in line with the conclusion that lack of endogenous TNF results in moderate sensitivity of BMDM to L. pneumophila infection is the observation that MyD88-/- BMDM, which fail to secrete TNF in response to L. pneumophila infection (S2 Fig and [36]), also have a similar susceptibility to L. pneumophila as TNFR1-/- BMDM (Fig 4A). Taken together, these data suggest that TNF activates an antibacterial mechanism in macrophages via TNFR1 that is independent of NLRC4 and NOX2. Furthermore, TNF production by BMDM in response to L. pneumophila is downstream of MyD88.
Fig 4. TNF mediates an antibacterial effect in macrophages via TNFR1, which is independent of NLRC4 and ROS.
(A-B) WT or knockout BMDM were infected with WT L. pneumophila at MOI 0.1. BMDM were either left untreated (left hand panels) or rTNF was added at the time of infection (right hand panels). 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. (C) WT BMDM were infected with WT L. pneumophila MOI 0.1, with or without the addition of TNFR1-Fc, anti-TNF Ab or anti-IL1β Ab. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 3–7 pooled experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to WT by Kruskal-Wallis test with Dunn's post test.
In vivo, TNF produced by neutrophils and monocytes enhances AM-mediated killing of L. pneumophila
In order to determine which cells produce TNF in vivo, we infected WT mice and TNF-/- mice with WT, ΔT and ΔFlaA L. pneumophila and stained BALF cells for TNF 30 hr p.i.. We found that neutrophils and monocytes produced TNF in response to L. pneumophila lung infection, suggesting that neutrophils and monocytes are the relevant TNF source (Fig 3B).
As has been shown in published results, we observed that NLRC4-/- mice were only moderately susceptible to infection, despite the well-recognized role of NLRC4 in inflammasome activation in response to L. pneumophila flagellin, and the high susceptibility of NLRC4-/- macrophages to L. pneumophila replication in vitro (Figs 1A and 4B, [55], [44]). The fact that NLRC4-/- BMDM are highly susceptible to L. pneumophila replication in vitro, but NLRC4-/- mice are only moderately susceptible in vivo, suggests that mechanisms that are only present in vivo are able to compensate for a lack of NLRC4. To determine if paracrine TNF compensates for a lack of NAIP5-NLRC4-mediated signaling in vivo, we infected MN-TNF NAIP5129S1 mice, which have a hypofunctional NAIP5 allele (NAIP5129S1) and are deficient in TNF in macrophages, monocytes and neutrophils, with WT L. pneumophila. We found that MN-TNF NAIP5129S1 mice were highly susceptible to L. pneumophila lung infection, with much greater bacterial burdens in the BALF 5 days p.i. compared to WT mice, and also compared to TNF-/- and NLRC4-/- mice (Figs 1A and 5B). Taken together, these results suggests that TNF produced by neutrophils and monocytes is essential for in vivo control of L. pneumophila lung infection. In addition, BMDM from MN-TNF NAIP5129S1 mice were almost as susceptible to L. pneumophila replication as NLRC4-/- BMDM, and this susceptibility could be abrogated by the addition of rTNF (Fig 4B). Taken together, these data suggest that neutrophil and monocyte derived TNF enhances AM-mediated L. pneumophila killing and partially compensates for a lack of NAIP5-NLRC4 signaling in AM in vivo.
Fig 5. NAIP5129S1 and TNF-deficiency in macrophages, monocytes and neutrophils are the genetic traits that render MN-TNF NAIP5129S1 mice susceptible to L. pneumophila infection in vitro and in vivo.
(A-B) WT, TNF-/-, MN-TNF NAIP5129S1 mice were infected intranasally with WT L. pneumophila, and analyzed at the indicated time points. (A) TNF was quantified in the BALF via CBA assay. (B) BALF CFU were quantified on CYE agar plates. (C) WT or MN-TNF NAIP5129S1 BMDM, or BMDM from the F2 offspring of MN-TNF NAIP5129S1 x C57BL/6 intercrosses were infected with WT L. pneumophila at MOI 0.1. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. (D) WT, TNF-/-, MN-TNF NAIP5129S1 mice, or the F2 offspring of MN-TNF NAIP5129S1 x C57BL/6 intercrosses were infected intranasally with WT L. pneumophila, and 5 days p.i. BALF CFU were quantified on CYE agar plates. All of the F2 offspring shown are Cre+. Panel A is from 1 experiment, panels B-D are from 2–3 pooled experiments. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 compared to WT by Kruskal-Wallis test with Dunn's post test.
To verify that TNF is indeed abrogated in MN-TNF NAIP5129S1 mice, we infected WT and MN-TNF NAIP5129S1 mice intranasally with WT L. pneumophila, and measured TNF in the BALF (Fig 5A). MN-TNF NAIP5129S1 mice had almost undetectable TNF in the BALF following intranasal L. pneumophila infection, confirming that in the context of intranasal L. pneumophila lung infection, neutrophils and monocytes are the primary source of TNF.
Since 129 mice have a documented mutation in caspase-11 [56], we sequenced this gene in MN-TNF NAIP5129S1 mice and found it to be WT. To rule out the possibility that further genes besides NAIP5 from the 129S1 genetic background influenced the phenotype of MN-TNF NAIP5129S1 mice in our experiments, we backcrossed them to C57BL/6 mice, and used the F2 offspring to conduct littermate controlled experiments. All the offspring we used for experiments were positive for MLys-Cre and the NAIP5 locus was sequenced for each individual mouse. We compared in vitro L. pneumophila replication in BMDM derived from the homozygous F2 offspring (TNF+/+/NAIP5B6, TNF+/+/NAIP5129S1, TNFfl/fl/NAIP5B6, TNFfl/fl/NAIP5129S1), C57BL/6 (WT), TNF-/- and MN-TNF NAIP5129S1 mice as well as in vivo bacterial loads in the BALF 5 days after intranasal L. pneumophila infection (Fig 5C and 5D). We observed that BMDM from F2 offspring that were TNF sufficient and carried the NAIP5B6 allele were as resistant to WT L. pneumophila infection as WT BMDM, indicating that these two genes were responsible for the enhanced susceptibility of MN-TNF NAIP5129S1 BMDM (Fig 5C). Furthermore, TNF+/+/NAIP5129S1 BMDM supported more L. pneumophila growth than did TNF+/+/NAIP5B6 BMDM, though this was not statistically significant, and TNFfl/fl/NAIP5129S1 BMDM were as susceptible as MN-TNF NAIP5129S1 BMDM (Fig 5C). These data suggest that MN-TNF NAIP5129S1 BMDM are more susceptible to L. pneumophila infection than WT BMDM due to defects in both NAIP5 signaling and TNF production.
In vivo, we found that TNF+/+ littermates with NAIP5129S1 were not more susceptible than TNF+/+ littermates with NAIP5B6, which is in line with our previous findings and published data indicating that reduced NAIP5-NLRC4 signaling has only a moderate impact on susceptibility to L. pneumophila lung infection in vivo (Fig 5D). In contrast, TNFfl/fl/NAIP5129S1 mice tended to have greater susceptibility to L. pneumophila lung infection, similar to MN-TNF NAIP5129S1 mice, while TNFfl/fl/NAIP5B6 mice had similar susceptibility to TNF-/- mice (Fig 5D). These data suggest that NAIP5129S1 and TNF deficiency in macrophages, monocytes and neutrophils are the genetic elements that mediate the enhanced susceptibility of MN-TNF NAIP5129S1 mice to L. pneumophila lung infection.
