Abstract
Escherichia coli is a bacterial species found ubiquitously in the intestinal flora of animals, although pathogenic variants cause major public health problems. Aptamers are short oligonucleotides that bind to targets with high affinity and specificity, and have great potential for use in diagnostics and therapy. We used cell-based Systematic Evolution of Ligands by EXponential enrichment (cell-SELEX) to isolate four single stranded DNA (ssDNA) aptamers that bind strongly to E. coli cells (ATCC generic strain 25922), with Kd values in the nanomolar range. Fluorescently labeled aptamers label the surface of E. coli cells, as viewed by fluorescent microscopy. Specificity tests with twelve different bacterial species showed that one of the aptamers–called P12-31—is highly specific for E. coli. Importantly, this aptamer binds to Meningitis/sepsis associated E. coli (MNEC) clinical isolates, and is the first aptamer described with potential for use in the diagnosis of MNEC-borne pathologies.
Introduction
Escherichia coli is a Gram-negative bacterial species found ubiquitously in the intestinal gut flora of animals, including humans, and can also survive and multiply in abiotic environments. Typically, it colonizes the infant gastro-intestinal tract within hours after birth, with lifelong benefits to the host [1]. However, E. coli can acquire specific virulence factors that allow it to adapt to new niches, causing diseases that are major public health issues, with high morbidity and mortality [1]. Pathogenic E. coli variants can cause three clinical syndromes: 1) sepsis/meningitis (meningitis/sepsis associated E. coli, or MNEC), 2) urinary tract infections (uropatogenic E. coli, or UPEC), 3) and intestinal and diarrheal diseases. Depending on the disease type, pathogenic E. coli in the intestinal tract can be further be classified in at least six different “pathotypes”: enterohemorragic (EHEC), enteropathogenic (EPEC), enterotoxigenic (ETEC), enteroaggregative (EAEC), diffusely-adherent (DAEC) and enteroinvasive E. coli (EIEC) [2].
Currently used E. coli detection methods normally require an enrichment step of bacterial culturing, which usually takes 2 to 3 days to yield results. This method does not permit rapid bacterial detection, which requires bacterial identification either by the polymerase chain reaction (PCR) [3, 4], or by the specific recognition of bacterial cell surface biomarkers using biological probes, including antibodies and aptamers [5], which enable bacterial detection in few hours.
Aptamers are short oligonucleotides that bind targets with high specificity and affinity (in the nanomolar or picomolar ranges) [6], and are isolated by Systematic Evolution of Ligands by EXponential enrichment (SELEX) procedures [6–8]. SELEX methodologies follow the same pattern: a large pool of random sequences is subjected to iterative steps of selection and amplification, obtaining a pool of molecules enriched for those with high affinity and specificity to the target. At the end of the process, sequences are cloned and evaluated individually for their binding properties [6–8].
Aptamers have been selected for targets with varying degrees of complexity, from small molecules to whole cells or tissues [9]. Cell SELEX methods have the advantage of selecting multiple aptamers that could potentially bind to different targets on the cells in their native conformation and physiological environment, without the requirement for target protein purification before selection. Cell SELEX also allows aptamer selection without previous knowledge of the cellular target, and eliminates the possibility of selecting aptamers that bind to targets that are not exposed on the native cell surface [10, 11].
Aptamers have high potential as diagnostic and therapeutic tools, with many advantages when compared with antibodies [12], including their smaller size–which improves access to biological environments ‘hidden’ from antibodies–their lack of immunogenicity, and the lower cost and higher reproducibility of nucleotide production. In addition, aptamers can be chemically modified to became more stable [13], labeled with fluorophores or other reporters [14], and can be easily truncated to eliminate sequences not important for interaction [15, 16]. These valuable properties make aptamers flexible and powerful tools for diagnostic and therapeutic purposes [5, 9, 12, 16–20].
Single strand DNA (ssDNA) aptamers have been selected against cells from different bacterial species, including Escherichia coli K88 [21] and NSM59 [22], a fecal E. coli isolate [23], Salmonella typhimurium [24–26], S. enteritidis [27, 28], S. paratyphi A [29], Salmonella O8 [30], Vibrio parahaemolyticus [31, 32], Listeria monocytogenes [33, 34], Shigella dysenteriae [35], Streptococcus mutans [36], Streptococcus pyogenes [37], Staphylococcus aureus [38], Proteus mirabilis [39], Pseudomonas aeruginosa [40], Mycobacterium tuberculosis [41], Francisella tularensis subspecies (subsp.) japonica [42] and Campylobacter jejuni [43]. Besides cell SELEX, surface molecules have been used for ssDNA aptamer selection, including peptidoglycan expressed in all bacterial cells [44], lipopolysaccharides found in Gram-negative cells [45], or specific surface proteins such as K88 frimbiae from E coli [46] or outer membrane proteins [47]. Aptamers that recognize bacterial cells have been used in biosensors devices allowing specific and rapid detection of bacterial cells [48]. Biosensors are composed of a biomolecule (typically an aptamer or antibody) that recognizes the target analyte and a transducer that converts the recognition event into a measurable signal. Thus far, biosensors using aptamers have been described that against S. enteriditis [49–51], S. typhimurium [52–56], Vibrio parahaemolyticus [52], Staphylococcus aureus [53, 57–59] and E. Coli [51, 60, 61] have been designed an binding have been measure by changes in electrical/electrochemical, optical, and mass sensitive parameter when the bacteria is bound. However, no biosensors described to date bind to MNEC species of E. coli.
In the present work, we describe the selection of ssDNA aptamers against the generic non-virulent strain E. coli ATCC 25922, using cell SELEX. We isolated four aptamers that bind to E. coli with high affinity, with Kd values in the nanomolar range. All four aptamers bind to the surface of E. coli, and one of the aptamers shows high specificity for E. coli cells. Importantly, this aptamer also binds to three MNEC clinical isolates from septic patients. To our knowledge, this is the first description of an aptamer capable of recognizing MNEC.
Materials and Methods
Bacterial strains and culture
The bacterial strain E. coli ATCC 25922 (American Type Culture Collection, Georgetown, DC, USA) was maintained in Luria-Bertani (LB) medium. Whole bacterial cells to be use as targets for selection were cultured at 37°C to a OD600 of 0.3 (equivalent to ~2.4 x 108 bacteria/mL), washed twice with PBS (NaCl2 137 mM, 2.7 mM KCl, 4.3 mM Na2HPO4.7H2O, 1.5 mM KH2PO4) and diluted in selection buffer (PBS containing 1.4 mM MgCl2).
The following bacterial strains were used in binding assays: Klebsiella pneumonia ATCC 27853, Enterobacter aerogenes ATCC 13048, Proteus mirabilis ATCC 00557, Pseudomonas aeruginosa ATCC 700603, Staphylococcus aureus ATCC 29213 and Enterococcus faecalis ATCC 29212, from ATCC; and Enterobacter cloacae, Proteus vulgaris, Morganella morganii, Citrobacter freundii, Acinetobacter baumannii, Enterococcus faecalis, Enterococcus faecium, from the bacterial collection of the Instituto Adolfo Lutz (São Paulo, SP, Brazil). The MNEC clinical isolates used here were obtained from the Clinical Hospital of the Federal University of Paraná (HC, Curitiba, PR, Brazil).
Random library, primers and aptamers
The single stranded DNA (ssDNA) library used here represents a collection of 88-mer nucleotides with a central random region of 40 nt (N40) flanked by two 24-nt primer-binding sites (5’ CATACGATTTAGGTGACACTATAG-N40-ATTTCTCCTACTGGGATAGGTGGA 3’) obtained from Integrated DNA Technologies (Coralville, IA, USA). The BR Forward (5’CATACGATTTAGGTGACACTATAG3’) and reverse (5’TCCACCTATCCCAGTAGGAGAAAT3’) primers, selected aptamers and the random oligonucleotide 5’CATACGATTTAGGTGACACTATAGTTCCAACATAGTGTCTGATTTTCTTAATGGTAGGCGAGTAATTTCTCCTACTGGGATAGGTGGA3’ (either unlabeled or 5’-labeled with 6-FAM) were synthesized by Integrated DNA Technologies. The 183-mer ssDNA AT1 (5’CCACTCATGTGAGAGCCAATTGTGAAGAGCACAAAAGGTGATTTCATTTCCTTTTGTGTAATTTGCATGTTTGAACAGACACTGTATCTGTATTGTTACAATGGATATTGATTTGGTGTTTGCAGGATCCTGGACAGAAGCAAAGGCAAAGGTATAAAAGATTTGATCCCATTAGTGTCCAAC3’) was used as an exogenous control, and was amplified using the AT1 forward (5’CTCATGTGAGAGCCAATTGTGAAG3’) and AT1 reverse (5’GGACACTAATGGGATCAAATCTTTTATACC3’) primers.
