Abstract
Alzheimer's disease (AD), the most common form of dementia among the elderly, manifests mostly late in adult life. However, it is presently unclear when the disease process starts and how long the pathobiochemical processes take to develop. Our goal is to address the timing and nature of triggers that lead to AD. To explain the etiology of AD, we have recently proposed a “Latent Early Associated Regulation” (LEARn) model, which postulates a latent expression of specific genes triggered at the developmental stage. This model integrates both the neuropathological features (e.g., amyloid-loaded plaques and tau-laden tangles) and environmental factors (e.g., diet, metal exposure, and hormones) associated with the disease. Environmental agents perturb gene regulation in a long-term fashion, beginning at early developmental stages, but these perturbations do not have pathological results until significantly later in life. The LEARn model operates through the regulatory region (promoter) of the gene and by affecting the methylation status within the promoter of specific genes.
Keywords: Latency, gene regulation, methylation, epigenetic, amyloid, lead
Neurochemical characteristics of Alzheimer's disease
The major symptoms of Alzheimer's disease (AD) include severe loss of memory, cognition, reasoning, and other intellectual abilities, and it is the most prominent form of dementia among the elderly in Western countries (Hebert et al. 2003). At the neuropathological level, the disease is characterized by excessive deposition of two major proteinaceous aggregates. They are hyperphosphorylated microtubule associated protein τ “tangles” and senile plaques formed mostly of the amyloid-β peptide (Aβ), which is derived from the β-amyloid precursor protein (APP). These two protein aggregates are the basis for the two currently-dominant models in the field, the “amyloid hypothesis” and the “τ hypothesis” (Sambamurti et al. 2006). According to the “amyloid model”, the neurotoxicity of Aβ dimers and oligomers and/or damage caused by Aβ plaque aggregation as the primary cause of AD. The “τ model” suggest that aggregation of hyperphosphorylated τ leads to neuronal cell death and resulting neuropathology. Aβ aggregation would be a result of cellular damage imposed by τ aggregation.
Risk factors and Genetics of AD
Only a minority of AD is attributable to autosomal familial AD (FAD) mutations in the coding sequences of AD-associated genes, such as APP and presenilin 1 (PSEN1), and this form of AD does not explain the more common sporadic late-onset AD (LOAD). The known risk factors for sporadic LOAD include age, limited education, head trauma, dietary cholesterol, the APOEε4 genotype, and further associations with additional proteins such as the insulin degrading enzyme (IDE), α2-macroglobulin and endothelin converting enzyme 2 (ECE2) (Lahiri et al. 2003). In addition, AD risk has been associated with promoter polymorphisms in the APOE and APP genes (Lahiri et al. 2005; Maloney et al. 2007). Oxidative stress and metals in the brain and inflammatory factors are likewise linked to AD (Bellingham et al. 2004). Nevertheless, none of the current etiological models have proven sufficient to explain the sporadic nature of AD and the “incomplete” effects of known risk factors.
Major questions in AD can be addressed by the LEARn model
AD manifests late in adult life, however, it is not clearly understood when the disease actually starts and how long the biochemical events and neuropathological processes take to develop the disease. Is AD an early/developmental or late phenomenon? What are the factors that trigger the cascade of pathobiochemical processes of the disease? Thus, the major unresolved questions relate to the timing and nature of triggering leading to AD. Any unifying hypothesis to explain the etiology of AD must take into account not only neuropathological features but also the various environmental factors associated with AD, such as dietary imbalance, toxicological exposure and hormonal factors. In order to address the interaction of environment and genes, we have recently proposed a “Latent Early-life Associated Regulation” (LEARn) model, which may explain the etiology of AD, and possibly other neuropsychiatric and developmental disorders (Lahiri et al. 2007).
The LEARn model states that environmental agents (e.g., heavy metals), intrinsic factors (e.g., inflammatory cytokines), and dietary factors (e.g., folate and cholesterol) could alter gene regulation in a long-term fashion, beginning at early developmental stages, but that these perturbations do not have pathological results until significantly later in life (Basha et al. 2005; Lahiri et al. 2007), such as development of AD-like pathology in aged monkeys after infantile exposure to environmental Pb (Wu et al. 2008). It should be mentioned that similar hypotheses were developed in the 1980s by Barker and colleagues (Barker et al. 2002). However, Barker's model is predicated upon low birth weight and rapid childhood weight gain. The LEARn model is based on the regulatory structure common to eukaryotic genes and the effect of methylation at certain specific sites within the promoter (regulatory) region of specific genes. Overall, the LEARn model based interaction is consistent with the classic “Barker model” of fetal origins of adult disease.