TNF induces the fusion of LCVs with lysosomal compartments in macrophages
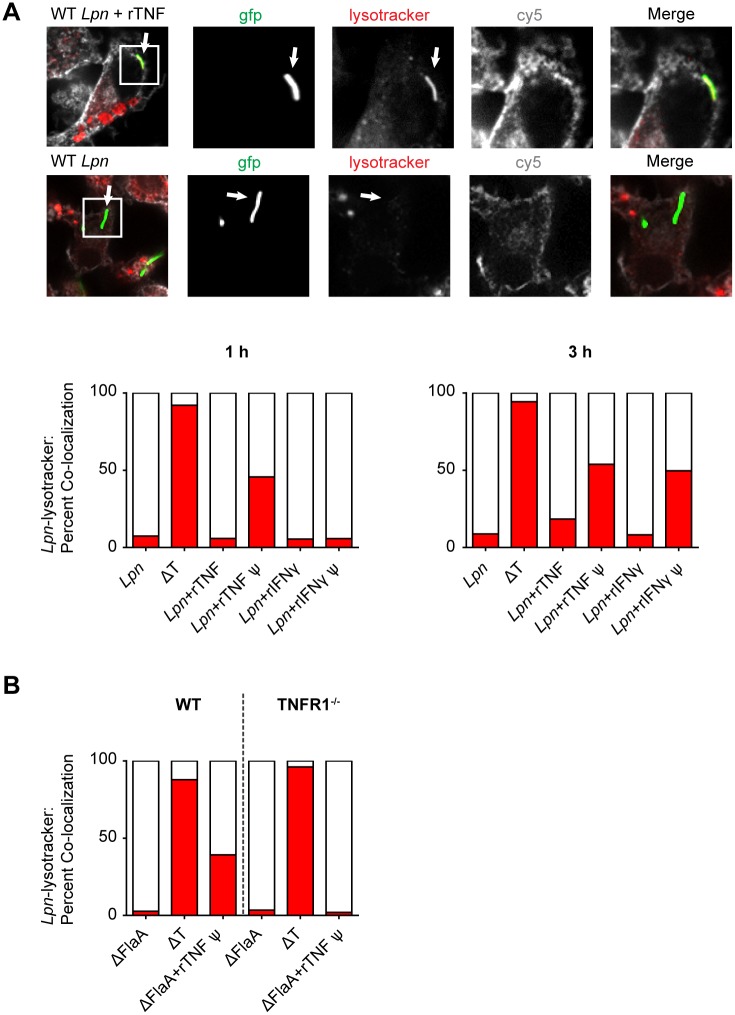
To gain further insight into the antibacterial mechanism mediated by TNF in macrophages, we infected MN-TNF NAIP5129S1 BMDM with Lpn-GFP, in the presence or absence of rTNF or rIFNγ as a positive control [57], and examined the fate of the LCV with respect to lysosomal fusion using confocal microscopy. By 3 hours p.i. neither 100 ng/ml rTNF nor 200 U/ml rIFNγ resulted in Lpn-GFP co-localization with lysosomal compartments as defined by lysotracker staining (Fig 6A). However, when MN-TNF NAIP5129S1 BMDM were pre-treated with rTNF or rIFNγ overnight, by 1 hour p.i. 50% of L. pneumophila in rTNF pre-treated MN-TNF NAIP5129S1 BMDM co-localized with lysosomal compartments, but not in rIFNγ pre-treated BMDM. By 3 hours p.i., Lpn-GFP co-localization with lysosomal compartments was observed in both rTNF and rIFNγ pre-treated MN-TNF NAIP5129S1 BMDM, suggesting that TNF induces the fusion of lysosomes with the LCV, but with different kinetics than IFNγ (Fig 6A). Furthermore, pre-treatment with rTNF was also shown to induce co-localization of ΔFlaA Lpn in WT BMDM but not TNFR1-/- BMDM after 1 hr, confirming TNF-mediated flagellin-independent induction of the fusion of lysosomes with LCVs in macrophages (Fig 6B).
Fig 6. TNF-mediated killing of L. pneumophila is associated with the fusion of LCVs with lysosomal compartments in macrophages.
(A) MN-TNF NAIP5129S1 BMDM were pre-treated overnight with rTNF (rTNFѱ), rIFNγ (rIFNγѱ), or were left untreated, and then infected with Lpn-GFP or ΔT-GFP at MOI 5 with simultaneous addition of rTNF or rIFNγ where indicated. 1 hr or 3 hr p.i. co-localization of Lpn-GFP with lysosomes (stained with lysotracker Red) was analyzed via confocal microscopy, and at least 100 bacteria were counted per group. BMDM cell membranes were stained with Cholera toxin B AF647 (cy5). Data are representative of 2 experiments. (B) WT or TNFR1-/- BMDM were pre-treated overnight with rTNF (rTNFѱ) or were left untreated, and then infected with ΔFlaA Lpn-GFP or ΔT-GFP at MOI 5 with simultaneous addition of rTNF where indicated. 1 hr p.i. co-localization of Lpn-GFP with lysosomes (stained with lysotracker Red) was analyzed via confocal microscopy as in A) and at least 100 bacteria were counted per group. Data is from 1 experiment.
The antibacterial mechanism mediated by TNF in macrophages is dependent on lysosomal acidification and caspase activity
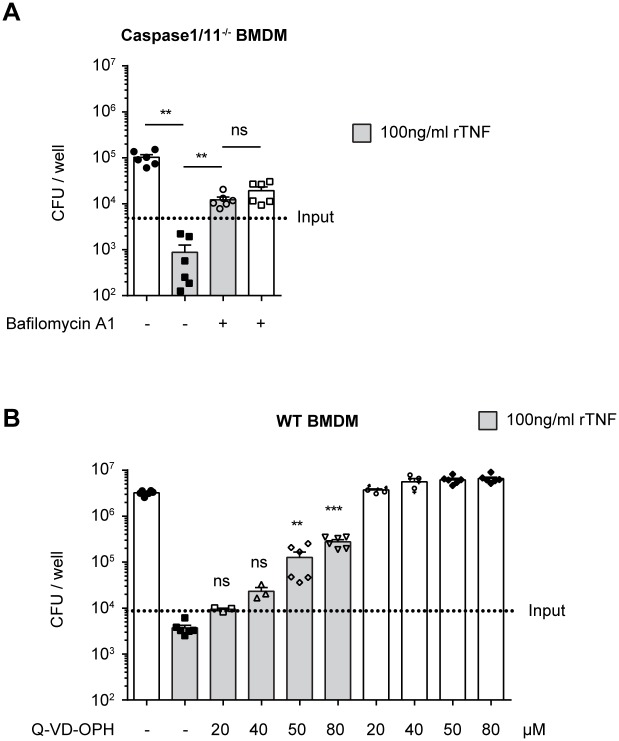
Given that the fusion of LCVs with lysosomes has been shown to be induced by caspase-11, as well as caspase-1 in conjunction with caspase-7 [42,58], we wished to determine if the antibacterial effect mediated by TNF is dependent on Caspase-1 or 11. We therefore infected caspase-1/11-/- BMDM with L. pneumophila, with or without the addition of rTNF, and CFU were quantified 3 days p.i.. The addition of rTNF prevented bacterial replication in Caspase-1/11-/- BMDM, demonstrating that the TNF-mediated antibacterial mechanism in BMDM is independent of Caspase 1 and 11 (Fig 7A). Since our co-localization experiments suggested that TNF redirected L. pneumophila to lysosomal compartments, we sought to determine if lysosomal acidification was required for the TNF-mediated mechanism. To test this we infected Caspase-1/11-/- BMDM with L. pneumophila with or without rTNF and in the presence or absence of bafilomycin A1, a vacuolar H+-ATPase (v-ATPase) inhibitor that blocks lysosomal acidification. We found that bafilomycin A1 abrogates the TNF-mediated inhibition of L. pneumophila, suggesting that lysosomal acidification is required downstream of TNF (Fig 7A).
Fig 7. TNF-mediated killing of L. pneumophila is dependent of lysosomal acidification and caspases other than caspase-1 and 11 in macrophages.
(A) Caspase-1/11-/- BMDM were infected with WT L. pneumophila at MOI 0.1. Where indicated rTNF and/or v-ATPase inhibitor bafilomycin A1 were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 2 pooled experiments. **p<0.01 by Mann-Whitney test. (B) WT BMDM were either pre-treated with rTNF overnight or left untreated, and then infected with ΔFlaA L. pneumophila at MOI 0.1. Where indicated rTNF and/or pan caspase inhibitor Q-VD-OPh were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 2 pooled experiments. **p<0.01, ***p<0.001 compared to WT+rTNF by Kruskal-Wallis test with Dunn's post test.
To determine if further caspases were involved, we tested if the TNF-mediated inhibition of L. pneumophila growth could be blocked using the third generation pan caspase inhibitor Q-VD-OPh, which is highly potent and specific for caspases [59]. We infected WT BMDM with ΔFlaA L. pneumophila, in the presence of increasing concentrations of Q-VD-OPh, with or without rTNF, and quantified CFU 3 days p.i.. Q-VD-OPh blocked TNF-mediated growth restriction in a dose dependent manner, suggesting that caspases other than caspase-1 and 11 are required for the TNF-mediated restriction of L. pneumophila growth in BMDM (Fig 7B). In summary, our data show that the antibacterial mechanism mediated by TNF in BMDM is dependent on at least one caspase and lysosomal acidification, but is independent of Caspase-1 and 11.