Cell-SELEX
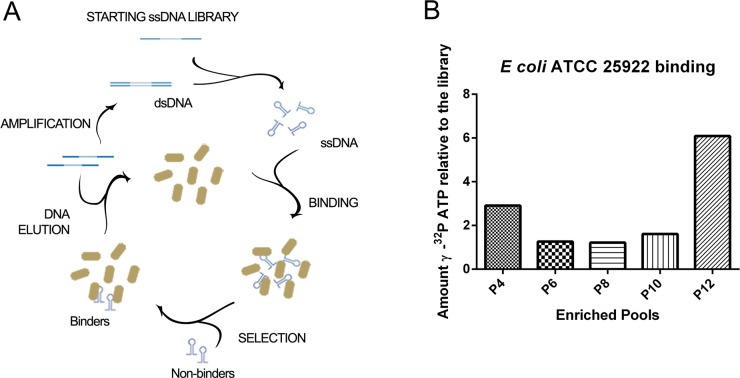
A scheme of the bacterial cell-SELEX process is shown in Fig 1A. Briefly, 1.5 nmol of random library nucleotides (approx 1014 molecules) diluted in selection buffer were denatured for 5 min at 95°C, cooled for 15 minutes at 25°C and incubated with 1 mL of 107 colony forming units (CFU)/mL of E. coli ATCC 25922 cells for 45 min, at room temperature, and under constant agitation (220 rpm). Then, the bacterial suspension was centrifuged for 6 minutes at 8,000 g. The supernatant was discarded, unbound ssDNA sequences were removed by washing with 1 mL of selection buffer, and bacterial cells were centrifuged for 6 min at 8,000 g. Bound ssDNA was eluted by resuspending the bacteria-aptamer complex in sterile water and heating at 95°C for 5 min. The supernatant was collected and amplified by asymmetric PCR in a 100 ul reaction, using GoTaq® Green Master Mix (Promega, Fitchburg, WI, USA), with 0.06 μM reverse BR primer and 2 μM forward BR primer. PCR was performed by forty cycles of 1 min of denaturation at 95°C, 30 seconds of annealing at 65°C, and 40 seconds of extension at 72°C. The ssDNA was extracted from 8% polyacrylamide native gels colored with SYBR® Gold Nucleic Acid Gel Stain (S1 Fig, Invitrogen, Carlsbad, CA, USA), precipitated with ethanol and resuspended in selection buffer to be used in the next selection round. A total of twelve rounds were performed and the selection conditions were changed during the cell-SELEX procedure in order to increase the selective pressure towards the last steps (S1 Table).
Fig 1. Enrichment of E. coli-binding aptamers by cell SELEX.
(A) Schematic representation of the in vitro selection of aptamers against E coli ATCC 25922. E coli bacterial cells were incubated with an ssDNA random library for 45 min at room temperature. Then, the bacterial suspension was centrifuged, washed repeatedly, and the molecules that remained bound to bacterial cells were eluted at 95°C. The recovered aptamers were amplified by asymmetric PCR, and then incubated with bacterial cells in the next round of selection. (B) Enrichment of E coli binding ssDNA pools during 12 iterative rounds of aptamer selection. Bacterial cells were incubated with γ 32P ATP-labeled ssDNA from rounds 4 (P4), 6 (P6), 8 (P8), 10 (P10) and 12 (P12) of selection, and then bound aptamers were eluted and quantified by scintillation counting. The graph represents the fold increase of bound radiolabeled ssDNA from each round relative to that of the starting library (P0).
PCR products obtained after twelve selection rounds were cloned using the TOPO® TA Cloning® Kit (Invitrogen), and positive clones were sequenced. Aptamers sequences were analyzed using the CLustal and Mfold structure prediction algorithms.
Pool binding assays
The ssDNA random library and enriched nucleotide pools from each round of selection were amplified by asymmetric PCR, purified from polyacrylamide native gels and radiolabeled with γ 32P ATP. The 5´end-labeling reactions were performed in a 15-μL reaction mix containing the T4 polynucleotide kinase (Invitrogen), following the manufacturer’s instructions. Samples were incubated for 1 hr at 37°C, and then the enzyme was inactivated by heating for 5 min at 80°C. Unincorporated γ 32P ATP was eliminated by DNA purification through sephadex G-25 columns (GE Healthcare, Little Chalfont, Buckinghamshire, UK).
To determine the binding capability of the pools 106 CFU/mL of E. coli cells were incubated with 50 nM of enriched pools or ssDNA random labeled library and agitated at room temperature for 45 min, in the presence of the selection buffer with 0.05 μg/μl of BSA. Following three washes with 1 mL of selection buffer, by centrifugation at 8,000 g, 6 min, the amount of radiolabeled ssDNA associated with bacterial cells was measured by scintillation counting. The amount of radiolabeled ssDNA bound to bacterial cells at each round of selection was normalized to the radiolabeled library signal.
Aptamer binding analysis by qPCR
For the identification of individual aptamers, 106 bacterial cells were incubated with 200 nM of aptamer or random library in the presence of selection buffer with 0.05% BSA, for 45 min at room temperature, and under agitation (at 220 rpm). The bacterial aptamer complexes were washed three times with 1 mL of selection buffer and resuspended in 25 μl of sterile water. Before the elution step, 0.016 pmol of the AT1 oligonucleotide, used as exogenous control, was added to each sample, and aptamers were eluted by heating for 5 min at 95°C, followed by centrifugation for 5 min at 8,000 g. ssDNA aptamers recovered in the supernatant were quantified by SYBR Green-based qPCR, using an Applied Biosystems 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). All reactions were done in 20 μl volume, and in triplicates in 96-well plates, using BR forward and reverse primers, for aptamer or random library amplification, or AT1 forward and reverse primers, for AT1 amplification.
Aptamer Kd determination was performed by qPCR of the aptamer bound to bacterial cells after the incubation of 106 bacterial cells with increasing concentration of the aptamer (6.25 nM, 12.5 nM, 25 nM, 50 nM, 100 nM, 200 nM and 400 nM), followed by washing steps. Apparent Kd values for each aptamer were determined by Linewaver-Burk analysis, using the formula:
1/[complex] = Kd/ [Cmax] x 1/[aptamer] + 1/[Cmax], where kd is the steady state dissociation constant, [Complex] is the concentration of the complex bacteria-aptamer, [Cmax] is the concentration of the complex at maximal binding capacity, where all the binding sites are occupied by the aptamer, and [aptamer] is the concentration of the aptamer.
The estimated number of binding sites/E Coli calculation was made according to [62].
Fluorescence assays
Bacterial cells were washed once and resuspended in PBS at a density of 109 CFU/ml, and then incubated for 45 min with 400 nM of 6-FAM-labeled aptamers (5’ labeling). Then, bacteria-aptamer complexes were adhered to poly-L-lysine-coated slides for 20 min (at room temperature), slides were washed 3 times in PBS and observed in a Nikon Eclipse E600 epifluorescence microscope, using a 100x objective.
Secondary structure prediction
To predict aptamer secondary structure, we used the mfold algorithm [63], set for the conditions used in binding assays (1.4 mM MgCl2 and 137 mM NaCl, at 25°C).
Results
Sequential steps of cell-SELEX yields aptamer pools with increased binding to E. coli
To select aptamers that bind to E. coli we performed a cell-SELEX strategy as previously described [38, 40, 64, 65] (Fig 1A). We chose to use in our experiments the E. coli strain ATCC 25922 (serotype O6 and biotype 1), because this strain—originally isolated from a human clinical sample (Seattle, WA, USA; 1946)—is commonly used for quality control of antibody sensitivity assays. Also, we used live, unfixed bacterial cells, since formaldheide, glutaraldeide or alcohol fixation may alter the conformation of cell wall molecules [66], the most likely aptamer targets. The composition of the bacterial cell wall changes during growth in culture [38, 67]. Thus, to minimize differences in cell surface composition, we used exponential-phase bacterial cells at in all the experiments. Also, we used non-modified nucleotides due to the lower costs and ease of production with conventional enzymes, and preferred ssDNA to RNA, due the higher stability of DNA molecules.