Biochemical basis of the LEARn model
The LEARn model presumes that the foundation of latent early regulation is due to epigenetic modification. It combines the already-present variation of the genetic substrate with long-term response to differences in environmental stresses. Human DNA is most commonly modified by DNA methylation, which involves the addition of a methyl group to cytosine residues at CpG dinucleotides. This reaction is catalyzed by DNA methyltransferase (DNMT) enzymes. In the DNA sequence, CpG dinucleotides are found in clusters called CpG islands. In normal tissues, CpG islands are primarily unmethylated, and the aberrant methylation of CpG islands is most likely related to disease. The basis of the LEARn model is on changing the methylation status of a gene as hypomethylation in the promoter region leads to increased gene expression, whereas hypermethylation results in decreased gene expression. For example, environmental factors, including metals and dietary factors, may operate by interfering with the interaction of methylated CpG clusters with binding proteins, such as MeCP2 and SP1. Thus, the LEARn model uses a “promoter methylation” mechanism as a switch to control gene expression.
AD and the LEARn model
The LEARn model explains developmental triggering and latent expression of the APP gene. The APP protein and Aβ peptide appear in healthy individuals. Studies from knockout animals have indicated that APP has necessary functions, although there is redundancy with other APP protein family members. Therefore, what would trigger APP and Aβ peptides to be overproduced in sporadic cases of AD? The LEARn model proposes that the initial APP triggering mechanism activates early in life, at developmental stages. Sites of action would be within the promoter of APP and associated genes. The trigger is primarily a property of genetic regulation. It is maintained through epigenetic means, such as DNA methylation.
Other Implications of the LEARn model
The LEARn model is based on environmentally-induced hypomethylation or oxidative damage as the physical mechanism that perturbs gene expression (Bolin et al. 2006) (Fig. 1). These perturbations are latent. They are not immediately apparent in the same manner found in conventional toxic responses. Thus, apparent reversal of the symptoms of acute exposure to environmental stressors such as Pb or poor nutrition need not mean that there will be no long-lasting repercussions of an environmental insult. This indicates that conventional anti-toxicity treatments may be insufficient, the relatively long term of latency suggests a possibility for biologically-based medical remediation. For examples, bans enacted upon lead (Pb) in gasoline in previous decades would significantly reduce levels of Pb in urban dwellers. However, the LEARn model would suggest that levels of AD would not be likely to decrease in response until 50-60 years after the bans were enacted, when individuals would begin to reach ages at risk of LOAD but would not have suffered higher levels of childhood Pb exposure.
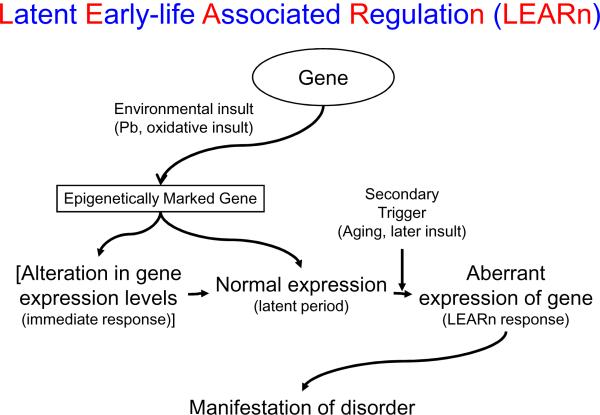
Fig. 1.
The LEARn Model. The LEARn model proposes a mechanism for interaction between genes and environment in early developmental etiology for AD. Promoter regions of genes, such as SP1, APP, and possibly BACE, provide the substrate for LEARn activity. Environmental factors such as exposure to Pb, nutritional deficiencies (e.g., folate or B12), or oxidative stress alter DNA epigenetically, by reducing the activity of enzymes such as DnMT, which would result in hypomethylation and reprogram gene expression. In some cases, this results in immediate alteration in gene expression levels. In LEARn systems, alterations are latent and effects are primarily notable later in life. In both immediate and LEARn systems, epigenetic changes in gene regulation result in disorder.