TNF-mediated restriction of L. pneumophila growth in macrophages is enhanced by cysteine-type cathepsins or calpains
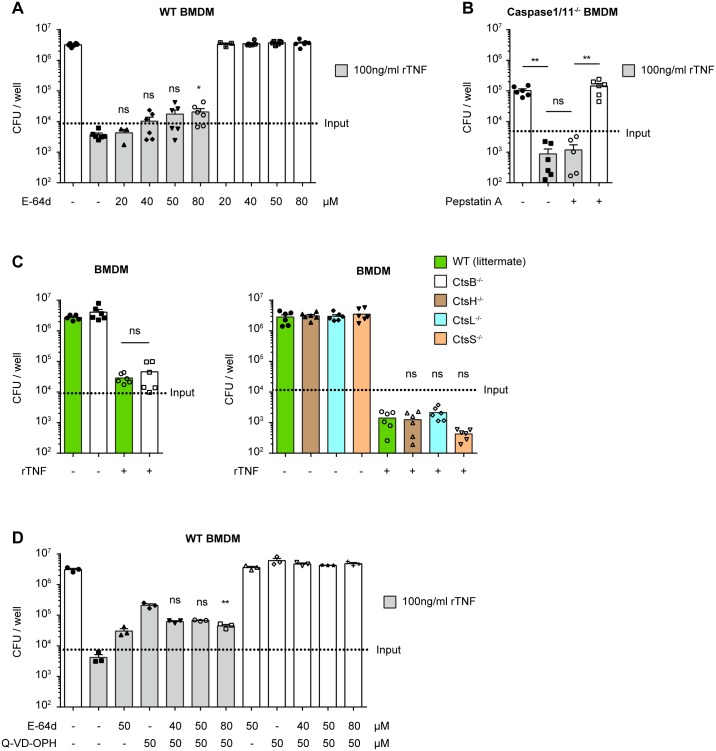
To gain further insight into the TNF-induced bactericidal mechanisms responsible for L. pneumophila degradation in acidic compartments, we investigated the involvement of lysosomal proteases in the cathepsin family. For this we titrated the inhibitor E-64d, which potently inhibits cysteine-type cathepsins and calpains but not caspases [60–64], in the presence or absence of rTNF on ΔFlaA L. pneumophila infected WT BMDM. We observed that E-64d modestly reduced TNF-mediated restriction of L. pneumophila growth only at high concentrations, suggesting that cathepsins or calpains are involved in the TNF-mediated restriction of L. pneumophila growth in BMDM, but play a minor role (Fig 8A).
Fig 8. TNF-mediated restriction of L. pneumophila growth in macrophages is enhanced by cysteine-type cathepsins or calpains.
(A, C, D) WT, CtsB-/-, CtsH-/-, CtsL-/- or CtsS-/- BMDM were either pre-treated with rTNF overnight or left untreated, and then infected with ΔFlaA L. pneumophila at MOI 0.1. Where indicated rTNF and/or E-64d and/or Q-VD-OPh were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. (B) Caspase-1/11-/- BMDM were infected with WT L. pneumophila at MOI 0.1. Where indicated rTNF and/or cathepsin D inhibitor pepstatin A were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. (A) Data are from 2 pooled experiments. *p<0.05 compared to WT+rTNF by Kruskal-Wallis test with Dunn's post test. (B) Data are from 2 pooled experiments. **p<0.01 by Mann-Whitney test. (C) Data are from 2 pooled experiments. *p<0.05 by Mann-Whitney test (left panel) and *p<0.05 compared to WT by Kruskal-Wallis test with Dunn's post test. (D) Data are from 1 experiment. **p<0.01 compared to WT+rTNF+Q-VD-OPh by Kruskal-Wallis test with Dunn's post test.
Next, we attempted to identify individual cathepsins involved in the TNF-mediated mechanism. Cathepsin D inhibition using pepstatin A did not interfere with the TNF-mediated restriction of L. pneumophila replication, suggesting that this aspartic protease is not required (Fig 8B). In follow up of the experiments with cysteine protease inhibitors described above, we observed that the pan caspase inhibitor Z-VAD-FMK partially blocked the TNF-mediated restriction of L. pneumophila growth, both in WT and caspase-1/11-/- BMDM (S4 Fig). Since Z-VAD-FMK is also known to inhibit cathepsin B, H, L and S [59,60], we tested their involvement using BMDM from the corresponding cathepsin knockout mice. We found that rTNF suppressed L. pneumophila replication to a similar degree as in WT BMDM, showing that cathepsin B, H, L and S are not critical for TNF-mediated restriction of L. pneumophila growth in macrophages (Fig 8C). This, however, does not rule out that these cathepsins contribute redundantly to TNF-mediated inhibition of L. pneumophila growth in BMDMs.
To test if caspases and cathepsins / calpains have a synergistic role in TNF-mediated restriction of L. pneumophila replication, which could indicate that they are involved in separate converging pathways, we tested if the effect of E64d and Q-VD-OPh was additive. However, the concomitant addition of E-64d with Q-VD-OPh did not increase the ability of Q-VD-OPh to block the TNF-mediated effect (Fig 8D). These data suggest that caspases and cathepsins / calpains do not synergize to enhance TNF-mediated restriction of L. pneumophila growth, and could be involved interdependently in the same pathway. In conclusion, our data show that cathepsins or calpains contribute somewhat to TNF-mediated restriction of L. pneumophila replication in macrophages, but there is not a non-redundant requirement for cathepsin B, D, H, L or S. Therefore, among the proteases tested, the major cysteine proteases reducing the replication of L. pneumophila upon TNF treatment appear to be the caspases.
Discussion
In this study, we identified cell-type specific key innate immune effector functions responsible for effective control of pulmonary L. pneumophila lung infection. Neutrophil-mediated mechanisms that lead to L. pneumophila clearance in vivo are twofold. On the one hand, neutrophils directly kill L. pneumophila via ROS-mediated mechanisms, and on the other hand, neutrophil and monocyte-derived TNF initiates microbicidal mechanisms in AM via TNFR1, which increase their capacity to inhibit L. pneumophila replication. The latter involves rerouting the bacteria to lysosomal compartments despite the presence of T4SS effectors, and requires at least one caspase other than caspase-1 or 11. The importance of TNF and NOX2-mediated mechanisms in the control of L. pneumophila infection are underscored by the marked susceptibility of TNF-/- and CYBB-/- mice to L. pneumophila infection.
The impact of TNF-mediated antimicrobial mechanisms directed against L. pneumophila cannot be fully appreciated by the study of macrophages in vitro. In accordance with other studies, we observed that TNFR1-/- BMDM only support moderate L. pneumophila growth in comparison to NAIP5-/- or NLRC4-/- BMDM which support several orders of magnitude more growth (Fig 4A and 4B, [36,55]). However, this difference is not observed when comparing the bacterial burden of TNFR1-/- and NLRC4-/- mice in vivo, where there is even a trend for TNF to play a more dominant role (Fig 1A). A possible explanation for these apparently incongruent results is that paracrine TNF produced in vivo by neutrophils and monocytes, rather than autocrine TNF produced by AM, mediates the increased resistance to L. pneumophila, and further that this TNF can compensate for a lack of NAIP5-NLRC4-mediated immune defense. Though we and others did observe modest endogenous TNF production by BMDM in response to L. pneumophila infection (S2 Fig, [36]), and found that this TNF accounted for the increased susceptibility of TNFR1-/- BMDMs (Fig 4C, [36]), this was not enough to compensate for lack of NAIP5-NLRC4 flagellin sensing (Figs 4B and S3B, [36]), arguing against a dominant role for autocrine TNF production by AM. In fact, NLRC4-/- BMDM were highly susceptible to infection despite secreting more TNF than WT BMDM in response to L. pneumophila infection, possibly due to increased bacterial burden or a failure to undergo pyroptosis (S2 Fig, [36]). However, we propose that in vivo, AM are exposed to much higher local concentrations of TNF than produced endogenously by BMDM. In vitro, 200–600 pg/ml TNF were observed in the supernatant of L. pneumophila infected WT BMDM (S2 Fig, [36]), in comparison to 1 ng/ml we observed in the BALF (Fig 5A) and up to 20 ng/ml reported in the BALF at peak concentration [65,66]. Making the conservative estimate of an epithelial lining fluid volume of 100 μl, or a 10–20 fold dilution in 1–2 ml BALF, the actual TNF concentration in the undiluted endothelial lining fluid would be 10–400 ng/ml. Indeed, the addition of 100 ng/ml rTNF markedly suppressed L. pneumophila replication in NLRC4-/- BMDM and increased cell viability (Figs 4B and S1). In addition, TNF has been shown to synergize with other cytokines such as IFNγ and type 1 interferons (IFN) in the restriction of L. pneumophila, which might also be present at higher concentrations in the epithelial lining fluid [34,36,53]. In line with this idea, bacterial burden is more severely impaired in TNF-/- and IFNAR/IFNγR-/- mice at later time points, which could reflect a shared mechanism of action (Fig 1B).