After each of the twelve rounds of positive selection, the enrichment of ssDNA molecules that bind bacterial cells specifically was monitored using γ 32P ATP 5’-end labeled oligonucleotide pools [68]. Radiolabeled ssDNA that binds to bacterial cells was quantified by scintillation counting, and the results were reported relative to the binding of the radiolabeled starting library binding (Fig 1B). The results show that the pool of oligonucleotides recovered from the twelfth round of selection (‘P12’) had approximately 6-fold higher binding to E coli cells than the starting library pool. These results indicate that P12 was the most enriched in E coli-binding ssDNA molecules.
The pool of molecules from the fourth round of selection (P4) displayed about 3-fold increase in binding to E coli relative to the starting library. Then this binding increase was lost in P6, P8 and P10 indicating that these pools were not enriched in E coli binding molecules than the library pool.
Round 12 of cell SELEX contains E. coli-binding aptamers
To identify aptamer sequences that specifically recognize E. coli ATCC 25922, we cloned and sequenced the aptamers found in pool P12 (Fig 1 and Table 1). We obtained 64 clones, and primary sequence similarity analysis identified eleven highly related families of aptamers within the group (Table 1), corresponding to the 34% (22 aptamers) of all cloned sequences, while the remaining 66% of sequences were not clearly related to each other.
Table 1. Sequence families in P12 aptamer pool.
| Aptamer clone | Central sequence of families of aptamers |
|---|---|
| P12-3 P12-34 | GCGGATGCGTGAGACCCCCACAAGCAGTGAGTAGGAGGGGACGGATGCGTGAGACCCCCACAAGCAGTGAGTAGGAGGGG |
| P12-9 a, P12-9 b | TGCGGGCGGAGGACACGGACCCGTATGGGAGCAATGCACG |
| P12-11 P12-31 | CCCTCCGGGGGGGGGGGTCATCGGGATACCTGGTAAGGATACCCTCCGGGGGGG———TCATCGGGATACCTGGTAAGGATA |
| P12-15 a, P12-15 b | GGCCACCCACAGGCACTCCGCTCATGAATCGTGGAGTCGG |
| 12–17 a, P12-17 b | GACGGTGGCAGGGAAAGGGGTCGGGCATATGGCGGAGGGG |
| P12-21 P12-55 | CCGGAGGTGGGTGAGGTCTGCGGCAGGCTGTGTGGGTGGACCGGAGGGGGGTGAGGTCTGCGGCAGGCTGTGTGGGTGGA |
| P12-30 a, P12-30 b | CACCACGGACACGATCCCAAGCTAGGAGGTGCGGCGGGGT |
| P12-48 a, P12-48 b | TGGCACAGCACGTCGCACGGTCCCCGGGAGGTGTTCACTG |
| P12-51 a, P12-51 b | TCGCGAGTGCGTGTACGCCACACATCACAAAAGGGGTGTG |
| P12-52 a, P12-52 b | CCGCCCAGCGGGGGTAGGGCCGGACGTAGGAGGAGCTGCG |
| P12-66 a, P12-66 b | GGCAACATTCAGACATACCAGTACCCACTCGGACTTCCCG |
a and b are clones who share the same sequence
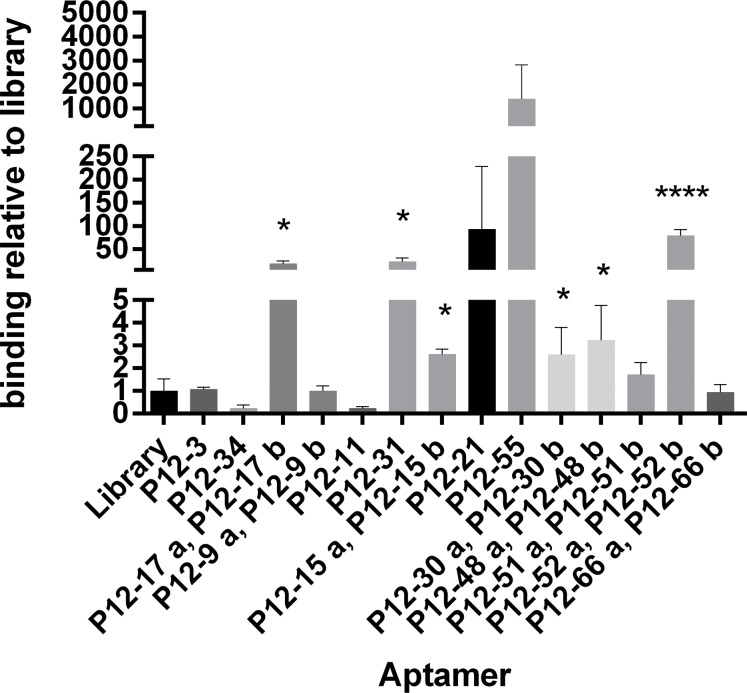
We tested the ability of related sequences to bind E. coli, compared with that of the random library nucleotides, by incubation with 106 E. coli cells, followed by elution and ssDNA quantification by qPCR (Fig 2A). Five aptamers, - P12-17, P12-31, P12-52, P12-21 and P12-55 –strongly bind to E. coli ATCC 25922. In particular, the binding of P12-21, P12-51 and P12-55 to E. coli cells was more than 100-fold stronger than that of the random library, while that of P12-31 and P12-17 was 10-fold stronger than that of the random library. Other sequences showed low (P12-15, P12-30 and P12-48) or no detectable binding to E. coli bacterial cells (Fig 2A).
Fig 2. Binding to E. coli of the individual sequences from round 12 cell SELEX.
Pool 12 (P12) aptamers with conserved sequences (within the pool) were incubated with E coli strain ATCC 25922 cells, and then bound aptamers were eluted and quantified by qPCR (using the ΔΔCt method). Results show the amount of bound aptamers relative to that of starting library nucleotides. Data represent mean ± SD of 3 independent experiments. *, p < 0.05 and ****, p<0.0001.
While the aptamers P12-17 and P12-52 were representatives of two groups of clones with identical sequences, the primary sequences of aptamers P12-31 and P12-55 are related to sequences P12-11 and P12-21, respectively (Table 1). P12-31 showed stronger binding than P12-11, and this difference could be explained by primary sequence changes. Relative to P12-11, P12-31 lacks four G nucleotides in positions 14 to 17 of the central region (Table 1). In contrast, the binding of P12-55 E. coli-binding was not significantly different to that of P12-21, in agreement with the near sequence identity between these oligonucleotides in the central region, with a single G-to-T substitution from P12-55 to P12-21 (Table 1).