On the other hand, the proposed mechanisms of LEARn, epigenetic hypomethylation and/or oxidative damage to DNA, do lend towards potential solutions to a LEARn-type environmental exposure. For example, fruit juices, such as concentrated apple juice, have been shown to reverse acute oxidative damage and be a useful source of s-adenylmethionine, reversing hypomethylation in mice (Chan and Shea 2006). Likewise, dietary melatonin supplementation reduced levels of Aβ in mouse cerebral cortex (Lahiri et al. 2004). This suggests investigation of the use of appropriate fruit and vegetable dietary supplementation early in life, as a prophylactic or treatment measure against possible latent response to environmental insult. Thus, the LEARn model presented here has far-reaching implications in personal health practices and public policy.
Acknowledgments
This work was supported in part by grants from Alzheimer's Associations (Zenith award) and the National Institutes of Health (AG18379 and AG18884).
References
- Barker DJ, Eriksson JG, Forsen T, et al. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Basha MR, Wei W, Bakheet SA, et al. The fetal basis of amyloidogenesis: exposure to lead and latent overexpression of amyloid precursor protein and beta-amyloid in the aging brain. J Neurosci. 2005;25:823–829. doi: 10.1523/JNEUROSCI.4335-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham SA, Lahiri DK, Maloney B, et al. Copper depletion down-regulates expression of the Alzheimer's disease amyloid-beta precursor protein gene. J Biol Chem. 2004;279:20378–20386. doi: 10.1074/jbc.M400805200. [DOI] [PubMed] [Google Scholar]
- Bolin CM, Basha R, Cox D, et al. Exposure to lead and the developmental origin of oxidative DNA damage in the aging brain. Faseb J. 2006;20:788–790. doi: 10.1096/fj.05-5091fje. [DOI] [PubMed] [Google Scholar]
- Chan A, Shea TB. Supplementation with apple juice attenuates presenilin-1 overexpression during dietary and genetically-induced oxidative stress. J Alzheimers Dis. 2006;10:353–358. doi: 10.3233/jad-2006-10401. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, et al. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Chen D, Ge YW, et al. Dietary supplementation with melatonin reduces levels of amyloid beta-peptides in the murine cerebral cortex. J Pineal Res. 2004;36:224–231. doi: 10.1111/j.1600-079X.2004.00121.x. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Farlow MR, Sambamurti K, et al. A critical analysis of new molecular targets and strategies for drug developments in Alzheimer's disease. Curr Drug Targets. 2003;4:97–112. doi: 10.2174/1389450033346957. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Maloney B, Basha MR, et al. How and when environmental agents and dietary factors affect the course of Alzheimer's disease: the “LEARn” model (Latent Early Associated Regulation) may explain the triggering of AD. Curr Alzheimer Res. 2007;4:219–228. doi: 10.2174/156720507780362164. [DOI] [PubMed] [Google Scholar]
- Lahiri DK, Wavrant De-Vrieze F, Ge Y-W, et al. Characterization of two APP gene promoter polymorphisms that appear to influence risk of late-onset Alzheimer's disease. Neurobiol Aging. 2005;26:1329–1341. doi: 10.1016/j.neurobiolaging.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Maloney B, Ge Y-W, Alley GM, et al. Important Differences between human and mouse APOE gene promoters with implications for Alzheimer's disease. J Neurochem. 2007;103:1237–1257. doi: 10.1111/j.1471-4159.2007.04831.x. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Suram A, Venugopal C, et al. A partial failure of membrane protein turnover may cause Alzheimer's disease: a new hypothesis. Curr Alzheimer Res. 2006;3:81–90. doi: 10.2174/156720506775697142. [DOI] [PubMed] [Google Scholar]
- Wu J, Basha MR, Brock B, et al. Alzheimer's disease (AD)-like pathology in aged monkeys after infantile exposure to environmental metal lead (Pb): evidence for a developmental origin and environmental link for AD. J Neurosci. 2008;28:3–9. doi: 10.1523/JNEUROSCI.4405-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]