In order to verify the hypothesis that TNF might compensate for reduced NAIP5-NLRC4 mediated mechanisms, we made use of MN-TNF NAIP5129S1 mice, in which TNF is ablated in macrophages, monocytes and neutrophils and which carry the NAIP5129S1 allele. BMDM from MN-TNF NAIP5129S1 mice were almost as susceptible to L. pneumophila infection as BMDM from NLRC4-/- mice, as expected in the absence of strong NAIP5 signaling (Fig 4B). Strikingly, MN-TNF NAIP5129S1 mice were also much more susceptible to L. pneumophila infection in vivo compared to either NLRC4-/- or TNF-/- mice, which in combination with the intracellular staining results suggests that neutrophil and monocyte derived TNF compensates to a large degree for weak NAIP5-NLRC4 flagellin sensing in vivo (Figs 1A, 3B and 5B). Together with the observation that TNF is important for AM but not neutrophil-mediated killing, these experiments highlight the importance of TNF-mediated antibacterial mechanisms in AM in the context of L. pneumophila lung infection.
Our results indicating the functionally relevant production of TNF by neutrophils and monocytes are in agreement with a study by Copenhaver et al. [67]. However, there it was found that AM and DCs are also important for TNF production in response to L. pneumophila lung infection. In contrast, we do not observe significant TNF production by AM, which may reflect differences in the strains of bacteria used between the studies. Though we also observed TNF production by DCs, our results with MN-TNF NAIP5129S1 mice suggest that neutrophil / monocyte-derived TNF is physiologically more relevant for the innate immune response to L. pneumophila (Fig 5A, [52]).
In light of the finding that neutrophil and monocyte-derived TNF mediates an essential AM-driven immune response that can compensate for weak NAIP5-NLRC4-mediated immunity, it is interesting to note that ΔFlaA L. pneumophila is able to replicate in AM within the first 2 days p.i., after which bacteria are cleared [23]. These kinetics fit with the observations that TNF peaks in the BALF 2 days p.i. [39,65], that macrophages require pre-activation of around 20 hours with TNF before they become restrictive for L. pneumophila replication (Fig 6A, [36]), and that failure to recruit neutrophils to the lung from 12 hours up to around 2 days p.i. does not greatly impact bacterial burden, though these kinetics may vary with the size of the inoculum [19,23]. In addition, anti-TNF Ab treatment of A/J mice resulted in an increase in lung bacterial burden only as of around day 3 p.i. [39]. Also consistent with a need for neutrophil-derived TNF is the observation that clearance of ΔFlaA L. pneumophila is delayed to 72 hours p.i. in IL1R-/- mice, in which neutrophil recruitment is delayed, and that in MyD88-/- mice clearance is postponed to 6 days p.i., or even abrogated [23]. Since MyD88-/- BMDM fail to secrete TNF in response to L. pneumophila [36,68], and neutrophils secrete TNF in a flagellin-independent manner (Fig 3B, [10]), it seems highly likely that impaired TNF production by neutrophils and monocytes contributes to the striking susceptibility of MyD88-/- mice to L. pneumophila lung infection. The fact that AM do not produce much TNF in response to L. pneumophila infection but instead rely mostly on neutrophils and monocytes, which must first be recruited to the airways to produce TNF, likely reflects a mechanism which limits overzealous lung inflammation. Indeed, TNF is a very potent cytokine, and it's leakage from the airspace to the circulation can on its own strongly contribute to anaphylactic shock, as shown by systemic anti-TNF treatment in a rabbit model of Pseudomonas aeruginosa pneumonia [69]. Congruent with this idea, though neutrophils are essential for the resolution of L. pneumophila lung infection, they are also associated with lung pathology in Legionnaires' disease [70,71]. This may in part be due to their role in TNF secretion.
We also show that neutrophils kill L. pneumophila in the lung directly by NOX2-dependent mechanisms. Interestingly, AM do not produce ROS in response to WT L. pneumophila. This is in line with a study demonstrating that L. pneumophila actively represses ROS in AM by a T4SS-dependent mechanism [54] and our observation that AM produce ROS in response to ΔT but not much ROS in response to WT L. pneumophila (Fig 3B). Why this mechanism is not active in neutrophils remains unclear, given that both neutrophils and AM are targeted by the T4SS and harbor live L. pneumophila in vivo (Fig 2A, [10]). In fact, for neutrophils the opposite is true, as our results show that ROS induction in neutrophils is T4SS-dependent. On a similar note, a recent study has shown differential responses between macrophages and neutrophils to Salmonella flagellin, in that NAIP5-NLRC4 triggered pyroptosis in macrophages but not neutrophils [72]. How L. pneumophila adapts to these two different intracellular environments also remains unknown. The differential activation of neutrophils and AM by L. pneumophila will likely yield interesting insights into this host-pathogen interaction in future investigations.
In this study, we show that the TNF-mediated antibacterial mechanism in AM is dependent on the rerouting of L. pneumophila to lysosomal compartments, where they are degraded via processes that involve acidification. This acidification likely occurs early in the infection cycle, since fusion of LCVs and lysosomes can be observed within an hour of infection in BMDM pre-treated with TNF. Consistent with this view, a previous study found that L. pneumophila has at least one T4SS effector, SidK, which inhibits the v-ATPase [73]. SidK is highly induced when L. pneumophila begins a new growth cycle, presumably counteracting the early acidification of LCVs [73]. The observation that bafilomycin A1 alone reduced L. pneumophila replication in BMDM is expected, since L. pneumophila requires the acidification of the LCV in late stages of infection for proper LCV maturation [74].
Our data implicate the involvement of at least one caspase other than caspase-1 or 11 in the TNF-mediated growth-restriction of L. pneumophila in macrophages, since the mechanism is active in caspase-1/11-/- BMDM and can be partially blocked by Q-VD-OPh. Of the eight remaining caspases encoded in the mouse genome, namely caspase-2, 3, 6, 7, 8, 9, 12 and 14, a number have been shown to be involved in non-apoptotic functions related to host defense [75]. Caspase-7 has been shown to mediate the fusion of LCVs with lysosomes, though this was dependent on caspase-1 activity [42]. However, caspase-7 has also been demonstrated to protect cells from plasma membrane damage with the pore-forming toxin Listeriolysin O, and this was caspase-1 independent [76]. Caspase-8 has also been shown to mediate innate immune responses involving NFκB activation in response to dsRNA, as well as cell motility [77,78]. Caspases 7 and 8 might therefore be good candidates for involvement in the TNF-mediated mechanism.
Our results also implicate modest involvement of cathepsins or calpains in the TNF-mediated restriction of L. pneumophila replication in macrophages, as demonstrated by the partial inhibition of the TNF-mediated effect by E-64d. However, we did not find a requirement for cathepsin B, D, H, L or S, though a redundant requirement among these cathepsins cannot be excluded. Of note, the cathepsin B inhibitor CA-074-Me partially blocked the TNF-mediated restriction of L. pneumophila growth, however this was shown to be non-specific as the compound blocked the effect equally well in WT and CtsB-/- BMDM (S3C Fig).
Further, we find that caspase and cathepsin or calpain activity may be interdependent. This may not be a surprising result, as cathepsins have been documented to have an involvement upstream of caspase activation in other biological contexts and in vitro [79,80]. Similarly, calpains have been shown to impact the activation of caspase-8, 9 and 12 [81–83]. Furthermore, the intracellular pathogen Francisella tularensis was shown to exploit this relationship to manipulate caspases and promote its survival in neutrophils [82]. Further investigation of these mechanisms will surely yield a better understanding of TNF-mediated host defense mechanisms directed at intracellular pathogens.