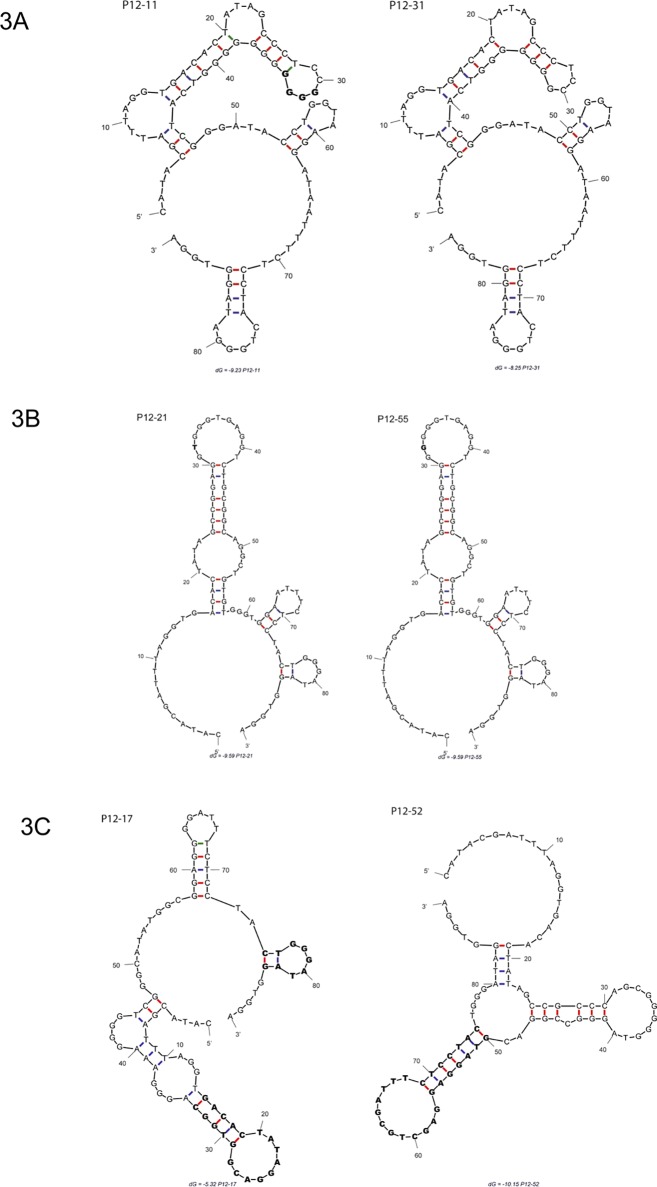
To understand the impact of aptamer sequences on binding, we generated predicted secondary structures for the most efficient E.coli-binding aptamers using the mfold algorithm [63]. P12-31 and P12-11 have similar predicted secondary structures, but the four G residues absent in P12-31 are predicted to alter the folding pattern of the main stem-loop region, changing the sequences of upper lateral and apical loops (Fig 3A). P12-55 and P12-21 have nearly identical predicted secondary structures, and the different nucleotide (G or T) is situated in the apical loop of the stem-loop structure (Fig 3B).
Fig 3. Prediction of pool 12 (P12) aptamer secondary structures.
The structures of aptamers with the highest E coli binding values were predicted using the mfold algorithm [63]. (A) Predicted structures of similar aptamers P12-11 and P12-31. The four G residues in P12-11 that are missing in P12-31 are depicted in bold. (B) P12-21 and P12-55 predicted structures. The different nucleotide (G or T) in the apical loop is in bold. (C) Structures of one conformer of aptamers P12-17 and P12-52, with conserved stem loops were depicted in bold.
For the aptamers P12-55 and P12-31, mfold yielded only one predicted secondary structure, while different alternative conformers were predicted for P12-17 and P12-52 (S2A and S2B Fig). In fact, P12-17 has four different possible secondary structures; however, all of them have two stables stem loops (Fig 3C). The mfold results for P12-52 include at least five thermodynamically stable predicted structures that share one conserved stem loop (Fig 3B). The presence of stable stem loops in the predicted secondary structures of P12-17 and P12-52 suggests that this feature could play an important role in the interaction of aptamers with theirs ligands in the bacterial cell wall.
Aptamers from the P12 pool have high affinity and are specific for E. coli ATCC 25922
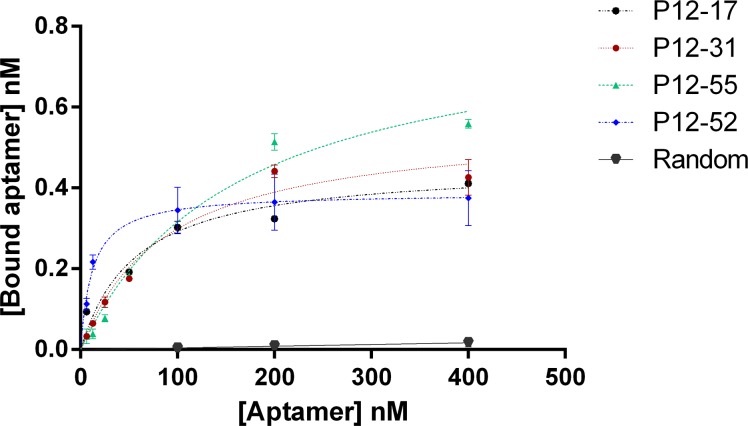
To evaluate aptamer binding in more detail, we determined the affinity for E. coli ATCC 25922 of the four P12 aptamers with the highest binding values relative to that of the original library. All of the four aptamers showed saturable binding to live bacterial cells, whereas a random oligonucleotide showed low or no detectable binding to E. coli (Fig 4). The dissociation constants (Kd) of the four aptamers were estimated to be between 11.97 (P12-52) and 161.0 nM (P12-31) (Table 2), and the Bmax values between 0.3874 (P12-52) to 0.8283 (P12-55) (Table 2). Based on the Bmax data, we calculated the estimated number of binding sites per bacterial cell [62]. Interestingly, P12-52 had the lowest estimated number of binding sites per E. coli cell (6996 ± 350), despite the fact that this aptamer had the highest affinity for these cells, with a low Kd value (11.97 nM).
Fig 4. Binding affinity of selected pool 12 aptamers to E coli ATCC 25922.
Binding affinity curves of aptamers P12-17, P12-31, P12-52 and P12-55 to live E coli ATCC 25922. Aptamer binding was quantified by qPCR. Data represent mean ± SD values of three independent experiments. The binding analysis was performed using GraphPad Prism 6, under the non-linear fit model for specific binding.
Table 2. Binding characteristics of the selected aptamers.
| Aptamer | Kd (nM) | Bmax (nM) | Estimated number of bindig sites/E coli |
|---|---|---|---|
| P12-17 | 56.33 ± 15.87 | 0.46 ± 0.03 | 8100 |
| P12-31 | 87.03 ± 17.32 | 0.56 ± 0.04 | 10113 |
| P12-52 | 11.97 ± 2.94 | 0.39 ± 0.02 | 6996 |
| P12-55 | 161.0 ± 34.74 | 0.83 ± 0.07 | 14950 |
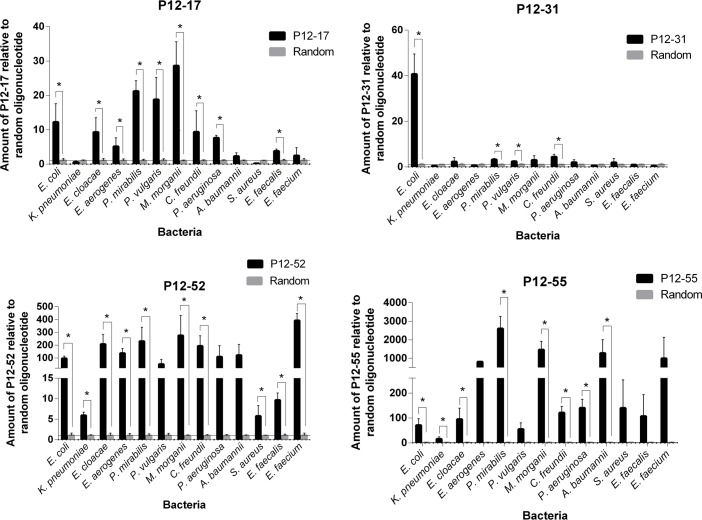
We also evaluated the specificity of aptamers to E. coli by comparing the ability of these molecules to bind to 12 different bacterial species, including nine Gram-negative and three Gram-positive strains (Fig 5). The results showed that P12-17, P12-52 and P12-55 showed strong binding to bacterial species other than E. coli. Although P12-31 could bind to other Gram-negative bacteria (P. mirabilis, P. vulgaris and C. freudini), its binding to those species was weaker than that observed towards E. coli (using the Dunnett’s test, α = 0.05; S2 Table). P12-17 and P12-52 could recognize Gram-negative and positive bacterial cells. While P12-17 could only bind to E. faecalis, P12-52 bound strongly to all three Gram-positive bacteria tested (S. aureus, E. faecalis and E. freudini).
Fig 5. Specificity of the aptamers to E. coli ATCC 25922.
Binding assays of P12-17, P12-31, P12-52 and P12-55 to the target species E. coli ATCC 25922 and to other (non-target) bacterial species. Bound aptamers were quantified by qPCR (ΔΔCt method) using a random oligonucleotide as a negative control, and AT1 as an exogenous reference control. Data represent mean ± SD of three independent experiments. * p<0.05.