Materials and Methods
Ethics statement
This study was conducted in accordance to the guidelines of the animal experimentation law (SR 455.163; TVV) of the Swiss Federal Government. The protocol was approved by Cantonal Veterinary Office of the canton Zurich, Switzerland (Permit number 125/2012).
Mice and L. pneumophila infections
All mice used in this study were bred at the Swiss Federal Institute of Technology Zürich or purchased (Janvier Labs, Le Genest Saint Isle, France) and used at 6–20 weeks of age (age- and sex-matched within experiments). All mice were backcrossed >9 generations on the C57BL/6 background with the exception of MN-TNF NAIP5129S1 mice. MemTNF KI mice and MN-TNF NAIP5129S1 mice have been previously described [52,84]. Sequencing of the MN-TNF NAIP5129S1 mice revealed the same mutations in 129S1 NAIP5 (NAIP5129S1) as previously described [49], with the exception of two mutations in exon 15, which matched the C57BL/6 DNA sequence. Bone marrow chimeric mice were generated as described previously [18], reconstituting with a total of 5 x 106 bone marrow cells and allowing at least 8 weeks for reconstitution of lethally irradiated Ly5.1+ WT recipient mice. Neutrophil and AM chimerism was around 40:60 in WT:WT mice, 35:65 in WT:CYBB-/- mice and 33:67 in WT:TNFR1-/- mice.
The L. pneumophila strains used in this study were the wildtype strain JR32 (Philadelphia-1) [85], as well as modifications of JR32 including an aflagellated mutant (ΔFlaA) [86], JR32-GFP [87], JR32-GFPind (pGS-GFP-04) [88], a deletion mutant lacking a functional Icm/Dot T4SS (ΔT) [89], and ΔT-GFP [87]. L. pneumophila was grown for 3 days at 37°C on charcoal yeast extract (CYE) agar plates before use, with chloramphenicol (5 mg/ml) added for selection of strains containing GFP-encoding plasmids.
For intranasal (i.n.) infections mice were anesthetized with an i.p. injection of 5 mg xylazine/100 mg ketamine per gram body weight, and 5 x 106 CFU L. pneumophila (unless otherwise specified) resuspended in 20 μl PBS were directly applied to one nostril using a Gilson pipette. Bacterial titers in bronchoalveolar lavage fluid (BALF) were determined by plating serial dilutions in PBS on CYE plates. For quantification of CFU from sorted AM and neutrophils, cells were lysed to release viable L. pneumophila by vortexing 30 seconds in 1 ml PBS with 0.7% Tween 20 prior to plating serial dilutions in PBS on CYE plates.
In vitro L. pneumophila infection of BMDM
Bone marrow-derived macrophages (BMDM) were generated by plating bone marrow in L929 conditioned medium containing M-CSF in 5 cm diameter non-cell culture treated Petri dishes as described previously [18]. On day 7, BMDM were harvested in ice cold PBS, 5% FBS, 2.5 mM EDTA by incubating 12 min in the fridge and resuspending by pipetting. The cells were then seeded at 1 x 105 cells/well in 96-well plates and rested overnight prior to infection. L. pneumophila used for infection was grown for 3 days at 37°C on CYE agar plates, then inoculated in ACES yeast extract medium at an OD600 of 0.1 and grown for 21 h at 37°C before use, with 5 mg/ml chloramphenicol added to maintain plasmids. BMDM were infected at MOI 0.1, synchronized by centrifugation, and incubated for 3 days at 37°C, 5% CO2. Intra- and extracellular CFU were quantified on day 3 by plating on CYE plates after a 10 min incubation in dH2O to lyse BMDM. Where indicated, 20 nM V-ATPase inhibitor bafilomycin A1 (Enzo Life Sciences, BML-CM110-0100), 25 μM cathepsin B inhibitor CA-074-Me (Enzo Life Sciences, BML-PI126-0001), 25 μM cathepsin D inhibitor pepstatin A (Enzo Life Sciences, ALX-260-085-M005), 2 μg/ml TNFR1-Fc (Adipogen, AG-40B-0074-C050), 25 μg/ml anti-IL1β (R&D, AB-401-NA), 25 μg/ml anti-TNF (Bioxcell, BE0058, clone XT3.11) or 100 ng/ml TNF (Peprotech, 315-01A) were added 15 min prior to infection.
Microscopy experiments
BMDM were seeded in 24-well plates containing 0.01% polylysine solution (Sigma P4707) coated 12 mm cover glasses (Faust 6080181) at 2.5 x 105 cells/well and rested overnight. Where indicated 100 ng/ml TNF (Peprotech, 315-01A) or 200 U/ml IFNγ was added to pre-activate the BMDM. Cells were infected with Lpn-GFP as described above at MOI 5 for 1 or 3 hours at 37°C, 5% CO2, with the simultaneous addition of100 ng/ml TNF or 200 U/ml IFNγ where indicated. For the final 30 minutes of incubation 1μM lysotracker Red DND-99 (Life Technologies, L7528) and 0.5 μg/ml Cholera toxin B AF647 (CTB-AF647, Life Technologies, C34778) were added to the cells. Cells were then washed with 1 ml PBS, and cover glasses were then placed on parafilm, and fixed 5–10 min at RT with 200 μl 4% PFA in PBS. Cells were washed 3 times with 200 μl PBS, incubating 2 min after applying each wash. Cover glasses were dipped in dH2O, blotted on paper towel to remove excess water and mounted on glass slides with cells facing downwards with 6 μl Mowiol (VWR, 475904–100). Z-stack images were acquired on a spinning-disk confocal microscope (Visitron confocal system) using a 100x objective, and analyzed with volocity software (PerkinElmer, Waltham, MA). To assess co-localization of L. pneumophila and lysosomes, at least 100 bacteria were scored per coverslip.
Antibodies and flow cytometry
BALF was recovered from mice at the specified timepoint in 1 ml sterile PBS containing 5 mM EDTA as previously described [90]. Cells were surface stained 30 min in cold FACS buffer (PBS with 2.5% FBS, 5 mM EDTA) with Siglec-F (clone E50-2440, Biolegend), CD11c (clone N418, Biolegend), Ly6G (clone 1A8, BD Biosciences), Ly6C (clone AL-21, BD Biosciences, Allschwil, Switzerland), CD11b (clone M1/70, Biolegend), CD45.1 (clone A20, BD Biosciences), CD45.2 (clone 104, BD Biosciences).
For intracellular staining of TNF (clone MP6-XT22, Biolegend), mice were injected i.p. with 50 μl of 5 mg/ml Brefeldin A in EtOH (diluted with 100 μl PBS) 3 hours prior to taking BALF. Lavage was performed with 1 ml PBS 5mM EDTA containing 5 μg/ml Brefeldin A, and was immediately placed on ice. After surface stain, cells were washed with FACS buffer and fixed, permeabilized and stained using the BD Biosciences Cytofix/Cytoperm Kit according to the manufacturer's instructions. Data were acquired on an LSRII (BD Biosciences) and analyzed with FlowJo software (TreeStar, Ashland, OR). An Aria III instrument (BD Biosciences) was used for cell sorting.
ROS assay
ROS was stained in BALF cells by collecting BALF as usual in 1 ml PBS 5 mM EDTA, washing with 2 ml RPMI 10% FBS at RT, and staining with 60 μM Dihydroethidium (Sigma, D7008) for 1 hour at 37°C, 5% CO2. For a positive control, cells were stimulated with PMA/ionomycin. Cells were then washed in 2 ml cold FACS buffer and stained as usual with fluorescence-labeled Abs. Data were acquired on an LSRII (BD Biosciences), Dihydroethidium was measured in the FITC channel.
Statistical analysis
Non-parametric tests, including the Kruskal-Wallis test with Dunn's post test, the Mann-Whitney test, or in the case of paired samples, the Wilcoxon test, were applied for statistical analysis using Prism GraphPad software (La Jolla, CA).
Supporting Information
WT BMDM were seeded in 96 well plates at 1x105 cells / well. After resting overnight, media was replaced with new media containing 20% L929 conditioned media containing M-CSF, with or without 100 ng/ml rTNF. After 3 days of incubation at 37°C, medium was replaced with 200 μl medium containing 20% L929 conditioned media and 10% alamar blue (Lucerna Chem AG, A1180), and incubated for 6.5 hr at 37°C. Conversion of alamar blue reagent by live cells was then measured with an ELISA plate reader and OD570-OD600 was calculated. Results are from one experiment.