Selected aptamers bind to the surface of live E. coli cells
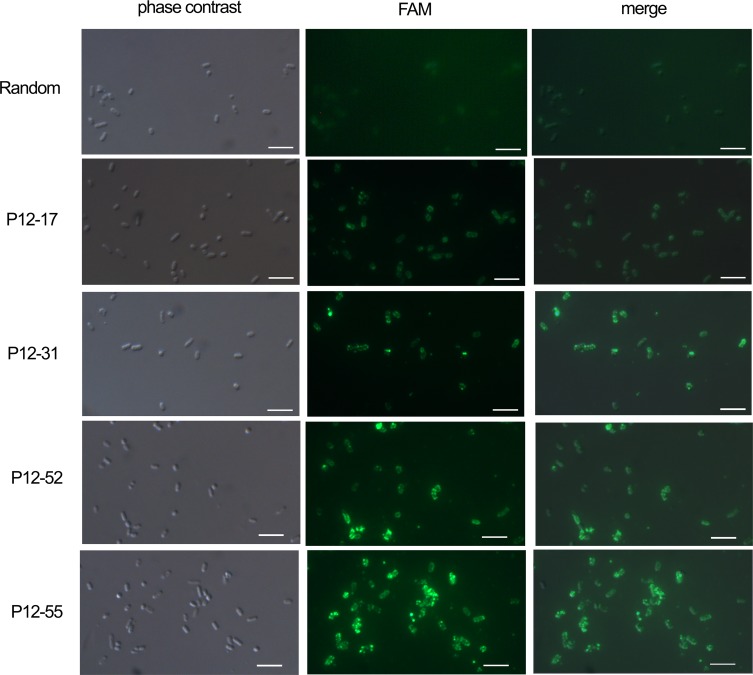
To confirm that the selected aptamers were capable of binding E. coli cells, we visualized by fluorescence microscopy the binding of aptamers P12-17, P12-31, P12-52 and P12-55 to E. coli ATCC 25922 cells. Bacterial cells were incubated with 6-FAM labeled aptamers, adhered to poly-L-lysine-coated slides, and then visualized by fluorescence microscopy. We observed that the four aptamers bound to bacterial cells, whereas no clear labeling was detected using random ssDNA (Fig 6). For all aptamers, labeling appeared as granules covering bacterial cells (Fig 6), likely corresponding to aptamer binding to components of the bacterial surface.
Fig 6. Fluorescence microscopic images of aptamer binding to E coli ATCC 25922.
Bacterial cells were incubated with aptamers (400 nM) 5’-labeled with 6-FAM, and then fixed and adhered to poly-L-lysine-coated slides. A random oligonucleotide sequence was used as a nonspecific binding control. Scale bar, 4 μm.
Aptamer recognition of E. coli isolates from septic patients
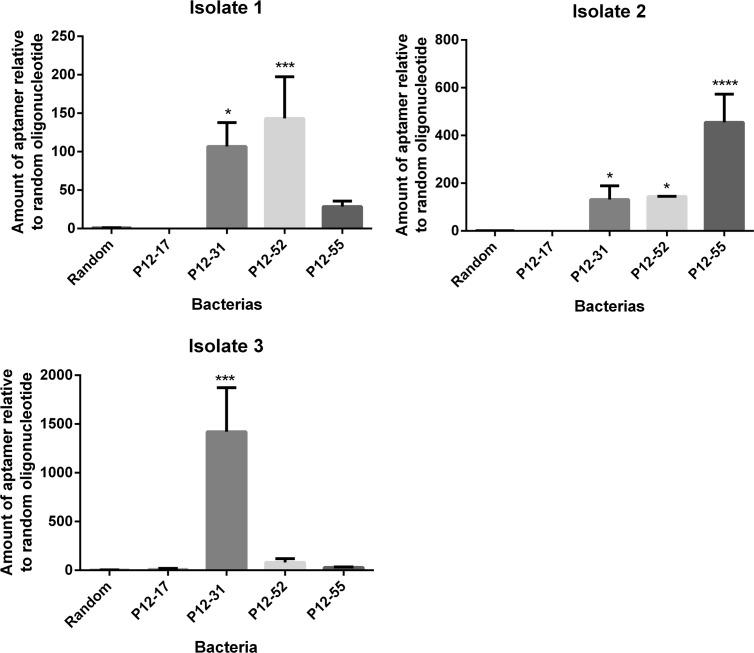
E. coli serotypes have different cell wall composition, which includes variation in cell wall receptors vary within different E. coli strains [1]. To assess whether the selected aptamers could recognize E. coli strains other than ATCC 25922 we evaluated the binding of aptamers P12-17, P12-31, P12-52 and P12-55 to three meningitis/sepsis associated E. coli (MNEC) clinical isolates. Aptamers P12-31, P12-52 and P12-55 showed higher binding to E. coli isolates relative to the random oligonucleotide, indicating that the aptamers are able to recognize pathogenic E. coli strains (Fig 7). On the other hand, P12-17 did not recognize different E. coli isolates. The aptamers had different binding strength to the distinct bacterial isolates. P12-31 shows stronger binding to isolates 1, 2 and 3 than to E. coli ATCC 25922. P12-52 show similar binding to isolates 1, 2 and E. coli ATCC 25922, while it didn’t bind to isolate 3. Finally, P12-55 showed very strong binding to isolate 2, strong binding to E. coli ATCC 25922 and no significant binding to isolates 1 and 3.
Fig 7. Aptamer binding to meningitis/sepsis-associated E. coli (MNEC).
Aptamers P12-17, P12-31, P12-52 and P12-55 were incubated with live cells from MNEC clinical isolates and then bound aptamers were quantified by qPCR. Data represent mean ± SD of three independent experiments. * p<0.05, *** p<0.001, **** p< 0.0001.
Discussion
E. coli strains generate numerous disease conditions with high social and economic impact. Nevertheless, E. coli detection in the clinic remains suboptimal, with urgent need for faster and more sensitive detection methods, which could be aided by the use of aptamers as diagnostic tools in biosensors devices. Here we used a whole cell-SELEX procedure to select ssDNA aptamers against live E. coli cells from strain ATCC 25922, and we isolate four aptamers - P12-17, P12-31, P12-52 and P12-55—that bind to these cells with high affinity (Fig 2, Table 2).
Fluorescence analysis suggested that the target molecules recognized by the aptamers described here localize to the bacterial surface. This localization pattern is interesting, and indicates that the aptamers could be used for E. coli recognition purposes. Also, surface localization is likely to facilitate bacterial enrichment for diagnostics. For numerous aptamers selected for cancer cells or conditioned media (from cancer cell culture), the molecular target recognized by the aptamer was identified, and represented a novel cancer biomarker [69–72]. Aptamers allowed the identification of the alkaline phosphatase placental-like 2 (ALPPL-2) as enriched in pancreatic cancer cells, and a biomarker of pancreatic cancer cell secretomes [69, 71]. Similarly, the use of aptamers showed that the protein tyrosine kinase 7 (PTK7) is enriched in T-cell acute lymphoblastic leukemia [72], and the immunoglobin heavy chain mu in Burkitt’s lymphoma [70]. In the case of E coli, aptamers such as those described here could aid in the identification of bacterial serotypes [1].
The aptamer P12-31 has at least ten-fold stronger binding to E. coli than to the other twelve bacterial species tested here (Fig 5), indicating that this aptamer is highly specific for E. coli. On the other hand, the selected aptamers P12-17, P12-52 and P12-55 show strong binding to different bacterial species, possibly due to the lack of a negative selection step to exclude aptamers that bind to Gram-negative or Gram-positive bacteria other than E. coli [65, 73]. Most specificity test for aptamers isolated against E. coli cells uses bacterial samples with as many as four bacterial species [46, 47, 64, 74]. It remains possible that cross reactivity accounts for part of the binding activity observed here towards non-E. coli species. Indeed, Bruno and co-workers (2010) reported cross reaction with S. enterica cells of aptamers selected for E. coli outer membrane proteins (OMPs) [74].
The fact that the four aptamers recognize different subsets of bacterial species (Fig 5), suggests that the aptamers P12-17, P12-31, P12-52 and P12-55 bind to different molecular markers. Interestingly P12-17 and P12-52 can also recognize Gram-positive bacteria (Fig 5). Gram-positive cells lack the outer lipopolysaccharide membrane that defines bacterial species as Gram-negative; thus, it is possible that P12-17 and P12-52 bind to common groups in different membrane receptors.
Besides being highly specific to E. coli, P12-31 recognizes other E. coli subtypes, such as MNEC clinical isolates (Fig 7). Thus, P12-31 is likely to recognize a molecule specific and probably common to the E. coli strains tested here, and may recognize other E. coli strains. Differences in the strength of aptamer binding to the E. coli isolates used here could be explained by differences in the level of expression of target molecules on the bacterial cell surface. In agreement with our results, the aptamer EcA5-27 –selected against the uropathogenic E. coli NSM59—shows between 8.5 and 56 fold higher affinity to seven uropathogenic E. coli clinical isolates than to E. coli laboratory strains such as DH5α, SM10 and BW20338 [22]. In addition, the aptamers selected against fecal E. coli isolate KCTC 2571 were capable of binding with different affinities to other fecal E. coli strains, such KCTC 1681, KCTC 2617 and KCTC 2618. [23].
Aptamers against E. coli have been isolated previously by SELEX against extracellular proteins, OMPs of E. coli ATCC 8739 or the K88 fimbriae protein of the enterotoxigenic (ETEC) K88, [46, 74], or by cell SELEX against other E. coli strains such as the laboratory strain DH5α, enterohemorrhagic O157:H7, uropathogenic NSM59 and fecal E. coli isolate KCTC 2571 [22, 23, 75–77]. However, to our knowledge, this is the first study to report the binding of aptamers to MNEC. Importantly, our results show that some of the aptamers described here recognize pathogenic strains with affinity and specificity compatible with their potential use in clinical diagnosis and therapheutic applications.
Supporting Information
PCR results of conventional PCR. Neg negative control from conventional PCR experiment. aPCR, results from asymmetric PCR (aPCR) performed in the conditions used during SELEX. The position of double stranded (88 bp) or singles stranded (88nt) product is indicated on the left.
(PDF)
The differences between P12-31 binding to E coli and to all other bacterial species were statistically significant (** p<0.005).
(PDF)
Conserved stem loops are highlighted in bold.
(PDF)
Conserved stem loops are highlighted in bold.
(PDF)
*Correspond to the library ssDNA population. ** Washes were done with selection buffer (PBS containing 1.4 mM MgCl2).
(PDF)
(PDF)
Acknowledgments
This investigation received financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), Coordenação de aperfeiçoamento de pessoal de nivel superior (CAPES) and Fundação Oswaldo Cruz (FIOCRUZ).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This investigation received financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP), Coordenação de aperfeiçoamento de pessoal de nivel superior (CAPES) and Fundação Oswaldo Cruz (FIOCRUZ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nature reviews. 2004. February;2(2):123–40. [DOI] [PubMed] [Google Scholar]
- 2.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clinical microbiology reviews. 1998. January;11(1):142–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajendhran J, Gunasekaran P. Microbial phylogeny and diversity: small subunit ribosomal RNA sequence analysis and beyond. Microbiological research. 2011. February 20;166(2):99–110. 10.1016/j.micres.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 4.Woo PC, Lau SK, Teng JL, Tse H, Yuen KY. Then and now: use of 16S rDNA gene sequencing for bacterial identification and discovery of novel bacteria in clinical microbiology laboratories. Clin Microbiol Infect. 2008. October;14(10):908–34. 10.1111/j.1469-0691.2008.02070.x [DOI] [PubMed] [Google Scholar]
- 5.Gopinath SC, Tang TH, Chen Y, Citartan M, Lakshmipriya T. Bacterial detection: from microscope to smartphone. Biosensors & bioelectronics. 2011. October 15;60:332–42. [DOI] [PubMed] [Google Scholar]
- 6.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990. August 30;346(6287):818–22. [DOI] [PubMed] [Google Scholar]
- 7.Green R, Ellington AD, Szostak JW. In vitro genetic analysis of the Tetrahymena self-splicing intron. Nature. 1990. September 27;347(6291):406–8. [DOI] [PubMed] [Google Scholar]
- 8.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science (New York, NY. 1990. August 3;249(4968):505–10. [DOI] [PubMed] [Google Scholar]
- 9.Weigand JE, Suess B. Aptamers and riboswitches: perspectives in biotechnology. Applied microbiology and biotechnology. 2009. November;85(2):229–36. 10.1007/s00253-009-2194-2 [DOI] [PubMed] [Google Scholar]
- 10.Pestourie C, Cerchia L, Gombert K, Aissouni Y, Boulay J, De Franciscis V, et al. Comparison of different strategies to select aptamers against a transmembrane protein target. Oligonucleotides. 2006. Winter;16(4):323–35. [DOI] [PubMed] [Google Scholar]
- 11.Cerchia L, Duconge F, Pestourie C, Boulay J, Aissouni Y, Gombert K, et al. Neutralizing aptamers from whole-cell SELEX inhibit the RET receptor tyrosine kinase. PLoS biology. 2005. April;3(4):e123 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010. July;9(7):537–50. 10.1038/nrd3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marton S, Reyes-Darias JA, Sanchez-Luque FJ, Romero-Lopez C, Berzal-Herranz A. In vitro and ex vivo selection procedures for identifying potentially therapeutic DNA and RNA molecules. Molecules (Basel, Switzerland). 2010. July;15(7):4610–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nutiu R, Li Y. Aptamers with fluorescence-signaling properties. Methods (San Diego, Calif. 2005. September;37(1):16–25. [DOI] [PubMed] [Google Scholar]
- 15.Ulrich H, Trujillo CA, Nery AA, Alves JM, Majumder P, Resende RR, et al. DNA and RNA aptamers: from tools for basic research towards therapeutic applications. Combinatorial chemistry & high throughput screening. 2006. September;9(8):619–32. [DOI] [PubMed] [Google Scholar]
- 16.White RR, Sullenger BA, Rusconi CP. Developing aptamers into therapeutics. The Journal of clinical investigation. 2000. October;106(8):929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marton S, Romero-Lopez C, Berzal-Herranz A. RNA aptamer-mediated interference of HCV replication by targeting the CRE-5BSL3.2 domain. Journal of viral hepatitis. 2013. February;20(2):103–12. 10.1111/j.1365-2893.2012.01629.x [DOI] [PubMed] [Google Scholar]
- 18.Sullenger BA, White RR, Rusconi CP. Therapeutic aptamers and antidotes: a novel approach to safer drug design. Ernst Schering Research Foundation workshop. 2003(43):217–23. [DOI] [PubMed] [Google Scholar]
- 19.Thiel KW, Giangrande PH. Therapeutic applications of DNA and RNA aptamers. Oligonucleotides. 2009. September;19(3):209–22. 10.1089/oli.2009.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimbres FM, Tarnok A, Ulrich H, Wrenger C. Aptamers: novel molecules as diagnostic markers in bacterial and viral infections? BioMed research international. 2013;2013:731516 10.1155/2013/731516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng Z, Ling M, Ning Y, Deng L. Rapid fluorescent detection of Escherichia coli K88 based on DNA aptamer library as direct and specific reporter combined with immuno-magnetic separation. Journal of fluorescence. 2014. July;24(4):1159–68. 10.1007/s10895-014-1396-x [DOI] [PubMed] [Google Scholar]