(TIF)
(A) WT, MN-TNF NAIP5129S1 or NLRC4-/- BMDM were infected with WT L. pneumophila at MOI 0.1 or left untreated. (A) 3 days p.i. supernatant was collected and TNF was quantified by cytometric bead array assay.
(TIF)
(A) Caspase-1/11-/- BMDM were infected with WT L. pneumophila at MOI 0.1. Where indicated rTNF and/or cathepsin B inhibitor CA-074-Me were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 2 pooled experiments. (B) WT or TNFR1-/- BMDM were either pre-treated with rTNF overnight or left untreated, and then infected with ΔFlaA L. pneumophila at MOI 0.1. Where indicated rTNF and/or cathepsin B inhibitor CA-074-Me were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 2 pooled experiments. (C) WT or CtsB-/- BMDM were either pre-treated with rTNF overnight or left untreated, and then infected with ΔFlaA L. pneumophila at MOI 0.1. Where indicated rTNF and/or cathepsin B inhibitor CA-074-Me was added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 2 pooled experiments. **p<0.01 by Mann-Whitney test.¨
(TIF)
WT BMDM were either pre-treated with rTNF overnight or left untreated, and then infected with ΔFlaA L. pneumophila at MOI 0.1. Where indicated rTNF and/or Z-VAD-FMK or Q-VD-OPh were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data is from 1 experiment.
(TIF)
Acknowledgments
We thank Dr. Irene Garcia for the kind donation of memTNF KI mice. We thank Dr. Pascal Schneider for the TNFR1-Fc reagent, discussion and advice and we would like to thank Nathalie Oetiker and Franziska Wagen for their help with sort experiments and genotyping. We also thank the members of the Oxenius lab for critical discussion.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Swiss Federal Institute of Technology (https://www.ethz.ch/de.html) (AO) and the Vontobel Foundation (https://www.vontobel-stiftung.ch/DE/Home) (AO). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Michard C, Sperandio D, Bailo N, Pizarro-Cerda J, LeClaire L, et al. (2015) The Legionella Kinase LegK2 Targets the ARP2/3 Complex To Inhibit Actin Nucleation on Phagosomes and Allow Bacterial Evasion of the Late Endocytic Pathway. MBio 6: e00354–00315. 10.1128/mBio.00354-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allombert J, Fuche F, Michard C, Doublet P (2013) Molecular mimicry and original biochemical strategies for the biogenesis of a Legionella pneumophila replicative niche in phagocytic cells. Microbes Infect 15: 981–988. 10.1016/j.micinf.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 3. Isberg RR, O'Connor TJ, Heidtman M (2009) The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat Rev Microbiol 7: 13–24. 10.1038/nrmicro1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabria M, Campins M (2003) Legionnaires' disease: update on epidemiology and management options. Am J Respir Med 2: 235–243. [DOI] [PubMed] [Google Scholar]
- 5. Parr A, Whitney EA, Berkelman RL (2014) Legionellosis on the Rise: A Review of Guidelines for Prevention in the United States. J Public Health Manag Pract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wickramasekaran RN, Sorvillo F, Kuo T (2015) Legionnaires' disease and associated comorbid conditions as causes of death in the U.S., 2000–2010. Public Health Rep 130: 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hubber A, Roy CR (2010) Modulation of host cell function by Legionella pneumophila type IV effectors. Annu Rev Cell Dev Biol 26: 261–283. 10.1146/annurev-cellbio-100109-104034 [DOI] [PubMed] [Google Scholar]
- 8. Schwake DO, Alum A, Abbaszadegan M (2015) Automobile windshield washer fluid: A potential source of transmission for Legionella. Sci Total Environ 526: 271–277. 10.1016/j.scitotenv.2015.03.122 [DOI] [PubMed] [Google Scholar]
- 9. Phin N, Parry-Ford F, Harrison T, Stagg HR, Zhang N, et al. (2014) Epidemiology and clinical management of Legionnaires' disease. Lancet Infect Dis 14: 1011–1021. 10.1016/S1473-3099(14)70713-3 [DOI] [PubMed] [Google Scholar]
- 10. Copenhaver AM, Casson CN, Nguyen HT, Fung TC, Duda MM, et al. (2014) Alveolar macrophages and neutrophils are the primary reservoirs for Legionella pneumophila and mediate cytosolic surveillance of type IV secretion. Infect Immun 82: 4325–4336. 10.1128/IAI.01891-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nash TW, Libby DM, Horwitz MA (1984) Interaction between the legionnaires' disease bacterium (Legionella pneumophila) and human alveolar macrophages. Influence of antibody, lymphokines, and hydrocortisone. J Clin Invest 74: 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Finsel I, Hilbi H (2015) Formation of a pathogen vacuole according to Legionella pneumophila: how to kill one bird with many stones. Cell Microbiol 17: 935–950. 10.1111/cmi.12450 [DOI] [PubMed] [Google Scholar]
- 13. Shin S, Roy CR (2008) Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell Microbiol 10: 1209–1220. 10.1111/j.1462-5822.2008.01145.x [DOI] [PubMed] [Google Scholar]
- 14. Losick VP, Isberg RR (2006) NF-kappaB translocation prevents host cell death after low-dose challenge by Legionella pneumophila. J Exp Med 203: 2177–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gaspar AH, Machner MP (2014) VipD is a Rab5-activated phospholipase A1 that protects Legionella pneumophila from endosomal fusion. Proc Natl Acad Sci U S A 111: 4560–4565. 10.1073/pnas.1316376111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akhter A, Caution K, Abu Khweek A, Tazi M, Abdulrahman BA, et al. (2012) Caspase-11 promotes the fusion of phagosomes harboring pathogenic bacteria with lysosomes by modulating actin polymerization. Immunity 37: 35–47. 10.1016/j.immuni.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fontana MF, Vance RE (2011) Two signal models in innate immunity. Immunol Rev 243: 26–39. 10.1111/j.1600-065X.2011.01037.x [DOI] [PubMed] [Google Scholar]
- 18. LeibundGut-Landmann S, Weidner K, Hilbi H, Oxenius A (2011) Nonhematopoietic cells are key players in innate control of bacterial airway infection. J Immunol 186: 3130–3137. 10.4049/jimmunol.1003565 [DOI] [PubMed] [Google Scholar]
- 19. Barry KC, Fontana MF, Portman JL, Dugan AS, Vance RE (2013) IL-1alpha signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. J Immunol 190: 6329–6339. 10.4049/jimmunol.1300100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tateda K, Moore TA, Deng JC, Newstead MW, Zeng X, et al. (2001) Early recruitment of neutrophils determines subsequent T1/T2 host responses in a murine model of Legionella pneumophila pneumonia. J Immunol 166: 3355–3361. [DOI] [PubMed] [Google Scholar]
- 21. Sporri R, Joller N, Hilbi H, Oxenius A (2008) A novel role for neutrophils as critical activators of NK cells. J Immunol 181: 7121–7130. [DOI] [PubMed] [Google Scholar]
- 22. Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, et al. (2001) Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun 69: 2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mascarenhas DP, Pereira MS, Manin GZ, Hori JI, Zamboni DS (2015) Interleukin 1 receptor-driven neutrophil recruitment accounts to MyD88-dependent pulmonary clearance of legionella pneumophila infection in vivo. J Infect Dis 211: 322–330. 10.1093/infdis/jiu430 [DOI] [PubMed] [Google Scholar]
- 24. Yiu HH, Graham AL, Stengel RF (2012) Dynamics of a cytokine storm. PLoS One 7: e45027 10.1371/journal.pone.0045027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen J (2002) The immunopathogenesis of sepsis. Nature 420: 885–891. [DOI] [PubMed] [Google Scholar]
- 26. Dinarello CA (1997) Proinflammatory and anti-inflammatory cytokines as mediators in the pathogenesis of septic shock. Chest 112: 321s–329s. [DOI] [PubMed] [Google Scholar]
- 27. Schuelein R, Ang DK, van Driel IR, Hartland EL (2011) Immune Control of Legionella Infection: An in vivo Perspective. Front Microbiol 2: 126 10.3389/fmicb.2011.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lanternier F, Tubach F, Ravaud P, Salmon D, Dellamonica P, et al. (2013) Incidence and risk factors of Legionella pneumophila pneumonia during anti-tumor necrosis factor therapy: a prospective French study. Chest 144: 990–998. 10.1378/chest.12-2820 [DOI] [PubMed] [Google Scholar]