- 22.Savory N, Nzakizwanayo J, Abe K, Yoshida W, Ferri S, Dedi C, et al. Selection of DNA aptamers against uropathogenic Escherichia coli NSM59 by quantitative PCR controlled Cell-SELEX. Journal of microbiological methods. 2014. September;104:94–100. 10.1016/j.mimet.2014.06.016 [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Song MY, Jurng J, Kim BC. Isolation and characterization of DNA aptamers against Escherichia coli using a bacterial cell-systematic evolution of ligands by exponential enrichment approach. Analytical biochemistry. 2013. May 1;436(1):22–8. 10.1016/j.ab.2013.01.014 [DOI] [PubMed] [Google Scholar]
- 24.Duan N, Wu S, Chen X, Huang Y, Xia Y, Ma X, et al. Selection and characterization of aptamers against Salmonella typhimurium using whole-bacterium Systemic Evolution of Ligands by Exponential Enrichment (SELEX). Journal of agricultural and food chemistry. 2013. April 3;61(13):3229–34. 10.1021/jf400767d [DOI] [PubMed] [Google Scholar]
- 25.Dwivedi HP, Smiley RD, Jaykus LA. Selection of DNA aptamers for capture and detection of Salmonella Typhimurium using a whole-cell SELEX approach in conjunction with cell sorting. Applied microbiology and biotechnology. 2013. April;97(8):3677–86. 10.1007/s00253-013-4766-4 [DOI] [PubMed] [Google Scholar]
- 26.Moon J, Kim G, Lee S, Park S. Identification of Salmonella Typhimurium-specific DNA aptamers developed using whole-cell SELEX and FACS analysis. Journal of microbiological methods. 2013. November;95(2):162–6. 10.1016/j.mimet.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 27.Kolovskaya OS, Savitskaya AG, Zamay TN, Reshetneva IT, Zamay GS, Erkaev EN, et al. Development of bacteriostatic DNA aptamers for salmonella. Journal of medicinal chemistry. 2013. February 28;56(4):1564–72. 10.1021/jm301856j [DOI] [PubMed] [Google Scholar]
- 28.Park HC, Baig IA, Lee SC, Moon JY, Yoon MY. Development of ssDNA aptamers for the sensitive detection of Salmonella typhimurium and Salmonella enteritidis. Applied biochemistry and biotechnology. 2014. September;174(2):793–802. 10.1007/s12010-014-1103-z [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Peng Z, Ning Y, Chen Y, Zhou Q, Deng L. Highly specific and cost-efficient detection of Salmonella Paratyphi A combining aptamers with single-walled carbon nanotubes. Sensors (Basel, Switzerland). 2013;13(5):6865–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu ZC, Zhao LJ, Zhang YF, Shi HL, Xie Y. [Screening and characterization of aptamers of Cepsilon3-Cepsilon4 protein]. Yao xue xue bao = Acta pharmaceutica Sinica. 2012. December;47(12):1605–11. [PubMed] [Google Scholar]
- 31.Duan N, Wu S, Chen X, Huang Y, Wang Z. Selection and identification of a DNA aptamer targeted to Vibrio parahemolyticus. Journal of agricultural and food chemistry. 2012. April 25;60(16):4034–8. 10.1021/jf300395z [DOI] [PubMed] [Google Scholar]
- 32.Tang X, Zheng J, Yan Q, Li Z, Li Y. Selection of aptamers against inactive Vibrio alginolyticus and application in a qualitative detection assay. Biotechnology letters. 2013. June;35(6):909–14. 10.1007/s10529-013-1154-1 [DOI] [PubMed] [Google Scholar]
- 33.Suh SH, Dwivedi HP, Choi SJ, Jaykus LA. Selection and characterization of DNA aptamers specific for Listeria species. Analytical biochemistry. 2014. August 15;459:39–45. 10.1016/j.ab.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 34.Suh SH, Jaykus LA. Nucleic acid aptamers for capture and detection of Listeria spp. Journal of biotechnology. 2013. September 20;167(4):454–61. 10.1016/j.jbiotec.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 35.Duan N, Ding X, Wu S, Xia Y, Ma X, Wang Z, et al. In vitro selection of a DNA aptamer targeted against Shigella dysenteriae. Journal of microbiological methods. 2013. September;94(3):170–4. 10.1016/j.mimet.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 36.Savory N, Takahashi Y, Tsukakoshi K, Hasegawa H, Takase M, Abe K, et al. Simultaneous improvement of specificity and affinity of aptamers against Streptococcus mutans by in silico maturation for biosensor development. Biotechnology and bioengineering. 2013. March;111(3):454–61. 10.1002/bit.25111 [DOI] [PubMed] [Google Scholar]
- 37.Hamula CL, Le XC, Li XF. DNA aptamers binding to multiple prevalent M-types of Streptococcus pyogenes. Analytical chemistry. 2011. May 15;83(10):3640–7. 10.1021/ac200575e [DOI] [PubMed] [Google Scholar]
- 38.Cao X, Li S, Chen L, Ding H, Xu H, Huang Y, et al. Combining use of a panel of ssDNA aptamers in the detection of Staphylococcus aureus. Nucleic acids research. 2009. August;37(14):4621–8. 10.1093/nar/gkp489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savory N, Lednor D, Tsukakoshi K, Abe K, Yoshida W, Ferri S, et al. In silico maturation of binding-specificity of DNA aptamers against Proteus mirabilis. Biotechnology and bioengineering. 2013. October;110(10):2573–80. 10.1002/bit.24922 [DOI] [PubMed] [Google Scholar]
- 40.Wang KY, Zeng YL, Yang XY, Li WB, Lan XP. Utility of aptamer-fluorescence in situ hybridization for rapid detection of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 2011. February;30(2):273–8. 10.1007/s10096-010-1074-0 [DOI] [PubMed] [Google Scholar]
- 41.Chen F, Zhou J, Luo F, Mohammed AB, Zhang XL. Aptamer from whole-bacterium SELEX as new therapeutic reagent against virulent Mycobacterium tuberculosis. Biochemical and biophysical research communications. 2007. June 8;357(3):743–8. [DOI] [PubMed] [Google Scholar]
- 42.Vivekananda J, Kiel JL. Anti-Francisella tularensis DNA aptamers detect tularemia antigen from different subspecies by Aptamer-Linked Immobilized Sorbent Assay. Laboratory investigation; a journal of technical methods and pathology. 2006. June;86(6):610–8. [DOI] [PubMed] [Google Scholar]
- 43.Dwivedi HP, Smiley RD, Jaykus LA. Selection and characterization of DNA aptamers with binding selectivity to Campylobacter jejuni using whole-cell SELEX. Applied microbiology and biotechnology. 2010. August;87(6):2323–34. 10.1007/s00253-010-2728-7 [DOI] [PubMed] [Google Scholar]
- 44.Ferreira IM, de Souza Lacerda CM, de Faria LS, Correa CR, de Andrade AS. Selection of peptidoglycan-specific aptamers for bacterial cells identification. Applied biochemistry and biotechnology. 2014. December;174(7):2548–56. 10.1007/s12010-014-1206-6 [DOI] [PubMed] [Google Scholar]
- 45.Kim SE, Su W, Cho M, Lee Y, Choe WS. Harnessing aptamers for electrochemical detection of endotoxin. Analytical biochemistry. 2012. May 1;424(1):12–20. 10.1016/j.ab.2012.02.016 [DOI] [PubMed] [Google Scholar]
- 46.Li H, Ding X, Peng Z, Deng L, Wang D, Chen H, et al. Aptamer selection for the detection of Escherichia coli K88. Canadian journal of microbiology. 2011. June;57(6):453–9. 10.1139/w11-030 [DOI] [PubMed] [Google Scholar]
- 47.Bruno JG, Carrillo MP, Phillips T. In vitro antibacterial effects of antilipopolysaccharide DNA aptamer-C1qrs complexes. Folia microbiologica. 2008;53(4):295–302. 10.1007/s12223-008-0046-6 [DOI] [PubMed] [Google Scholar]
- 48.Hong KL, Sooter LJ. Single-Stranded DNA Aptamers against Pathogens and Toxins: Identification and Biosensing Applications. BioMed research international. 2015;2015:419318 10.1155/2015/419318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang Z, Wu W, Lu X, Zeng L. Lateral flow biosensor for DNA extraction-free detection of Salmonella based on aptamer mediated strand displacement amplification. Biosensors & bioelectronics. 2014. June 15;56:192–7. [DOI] [PubMed] [Google Scholar]