- 29. Wondergem MJ, Voskuyl AE, van Agtmael MA (2004) A case of legionellosis during treatment with a TNFalpha antagonist. Scand J Infect Dis 36: 310–311. [DOI] [PubMed] [Google Scholar]
- 30. Giassi Kde S, Furlanetto V Jr., Fialho S, Gomes Ribeiro G, Pereira IA (2014) [Legionella pneumonia after infliximab in a patient with rheumatoid arthritis]. Rev Bras Reumatol 54: 397–399. 10.1016/j.rbr.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 31. Tubach F, Ravaud P, Salmon-Ceron D, Petitpain N, Brocq O, et al. (2006) Emergence of Legionella pneumophila pneumonia in patients receiving tumor necrosis factor-alpha antagonists. Clin Infect Dis 43: e95–100. [DOI] [PubMed] [Google Scholar]
- 32. Shin S, Case CL, Archer KA, Nogueira CV, Kobayashi KS, et al. (2008) Type IV secretion-dependent activation of host MAP kinases induces an increased proinflammatory cytokine response to Legionella pneumophila. PLoS Pathog 4: e1000220 10.1371/journal.ppat.1000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fontana MF, Banga S, Barry KC, Shen X, Tan Y, et al. (2011) Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog 7: e1001289 10.1371/journal.ppat.1001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skerrett SJ, Martin TR (1996) Roles for tumor necrosis factor alpha and nitric oxide in resistance of rat alveolar macrophages to Legionella pneumophila. Infect Immun 64: 3236–3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu M, Conover GM, Isberg RR (2008) Legionella pneumophila EnhC is required for efficient replication in tumour necrosis factor alpha-stimulated macrophages. Cell Microbiol 10: 1906–1923. 10.1111/j.1462-5822.2008.01180.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coers J, Vance RE, Fontana MF, Dietrich WF (2007) Restriction of Legionella pneumophila growth in macrophages requires the concerted action of cytokine and Naip5/Ipaf signalling pathways. Cell Microbiol 9: 2344–2357. [DOI] [PubMed] [Google Scholar]
- 37. Fujita M, Ikegame S, Harada E, Ouchi H, Inoshima I, et al. (2008) TNF receptor 1 and 2 contribute in different ways to resistance to Legionella pneumophila-induced mortality in mice. Cytokine 44: 298–303. 10.1016/j.cyto.2008.08.015 [DOI] [PubMed] [Google Scholar]
- 38. Skerrett SJ, Bagby GJ, Schmidt RA, Nelson S (1997) Antibody-mediated depletion of tumor necrosis factor-alpha impairs pulmonary host defenses to Legionella pneumophila. J Infect Dis 176: 1019–1028. [DOI] [PubMed] [Google Scholar]
- 39. Brieland JK, Remick DG, Freeman PT, Hurley MC, Fantone JC, et al. (1995) In vivo regulation of replicative Legionella pneumophila lung infection by endogenous tumor necrosis factor alpha and nitric oxide. Infect Immun 63: 3253–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lightfield KL, Persson J, Brubaker SW, Witte CE, von Moltke J, et al. (2008) Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat Immunol 9: 1171–1178. 10.1038/ni.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tenthorey JL, Kofoed EM, Daugherty MD, Malik HS, Vance RE (2014) Molecular basis for specific recognition of bacterial ligands by NAIP/NLRC4 inflammasomes. Mol Cell 54: 17–29. 10.1016/j.molcel.2014.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akhter A, Gavrilin MA, Frantz L, Washington S, Ditty C, et al. (2009) Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog 5: e1000361 10.1371/journal.ppat.1000361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, et al. (2010) Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11: 1136–1142. 10.1038/ni.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pereira MS, Morgantetti GF, Massis LM, Horta CV, Hori JI, et al. (2011) Activation of NLRC4 by Flagellated Bacteria Triggers Caspase-1-Dependent and -Independent Responses To Restrict Legionella pneumophila Replication in Macrophages and In Vivo. J Immunol 187: 6447–6455. 10.4049/jimmunol.1003784 [DOI] [PubMed] [Google Scholar]
- 45. Katagiri N, Shobuike T, Chang B, Kukita A, Miyamoto H (2012) The human apoptosis inhibitor NAIP induces pyroptosis in macrophages infected with Legionella pneumophila. Microbes Infect 14: 1123–1132. 10.1016/j.micinf.2012.03.006 [DOI] [PubMed] [Google Scholar]
- 46. Vinzing M, Eitel J, Lippmann J, Hocke AC, Zahlten J, et al. (2008) NAIP and Ipaf control Legionella pneumophila replication in human cells. J Immunol 180: 6808–6815. [DOI] [PubMed] [Google Scholar]
- 47. Kortmann J, Brubaker SW, Monack DM (2015) Cutting Edge: Inflammasome Activation in Primary Human Macrophages Is Dependent on Flagellin. J Immunol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Casson CN, Yu J, Reyes VM, Taschuk FO, Yadav A, et al. (2015) Human caspase-4 mediates noncanonical inflammasome activation against gram-negative bacterial pathogens. Proc Natl Acad Sci U S A 112: 6688–6693. 10.1073/pnas.1421699112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wright EK, Goodart SA, Growney JD, Hadinoto V, Endrizzi MG, et al. (2003) Naip5 affects host susceptibility to the intracellular pathogen Legionella pneumophila. Curr Biol 13: 27–36. [DOI] [PubMed] [Google Scholar]
- 50. Diez E, Lee SH, Gauthier S, Yaraghi Z, Tremblay M, et al. (2003) Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat Genet 33: 55–60. [DOI] [PubMed] [Google Scholar]
- 51. Lamkanfi M, Amer A, Kanneganti TD, Munoz-Planillo R, Chen G, et al. (2007) The Nod-like receptor family member Naip5/Birc1e restricts Legionella pneumophila growth independently of caspase-1 activation. J Immunol 178: 8022–8027. [DOI] [PubMed] [Google Scholar]
- 52. Grivennikov SI, Tumanov AV, Liepinsh DJ, Kruglov AA, Marakusha BI, et al. (2005) Distinct and nonredundant in vivo functions of TNF produced by t cells and macrophages/neutrophils: protective and deleterious effects. Immunity 22: 93–104. [DOI] [PubMed] [Google Scholar]
- 53. Lippmann J, Muller HC, Naujoks J, Tabeling C, Shin S, et al. (2011) Dissection of a type I interferon pathway in controlling bacterial intracellular infection in mice. Cell Microbiol 13: 1668–1682. 10.1111/j.1462-5822.2011.01646.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harada T, Miyake M, Imai Y (2007) Evasion of Legionella pneumophila from the bactericidal system by reactive oxygen species (ROS) in macrophages. Microbiol Immunol 51: 1161–1170. [DOI] [PubMed] [Google Scholar]
- 55. Lightfield KL, Persson J, Trinidad NJ, Brubaker SW, Kofoed EM, et al. (2011) Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect Immun 79: 1606–1614. 10.1128/IAI.01187-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, et al. (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121. 10.1038/nature10558 [DOI] [PubMed] [Google Scholar]
- 57. Santic M, Molmeret M, Abu Kwaik Y (2005) Maturation of the Legionella pneumophila-containing phagosome into a phagolysosome within gamma interferon-activated macrophages. Infect Immun 73: 3166–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Caution K, Gavrilin MA, Tazi M, Kanneganti A, Layman D, et al. (2015) Caspase-11 and caspase-1 differentially modulate actin polymerization via RhoA and Slingshot proteins to promote bacterial clearance. Sci Rep 5: 18479 10.1038/srep18479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E (2007) Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ 14: 387–391. [DOI] [PubMed] [Google Scholar]
- 60. Rozman-Pungercar J, Kopitar-Jerala N, Bogyo M, Turk D, Vasiljeva O, et al. (2003) Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: when reaction mechanism is more important than specificity. Cell Death Differ 10: 881–888. [DOI] [PubMed] [Google Scholar]
- 61. Spes A, Sobotic B, Turk V, Turk B (2012) Cysteine cathepsins are not critical for TRAIL- and CD95-induced apoptosis in several human cancer cell lines. Biol Chem 393: 1417–1431. [DOI] [PubMed] [Google Scholar]