- 50.Wu S, Duan N, Zhang H, Wang Z. Simultaneous detection of microcysin-LR and okadaic acid using a dual fluorescence resonance energy transfer aptasensor. Analytical and bioanalytical chemistry. 2014. February;407(5):1303–12. 10.1007/s00216-014-8378-3 [DOI] [PubMed] [Google Scholar]
- 51.Wu WH, Li M, Wang Y, Ouyang HX, Wang L, Li CX, et al. Aptasensors for rapid detection of Escherichia coli O157:H7 and Salmonella typhimurium. Nanoscale research letters. 2012;7(1):658 10.1186/1556-276X-7-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duan N, Wu S, Yu Y, Ma X, Xia Y, Chen X, et al. A dual-color flow cytometry protocol for the simultaneous detection of Vibrio parahaemolyticus and Salmonella typhimurium using aptamer conjugated quantum dots as labels. Analytica chimica acta. 2013. December 4;804:151–8. 10.1016/j.aca.2013.09.047 [DOI] [PubMed] [Google Scholar]
- 53.Duan N, Wu S, Zhu C, Ma X, Wang Z, Yu Y, et al. Dual-color upconversion fluorescence and aptamer-functionalized magnetic nanoparticles-based bioassay for the simultaneous detection of Salmonella Typhimurium and Staphylococcus aureus. Analytica chimica acta. 2012. April 20;723:1–6. 10.1016/j.aca.2012.02.011 [DOI] [PubMed] [Google Scholar]
- 54.Labib M, Zamay AS, Kolovskaya OS, Reshetneva IT, Zamay GS, Kibbee RJ, et al. Aptamer-based impedimetric sensor for bacterial typing. Analytical chemistry. 2012. October 2;84(19):8114–7. 10.1021/ac302217u [DOI] [PubMed] [Google Scholar]
- 55.Ma X, Jiang Y, Jia F, Yu Y, Chen J, Wang Z. An aptamer-based electrochemical biosensor for the detection of Salmonella. Journal of microbiological methods. 2014. March;98:94–8. 10.1016/j.mimet.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 56.Wu W, Li J, Pan D, Li J, Song S, Rong M, et al. Gold nanoparticle-based enzyme-linked antibody-aptamer sandwich assay for detection of Salmonella Typhimurium. ACS applied materials & interfaces. 2014. October 8;6(19):16974–81. [DOI] [PubMed] [Google Scholar]
- 57.Hernandez R, Valles C, Benito AM, Maser WK, Rius FX, Riu J. Graphene-based potentiometric biosensor for the immediate detection of living bacteria. Biosensors & bioelectronics. 2014. April 15;54:553–7. [DOI] [PubMed] [Google Scholar]
- 58.Yuan J, Wu S, Duan N, Ma X, Xia Y, Chen J, et al. A sensitive gold nanoparticle-based colorimetric aptasensor for Staphylococcus aureus. Talanta. 2014. September;127:163–8. 10.1016/j.talanta.2014.04.013 [DOI] [PubMed] [Google Scholar]
- 59.Zelada-Guillen GA, Sebastian-Avila JL, Blondeau P, Riu J, Rius FX. Label-free detection of Staphylococcus aureus in skin using real-time potentiometric biosensors based on carbon nanotubes and aptamers. Biosensors & bioelectronics. 2012. January 15;31(1):226–32. [DOI] [PubMed] [Google Scholar]
- 60.Kim YS, Chung J, Song MY, Jurng J, Kim BC. Aptamer cocktails: enhancement of sensing signals compared to single use of aptamers for detection of bacteria. Biosensors & bioelectronics. 2014. April 15;54:195–8. [DOI] [PubMed] [Google Scholar]
- 61.Zelada-Guillen GA, Bhosale SV, Riu J, Rius FX. Real-time potentiometric detection of bacteria in complex samples. Analytical chemistry. 2010. November 15;82(22):9254–60. 10.1021/ac101739b [DOI] [PubMed] [Google Scholar]
- 62.Nagarkatti R, Bist V, Sun S, Fortes de Araujo F, Nakhasi HL, Debrabant A. Development of an aptamer-based concentration method for the detection of Trypanosoma cruzi in blood. PloS one. 2012;7(8):e43533 10.1371/journal.pone.0043533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic acids research. 2003. July 1;31(13):3406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee YJ, Han SR, Maeng JS, Cho YJ, Lee SW. In vitro selection of Escherichia coli O157:H7-specific RNA aptamer. Biochemical and biophysical research communications. 2011. January 6;417(1):414–20. 10.1016/j.bbrc.2011.11.130 [DOI] [PubMed] [Google Scholar]
- 65.Sefah K, Shangguan D, Xiong X, O'Donoghue MB, Tan W. Development of DNA aptamers using Cell-SELEX. Nature protocols. 2010. June;5(6):1169–85. 10.1038/nprot.2010.66 [DOI] [PubMed] [Google Scholar]
- 66.Park JP, Shin HJ, Park SG, Oh HK, Choi CH, Park HJ, et al. Screening and Development of DNA Aptamers Specific to Several Oral Pathogens. Journal of microbiology and biotechnology. 2014. October 1. [DOI] [PubMed] [Google Scholar]
- 67.Bronner S, Monteil H, Prevost G. Regulation of virulence determinants in Staphylococcus aureus: complexity and applications. FEMS microbiology reviews. 2004. May;28(2):183–200. [DOI] [PubMed] [Google Scholar]
- 68.Mayer G, Ahmed MS, Dolf A, Endl E, Knolle PA, Famulok M. Fluorescence-activated cell sorting for aptamer SELEX with cell mixtures. Nature protocols. 2010. December;5(12):1993–2004. 10.1038/nprot.2010.163 [DOI] [PubMed] [Google Scholar]
- 69.Dua P, Kang HS, Hong SM, Tsao MS, Kim S, Lee DK. Alkaline phosphatase ALPPL-2 is a novel pancreatic carcinoma-associated protein. Cancer research. 2013. March 15;73(6):1934–45. 10.1158/0008-5472.CAN-12-3682 [DOI] [PubMed] [Google Scholar]
- 70.Mallikaratchy P, Tang Z, Kwame S, Meng L, Shangguan D, Tan W. Aptamer directly evolved from live cells recognizes membrane bound immunoglobin heavy mu chain in Burkitt's lymphoma cells. Mol Cell Proteomics. 2007. December;6(12):2230–8. [DOI] [PubMed] [Google Scholar]
- 71.Ray P, Rialon-Guevara KL, Veras E, Sullenger BA, White RR. Comparing human pancreatic cell secretomes by in vitro aptamer selection identifies cyclophilin B as a candidate pancreatic cancer biomarker. The Journal of clinical investigation. 2012. May;122(5):1734–41. 10.1172/JCI62385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shangguan D, Cao Z, Meng L, Mallikaratchy P, Sefah K, Wang H, et al. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. Journal of proteome research. 2008. May;7(5):2133–9. 10.1021/pr700894d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shamah SM, Healy JM, Cload ST. Complex target SELEX. Accounts of chemical research. 2008. January;41(1):130–8. 10.1021/ar700142z [DOI] [PubMed] [Google Scholar]
- 74.Bruno JG, Carrillo MP, Phillips T, Andrews CJ. A novel screening method for competitive FRET-aptamers applied to E. coli assay development. Journal of fluorescence. 2010. November;20(6):1211–23. 10.1007/s10895-010-0670-9 [DOI] [PubMed] [Google Scholar]
- 75.So HM, Park DW, Jeon EK, Kim YH, Kim BS, Lee CK, et al. Detection and titer estimation of Escherichia coli using aptamer-functionalized single-walled carbon-nanotube field-effect transistors. Small (Weinheim an der Bergstrasse, Germany). 2008. February;4(2):197–201. [DOI] [PubMed] [Google Scholar]
- 76.Homann M, Goringer HU. Combinatorial selection of high affinity RNA ligands to live African trypanosomes. Nucleic acids research. 1999. May 1;27(9):2006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morris KN, Jensen KB, Julin CM, Weil M, Gold L. High affinity ligands from in vitro selection: complex targets. Proceedings of the National Academy of Sciences of the United States of America. 1998. March 17;95(6):2902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PCR results of conventional PCR. Neg negative control from conventional PCR experiment. aPCR, results from asymmetric PCR (aPCR) performed in the conditions used during SELEX. The position of double stranded (88 bp) or singles stranded (88nt) product is indicated on the left.
(PDF)
The differences between P12-31 binding to E coli and to all other bacterial species were statistically significant (** p<0.005).
(PDF)
Conserved stem loops are highlighted in bold.
(PDF)
Conserved stem loops are highlighted in bold.
(PDF)
*Correspond to the library ssDNA population. ** Washes were done with selection buffer (PBS containing 1.4 mM MgCl2).
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.