- 62. Kagedal K, Zhao M, Svensson I, Brunk UT (2001) Sphingosine-induced apoptosis is dependent on lysosomal proteases. Biochem J 359: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jin QH, Zhao B, Zhang XJ (2004) Cytochrome c release and endoplasmic reticulum stress are involved in caspase-dependent apoptosis induced by G418. Cell Mol Life Sci 61: 1816–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Oberle C, Huai J, Reinheckel T, Tacke M, Rassner M, et al. (2010) Lysosomal membrane permeabilization and cathepsin release is a Bax/Bak-dependent, amplifying event of apoptosis in fibroblasts and monocytes. Cell Death Differ 17: 1167–1178. 10.1038/cdd.2009.214 [DOI] [PubMed] [Google Scholar]
- 65. Chen Y, Tateda K, Fujita K, Ishii T, Ishii Y, et al. (2010) Sequential changes of Legionella antigens and bacterial load in the lungs and urines of a mouse model of pneumonia. Diagn Microbiol Infect Dis 66: 253–260. 10.1016/j.diagmicrobio.2009.11.001 [DOI] [PubMed] [Google Scholar]
- 66. Ader F, Le Berre R, Fackeure R, Raze D, Menozzi FD, et al. (2008) In vivo effect of adhesion inhibitor heparin on Legionella pneumophila pathogenesis in a murine pneumonia model. Intensive Care Med 34: 1511–1519. 10.1007/s00134-008-1063-2 [DOI] [PubMed] [Google Scholar]
- 67. Copenhaver AM, Casson CN, Nguyen HT, Duda MM, Shin S (2015) IL-1R signaling enables bystander cells to overcome bacterial blockade of host protein synthesis. Proc Natl Acad Sci U S A 112: 7557–7562. 10.1073/pnas.1501289112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lapaque N, Takeuchi O, Corrales F, Akira S, Moriyon I, et al. (2006) Differential inductions of TNF-alpha and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell Microbiol 8: 401–413. [DOI] [PubMed] [Google Scholar]
- 69. Kurahashi K, Kajikawa O, Sawa T, Ohara M, Gropper MA, et al. (1999) Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest 104: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Winn WC Jr., Myerowitz RL (1981) The pathology of the Legionella pneumonias. A review of 74 cases and the literature. Hum Pathol 12: 401–422. [DOI] [PubMed] [Google Scholar]
- 71. Yu H, Higa F, Koide M, Haranaga S, Yara S, et al. (2009) Lung abscess caused by Legionella species: implication of the immune status of hosts. Intern Med 48: 1997–2002. [DOI] [PubMed] [Google Scholar]
- 72. Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, et al. (2014) The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep 8: 570–582. 10.1016/j.celrep.2014.06.028 [DOI] [PubMed] [Google Scholar]
- 73. Xu L, Shen X, Bryan A, Banga S, Swanson MS, et al. (2010) Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog 6: e1000822 10.1371/journal.ppat.1000822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sturgill-Koszycki S, Swanson MS (2000) Legionella pneumophila replication vacuoles mature into acidic, endocytic organelles. J Exp Med 192: 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rodrigue-Gervais IG, Saleh M (2013) Caspases and immunity in a deadly grip. Trends Immunol 34: 41–49. 10.1016/j.it.2012.09.005 [DOI] [PubMed] [Google Scholar]
- 76. Cassidy SK, Hagar JA, Kanneganti TD, Franchi L, Nunez G, et al. (2012) Membrane damage during Listeria monocytogenes infection triggers a caspase-7 dependent cytoprotective response. PLoS Pathog 8: e1002628 10.1371/journal.ppat.1002628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Takahashi K, Kawai T, Kumar H, Sato S, Yonehara S, et al. (2006) Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol 176: 4520–4524. [DOI] [PubMed] [Google Scholar]
- 78. Helfer B, Boswell BC, Finlay D, Cipres A, Vuori K, et al. (2006) Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res 66: 4273–4278. [DOI] [PubMed] [Google Scholar]
- 79. Appelqvist H, Waster P, Eriksson I, Rosdahl I, Ollinger K (2013) Lysosomal exocytosis and caspase-8-mediated apoptosis in UVA-irradiated keratinocytes. J Cell Sci 126: 5578–5584. 10.1242/jcs.130633 [DOI] [PubMed] [Google Scholar]
- 80. Hentze H, Lin XY, Choi MS, Porter AG (2003) Critical role for cathepsin B in mediating caspase-1-dependent interleukin-18 maturation and caspase-1-independent necrosis triggered by the microbial toxin nigericin. Cell Death Differ 10: 956–968. [DOI] [PubMed] [Google Scholar]
- 81. Vaisid T, Barnoy S, Kosower NS (2009) Calpain activates caspase-8 in neuron-like differentiated PC12 cells via the amyloid-beta-peptide and CD95 pathways. Int J Biochem Cell Biol 41: 2450–2458. 10.1016/j.biocel.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 82. McCracken JM, Kinkead LC, McCaffrey RL, Allen LH (2016) Francisella tularensis Modulates a Distinct Subset of Regulatory Factors and Sustains Mitochondrial Integrity to Impair Human Neutrophil Apoptosis. J Innate Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nakagawa T, Yuan J (2000) Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol 150: 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Olleros ML, Vesin D, Bisig R, Santiago-Raber ML, Schuepbach-Mallepell S, et al. (2012) Membrane-bound TNF induces protective immune responses to M. bovis BCG infection: regulation of memTNF and TNF receptors comparing two memTNF molecules. PLoS One 7: e31469 10.1371/journal.pone.0031469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sadosky AB, Wiater LA, Shuman HA (1993) Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect Immun 61: 5361–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Weber SS, Joller N, Kuntzel AB, Sporri R, Tchang VS, et al. (2012) Identification of protective B cell antigens of Legionella pneumophila. J Immunol 189: 841–849. 10.4049/jimmunol.1200794 [DOI] [PubMed] [Google Scholar]
- 87. Tiaden A, Spirig T, Weber SS, Bruggemann H, Bosshard R, et al. (2007) The Legionella pneumophila response regulator LqsR promotes host cell interactions as an element of the virulence regulatory network controlled by RpoS and LetA. Cell Microbiol 9: 2903–2920. [DOI] [PubMed] [Google Scholar]
- 88. Hilbi H, Segal G, Shuman HA (2001) Icm/dot-dependent upregulation of phagocytosis by Legionella pneumophila. Mol Microbiol 42: 603–617. [DOI] [PubMed] [Google Scholar]
- 89. Segal G, Purcell M, Shuman HA (1998) Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc Natl Acad Sci U S A 95: 1669–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Weber SS, Joller N, Oxenius A (2013) Assessment of legionella-specific immunity in mice. Methods Mol Biol 954: 505–520. 10.1007/978-1-62703-161-5_31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
WT BMDM were seeded in 96 well plates at 1x105 cells / well. After resting overnight, media was replaced with new media containing 20% L929 conditioned media containing M-CSF, with or without 100 ng/ml rTNF. After 3 days of incubation at 37°C, medium was replaced with 200 μl medium containing 20% L929 conditioned media and 10% alamar blue (Lucerna Chem AG, A1180), and incubated for 6.5 hr at 37°C. Conversion of alamar blue reagent by live cells was then measured with an ELISA plate reader and OD570-OD600 was calculated. Results are from one experiment.
(TIF)
(A) WT, MN-TNF NAIP5129S1 or NLRC4-/- BMDM were infected with WT L. pneumophila at MOI 0.1 or left untreated. (A) 3 days p.i. supernatant was collected and TNF was quantified by cytometric bead array assay.
(TIF)
(A) Caspase-1/11-/- BMDM were infected with WT L. pneumophila at MOI 0.1. Where indicated rTNF and/or cathepsin B inhibitor CA-074-Me were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 2 pooled experiments. (B) WT or TNFR1-/- BMDM were either pre-treated with rTNF overnight or left untreated, and then infected with ΔFlaA L. pneumophila at MOI 0.1. Where indicated rTNF and/or cathepsin B inhibitor CA-074-Me were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 2 pooled experiments. (C) WT or CtsB-/- BMDM were either pre-treated with rTNF overnight or left untreated, and then infected with ΔFlaA L. pneumophila at MOI 0.1. Where indicated rTNF and/or cathepsin B inhibitor CA-074-Me was added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data are from 2 pooled experiments. **p<0.01 by Mann-Whitney test.¨
(TIF)
WT BMDM were either pre-treated with rTNF overnight or left untreated, and then infected with ΔFlaA L. pneumophila at MOI 0.1. Where indicated rTNF and/or Z-VAD-FMK or Q-VD-OPh were added at the time of infection. 3 days p.i. BMDM were lysed and CFU were quantified on CYE agar plates. Data is from 1 experiment.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.